Abstract
Little is known about the white matter integrity of cerebellar-cortical pathways in individuals with dyslexia. Building on previous findings of decreased volume in the anterior lobe of the cerebellum, we utilized novel cerebellar segmentation procedures and probabilistic tractography to examine tracts that connect the anterior lobe of the cerebellum and cortical regions typically associated with reading: the temporoparietal (TP), occipitotemporal (OT), and inferior frontal (IF) regions. The sample included 29 reading impaired children and 27 typical readers. We found greater fractional anisotropy (FA) for the poor readers in tracts connecting the cerebellum with TP and IF regions relative to typical readers. In the OT region, FA was greater for the older poor readers, but smaller for the younger ones. This study provides evidence for discrete, regionally-bound functions of the cerebellum and suggests that projections from the anterior cerebellum appear to have a regulatory effect on cortical pathways important for reading.
Keywords: Cerebellum, DTI, Tractography, Dyslexia, Reading, Children
1. Introduction
Reports of structural differences, reduced functional activation, and atypical connectivity (functional and structural) in adults and children with dyslexia relative to typical readers have contributed to the idea of dyslexia as a “brain based” disorder with genetic and environmental components (Fletcher, Lyon, Fuchs, & Barnes, 2007; Gabrieli, 2009). Studies of children and adults with dyslexia have reliably documented the contributions of left hemisphere regions typically associated with language and sensory processing: temporoparietal (TP), occipitotemporal (OT), inferior frontal (IF), and to a lesser extent subregions of the corpus callosum, in accurate and fluent word reading (Fletcher et al., 2007; Pugh et al., 2010). Despite the growing body of literature on the role of the cerebellum in speech and language (Ackermann, 2013), and reports of regional structural differences in the cerebellum of children with dyslexia (Fernandez et al., 2013; Eckert et al., 2003, 2005; Kibby & Hynd, 2008; Leonard, Eckert, Given, Berninger, & Eden, 2006), very little is known about the integrity of cerebellar-cortical white matter in individuals with dyslexia relative to typical readers. The lack of empirical data is especially noteworthy because a cerebellar theory of dyslexia has been proposed (Nicolson & Fawcett, 2005). The present study examined cerebellar-cortical white matter pathways in children with dyslexia relative to typical readers to evaluate measures of white matter integrity which may help to explain the role of regional volumetric differences in the cerebellum.
1.1. Cerebellar theory of dyslexia
The cerebellar theory of dyslexia suggests that cerebellar abnormalities reduce the automaticity of decoding skills in children with dyslexia. Proponents of the theory argue that the cerebellum is active during early stages of skill acquisition (Nicolson & Fawcett, 2005, 2007), a process with which some children with dyslexia have difficulty (Reynolds, Nicolson, & Hambly, 2003). Theoretically, associated procedural learning deficits could prevent automatization of accurate word decoding and phonological processing in children with dyslexia.
This hypothesis has been evaluated behaviorally by comparing children with dyslexia and typically developing children on neuropsychological and cognitive tasks presumably associated with the cerebellum (Nicolson & Fawcett, 1994, 1999). There have been efforts to train the cerebellum through physical exercise, with claims that reading and cerebellar functions improved (Reynolds et al., 2003). However, these findings have been controversial because, among other issues, the children with dyslexia were defined in part by performance on these tasks (Bishop, 2007). In other studies, the same cerebellar tasks have not been strongly related to reading or to reading difficulties (Barth et al., 2010). Finally, it is not clear why the cerebellum would be involved in accurate decoding, a skill typically measured by untimed single-word reading tests without regard for efficiency, as opposed to problems with reading fluency, a skill that requires automaticity as proposed by the theory.
Despite these issues, there is evidence from postmortem studies and from quantitative MRI analyses for differences in cerebellar structure and volume between children and adults with dyslexia and typically developing comparison groups. To appreciate these differences, we will first review structural MRI studies of the cerebellum and then diffusion tensor imaging (DTI) studies of dyslexia more generally. Thus far, there are no published DTI studies of white matter integrity involving the cerebellum in individuals with dyslexia.
1.2. Structural MRI studies of the cerebellum
Structural imaging studies have been conducted that yielded findings concerning the cerebellum in children and adults with dyslexia. This area of research was influenced by early, small-sample postmortem studies (5 with dyslexia, 7 controls) in which abnormalities in the medial (Galaburda, Menard, & Rosen, 1994) and lateral geniculate nuclei (Livingstone, Rosen, Drislane, & Galaburda, 1991), auditory cortex (Galaburda & Kemper, 1979), and primary visual cortex (Eckert, 2004; Jenner, Rosen, & Galaburda, 1999) were identified.
Subsequent volumetric MRI studies of the cerebellum have yielded inconsistent findings in adult samples. Some have found differences in regional cerebellar volume between adults identified as having dyslexia (either by testing or history) and those without such problems (Brambati et al., 2004; Brown et al., 2001; Leonard et al., 2001; Rae et al., 2002). Other studies have found no differences in cerebellar volume using similar methods (Menghini et al., 2008; Pernet, Poline, Demonet, & Rousselet, 2009; ; Laylock et al., 2008). However, reduced volume in the right anterior lobe of the cerebellum has been more reliably identified in reading impaired children relative to typical readers (Eckert et al., 2003, 2005; Kibby & Hynd, 2008; Leonard et al., 2006).
Fernandez et al. (2013) investigated the role of regional variation in cerebellar anatomy in children with single-word decoding impairments (N = 23), children with impairment in fluency alone (N = 8), and typically developing children (N = 16). Children with single-word decoding impairments demonstrated no statistically significant differences in overall gray and white matter volumes or cerebellar asymmetry; however, reduced volume bilaterally in the anterior lobe of the cerebellum relative to typical readers was observed. These results supported previous findings in the child literature suggesting cerebellar involvement in dyslexia, and provided additional groundwork for the proposed study on the integrity of white matter connecting the cerