Abstract
Protein phosphatase 2A is a serine/threonine phosphatase involved in the regulation of many cellular processes. A confirmed tumor suppressor protein, PP2A is genetically altered or functionally inactivated in many cancers highlighting a need for its therapeutic reactivation. In this review we will discuss recent literature on PP2A: the elucidation of its structure and the functions of its subunits, and the identification of molecular lesions and post-translational modifications leading to its dysregulation in cancer. A final section will discuss the proteins and small molecules that modulate PP2A and how these might be used to target dysregulated forms of PP2A to treat cancers and other diseases.
Introduction
Complex processes in cell signaling require a set of molecular tools to modulate the activity and localization of specific proteins. Many of these responsibilities are regulated by kinases and phosphatases, whose opposite actions reversibly phosphorylate proteins. Due to their involvement in the progression of many cancers, the study of kinases has become an important field and one that has drawn considerable attention from the pharmaceutical industry over the past decade and a half. Despite their abundance and variety of substrates, the 518 human kinases display a high degree of similarity, and most features of their structure are conserved. In contrast, phosphatases exhibit considerably more structural variety with only a few enzymes performing the majority of the work. In the past, phosphatases were considered to play a more passive, housekeeping role and received less attention. Recent studies solidify their importance but reveal the inherent difficulties in designing tools to improve their function.
There are multiple families of phosphatases with diverse active sites and mechanisms. The major classes of phosphatases are protein tyrosine phosphatases (PTPs) and protein serine/threonine phosphatases (PSPs). The protein serine/threonine phosphatases consist of three families: phospho-protein phosphatases (PPPs), metal-dependent protein phosphatases (PPM), and DxDxT phosphatases [1, 2]. PPP, the phosphoprotein phosphatase family, is the largest containing several members including PP1, PP2A, PP2B, and PP4.
Structure of the PP2A holoenzyme
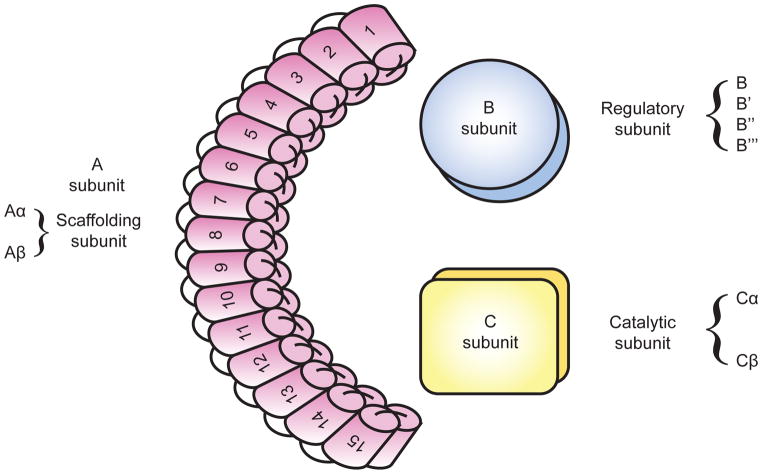
PP2A is a heterotrimeric complex. It consists of a scaffolding subunit (A), a regulatory subunit (B), and a catalytic subunit (C) (Fig. 1). The A and C subunits each exist with two possible variants α and β, with Aα and Cα accounting for the majority of each subunit in most cells [3–5]. The four classes of the B subunit are: B (B55/PR55), B′ (B56/PR61), B″(PR48/PR72/PR130), and B‴(PR93/PR110)/Striatin. Each class contains 2–5 isoforms and additional splice variants. This predicts over 80 distinct combinations of the PP2A holoenzyme. This multitude of forms regulates PP2A’s activity and cellular localization and imparts specificity towards different substrates.
Figure 1.
PP2A is a heterotrimeric complex consisting of a scaffolding subunit (A), a regulatory subunit (B), and a catalytic subunit (C). PP2A A subunit is composed of 15 tandem HEAT repeats in two isoforms α and β. PP2A C subunit also exists in two possible isoforms α and β. PP2A B subunit consists of four classes: B (B55/PR55), B′ (B56/PR61), B″(PR48/PR72/PR130) and B‴(PR93/PR110).
Structural contributions to PP2A activity
The PP2A A subunit is composed of 15 tandem HEAT repeats (Fig. 1). HEAT repeats are named for the set of four cytoplasmic proteins first recognized to contain them (Huntingtin, EF3, PP2A A subunit, and TOR1). Repeats contain approximately 40 amino acid residues organized into two anti-parallel α-helices. The helices are hydrophobic in nature enforcing their mutual attraction. In PP2A, the combination of these repeats forms a characteristic crescent structure [6]. The C subunit binds to HEAT repeats 11–15 of the A subunit [7]. The C subunit embodies a globular structure with an α/β fold. This structure is homologous to other PPP catalytic subunits, however, the subunits are not interchangeable among the different enzyme types [8]. The molecular basis for these interactions was described when the AC dimer, also known as the “Core Enzyme” crystal structure was solved [9]. The C subunit active site contains 2 manganese atoms that bind to phosphate and facilitate the hydrolysis of serine/threonine phosphate esters. The active site is positioned away from the ridge of the A subunit HEAT repeats and proximal to the site where the B subunits bind.
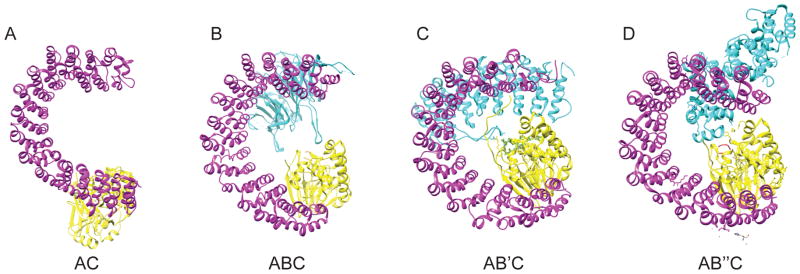
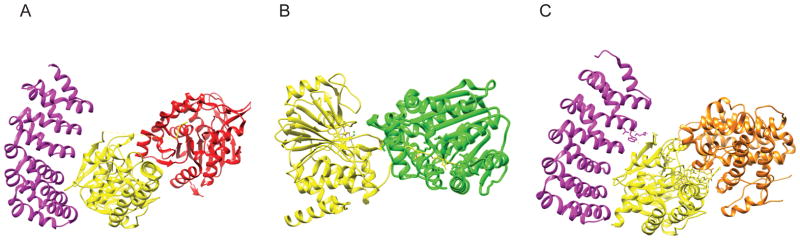
The core enzyme interacts differently with each class of B subunit (Fig. 2A). Crystal structures have been solved for the B, B′, B″ family of subunits while less is known about the B‴ family. The B/PR55 family contacts the scaffolding subunit via two extended interfaces (Fig. 2B). The first is a seven bladed propeller, each composed of a WD40 repeat. The second is a β-hairpin handle with additional secondary structures. The bottom face of the propeller binds to the HEAT Domains 3–7 of the A subunit, and the β-hairpin handle interacts with HEAT repeats 1 and 2. The top face of the propeller is the proposed substrate binding site. While proximal to it, the B/PR55 subunit makes very few contacts with the C subunit [10–12]. The structure of the B′/PR61 family of subunits is strikingly similar to the A subunit, containing 8 HEAT-like repeats (Fig. 2C). These interact with the A subunit at HEAT repeats 2–8 and also interact with the C subunit. The B/PR55 and B′/PR61 subunits bind similarly to the core enzyme such that the substrate binding site lies on the top face of the B subunit proximal to C subunit active site. The proximity of these B subunits to the active site explains their role in conferring specificity for substrate proteins [9, 13].
Figure 2.
Structures of PP2A core enzyme and holoenzyme. (A) (PDB code: 2IE3) Core Enzyme consisting of Aα (in magenta) subunit and Cα (in yellow) subunit. The C subunit binds A at Heat Repeats 11–15. The active site of the C subunit consists of 2 manganese atoms and is positioned away from the ridge of the A subunit HEAT Repeats. Binding with the catalytic subunit shifts HEAT repeats 13–15 by 20–30 Å (B) (PDB code: 3DW8) Core Enzyme binding to B family subunit (in cyan), Bα/PR55α. Members of this subunit family bind the A subunit at two interfaces. The first is via a seven bladed propeller, composed of WD40 repeats. The bottom face of the propeller binds to A subunit HEAT domains 3–7. The second is through a β-hairpin handle that interacts with A subunit HEAT repeats 1 and 2. Upon binding to the holoenzyme, the B subunit substrate binding site lies on the top face proximal to the active site of the catalytic subunit. (C) (PDB code: 2IAE) Core enzyme binding to B′ family subunit (in cyan), Bγ1/PR61γ1. The B′ structures are similar to the A subunit, composed of 8 HEAT-like repeats. These interact with HEAT repeats 2–8 of the A subunit, and with the C subunit. Much like binding to B family subunits, the substrate binding site of B′ is proximal to the active site of the catalytic subunit upon holoenzyme formation. Binding to the B′ subunits forces the N-terminal repeat of the A subunit to twist 50–60 Å, rearranging the hydrophobic core of the scaffolding subunit. (D) (PDB code: 4I5L) Core Enzyme binding to B″ family subunit PR72 (in cyan). B″ subunits consist of a linear arrangement of different functional motifs that include an N-terminal hydrophobic motif and 2 EF hand calcium binding motifs. The N-terminal hydrophobic motif and one EF hand bind to the A subunit at HEAT repeats 1–7 and binds to the catalytic subunit via a helix on the subunit at residues 439–446 near the active site, positioning the substrate binding site near the active site. The resulting conformation of this holoenzyme is wider and taller than that which forms with B or B′ subunits.
The structures of the holoenzyme with B″ subunits (PR70/PR72) were solved recently (Fig. 2D). The B″ family consists of linear arrangements of different functional motifs with a substrate binding region near the C-terminus. This arrangement differs from the B and B′ whose substrate binding sites are found on their top surfaces and involve their structural repeats. The B″ family also includes an N-terminal hydrophobic motif with two EF hand calcium-binding motifs that bind to the A subunit at HEAT repeats 1–7. The subunit also contacts the C subunit near the active site via a helix (439–446) positioning the substrate binding site next to the active site [14].
The A subunit orchestrates formation of the active holoenzyme through its conformational flexibility. Binding with the C subunit shifts HEAT repeats 13–15 by 20–30 Å, while binding with the B′ subunit forces an N-terminal repeat to twist up to 50–60 Å and rearranges the hydrophobic core within A [9, 13]. For the B″ family, the A subunit adopts a compact conformation relative to other holoenzymes, reducing its width and increasing its height. An additional helix domain of PR70 extends beyond the A subunit making it wider than the other holoenzymes [14].
Subunit stoichiometry and activity
PP2A constitutes approximately 1% of total cellular protein. The nature of its production, assembly, and subunit stoichiometry is still unclear. To address these questions, studies were conducted in yeast, which contain a smaller repertoire of subunit variants. The ratio of subunits (A:B:C) in yeast was originally determined to be 1:4:8, suggesting that production of A is a limiting factor [15, 16]. However, another study looking at global protein expression suggested the ratio was quite different; the ratio of subunits A:B:C instead being 17:9:10 [16, 17]. In mammalian cells, the A subunit is expressed in excess of the other subunits [18]. There is consensus that the monomeric C subunit is unstable and requires binding to the A subunit or other non-canonical B subunits to preserve its activity [19–21]. Moreover, binding to the A subunit may enhance the stability of the B subunits and fulfill other housekeeping roles [21–23].
Dysregulation of PP2A in diseases
PP2A related pathways are perturbed in many diseases. In both cancer and neurodegeneration, the common pathological mechanism involves activated kinase signaling pathways combined with loss of PP2A activity. In the case of neurodegeneration, PP2A dysfunction leads to increases in hyperphosphorylated tau. Tau protein normally stabilizes microtubules. Hyperphosphorylated tau is thought to play an important role in the etiology of Alzheimer’s disease by forming neurofibrillary tangles. The PP2A Bα isoform is the primary tau binding phosphatase. PP2A mediates ~71% of total tau phosphatase activity in the human brain [24] and dephosphorylates abnormally phosphorylated tau at Ser46, Ser199, Ser202, Ser396, and Ser404 [25]. Alterations in PP2A regulating proteins, its catalytic activity, subunit expression, methylation and phosphorylation patterns have been reported in Alzheimer’s disease-affected brain regions. For example, reduced PP2A mRNA [26], protein levels [27], and phosphatase activity [25, 28] were observed in the brains of Alzheimer’s patients. Increased phosphorylation of PP2A at Tyr307 has been found in phospho-tau-rich, tangle-bearing neurons [29]. Attempts to reduce this phosphorylation through kinase inhibition generated limited success clinically. Consequently, there is growing interest in developing PP2A-targeted therapies for Alzheimer’s disease. These include disruption of inhibitory protein-protein interactions, modulation of disease relevant post-translational modifications, and allosteric activation [30, 31].
In contrast, PP2A performs the opposite role in diabetes. Where these pathways are functioning normally, glucose homeostasis requires the insulin-mediated activation of PI3K-AKT and downregulation of PP2A [32]. This leads to stimulation of the GLUT4 transporter resulting in uptake of glucose into skeletal muscle tissues. This decreased PP2A activity is absent in skeletal muscle tissues of individuals with type II diabetes and leads to impaired insulin sensitivity [33]. A plant derived natural product, carnosic acid, also discussed below, stimulates proper glucose metabolism by activation of AKT via C subunit demethylation [34]. Therefore, PP2A deactivation might be a useful target for ameliorating dysregulated glucose metabolism that is characteristic of type II diabetes.
There are additional reports that implicate a positive role for PP2A in inflammatory lung diseases like asthma and COPD [35] and in heart function [36]. These effects are attributed to PP2A’s inhibitory effects on the mediators of inflammation, and on a variety of substrates involved in cardiac muscle contraction, respectively. Given that cancer, Alzheimer’s disease, diabetes, asthma, and COPD claim millions of lives each year and cost billions to treat in the United States alone, developing drugs that modulate relevant targets such as PP2A in these indications is an attractive option.
Mechanisms of PP2A inactivation in cancer
PP2A’s essential role in homeostasis can be inferred from the increasing number of disease states in which it is functionally inactivated. Numerous studies highlight the role of PP2A as a tumor suppressor and suggest that disruption of the PP2A holoenzyme may contribute to the development of cancer. In cancer, PP2A is inactivated through several mechanisms including: somatic mutation, phosphorylation and/or methylation of the C terminal tail of the catalytic subunit (Fig. 3) and through increased expression of endogenous PP2A inhibitors (Fig. 6) [37]. Several of the genetic alterations prevent the A subunit from binding to the B and/or C subunits, resulting in disruption of the core enzyme and complex [5, 38–40]. Understanding these defects will enhance the future development of PP2A targeted therapeutics by facilitating selection of the correct patient cohorts and suggesting effective combination therapies.
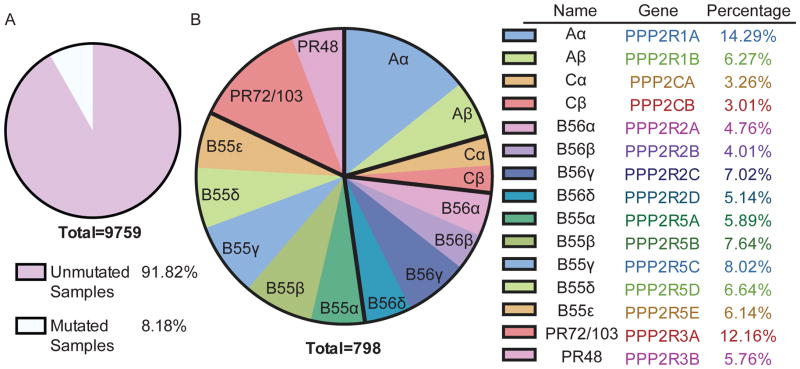
Figure 3.
(A) Pie chart of the frequency of PP2A mutations across 9,759 tumor samples. Mutational information was analyzed from Cbioportal.org, which includes 85 different sequencing studies, including TCGA data. Studies with targeted sequencing or expression only data were excluded from the total number. (B) Pie chart of the frequency of PP2A mutations divided by PP2A subunit families: A, B, B′, B″, and C. Bold black lines divide each subunit. Mutational information was analyzed from Cbioportal.org, which includes 85 different sequencing studies, including TCGA data. Studies with targeted sequencing or expression only data were excluded from the total number. The results shown here are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
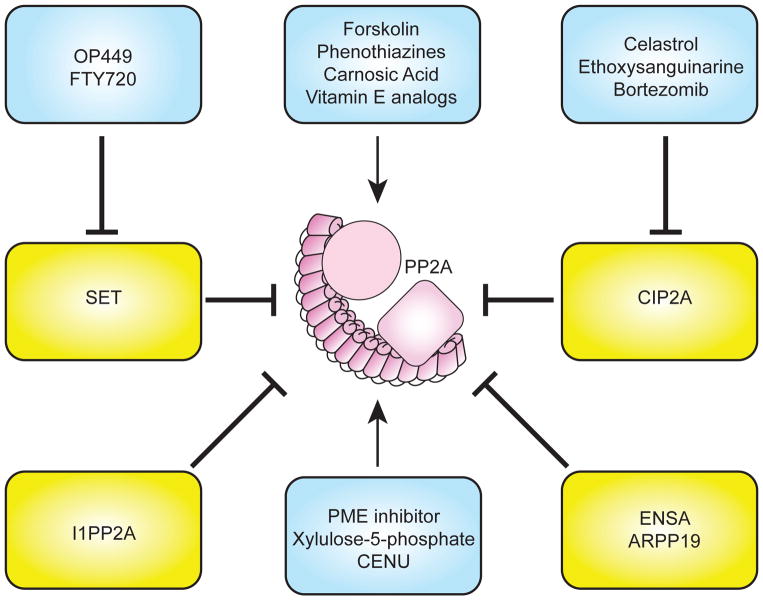
Figure 6.
Activators of PP2A. Several strategies to activate PP2A include: decreasing PP2A Y307 phosphorylation, inhibiting endogenous inhibitors (SET and CIP2A), inhibiting PME-1, and using promethylating agents.
PP2A subunits and cancer: Mutation, deletion, inactivation, and aberrant expression
Scaffolding subunit: PP2A Aα and Aβ
Mutations have been detected in all subunits of PP2A in cancer, but the gene encoding the Aα subunit, PPP2R1A, has the highest mutation rate. PP2A is an essential enzyme, therefore cancer-associated PP2A Aα mutations in clinical specimens typically involve only a single allele [23, 41]. These mutations create a state of haploinsufficiency. Point mutations most commonly occur in the Aα subunit and ~30% of these mutations occur at a mutational hotspot within HEAT repeat 5. While the functional relevance of some of the identified Aα mutations have been studied, the significance of the mutations in this hot spot region has yet to be elucidated [39, 42]. Most reported PP2A Aα mutants are unable to bind the regulatory subunits, and, in particular, to members of the B′ family (B56) [38]. For example, when R418W and Δ171–589, two point mutations in Aα that were first detected in melanoma and breast carcinomas respectively, were studied, these mutations led to reduced binding to the C subunit and to all of the B subunits tested. Two additional mutants, E64D and E64G, specifically lost efficient binding to the B56 family subunits [5, 39] and resulted in a state of happloinsufficiency in transgenic mice [23]. In addition, mice containing the E64D mutation showed an increased incidence of lung cancer when exposed to benzopyrene and decreased survival when crossed with KRASG12D mice [39]. To date, mutations in PPP2R1A have been identified in breast, lung, melanoma, ovarian, endometrial, uterine, and colon cancers [41, 43–47] and decreased expression of Aα was detected in human gliomas [48]. (Table 1)
Table 1.
PP2A subunit alterations in cancer
| Subunit | Gene | Isoform | Alteration | Disease | Reference |
|---|---|---|---|---|---|
| A | PPP2R1A | Aα | Point mutation | breast, lung, melanoma, ovarian, endometrial, uterine, colon | [41], [43], [44], [45], [46], [47] |
| Deletion | Breast | [41] | |||
| Decreased expression | Glioma | [48] | |||
| A | PPP2R1B | Aβ | Missense mutation | breast, colon, lung | [41], [49], [50], [51], [40] |
| In-frame deletion | breast | [40] | |||
| LOH | breast, lung, ovarian, cervical, melanoma, NHL, CLL | [49], [52] | |||
| Decreased expression | AML | [53] | |||
| Abberant transcription | HCC, B-CLL | [54],[55] | |||
|
| |||||
| B | PPP2R2A | B55α | Deletion | breast, prostate, myeloma | [56], [57], [58] |
| Decreased expression | AML | [59] | |||
|
| |||||
| B | PPP2R2B | B55β | DNA hypermethylation | breast, colon | [60], [61] |
|
| |||||
| B | PPP2R2C | B55γ | Decreased expression | breast, prostate | [62], [63], [64] |
|
| |||||
| B′ | PPP2R5A | B56α | Decreased expression | melanoma | [65] |
|
| |||||
| B′ | PPP2R5C | B56γ | Decreased expression | melanoma | [66] |
| Point mutation | lung | [67] | |||
|
| |||||
| B′ | PPP2R5E | B55ε | SNP | soft tissue sarcoma | [68] |
|
| |||||
| C | PPP2CA | Cα | Decreased expression | AML, prostate | [53], [69], [70], [71] |
Akin to PP2A Aα mutations, cancer-associated Aβ mutations also induce haploinsufficiency and impaired binding to the B and C subunits [38–40]. The gene that encodes the β isoform of the A subunit, PPP2R1B, is located on a chromosomal band frequently deleted in cancer cells, 11q23 [72, 73]. According to a seminal paper by Wang et al., which was the first to demonstrate PPP2R1B to be mutated in human cancers, the 11q23 gene locus displayed loss of heterozygosity in 30–50% of breast, lung, ovary, cervical carcinomas, melanomas, and in 15% of non-Hodgkin’s lymphomas (NHL) and chronic lymphocytic leukemias (CLL) [49]. Many of the mutations in PPP2R1B are missense mutations including G8R, P65S, G90D, L101P, K343E, D504G, V545A, V448A. One contains the double mutant L101P/V448A and one contains an in frame deletion ΔE344–E388 [40, 74]. The ΔE344–E388 mutant was found to be incapable of binding to any of the B subunits tested [40, 74]. One mechanism of inactivation unique to the β isoform of the scaffolding subunit is abnormal RNA splicing leading to aberrant transcripts of PPP2R1B. Alternative splice variants of PP2A Aβ were observed in B-cell chronic lymphocytic leukemia (B-CLL). These aberrant transcripts were incapable of binding B and C subunits, which subsequently led to a loss of PP2A activity [54]. In addition, 29% of hepatocellular carcinoma (HCC) tumors and 3% of corresponding non-tumor tissues tested showed co-expression of wild-type and aberrant mRNA of PPP2R1B, suggesting that alternative splicing may facilitate the development of HCC [55].
Analogous to AKT, the GTPase RalA participates in transcription, migration, transport, apoptosis, and cell proliferation. PP2A Aβ binds and regulates the activity of RalA by dephosphorylating RalA at Ser183 and Ser194. This dephosphorylation leads to inactivation of RalA, again highlighting that PP2A Aβ functions as a tumor suppressor. Mutations in Aβ disrupt this interaction leading to a constitutive activation of RalA that results in transformation [74].
In summary, extensive sequencing of human samples and cancer cell lines has revealed PPP2R1B to be mutated in many solid cancers including breast, lung, colon, melanoma, ovarian, cervical, HCC, NHL, CLL, and B-CLL. Although it is 40 times less abundant than Aα [75], PP2A Aβ clearly has a role in the tumor suppressor capabilities of PP2A as mutations and loss of expression of the PPP2R1B gene inhibit this activity. There has been some speculation that PP2A Aα can compensate for Aβ mutation and or loss of expression in cancer. However, several reports suggest that this is unlikely. While Aα and Aβ are 85% identical, they have vastly different B and C subunit affinities and have distinct biochemical properties [13, 40]. For instance, Aβ mutations show decreased binding to the B and some of the B′ regulatory family members but predominantly affect processes involving the catalytic subunit and regulatory PR72-containing holoenzyme. In contrast, Aα subunit mutations mostly affect pathways where B′-containing holoenzymes are instrumental [5, 38, 40]. In addition to this, complexes involving Aβ and regulatory subunits regulate the phosphorylation of specific substrates involved in cellular transformation that are distinct from pathways regulated by the Aα-regulatory subunit complexes [38]. Lastly, in experiments in transgenic mice, overexpression of Aα could not revert tumorigenesis that was induced by suppression of Aβ [5]. Together, this evidence strongly suggests that PP2A Aα and Aβ are functionally different and cannot compensate for each other.
Regulatory subunits: PP2A B, B′, B″, and B‴
Mutations in the regulatory subunits of PP2A occur at much lower frequencies than those in the A subunit and most commonly result in decreased expression of the B subunits. Other methods of inactivation include deletion, DNA hypermethylation, and one point mutation found in lung cancer. The F395C mutation detected in lung cancer occurs in a region necessary for PP2A-B56γ-p53 interaction. In cell culture based studies, the B56γ mutant protein was unable to interact with p53, thereby inhibiting its p53 dependent tumor suppressive functions [67]. A study of 141 prostate cancer samples using an Affymetrix SNP array found that PPP2R2A, which encodes the regulatory subunit, B55α, was deleted in 67.1% of the tumor samples tested. Moreover, homozygous deletions occurred in three of the prostate cancer samples [57]. Deletions in the PPP2R2A gene have also been reported in breast cancer and myeloma [56, 58].
In addition to mutations, loss of B subunit protein expression has been linked to cancer progression. Decreased expression of both PPP2R5A and PPP2R5C, which encode B56α and B56γ, respectively, were reported in melanoma, with the lowest levels of expression in metastatic tissues [65, 66, 76]. In addition, immunohistochemical analysis of PPP2R2C protein levels in primary prostate tumors determined that loss of PPP2R2C, which encodes B55γ, was highly correlated with metastasis and prostate cancer specific mortality (PCSM) [62]. Furthermore, in a cohort of 231 patients with acute AML, B55α expression was inhibited and was associated with increased AKT phosphorylation at threonine 308 and loss of complete hematological remission. B55α dephosphorylates AKT at T308 and when suppressed, it leads to constitutive activation of AKT and enhanced proliferation. In a study by Ruvolo et al., remission duration was evaluated in 231 newly diagnosed AML patients evaluated at the MD Anderson Cancer Center. Patients were divided into two groups based on their B55α protein expression level: B55α-high and B55α-low. Kaplan–Meier survival curves illustrated the effect of B55α expression level on remission duration. Patients in the B55α-low group experienced significantly shorter complete remission duration than those in the B55α-high group [59]. The identification of B55α as a specific regulator of AKT phosphorylation at Thr308 as well as B55α expression’s correlation with remission duration highlight its potential to serve as a biomarker in AML.
Lastly, epigenetic alterations are a method of PP2A inactivation that is unique to the regulatory subunits. PPP2R2B, which encodes B55β, may be inactivated through epigenetic silencing according to a study by Muggerud et al., which detected an increase in DNA methylation of the PPP2R2B gene in ductal carcinoma in situ and locally advanced breast tumors [60]. Furthermore, B55β is epigenetically inactivated by DNA hypermethylation in colorectal cancer (CRC). This inactivation was shown to effect MYC signaling. A study by Tan et al. demonstrated that epigenetic inactivation of PPP2R2B occurred in >90% of patient derived CRC tumor samples tested. They found that loss of PPP2R2B expression led to the induction of PDK-1 dependent MYC phosphorylation at serine 62 by the mTOR inhibitor, rapamycin, which subsequently led to resistance. Restoration of PPP2R2B expression abrogated MYC phosphorylation, resensitizing CRC cells to rapamycin. As clinical responses to rapamycin are quite variable, better biomarkers are needed to predict which patients are most likely to respond to treatment. Tan’s study highlighted the potential of PPP2R2B to act as such a biomarker for selecting patients who may respond best to rapamycin treatment [61]. In summary, given the critical role the regulatory subunits play in determining the substrate specificity of the PP2A heterotrimeric complex, identifying mutations in these subunits and understanding their functional implications remains an active area of research.
Catalytic subunit: PP2A Cα and Cβ
To date, the only reports of mutations in the C subunit have been in prostate cancer and AML. A genome wide expression study identified significant downregulation of PPP2CA, which encodes the α isoform of the catalytic subunit, in androgen insensitive prostate cancer cell lines compared with androgen sensitive lines [71]. This finding was subsequently confirmed at the protein level and in human clinical samples [70]. In addition, PPP2CA was found to be downregulated in a cohort of patients with P53 mutant AML[53, 69]. There have been no reports to date of mutations in PPP2CB, the gene that encodes the β isoform of the catalytic subunit.
Post-translational modifications critical to PP2A activity
Methylation at L309 on the C subunit C-terminal tail
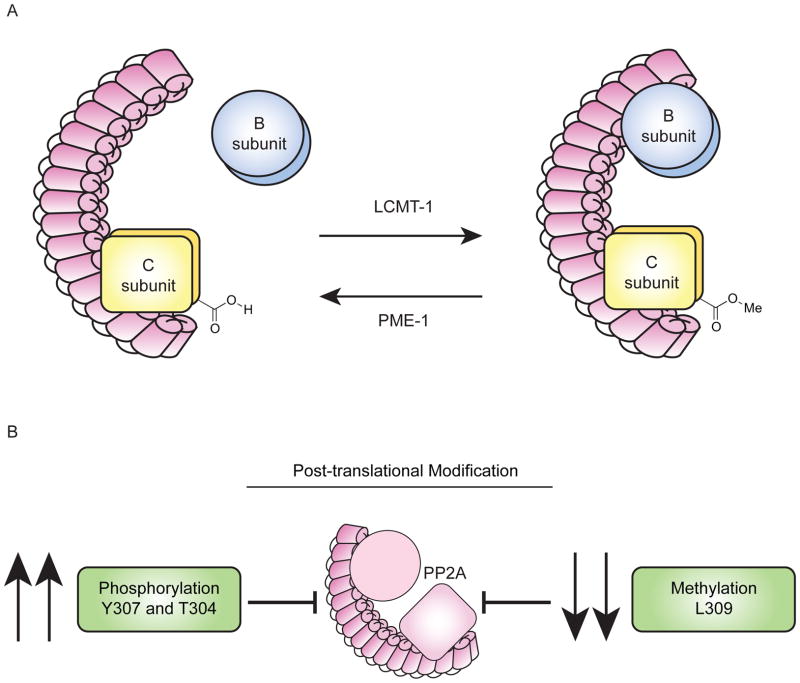
The C subunit C-terminus undergoes methylation at L309 (Fig. 4A and 4B). This modification is regulated by two enzymes: LCMT-1, an S-adenosylmethionine (SAM)-dependent methyltransferase that is expressed in the cytoplasm and PME-1 (Fig. 5A), a lipase-like methylesterase, that is expressed in the nucleus. While LCMT-1 mediated activation of PP2A is reversible (Fig. 5B), PME-1 mediated demethylation is not truly reversible because it denatures the active site [77–79]. The role of this methyl modification is complicated, and both enzymes are essential [80, 81], and likely exhibit control on cell cycle and development [82]. Decreased methylation typically corresponds with increases in cancer progression because methylation enhances holoenzyme assembly, specifically of the preformed AC core enzyme with B subunits. L309 methylation is not essential for every variant (PR61/B′, PR72/B″), but is essential for some (PR55/B) [2, 10, 83–88].
Figure 4.
(A) The C-terminus of the catalytic C subunit undergoes methylation at L309 via LCMT, a SAM-dependent methyltransferase. PME-1 mediates demethylation. (B) Decreased methylation at L309 and increased phosphorylation of Y307 and T304 of the catalytic C subunit are posttranslational modifications that inhibit PP2A.
Figure 5.
(A) (PDB code: 3C5W) Structure of PP2A and PME-1 complex with scaffold subunit in magenta, catalytic subunit in yellow, and PME-1 in red. (B) (PDB code: 3P71) Structure of PP2A and LCMT complex with catalytic subunit in yellow and LCMT in green (C) (PDB code: 4LAC) Structure of PP2A and PTPA complex with scaffold subunit in magenta, catalytic subunit in yellow, and PTPA in orange.
The role of L309 methylation gained in popularity in part due to its putative role in neurodegeneration. PP2A/PR55α is a predominant brain expressed phosphatase that requires L309 methylation for holoenzyme formation. Folate is a key nutrient in the production of the LCMT-1 cofactor, S-adenosylmethionine (SAM), which supplies the enzyme’s methyl donor. In folate deprived neuroblastoma cells, both PR55α and LCMT-1 expression levels are diminished, and PP2A exists in demethylated forms. This state leads to tau hyperphosphorylation and cell death, a signature of neurodegenerative diseases [89]. In mouse models of Alzheimer’s, levels of LCMT-1, methylated C subunit, and PR55α are decreased. Restoration of methylation by overexpression of LCMT-1, and induced expression of PR55α restores neurite outgrowth, a signature of disease remission [90]. Methylated PP2A is typically found associated with unphosphorylated tau, localized to the plasma membrane. In contrast, demethylated PP2A is improperly localized and not associated with tau [91]. An herb-derived compound, cornel iridoid glycoside, reverses tau phosphorylation by inhibiting C subunit demethylation [92]. The development of natural products and small molecules to modulate tau hyperphosphorylation would provide much needed tools and therapeutics for Alzheimer’s disease.
There is also evidence that PME-1 inhibits PP2A independently of its role on C subunit demethylation. Association with PME-1 possibly stabilizes PP2A in an inactive conformation and creates a cellular pool of enzyme that can be activated when required. This association is regulated by the interplay of PP2A with another protein, Phosphatase Activator (PTPA) which activates the PME-1 bound form of PP2A (Fig 5C) [93]. PTPA is a protein with an elusive functional role; however, it is commonly associated with PP2A and possesses a chaperone-like function for the correctly folded C subunit [94]. It also possesses a peptidyl-prolyl cis / trans isomerase activity that acts on Pro190 of the C subunit, inducing a conformational change that may contribute to the reactivation of PME-1 bound PP2A [95, 96]. Additionally, like PME-1 and PTPA, several other proteins associate with the PP2A core or trimer that are not classifed as B subunits. The α4 protein is a PP2A binding protein that stabilizes PP2A and other PPP enzymes by binding to the C subunit and preventing its degradation [97, 98]. The α4 protein is essential and plays a role in cellular adaptation to stress by preserving stocks of PP2A. This PP2A store ultimately dephosphorylates the accumulated products of stress responses [17].
Phosphorylation of the C subunit C-terminal tail
The phosphorylation of multiple residues on the C subunit C-terminus is also critical for modulating PP2A activity via B subunit interactions (Fig. 4B). Y307 phosphorylation inhibits methylation of L309 by LCMT-1 and limits specific B subunit binding to the core enzyme. Phosphorylation of Y307 itself increases T304 phosphorylation. T304 phosphorylation does not appear to influence methylation, but, like Y307, it may selectively inhibit B subunit binding and dissociate B subunits from fully formed trimers [10]. Y307 and T304 phosphorylation selectively inhibit the binding of all PR55 subunits. Phosphorylation of Y307 prevents assembly of the holoenzyme with PR61/B′αβγε subunits, but not with the B′δ, PR72/B″, or PR70/B″ subunits. As a note, these latter experiments were performed with uncharged (Y307F) or phosphomimetic (Y307D) point mutants, which could not undergo L309 methylation [10, 85, 99].
Exogenous inhibitors of PP2A
There are a number of microbial, marine, and insect derived natural products that bind and inhibit PP2A and other PPP members. These include okadaic acid, fostriecin, microcystins, calyculins, cantharidin, and dragmacidins. Many were isolated from screens of natural product extracts for cytotoxins. Others were discovered in pulldown studies of biologically active extracts, as PP2A binding small molecules [100]. While it seems counterintuitive to inhibit a tumor suppressor like PP2A, at the time, the potency of these compounds in cytotoxicity assays generated much interest in their potential clinical uses for cancer. Their toxicity underscores PP2A’s essential role in regulation, and several of these compounds provided extremely useful tools for exploring PP2A’s functions. The natural product toxins bind into or adjacent to and obstruct the C subunit active site. [9, 101] The same could be said for the Simian Virus 40 (SV40) small t antigens (ST). SV40 ST consists of an N-terminal J domain and a C-terminal unique domain that contains two separate zinc-binding motifs. SV40 ST interacts with the core enzyme by binding to the B56 subunit binding site on PP2A Aα (HEAT repeats 3–7), causing displacement of the B subunits [102]. This displacement perturbs the function of PP2A and its activity towards multiple substrates [103, 104]. While not directly tumorigenic in humans, these viruses transform cells and can promote tumor growth.
Endogenous inhibitors of PP2A
PP2A is commonly inactivated in cancer by the overexpression of its endogenous inhibitors. The most prominent deactivation mechanism, this occurs in up to 90% of cases in lung and breast cancers and is often associated with poor response to current therapies. There are several endogenous inhibitory proteins that inactivate PP2A (Fig. 6). Inhibitor 1 of PP2A (I1PP2A), also known as ANP32A, inhibits PP2A activity in human umbilical vein endothelial cells [105, 106]. I1PP2A also binds to sphingosine, and this interaction abrogates its binding to PP2A resulting in PP2A activation [107]. Additionally, the greatwall kinase (Gwl) might function as an inhibitor of PP2A. Gwl activates ENSA and Arpp19, which are phosphorylation-dependent inhibitors of PP2A [108, 109]. GWL mediated inhibition of PP2A-B55 leads to phosphorylation of Cdk1 substrates and mitotic entry. Furthermore studies have shown that HOX11, a homeobox gene rearranged in T-cell leukemia by chromosomal translocation, inhibits PP2A [110]. However, the two endogenous inhibitors of PP2A most overexpressed in human cancers and best characterized are SET and CIP2A [106, 111]. SET, also known as inhibitor-2 of PP2A (I2PP2A), binds to the C subunit of PP2A. It was discovered as a chimeric protein in a patient with acute undifferentiated leukemia. In this case, SET was a translocated gene fused with nucleoporin (CAN gene) [112–114]. SET displays increased expression or increased activity in several cancers such as CML, AML, and B-cell CLL, colorectal cancer, breast cancer, and lung cancer [115–121]. In addition to its overexpression, altered phosphorylation of SET also inactivates PP2A [122, 123]. Studies in Alzheimer’s disease have elucidated that Val92 at the amino-terminal fragment and the amino acids 176–277 on the C-terminal region of SET are important for PP2A binding. Furthermore, accumulation of SET in the cytoplasm is regulated by phosphorylation of Ser9 in the nuclear localization signal [124–126].
The association of SET with cancer inspired several attempts to target this inhibitor for PP2A activation. One strategy to inhibit SET involves ApoE (apolipoprotein E), a multifunctional holoprotein with a role in cholesterol transport [127–129] and immunoregulatory functions [130–132]. ApoE and apoE-mimetic peptides, COG112 and COG449 (OP449), bind to SET resulting in activation of PP2A [133–136]. SET antagonism with OP449 results in cytotoxic activity with demonstrable efficacy in the treatment of CML and AML [133].
FTY720 (Fingolimod/Gilenya®), originally approved for use in multiple sclerosis by Novartis, activates PP2A via inhibition of SET. FTY720 was derived from a fungal metabolite [137, 138] and acts as an immunosuppressant by modulating the sphingosine-1-phosphate (SIP) receptor [139–141]. FTY720 exerts anti-tumor activity in breast, HCC, glioma, and multiple myeloma models. Specifically in CML, activation of PP2A [117, 142–144] by FTY720 induces apoptosis through the inactivation of BCR-ABL1 and negative regulation of several survival factors including ERK. Finally, ceramide is a sphingolipid that activates PP2A in several cancers and induces apoptosis [145–149]. Some reports implicate a direct interaction of ceramide with PP2A. Others suggest that ceramide activates PP2A by inhibiting the interaction between PP2A and SET [150]. Ceramide induces apoptosis in prostate cancer cells through PP2A mediated induction of p27 [147].
CIP2A (Cancerous Inhibitor of PP2A) is a PP2A interacting protein. CIP2A is most strongly associated with inhibiting the activity of PP2A on c-MYC resulting in c-MYC stabilization and consequential proliferation. Inhibition of PP2A by CIP2A is also associated with the stabilization of other pro-survival and pro-growth proteins including E2F1, mTOR, and DAPk, resulting in the inhibition of senescence, autophagy and apoptotic pathways respectively [151–153]. Conversely, the depletion of CIP2A results in a decrease in cancer cell viability. It is not understood how CIP2A inhibits PP2A but some reports suggest that it interacts with the A subunit and perhaps the C subunit, preventing the interaction of the active site with target proteins [154]. Encoded by the KIAA1524 gene, CIP2A is overexpressed and may be prognostic in lung cancer, breast cancer, pancreatic cancer, bladder cancer, osteosarcoma, esophageal cancer, gastric cancer, ovarian cancer, cervical cancer, prostate cancer, hepatocellular carcinoma, and colorectal cancer [155–172]. This abundant clinical relevance makes CIP2A an important therapeutic target.
Several natural products possess activities that are relevant to PP2A activation via CIP2A inhibition. Celastrol (tripterine) caused a proteasome-mediated degradation of CIP2A resulting in inhibition of proliferation and induction of apoptosis in lung cancer [173, 174]. Celastrol induced rapid degradation of CIP2A through the interaction of the E3 ligase, CHIP. In vivo studies showed that celastrol potentiated the effects of cisplatin suggesting that celastrol could have therapeutic implications in lung cancer. Ethoxysanguinarine (ESG), a benzophenanthridine alkaloid, downregulates CIP2A resulting in an increase in PP2A activity and a consequential downregulation of c-MYC and AKT in lung cancer. The downregulation of CIP2A subsequently results in inhibition of proliferation and induction of apoptosis in lung cancer [175, 176]. Combined treatment with ESG enhanced the effects of cisplatin in lung cancer.
The anti-cancer drug bortezomib might provide another strategy for activating PP2A via CIP2A inhibition. Bortezomib, a dipeptidyl boronic acid, is a proteasome inhibitor first approved for treatment of multiple myeloma. It blocks degradation of IκB, an inhibitor of NF-κB [177, 178]. In subsequent studies, bortezomib negatively regulated transcription of CIP2A resulting in decreased AKT phosphorylation and induction of apoptosis in breast cancer [179]. It enhances PP2A activity in HCC [180]. Finally, derivatives of erlotinib that were devoid of anti-EGFR activity, inhibited production of CIP2A causing subsequent decreases in AKT phosphorylation and cell growth inhibition [181].
Promethylating agents
One strategy to activate PP2A could be the induction of methylation. As mentioned previously, PP2A methylation at residue Leu309 enhances the affinity of the PP2A core enzyme for the regulatory subunit [79, 182–186]. Some reports suggest that PP2A methylation is linked to PP2A activity and that PP2A methylation induced by an agonist, such as xylulose-5-phosphate, would cause an increase in PP2A activity [185, 187, 188]. This increase in PP2A activity results in a decrease in AKT and c-MYC expression and a decrease in proliferation [189]. Furthermore, DNA damaging agents such as chloroethylnitrosourea (CENU), induce PP2A methylation, increasing PP2A activity resulting in inhibition of AKT and c-MYC [189, 190].
PME-1 inhibitors
PME-1 garnered increasing attention due to its role in cancer and its association with PP2A [191]. To develop probes for annotating enzymatic function, the Cravatt laboratory used its activity based protein profiling technique to screen for inhibitors of serine hydrolases. Their methods generated two independent small molecule inhibitors of PME-1. The first, an azalactam (ABL127, IC50 = 10 nM) [192], and the second, a sulfonyl acrylonitrile covalent inhibitor derived from a complementary screening library [193]. These will hopefully provide useful leads to validate PME-1 as a drug target.
Agents with undefined mechanism of action
Several compounds activate PP2A by unknown mechanisms. Forskolin, derived from the root of Coleus forskohlii, is a diterpenoid natural product used for many conditions including cancer. By activating adenylate cyclase, forskolin increases intracellular concentration of cyclic adenosine monophosphate (cAMP) [194]. Forskolin treatment reduces phosphorylation at Y307 on the C-terminal tail of PP2A C thereby activating PP2A [195–197]. In AML, forskolin increased PP2A activity resulting in decreases in proliferation, induction of apoptosis, and changes in the phosphorylation of AKT and ERK [195]. Perphenazine and other phenothiazine containing tricyclic neuroleptics, activate PP2A in T-ALL cells through direct binding of the PP2A Aα subunit [198]. Carnosic acid is a polyphenolic diterpene that activates PP2A in prostate cancer. This activation results in negative regulation of the AKT/IKK/NF-κB pathway [199]. Vitamin E analogs, such as α-tocopheryl succinate, inhibit proliferation in several cancers [200–210]. α-Tocopheryl succinate promoted activation of PP2A inactivates JNK signaling [211, 212].
Conclusion
PP2A is one of the most abundant cellular proteins, a prominent phosphatase tumor suppressor that regulates the activity of numerous kinases. With a high degree of sequence conservation among yeast, drosophila, and mammals, PP2A controls many cellular functions ranging from metabolism, cell cycle, DNA replication, growth, and apoptosis. It is commonly dysregulated and deactivated in a variety of cancers and other diseases. The biochemical and clinical studies presented here demonstrate that each of its three subunits can be genetically altered and functionally inactivated. Where achievable, restoration of PP2A function inhibits cancer progression, and notably, by a mediator that is downstream of the oncogenic kinases that initiate and drive cancer progression. Several strategies in development accomplish this goal by affecting PP2A’s posttranslational modifications and its endogenous inhibitory proteins. There are likely small molecules capable of direct activation of the protein. These will provide additional tools for determining PP2A targets and as potential therapeutics. Further development and combinations of these with other targeted therapies will enable the translation of these findings to patients.
Acknowledgments
Goutham Narla is a recipient of a Howard Hughes Medical Institute (HHMI) Physician-Scientist Early Career Award and a Case Comprehensive Cancer Center Pilot Award. He is a Harrington Distinguished Scholar (Early Career Award). This work was supported by a R01 from NCI R01CA181654. We wish to thank Caitlin O’Connor and Abbey Perl for their assistance with the figures.
Abbreviations
- PP2A
protein phosphatase 2A
- PTP
protein tyrosine phosphatase
- PSP
protein serine/threonine phosphatase
- PPP
phospho-protein phosphatase
- PPM
metal-dependent protein phosphatase
- HCC
hepatocellular carcinoma
- NHL
non-Hodgkin’s lymphoma
- CLL
chronic lymphocytic leukemia
- B-CLL
B-cell chronic lymphocytic leukemia
- CRC
colorectal cancer
- LCMT-1
leucine carboxyl methyltransferase-1
- PME-1
Protein phosphatase methylesterase-1
- SAM
S-adenosyl methionine
- PTPA
PP2A phosphatase activator
- I1PP2A/ANP32A
Inhibitor 1 of PP2A
- ANP32A
acidic (leucine-rich) nuclear phosphoprotein 32 family member A
- Gwl kinase
greatwall kinase
- ENSA
endosulfine alpha
- Arpp19
cAMP-regulated phosphoprotein, 19kDa
- CIP2A
cancerous inhibitor of PP2A
- SET/I2PP2A
inhibitor-2 of PP2A
- ApoE
apolipoprotein E
- ERK
extracellular-signal-regulated kinase
- mTOR
mammalian target of rapamycin
- ESG
Ethoxysanguinarine
- EGFR
epidermal growth factor receptor
- CENU
chloroethylnitrosourea
- cAMP
cyclic adenosine monophosphate
References
- 1.Brautigan DL. Protein Ser/Thr phosphatases--the ugly ducklings of cell signalling. FEBS J. 2013;280:324–345. doi: 10.1111/j.1742-4658.2012.08609.x. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Stone SR, Hofsteenge J, Hemmings BA. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987;26:7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- 4.Khew-Goodall Y, Hemmings BA. Tissue-specific expression of mRNAs encoding alpha- and beta-catalytic subunits of protein phosphatase 2A. FEBS Lett. 1988;238:265–268. doi: 10.1016/0014-5793(88)80493-9. [DOI] [PubMed] [Google Scholar]
- 5.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Andrade MA, Bork P. HEAT repeats in the Huntington’s disease protein. Nat Genet. 1995;11:115–116. doi: 10.1038/ng1095-115. [DOI] [PubMed] [Google Scholar]
- 7.Groves MR, Hanlon N, Turowski P, Hemmings BA, Barford D. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell. 1999;96:99–110. doi: 10.1016/s0092-8674(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 8.Wozniak E, Oldziej S, Ciarkowski J. Molecular modeling of the catalytic domain of serine/threonine phosphatase-1 with the Zn2+ and Mn2+ di-nuclear ion centers in the active site. Comput Chem. 2000;24:381–390. doi: 10.1016/s0097-8485(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 9.Xing Y, Xu Y, Chen Y, Jeffrey PD, Chao Y, Lin Z, Li Z, Strack S, Stock JB, Shi Y. Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell. 2006;127:341–353. doi: 10.1016/j.cell.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Longin S, Zwaenepoel K, Louis JV, Dilworth S, Goris J, Janssens V. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic Subunit. J Biol Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- 11.Kamibayashi C, Lickteig RL, Estes R, Walter G, Mumby MC. Expression of the A subunit of protein phosphatase 2A and characterization of its interactions with the catalytic and regulatory subunits. J Biol Chem. 1992;267:21864–21872. [PubMed] [Google Scholar]
- 12.McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 13.Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 14.Wlodarchak N, Guo F, Satyshur KA, Jiang L, Jeffrey PD, Sun T, Stanevich V, Mumby MC, Xing Y. Structure of the Ca2+-dependent PP2A heterotrimer and insights into Cdc6 dephosphorylation. Cell Res. 2013;23:931–946. doi: 10.1038/cr.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry MS, Hallberg RL. Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol Biol Cell. 2002;13:3477–3492. doi: 10.1091/mbc.02-05-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 17.Sents W, Ivanova E, Lambrecht C, Haesen D, Janssens V. The biogenesis of active protein phosphatase 2A holoenzymes: a tightly regulated process creating phosphatase specificity. FEBS J. 2013;280:644–661. doi: 10.1111/j.1742-4658.2012.08579.x. [DOI] [PubMed] [Google Scholar]
- 18.Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R. The quantitative proteome of a human cell line. Mol Syst Biol. 2011;7:549. doi: 10.1038/msb.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Scuderi A, Letsou A, Virshup DM. B56-associated protein phosphatase 2A is required for survival and protects from apoptosis in Drosophila melanogaster. Mol Cell Biol. 2002;22:3674–3684. doi: 10.1128/MCB.22.11.3674-3684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci USA. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strack S, Cribbs JT, Gomez L. Critical role for protein phosphatase 2A heterotrimers in mammalian cell survival. J Biol Chem. 2004;279:47732–47739. doi: 10.1074/jbc.M408015200. [DOI] [PubMed] [Google Scholar]
- 22.Janssens V, Jordens J, Stevens I, Van Hoof C, Martens E, De Smedt H, Engelborghs Y, Waelkens E, Goris J. Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B″/PR72 subunit of protein phosphatase 2A. J Biol Chem. 2003;278:10697–10706. doi: 10.1074/jbc.M211717200. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Arroyo JD, Timmons JC, Possemato R, Hahn WC. Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 2005;65:8183–8192. doi: 10.1158/0008-5472.CAN-05-1103. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 25.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 26.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 27.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., 3rd Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 28.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Zhou XW, Tanila H, Bjorkdahl C, Wang JZ, Guan ZZ, Cao Y, Gustafsson JA, Winblad B, Pei JJ. Phosphorylated PP2A (tyrosine 307) is associated with Alzheimer neurofibrillary pathology. J Cell Mol Med. 2008;12:241–257. doi: 10.1111/j.1582-4934.2008.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voronkov M, Braithwaite SP, Stock JB. Phosphoprotein phosphatase 2A: a novel druggable target for Alzheimer’s disease. Future Med Chem. 2011;3:821–833. doi: 10.4155/fmc.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer’s disease. Neurosignals. 2002;11:262–269. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- 32.Begum N, Ragolia L. cAMP counter-regulates insulin-mediated protein phosphatase-2A inactivation in rat skeletal muscle cells. J Biol Chem. 1996;271:31166–31171. doi: 10.1074/jbc.271.49.31166. [DOI] [PubMed] [Google Scholar]
- 33.Hojlund K, Poulsen M, Staehr P, Brusgaard K, Beck-Nielsen H. Effect of insulin on protein phosphatase 2A expression in muscle in type 2 diabetes. Eur J Clin Invest. 2002;32:918–923. doi: 10.1046/j.1365-2362.2002.01098.x. [DOI] [PubMed] [Google Scholar]
- 34.Lipina C, Hundal HS. Carnosic acid stimulates glucose uptake in skeletal muscle cells via a PME-1/PP2A/PKB signalling axis. Cell Signal. 2014;26:2343–2349. doi: 10.1016/j.cellsig.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Wallace AM, Hardigan A, Geraghty P, Salim S, Gaffney A, Thankachen J, Arellanos L, D’Armiento JM, Foronjy RF. Protein phosphatase 2A regulates innate immune and proteolytic responses to cigarette smoke exposure in the lung. Toxicol Sci. 2012;126:589–599. doi: 10.1093/toxsci/kfr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchhefer U, Brekle C, Eskandar J, Isensee G, Kucerova D, Muller FU, Pinet F, Schulte JS, Seidl MD, Boknik P. Cardiac function is regulated by B56alpha-mediated targeting of protein phosphatase 2A (PP2A) to contractile relevant substrates. J Biol Chem. 2014;289:33862–33873. doi: 10.1074/jbc.M114.598938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013;14:e229–238. doi: 10.1016/S1470-2045(12)70558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sablina AA, Hahn WC. SV40 small T antigen and PP2A phosphatase in cell transformation. Cancer Metastasis Rev. 2008;27:137–146. doi: 10.1007/s10555-008-9116-0. [DOI] [PubMed] [Google Scholar]
- 39.Ruediger R, Ruiz J, Walter G. Human cancer-associated mutations in the Aalpha subunit of protein phosphatase 2A increase lung cancer incidence in Aalpha knock-in and knockout mice. Mol Cell Biol. 2011;31:3832–3844. doi: 10.1128/MCB.05744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruediger R, Pham HT, Walter G. Alterations in protein phosphatase 2A subunit interaction in human carcinomas of the lung and colon with mutations in the A beta subunit gene. Oncogene. 2001;20:1892–1899. doi: 10.1038/sj.onc.1204279. [DOI] [PubMed] [Google Scholar]
- 41.Calin GA, di Iasio MG, Caprini E, Vorechovsky I, Natali PG, Sozzi G, Croce CM, Barbanti-Brodano G, Russo G, Negrini M. Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene. 2000;19:1191–1195. doi: 10.1038/sj.onc.1203389. [DOI] [PubMed] [Google Scholar]
- 42.Ruediger R, Pham HT, Walter G. Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the A alpha subunit gene. Oncogene. 2001;20:10–15. doi: 10.1038/sj.onc.1204059. [DOI] [PubMed] [Google Scholar]
- 43.McConechy MK, Anglesio MS, Kalloger SE, Yang W, Senz J, Chow C, Heravi-Moussavi A, Morin GB, Mes-Masson AM, Carey MS, McAlpine JN, Kwon JS, Prentice LM, Boyd N, Shah SP, Gilks CB, Huntsman DG Australian Ovarian Cancer Study Group. Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas. J Pathol. 2011;223:567–573. doi: 10.1002/path.2848. [DOI] [PubMed] [Google Scholar]
- 44.Shih Ie M, Wang TL. Mutation of PPP2R1A: a new clue in unveiling the pathogenesis of uterine serous carcinoma. J Pathol. 2011;224:1–4. doi: 10.1002/path.2884. [DOI] [PubMed] [Google Scholar]
- 45.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, Guillory J, Ha C, Dijkgraaf GJ, Stinson J, Gnad F, Huntley MA, Degenhardt JD, Haverty PM, Bourgon R, Wang W, Koeppen H, Gentleman R, Starr TK, Zhang Z, Largaespada DA, Wu TD, de Sauvage FJ. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagendra DC, Burke J, 3rd, Maxwell GL, Risinger JI. PPP2R1A mutations are common in the serous type of endometrial cancer. Mol Carcinog. 2012;51:826–831. doi: 10.1002/mc.20850. [DOI] [PubMed] [Google Scholar]
- 48.Colella S, Ohgaki H, Ruediger R, Yang F, Nakamura M, Fujisawa H, Kleihues P, Walter G. Reduced expression of the Aalpha subunit of protein phosphatase 2A in human gliomas in the absence of mutations in the Aalpha and Abeta subunit genes. Int J Cancer. 2001;93:798–804. doi: 10.1002/ijc.1423. [DOI] [PubMed] [Google Scholar]
- 49.Wang SS, Esplin ED, Li JL, Huang L, Gazdar A, Minna J, Evans GA. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998;282:284–287. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- 50.Takagi Y, Futamura M, Yamaguchi K, Aoki S, Takahashi T, Saji S. Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut. 2000;47:268–271. doi: 10.1136/gut.47.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esplin ED, Ramos P, Martinez B, Tomlinson GE, Mumby MC, Evans GA. The glycine 90 to aspartate alteration in the Abeta subunit of PP2A (PPP2R1B) associates with breast cancer and causes a deficit in protein function. Genes Chromosomes Cancer. 2006;45:182–190. doi: 10.1002/gcc.20284. [DOI] [PubMed] [Google Scholar]
- 52.Baysal BE, Willett-Brozick JE, Taschner PE, Dauwerse JG, Devilee P, Devlin B. A high-resolution integrated map spanning the SDHD gene at 11q23: a 1.1-Mb BAC contig, a partial transcript map and 15 new repeat polymorphisms in a tumour-suppressor region. Eur J Hum Genet. 2001;9:121–129. doi: 10.1038/sj.ejhg.5200585. [DOI] [PubMed] [Google Scholar]
- 53.Ramaswamy K, Spitzer B, Kentsis A. Therapeutic Re-Activation of Protein Phosphatase 2A in Acute Myeloid Leukemia. Front Oncol. 2015;5:16. doi: 10.3389/fonc.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalla C, Scheuermann MO, Kube I, Schlotter M, Mertens D, Dohner H, Stilgenbauer S, Lichter P. Analysis of 11q22-q23 deletion target genes in B-cell chronic lymphocytic leukaemia: evidence for a pathogenic role of NPAT, CUL5, and PPP2R1B. Eur J Cancer. 2007;43:1328–1335. doi: 10.1016/j.ejca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Chou H-C, Chen C-H, Lee H-S, Lee C-Z, Huang G-T, Yang P-M, Lee P-H, Sheu J-C. Alterations of tumour suppressor PP2R1B in hepatocellular carcinoma. Cancer Lett. 2007;253:138–143. doi: 10.1016/j.canlet.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 56.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Gräf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, Langerød A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Børresen-Dale AL, Brenton JD, Tavaré S, Caldas C, Aparicio S METABRIC Group. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng Y, Liu W, Kim ST, Sun J, Lu L, Zheng SL, Isaacs WB, Xu J. Evaluation of PPP2R2A as a prostate cancer susceptibility gene: a comprehensive germline and somatic study. Cancer Genet. 2011;204:375–381. doi: 10.1016/j.cancergen.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosca L, Musto P, Todoerti K, Barbieri M, Agnelli L, Fabris S, Tuana G, Lionetti M, Bonaparte E, Sirchia SM, Grieco V, Bianchino G, D’Auria F, Statuto T, Mazzoccoli C, De Luca L, Petrucci MT, Morabito F, Offidani M, Di Raimondo F, Falcone A, Caravita T, Omedè P, Boccadoro M, Palumbo A, Neri A. Genome-wide analysis of primary plasma cell leukemia identifies recurrent imbalances associated with changes in transcriptional profiles. Am J Hematol. 2013;88:16–23. doi: 10.1002/ajh.23339. [DOI] [PubMed] [Google Scholar]
- 59.Ruvolo PP, Qui YH, Coombes KR, Zhang N, Ruvolo VR, Borthakur G, Konopleva M, Andreeff M, Kornblau SM. Low expression of PP2A regulatory subunit B55alpha is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patients. Leukemia. 2011;25:1711–1717. doi: 10.1038/leu.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muggerud AA, Ronneberg JA, Warnberg F, Botling J, Busato F, Jovanovic J, Solvang H, Bukholm I, Borresen-Dale AL, Kristensen VN, Sørlie T, Tost J. Frequent aberrant DNA methylation of ABCB1, FOXC1, PPP2R2B and PTEN in ductal carcinoma in situ and early invasive breast cancer. Breast cancer research: BCR. 2010;12:R3. doi: 10.1186/bcr2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan J, Lee PL, Li Z, Jiang X, Lim YC, Hooi SC, Yu Q. B55beta-associated PP2A complex controls PDK1-directed myc signaling and modulates rapamycin sensitivity in colorectal cancer. Cancer Cell. 2010;18:459–471. doi: 10.1016/j.ccr.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Spencer ES, Bluemn EG, Johnston R, Zhang X, Gordon RR, Lewinshtein D, Lucas J, Nelson P, Porter CR. Association of decreased expression of protein phosphatase 2A subunit PR55γ (PPP2R2C) with an increased risk of metastases and prostate cancer-specific mortality. J Clin Oncol. 2012;30(suppl):abstr 4669. [Google Scholar]
- 63.Bluemn EG, Spencer ES, Mecham B, Gordon RR, Coleman I, Lewinshtein D, Mostaghel E, Zhang X, Annis J, Grandori C, Porter C, Nelson PS. PPP2R2C loss promotes castration-resistance and is associated with increased prostate cancer-specific mortality. Mol Cancer Res. 2013;11:568–578. doi: 10.1158/1541-7786.MCR-12-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan YL, Chen L, Wang J, Yao Q, Wan JQ. Over expression of PPP2R2C inhibits human glioma cells growth through the suppression of mTOR pathway. FEBS Lett. 2013;587:3892–3897. doi: 10.1016/j.febslet.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Mannava S, Omilian AR, Wawrzyniak JA, Fink EE, Zhuang D, Miecznikowski JC, Marshall JR, Soengas MS, Sears RC, Morrison CD, Nikiforov MA. PP2A-B56alpha controls oncogene-induced senescence in normal and tumor human melanocytic cells. Oncogene. 2012;31:1484–1492. doi: 10.1038/onc.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deichmann M, Thome M, Benner A, Egner U, Hartschuh W, Naher H. PTEN/MMAC1 expression in melanoma resection specimens. Br J Cancer. 2002;87:1431–1436. doi: 10.1038/sj.bjc.6600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shouse GP, Nobumori Y, Liu X. A B56gamma mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A. Oncogene. 2010;29:3933–3941. doi: 10.1038/onc.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grochola LF, Vazquez A, Bond EE, Wurl P, Taubert H, Muller TH, Levine AJ, Bond GL. Recent natural selection identifies a genetic variant in a regulatory subunit of protein phosphatase 2A that associates with altered cancer risk and survival. Clin Cancer Res. 2009;15:6301–6308. doi: 10.1158/1078-0432.CCR-09-0797. [DOI] [PubMed] [Google Scholar]
- 69.Sallman DA, Wei S, List A. PP2A: The Achilles Heal in MDS with 5q Deletion. Front Oncol. 2014;4:264. doi: 10.3389/fonc.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh AP, Bafna S, Chaudhary K, Venkatraman G, Smith L, Eudy JD, Johansson SL, Lin MF, Batra SK. Genome-wide expression profiling reveals transcriptomic variation and perturbed gene networks in androgen-dependent and androgen-independent prostate cancer cells. Cancer Lett. 2008;259:28–38. doi: 10.1016/j.canlet.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhardwaj A, Singh S, Srivastava SK, Honkanen RE, Reed E, Singh AP. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol Cancer Ther. 2011;10:720–731. doi: 10.1158/1535-7163.MCT-10-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baysal BE, Farr JE, Goss JR, Devlin B, Richard CW., 3rd Genomic organization and precise physical location of protein phosphatase 2A regulatory subunit A beta isoform gene on chromosome band 11q23. Gene. 1998;217:107–116. doi: 10.1016/s0378-1119(98)00350-3. [DOI] [PubMed] [Google Scholar]
- 73.Kiely M, Kiely PA. PP2A: The Wolf in Sheep’s Clothing? Cancers (Basel) 2015;7:648–669. doi: 10.3390/cancers7020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, DeCaprio JA, Hahn WC. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hemmings BA, Adams-Pearson C, Maurer F, Muller P, Goris J, Merlevede W, Hofsteenge J, Stone SR. alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry. 1990;29:3166–3173. doi: 10.1021/bi00465a002. [DOI] [PubMed] [Google Scholar]
- 76.Ito A, Kataoka TR, Watanabe M, Nishiyama K, Mazaki Y, Sabe H, Kitamura Y, Nojima H. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. Embo J. 2000;19:562–571. doi: 10.1093/emboj/19.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Baere I, Derua R, Janssens V, Van Hoof C, Waelkens E, Merlevede W, Goris J. Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry. 1999;38:16539–16547. doi: 10.1021/bi991646a. [DOI] [PubMed] [Google Scholar]
- 78.Ogris E, Du X, Nelson KC, Mak EK, Yu XX, Lane WS, Pallas DC. A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J Biol Chem. 1999;274:14382–14391. doi: 10.1074/jbc.274.20.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xing YN, Li Z, Chen Y, Stock JB, Jeffrey PD, Shi YG. Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell. 2008;133:154–163. doi: 10.1016/j.cell.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 80.Ortega-Gutierrez S, Leung D, Ficarro S, Peters EC, Cravatt BF. Targeted disruption of the PME-1 gene causes loss of demethylated PP2A and perinatal lethality in mice. Plos One. 2008;3:e2486. doi: 10.1371/journal.pone.0002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JA, Pallas DC. Leucine carboxyl methyltransferase-1 is necessary for normal progression through mitosis in mammalian cells. J Biol Chem. 2007;282:30974–30984. doi: 10.1074/jbc.M704861200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xia X, Gholkar A, Senese S, Torres JZ. A LCMT1-PME-1 Methylation Equilibrium Controls Mitotic Spindle Size. Cell Cycle. 2015;14:1938–1947. doi: 10.1080/15384101.2015.1026487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ikehara T, Ikehara S, Imamura S, Shinjo F, Yasumoto T. Methylation of the C-terminal leucine residue of the PP2A catalytic subunit is unnecessary for the catalytic activity and the binding of regulatory subunit (PR55/B) Biochem Bioph Res Co. 2007;354:1052–1057. doi: 10.1016/j.bbrc.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 84.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Gentry MS, Li Y, Wei H, Syed FF, Patel SH, Hallberg RL, Pallas DC. A novel assay for protein phosphatase 2A (PP2A) complexes in vivo reveals differential effects of covalent modifications on different Saccharomyces cerevisiae PP2A heterotrimers. Eukaryot Cell. 2005;4:1029–1040. doi: 10.1128/EC.4.6.1029-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu J, Tolstykh T, Lee J, Boyd K, Stock JB, Broach JR. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. Embo J. 2000;19:5672–5681. doi: 10.1093/emboj/19.21.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu XX, Du X, Moreno CS, Green RE, Ogris E, Feng Q, Chou L, McQuoid MJ, Pallas DC. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Balpha regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol Biol Cell. 2001;12:185–199. doi: 10.1091/mbc.12.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu Y, Chen Y, Zhang P, Jeffrey PD, Shi Y. Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol Cell. 2008;31:873–885. doi: 10.1016/j.molcel.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sontag JM, Nunbhakdi-Craig V, Montgomery L, Arning E, Bottiglieri T, Sontag E. Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A B(alpha) subunit expression that correlate with enhanced tau phosphorylation. J Neurosci. 2008;28:11477–11487. doi: 10.1523/JNEUROSCI.2816-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sontag JM, Nunbhakdi-Craig V, Mitterhuber M, Ogris E, Sontag E. Regulation of protein phosphatase 2A methylation by LCMT1 and PME-1 plays a critical role in differentiation of neuroblastoma cells. J Neurochem. 2010;115:1455–1465. doi: 10.1111/j.1471-4159.2010.07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sontag JM, Nunbhakdi-Craig V, Sontag E. Leucine carboxyl methyltransferase 1 (LCMT1)-dependent methylation regulates the association of protein phosphatase 2A and Tau protein with plasma membrane microdomains in neuroblastoma cells. J Biol Chem. 2013;288:27396–27405. doi: 10.1074/jbc.M113.490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang CC, Kuai XX, Li YL, Zhang L, Yu JC, Li L. Cornel Iridoid Glycoside Attenuates Tau Hyperphosphorylation by Inhibition of PP2A Demethylation. Evid Based Complement Alternat Med. 2013;2013:108486. doi: 10.1155/2013/108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Longin S, Jordens J, Martens E, Stevens I, Janssens V, Rondelez E, De Baere I, Derua R, Waelkens E, Goris J, Van Hoof C. An inactive protein phosphatase 2A population is associated with methylesterase and can be re-activated by the phosphotyrosyl phosphatase activator. Biochem J. 2004;380:111–119. doi: 10.1042/BJ20031643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fellner T, Lackner DH, Hombauer H, Piribauer P, Mudrak I, Zaragoza K, Juno C, Ogris E. A novel and essential mechanism determining specificity and activity of protein phosphatase 2A (PP2A) in vivo. Genes Dev. 2003;17:2138–2150. doi: 10.1101/gad.259903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordens J, Janssens V, Longin S, Stevens I, Martens E, Bultynck G, Engelborghs Y, Lescrinier E, Waelkens E, Goris J, Van Hoof C. The protein phosphatase 2A phosphatase activator is a novel peptidyl-prolyl cis/trans-isomerase. J Biol Chem. 2006;281:6349–6357. doi: 10.1074/jbc.M507760200. [DOI] [PubMed] [Google Scholar]
- 96.Leulliot N, Vicentini G, Jordens J, Quevillon-Cheruel S, Schiltz M, Barford D, van Tilbeurgh H, Goris J. Crystal structure of the PP2A phosphatase activator: implications for its PP2A-specific PPIase activity. Mol Cell. 2006;23:413–424. doi: 10.1016/j.molcel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 97.Kong M, Fox CJ, Mu J, Solt L, Xu A, Cinalli RM, Birnbaum MJ, Lindsten T, Thompson CB. The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science. 2004;306:695–698. doi: 10.1126/science.1100537. [DOI] [PubMed] [Google Scholar]
- 98.LeNoue-Newton M, Watkins GR, Zou P, Germane KL, McCorvey LR, Wadzinski BE, Spiller BW. The E3 ubiquitin ligase- and protein phosphatase 2A (PP2A)-binding domains of the Alpha4 protein are both required for Alpha4 to inhibit PP2A degradation. J Biol Chem. 2011;286:17665–17671. doi: 10.1074/jbc.M111.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nunbhakdi-Craig V, Schuechner S, Sontag JM, Montgomery L, Pallas DC, Juno C, Mudrak I, Ogris E, Sontag E. Expression of protein phosphatase 2A mutants and silencing of the regulatory B alpha subunit induce a selective loss of acetylated and detyrosinated microtubules. J Neurochem. 2007;101:959–971. doi: 10.1111/j.1471-4159.2007.04503.x. [DOI] [PubMed] [Google Scholar]
- 100.McCluskey A, Sim AT, Sakoff JA. Serine-threonine protein phosphatase inhibitors: development of potential therapeutic strategies. J Med Chem. 2002;45:1151–1175. doi: 10.1021/jm010066k. [DOI] [PubMed] [Google Scholar]
- 101.Swingle MR, Amable L, Lawhorn BG, Buck SB, Burke CP, Ratti P, Fischer KL, Boger DL, Honkanen RE. Structure-activity relationship studies of fostriecin, cytostatin, and key analogs, with PP1, PP2A, PP5, and (beta12-beta13)-chimeras (PP1/PP2A and PP5/PP2A), provide further insight into the inhibitory actions of fostriecin family inhibitors. J Pharmacol Exper Ther. 2009;331:45–53. doi: 10.1124/jpet.109.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W. Structural basis of PP2A inhibition by small t antigen. PLoS Biol. 2007;5:e202. doi: 10.1371/journal.pbio.0050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL, Roberts TM. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 104.Kamibayashi C, Estes R, Lickteig RL, Yang SI, Craft C, Mumby MC. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J Biol Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 105.Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–1996. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- 106.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 107.Habrukowich C, Han DK, Le A, Rezaul K, Pan W, Ghosh M, Li ZG, Dodge-Kafka K, Jiang XJ, Bittman R, Hla T. Sphingosine Interaction with Acidic Leucine-rich Nuclear Phosphoprotein-32A (ANP32A) Regulates PP2A Activity and Cyclooxygenase (COX)-2 Expression in Human Endothelial Cells. J Biol Chem. 2010;285:26825–26831. doi: 10.1074/jbc.M110.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The Substrate of Greatwall Kinase, Arpp19, Controls Mitosis by Inhibiting Protein Phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 109.Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 110.Kawabe T, Muslin AJ, Korsmeyer SJ. HOX11 interacts with protein phosphatases PP2A and PP1 and disrupts a G2/M cell-cycle checkpoint. Nature. 1997;385:454–458. doi: 10.1038/385454a0. [DOI] [PubMed] [Google Scholar]
- 111.Saito S, Miyaji-Yamaguchi M, Shimoyama T, Nagata K. Functional domains of template-activating factor-I as a protein phosphatase 2A inhibitor. Biochem Bioph Res Co. 1999;259:471–475. doi: 10.1006/bbrc.1999.0790. [DOI] [PubMed] [Google Scholar]
- 112.Adachi Y, Pavlakis GN, Copeland TD. Identification and Characterization of Set, a Nuclear Phosphoprotein Encoded by the Translocation Break Point in Acute Undifferentiated Leukemia. J Biol Chem. 1994;269:2258–2262. [PubMed] [Google Scholar]
- 113.Saito S, Miyaji-Yamaguchi M, Nagata K. Aberrant intracellular localization of set-can fusion protein, associated with a leukemia, disorganizes nuclear export. Int J Cancer. 2004;111:501–507. doi: 10.1002/ijc.20296. [DOI] [PubMed] [Google Scholar]
- 114.Vonlindern M, Vanbaal S, Wiegant J, Raap A, Hagemeijer A, Grosveld G. Can, a Putative Oncogene Associated with Myeloid Leukemogenesis, May Be Activated by Fusion of Its 3′ 1/2 to Different Genes - Characterization of the Set Gene. Mol Cell Biol. 1992;12:3346–3355. doi: 10.1128/mcb.12.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Christensen DJ, Chen YW, Oddo J, Matta KM, Neil J, Davis ED, Volkheimer AD, Lanasa MC, Friedman DR, Goodman BK, Gockerman JP, Diehl LF, de Castro CM, Moore JO, Vitek MP, Weinberg JB. SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target. Blood. 2011;118:4150–4158. doi: 10.1182/blood-2011-04-351072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cristobal I, Garcia-Orti L, Cirauqui C, Cortes-Lavaud X, Garcia-Sanchez MA, Calasanz MJ, Odero MD. Overexpression of SET is a recurrent event associated with poor outcome and contributes to protein phosphatase 2A inhibition in acute myeloid leukemia. Haematol-Hematol J. 2012;97:543–550. doi: 10.3324/haematol.2011.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, Mao H, Chang JS, Galietta A, Uttam A, Roy DC, Valtieri M, Bruner-Klisovic R, Caligiuri MA, Bloomfield CD, Marcucci G, Perrotti D. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 118.Van Vlierberghe P, van Grotel M, Tchinda J, Lee C, Beverloo HB, van der Spek PJ, Stubbs A, Cools J, Nagata K, Fornerod M, Buijs-Gladdines J, Horstmann M, van Wering ER, Soulier J, Pieters R, Meijerink JP. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood. 2008;111:4668–4680. doi: 10.1182/blood-2007-09-111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu H, Gu Y, Wang H, Yin J, Zheng G, Zhang Z, Lu M, Wang C, He Z. Overexpression of PP2A inhibitor SET oncoprotein is associated with tumor progression and poor prognosis in human non-small cell lung cancer. Oncotarget. 2015;6:14913–14925. doi: 10.18632/oncotarget.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Janghorban M, Farrell AS, Allen-Petersen BL, Pelz C, Daniel CJ, Oddo J, Langer EM, Christensen DJ, Sears RC. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proc Natl Acad Sci USA. 2014;111:9157–9162. doi: 10.1073/pnas.1317630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cristobal I, Rincon R, Manso R, Carames C, Zazo S, Madoz-Gurpide J, Rojo F, Garcia-Foncillas J. Deregulation of the PP2A inhibitor SET shows promising therapeutic implications and determines poor clinical outcome in patients with metastatic colorectal cancer. Clin Cancer Res. 2015;21:347–356. doi: 10.1158/1078-0432.CCR-14-0724. [DOI] [PubMed] [Google Scholar]
- 122.Anazawa Y, Nakagawa H, Furihara M, Ashida S, Tamura K, Yoshioka H, Shuin T, Fujioka T, Katagiri T, Nakamura Y. PCOTH, a novel gene overexpressed in prostate cancers, promotes prostate cancer cell growth through phosphorylation of oncoprotein TAF-l beta/SET. Cancer Res. 2005;65:4578–4586. doi: 10.1158/0008-5472.CAN-04-4564. [DOI] [PubMed] [Google Scholar]
- 123.Irie A, Harada K, Araki N, Nishimura Y. Phosphorylation of SET Protein at Ser171 by Protein Kinase D2 Diminishes Its Inhibitory Effect on Protein Phosphatase 2A. Plos One. 2012;7:e51242. doi: 10.1371/journal.pone.0051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arnaud L, Chen S, Liu F, Li B, Khatoon S, Grundke-Iqbal I, Iqbal K. Mechanism of inhibition of PP2A activity and abnormal hyperphosphorylation of tau by I2(PP2A)/SET. FEBS Lett. 2011;585:2653–2659. doi: 10.1016/j.febslet.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu G, Yan T, Feng Y, Liu X, Xia Y, Luo H, Wang JZ, Wang X. Ser9 phosphorylation causes cytoplasmic detention of I2PP2A/SET in Alzheimer disease. Neurobiol Aging. 2013;34:1748–1758. doi: 10.1016/j.neurobiolaging.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 126.Saito S, Miyaji-Yamaguchi M, Shimoyama T, Nagata K. Functional domains of template-activating factor-I as a protein phosphatase 2A inhibitor. Biochem Bioph Res Co. 1999;259:471–475. doi: 10.1006/bbrc.1999.0790. [DOI] [PubMed] [Google Scholar]
- 127.Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The Ldl Receptor Related Protein, Lrp, Is an Apolipoprotein-E-Binding Protein. Nature. 1989;341:162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- 128.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low-Density Lipoprotein Receptor-Related Protein Mediates Uptake of Cholesteryl Esters Derived from Apoprotein-E-Enriched Lipoproteins. Proc Natl Acad Sci USA. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brown MS, Goldstein JL. A Receptor-Mediated Pathway for Cholesterol Homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 130.Avila EM, Holdsworth G, Sasaki N, Jackson RL, Harmony JAK. Apoprotein-E Suppresses Phytohemagglutinin-Activated Phospholipid Turnover in Peripheral-Blood Mononuclear-Cells. J Biol Chem. 1982;257:5900–5909. [PubMed] [Google Scholar]
- 131.Curtiss LK, Edgington TS. The Biologic Activity of the Immunoregulatory Lipoprotein, Ldl-in, Is Independent of Its Free Fatty-Acid Content. J Immunol. 1981;126:1382–1386. [PubMed] [Google Scholar]
- 132.Pepe MG, Curtiss LK. Apolipoprotein-E Is a Biologically-Active Constituent of the Normal Immunoregulatory Lipoprotein, Ldl-In. J Immunol. 1986;136:3716–3723. [PubMed] [Google Scholar]
- 133.Agarwal A, MacKenzie RJ, Pippa R, Eide CA, Oddo J, Tyner JW, Sears R, Vitek MP, Odero MD, Christensen DJ, Druker BJ. Antagonism of SET Using OP449 Enhances the Efficacy of Tyrosine Kinase Inhibitors and Overcomes Drug Resistance in Myeloid Leukemia. Clin Cancer Res. 2014;20:2092–2103. doi: 10.1158/1078-0432.CCR-13-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Agarwal A, MacKenzie R, Oddo J, Vitek MP, Christensen DJ, Druker BJ. A Novel SET Antagonist (OP449) Is Cytotoxic to CML Cells, Including the Highly-Resistant BCR-ABL(T315I) Mutant, and Demonstrates Enhanced Efficacy in Combination with ABL Tyrosine Kinase Inhibitors. Blood. 2011;118:1603–1604. [Google Scholar]
- 135.Christensen DJ, Ohkubo N, Oddo J, Van Kanegan MJ, Neil J, Li FQ, Colton CA, Vitek MP. Apolipoprotein E and Peptide Mimetics Modulate Inflammation by Binding the SET Protein and Activating Protein Phosphatase 2A. J Immunol. 2011;186:2535–2542. doi: 10.4049/jimmunol.1002847. [DOI] [PubMed] [Google Scholar]
- 136.Switzer CH, Cheng RYS, Vitek TM, Christensen DJ, Wink DA, Vitek MP. Targeting SET/I(2)PP2A oncoprotein functions as a multi-pathway strategy for cancer therapy. Oncogene. 2011;30:2504–2513. doi: 10.1038/onc.2010.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suzuki S, Li XK, Enosawa S, Shinomiya T. A new immunosuppressant, FTY720, induces bcl-2-associated apoptotic cell death in human lymphocytes. Immunology. 1996;89:518–523. doi: 10.1046/j.1365-2567.1996.d01-777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suzuki S, Enosawa S, Kakefuda T, Shinomiya T, Amari M, Naoe S, Hoshino Y, Chiba K. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation. Transplantation. 1996;61:200–205. doi: 10.1097/00007890-199601270-00006. [DOI] [PubMed] [Google Scholar]
- 139.Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Brit J Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 141.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 142.Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Brit J Pharmacol. 2003;138:1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen CS, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roberts KG, Smith AM, McDougall F, Carpenter H, Horan M, Neviani P, Powell JA, Thomas D, Guthridge MA, Perrotti D, Sim AT, Ashman LK, Verrills NM. Essential Requirement for PP2A Inhibition by the Oncogenic Receptor c-KIT Suggests PP2A Reactivation as a Strategy to Treat c-KIT+ Cancers. Cancer Res. 2010;70:5438–5447. doi: 10.1158/0008-5472.CAN-09-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide Activates Heterotrimeric Protein Phosphatase-2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 146.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Bio. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 147.Kim SW, Kim HJ, Chun YJ, Kim MY. Ceramide Produces Apoptosis Through Induction of p27kip1 by Protein Phosphatase 2A-dependent Akt Dephosphorylation in PC-3 Prostate Cancer Cells. J Toxicol Env Heal A. 2010;73:1465–1476. doi: 10.1080/15287394.2010.511553. [DOI] [PubMed] [Google Scholar]
- 148.Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, Lin YS. GSK-3 beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci. 2007;120:2935–2943. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- 149.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed Cell-Death Induced by Ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 150.Mukhopadhyay A, Saddoughi SA, Song PF, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, Ogretmen B. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. Faseb J. 2009;23:751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Laine A, Sihto H, Come C, Rosenfeldt MT, Zwolinska A, Niemela M, Khanna A, Chan EK, Kahari VM, Kellokumpu-Lehtinen PL, Sansom OJ, Evan GI, Junttila MR, Ryan KM, Marine JC, Joensuu H, Westermarck J. Senescence sensitivity of breast cancer cells is defined by positive feedback loop between CIP2A and E2F1. Cancer Discov. 2013;3:182–197. doi: 10.1158/2159-8290.CD-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Puustinen P, Rytter A, Mortensen M, Kohonen P, Moreira JM, Jaattela M. CIP2A oncoprotein controls cell growth and autophagy through mTORC1 activation. J Cell Biol. 2014;204:713–727. doi: 10.1083/jcb.201304012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guenebeaud C, Goldschneider D, Castets M, Guix C, Chazot G, Delloye-Bourgeois C, Eisenberg-Lerner A, Shohat G, Zhang M, Laudet V, Kimchi A, Bernet A, Mehlen P. The dependence receptor UNC5H2/B triggers apoptosis via PP2A-mediated dephosphorylation of DAP kinase. Mol Cell. 2010;40:863–876. doi: 10.1016/j.molcel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 154.Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, Ala-Aho R, Nielsen C, Ivaska J, Taya Y, Lu SL, Lin S, Chan EK, Wang XJ, Grènman R, Kast J, Kallunki T, Sears R, Kähäri VM. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 155.Bockelman C, Koskensalo S, Hagstrom J, Lundin M, Ristimaki A, Haglund C. CIP2A overexpression is associated with c-Myc expression in colorectal cancer. Cancer Biol Ther. 2012;13:289–295. doi: 10.4161/cbt.18922. [DOI] [PubMed] [Google Scholar]
- 156.Bockelman C, Lassus H, Hemmes A, Leminen A, Westermarck J, Haglund C, Butzow R, Ristimaki A. Prognostic role of CIP2A expression in serous ovarian cancer. Br J Cancer. 2011;105:989–995. doi: 10.1038/bjc.2011.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu XL, Jonkers J, Ivaska J, Isola J, Darbon JM, Kallioniemi O, Thézenas S, Westermarck J. CIP2A Is Associated with Human Breast Cancer Aggressivity. Clin Cancer Res. 2009;15:5092–5100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 158.Dong QZ, Wang Y, Dong XJ, Li ZX, Tang ZP, Cui QZ, Wang EH. CIP2A is Overexpressed in Non-Small Cell Lung Cancer and Correlates with Poor Prognosis. Ann Surg Oncol. 2011;18:857–865. doi: 10.1245/s10434-010-1313-8. [DOI] [PubMed] [Google Scholar]
- 159.Fang YY, Li ZT, Wang XX, Zhang SL. CIP2A is overexpressed in human ovarian cancer and regulates cell proliferation and apoptosis. Tumor Biol. 2012;33:2299–2306. doi: 10.1007/s13277-012-0492-2. [DOI] [PubMed] [Google Scholar]
- 160.He H, Wu G, Li WJ, Cao YC, Liu YF. CIP2A Is Highly Expressed in Hepatocellular Carcinoma and Predicts Poor Prognosis. Diagn Mol Pathol. 2012;21:143–149. doi: 10.1097/PDM.0b013e318249fd8b. [DOI] [PubMed] [Google Scholar]
- 161.Huang LP, Adelson ME, Mordechai E, Trama JP. CIP2A expression is elevated in cervical cancer. Cancer Biomark. 2010;8:309–317. doi: 10.3233/CBM-2011-0220. [DOI] [PubMed] [Google Scholar]
- 162.Huang LP, Savoly D, Sidi AA, Adelson ME, Mordechai E, Trama JP. CIP2A protein expression in high-grade, high-stage bladder cancer. Cancer Medicine. 2012;1:76–81. doi: 10.1002/cam4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khanna A, Bockelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, Junnila S, Murphy DJ, Evan GI, Haglund C, Westermarck J, Ristimäki A. MYC-Dependent Regulation and Prognostic Role of CIP2A in Gastric Cancer. J Natl Cancer Inst. 2009;101:793–805. doi: 10.1093/jnci/djp103. [DOI] [PubMed] [Google Scholar]
- 164.Kikuno R, Nagase T, Ishikawa K, Hirosawa M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6:197–205. doi: 10.1093/dnares/6.3.197. [DOI] [PubMed] [Google Scholar]
- 165.Li WJ, Ge Z, Liu C, Liu ZF, Bjorkholm M, Jia JH, Xu DW. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14:3722–3728. doi: 10.1158/1078-0432.CCR-07-4137. [DOI] [PubMed] [Google Scholar]
- 166.Qu W, Li WJ, Wei L, Xing LG, Wang XW, Yu JM. CIP2A is overexpressed in esophageal squamous cell carcinoma. Med Oncol. 2012;29:113–118. doi: 10.1007/s12032-010-9768-9. [DOI] [PubMed] [Google Scholar]
- 167.Rantanen T, Kauttu T, Akerla J, Honkanen T, Krogerus L, Salo J, Paavonen T, Oksala N. CIP2A expression and prognostic role in patients with esophageal adenocarcinoma. Med Oncol. 2013;30:684. doi: 10.1007/s12032-013-0684-7. [DOI] [PubMed] [Google Scholar]
- 168.Sung WW, Wang YC, Lin PL, Cheng YW, Chen CY, Wu TC, Lee H. IL-10 Promotes Tumor Aggressiveness via Upregulation of CIP2A Transcription in Lung Adenocarcinoma. Clin Cancer Res. 2013;19:4092–4103. doi: 10.1158/1078-0432.CCR-12-3439. [DOI] [PubMed] [Google Scholar]
- 169.Vaarala MH, Vaisanen MR, Ristimaki A. CIP2A expression is increased in prostate cancer. J Exp Clin Canc Res. 2010;29:136. doi: 10.1186/1756-9966-29-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wiegering A, Pfann C, Uthe FW, Otto C, Rycak L, Mader U, Gasser M, Waaga-Gasser AM, Eilers M, Germer CT. CIP2A Influences Survival in Colon Cancer and Is Critical for Maintaining Myc Expression. Plos One. 2013;8:e75292. doi: 10.1371/journal.pone.0075292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu GZ, Liu GH, Dong J, Jin YY. Clinical implications of CIP2A protein expression in breast cancer. Med Oncol. 2013;30:524. doi: 10.1007/s12032-013-0524-9. [DOI] [PubMed] [Google Scholar]
- 172.Zhai M, Cong L, Han YX, Tu GJ. CIP2A is overexpressed in osteosarcoma and regulates cell proliferation and invasion. Tumor Biol. 2014;35:1123–1128. doi: 10.1007/s13277-013-1150-z. [DOI] [PubMed] [Google Scholar]
- 173.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu Z, Ma L, Wen ZS, Hu Z, Wu FQ, Li W, Liu JS, Zhou GB. Cancerous inhibitor of PP2A is targeted by natural compound celastrol for degradation in non-small-cell lung cancer. Carcinogenesis. 2014;35:905–914. doi: 10.1093/carcin/bgt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Z, Ma L, Wen ZS, Cheng YX, Zhou GB. Ethoxysanguinarine Induces Inhibitory Effects and Downregulates CIP2A in Lung Cancer Cells. ACS Med Chem Lett. 2014;5:113–118. doi: 10.1021/ml400341k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li WG, Dai FY, Cheng YX, Yin GF, Bi JL, Li DP. Identification of Porcine Reproductive and Respiratory Syndrome Virus Inhibitors Through an Oriented Screening on Natural Products. Chem Res Chinese U. 2013;29:290–293. doi: 10.1007/s40242-013-2300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: A novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 178.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: Lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 179.Tseng LM, Liu CY, Chang KC, Chu PY, Shiau CW, Chen KF. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 2012;14:R68. doi: 10.1186/bcr3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen KF, Yeh PY, Hsu C, Hsu CH, Lu YS, Hsieh HP, Chen PJ, Cheng AL. Bortezomib Overcomes Tumor Necrosis Factor-related Apoptosis-inducing Ligand Resistance in Hepatocellular Carcinoma Cells in Part through the Inhibition of the Phosphatidylinositol 3-Kinase/Akt Pathway. J Biol Chem. 2009;284:11121–11133. doi: 10.1074/jbc.M806268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen KF, Pao KC, Su JC, Chou YC, Liu CY, Chen HJ, Huang JW, Kim I, Shiau CW. Development of erlotinib derivatives as CIP2A-ablating agents independent of EGFR activity. Bioorgan Med Chem. 2012;20:6144–6153. doi: 10.1016/j.bmc.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 182.Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–7755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 183.Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory B alpha subunit. Biochem J. 1999;339:241–246. [PMC free article] [PubMed] [Google Scholar]
- 184.Ogris E, Gibson DM, Pallas DC. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene. 1997;15:911–917. doi: 10.1038/sj.onc.1201259. [DOI] [PubMed] [Google Scholar]
- 185.Lee J, Chen Y, Tolstykh T, Stock J. A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc Natl Acad Sci USA. 1996;93:6043–6047. doi: 10.1073/pnas.93.12.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wei HJ, Ashby DG, Moreno CS, Ogris E, Yeong FM, Corbett AH, Pallas DC. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits CDC55p and RTS1p. J Biol Chem. 2001;276:1570–1577. doi: 10.1074/jbc.M008694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 188.Lee J, Stock J. Protein Phosphatase-2a Catalytic Subunit Is Methyl-Esterified at Its Carboxyl-Terminus by a Novel Methyltransferase. J Biol Chem. 1993;268:19192–19195. [PubMed] [Google Scholar]
- 189.Guenin S, Schwartz L, Morvan D, Steyaert JM, Poignet A, Madelmont JC, Demidem A. PP2A activity is controlled by methylation and regulates oncoprotein expression in melanoma cells: A mechanism which participates in growth inhibition induced by chloroethylnitrosourea treatment. Int J Oncol. 2008;32:49–57. [PubMed] [Google Scholar]
- 190.Godeneche D, Rapp M, Thierry A, Laval F, Madelmont JC, Chollet P, Veyre A. DNA Damage Induced by a New 2-Chloroethyl Nitrosourea on Malignant-Melanoma Cells. Cancer Res. 1990;50:5898–5903. [PubMed] [Google Scholar]
- 191.Puustinen P, Junttila MR, Vanhatupa S, Sablina AA, Hector ME, Teittinen K, Raheem O, Ketola K, Lin S, Kast J, Haapasalo H, Hahn WC, Westermarck J. PME-1 protects extracellular signal-regulated kinase pathway activity from protein phosphatase 2A-mediated inactivation in human malignant glioma. Cancer Res. 2009;69:2870–2877. doi: 10.1158/0008-5472.CAN-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bachovchin DA, Mohr JT, Speers AE, Wang C, Berlin JM, Spicer TP, Fernandez-Vega V, Chase P, Hodder PS, Schurer SC, Nomura DK, Rosen H, Fu GC, Cravatt BF. Academic cross-fertilization by public screening yields a remarkable class of protein phosphatase methylesterase-1 inhibitors. Proc Natl Acad Sci USA. 2011;108:6811–6816. doi: 10.1073/pnas.1015248108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bachovchin DA, Zuhl AM, Speers AE, Wolfe MR, Weerapana E, Brown SJ, Rosen H, Cravatt BF. Discovery and optimization of sulfonyl acrylonitriles as selective, covalent inhibitors of protein phosphatase methylesterase-1. J Med Chem. 2011;54:5229–5236. doi: 10.1021/jm200502u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cristobal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD. PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia. 2011;25:606–614. doi: 10.1038/leu.2010.294. [DOI] [PubMed] [Google Scholar]
- 196.Cristobal I, Rincon R, Manso R, Madoz-Gurpide J, Carames C, del Puerto-Nevado L, Rojo F, Garcia-Foncillas J. Hyperphosphorylation of PP2A in colorectal cancer and the potential therapeutic value showed by its forskolin-induced dephosphorylation and activation. Biochim Biophys Acta. 2014;1842:1823–1829. doi: 10.1016/j.bbadis.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 197.Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ. A novel cAMP-stimulated pathway in protein phosphatase 2A activation. J Pharmacol Exp Ther. 2002;302:111–118. doi: 10.1124/jpet.302.1.111. [DOI] [PubMed] [Google Scholar]
- 198.Gutierrez A, Pan L, Groen RW, Baleydier F, Kentsis A, Marineau J, Grebliunaite R, Kozakewich E, Reed C, Pflumio F, Poglio S, Uzan B, Clemons P, VerPlank L, An F, Burbank J, Norton S, Tolliday N, Steen H, Weng AP, Yuan H, Bradner JE, Mitsiades C, Look AT, Aster JC. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J Clin Invest. 2014;124:644–655. doi: 10.1172/JCI65093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kar S, Palit S, Ball WB, Das PK. Carnosic acid modulates Akt/IKK/NF-kappa B signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis. 2012;17:735–747. doi: 10.1007/s10495-012-0715-4. [DOI] [PubMed] [Google Scholar]
- 200.Birringer M, EyTina JH, Salvatore BA, Neuzil J. Vitamin E analogues as inducers of apoptosis: structure-function relation. Br J Cancer. 2003;88:1948–1955. doi: 10.1038/sj.bjc.6600981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Burton GW, Traber MG. Vitamin-E - Antioxidant Activity, Biokinetics, and Bioavailability. Annu Rev Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 202.Gu XB, Song XD, Dong YH, Cai H, Walters E, Zhang RS, Pang XW, Xie TP, Guo YH, Sridhar R, Califano JA. Vitamin E succinate induces ceramide-mediated apoptosis in head and neck squamous cell carcinoma in vitro and in vivo. Clin Cancer Res. 2008;14:1840–1848. doi: 10.1158/1078-0432.CCR-07-1811. [DOI] [PubMed] [Google Scholar]
- 203.Lawson KA, Anderson K, Menchaca M, Atkinson J, Sun LZ, Knight V, Gilbert BE, Conti C, Sanders BG, Kline K. Novel vitamin E analogue decreases syngeneic mouse mammary tumor burden and reduces lung metastasis. Mol Cancer Ther. 2003;2:437–444. [PubMed] [Google Scholar]
- 204.Neuzil J, Svensson I, Weber T, Weber C, Brunk UT. alpha-tocopheryl succinate-induced apoptosis in Jurkat T cells involves caspase-3 activation, and both lysosomal and mitochondrial destabilisation. FEBS Lett. 1999;445:295–300. doi: 10.1016/s0014-5793(99)00141-6. [DOI] [PubMed] [Google Scholar]
- 205.Neuzil J, Tomasetti M, Zhao Y, Dong LF, Birringer M, Wang XF, Low P, Wu K, Salvatore BA, Ralph SJ. Vitamin E analogs, a novel group of “mitocans,” as anticancer agents: The importance of being redox-silent. Mol Pharmacol. 2007;71:1185–1199. doi: 10.1124/mol.106.030122. [DOI] [PubMed] [Google Scholar]
- 206.Qian M, Kralova J, Yu WP, Bose HR, Dvorak M, Sanders BG, Kline K. c-Jun involvement in vitamin E succinate induced apoptosis of reticuloendotheliosis virus transformed avian lymphoid cells. Oncogene. 1997;15:223–230. doi: 10.1038/sj.onc.1201181. [DOI] [PubMed] [Google Scholar]
- 207.Wang XF, Dong LF, Zhao Y, Tomasetti M, Wu K, Neuzil J. Vitamin E analogues as anticancer agents: Lessons from studies with alpha-tocopheryl succinate. Mol Nutr Food Res. 2006;50:675–685. doi: 10.1002/mnfr.200500267. [DOI] [PubMed] [Google Scholar]
- 208.You HH, Yu WP, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induces MDA-MB-435 and MCF-7 human breast cancer cells to undergo differentiation. Cell Growth Differ. 2001;12:471–480. [PubMed] [Google Scholar]
- 209.Yu WP, Liao QY, Hantash FM, Sanders BG, Kline K. Activation of extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001;61:6569–6576. [PubMed] [Google Scholar]
- 210.Zhang Y, Ni J, Messing EM, Chang E, Yang CR, Yeh SY. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. Proc Natl Acad Sci USA. 2002;99:7408–7413. doi: 10.1073/pnas.102014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Neuzil J, Weber T, Schroder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Negre-Salvayre A, Sticha M, Coffey RJ, Weber C. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. Faseb J. 2001;15:403–415. doi: 10.1096/fj.00-0251com. [DOI] [PubMed] [Google Scholar]
- 212.Huang PH, Wang DS, Chuang HC, Wei S, Kulp SK, Chen CS. alpha-Tocopheryl succinate and derivatives mediate the transcriptional repression of androgen receptor in prostate cancer cells by targeting the PP2A-JNK-Sp1-signaling axis. Carcinogenesis. 2009;30:1125–1131. doi: 10.1093/carcin/bgp112. [DOI] [PMC free article] [PubMed] [Google Scholar]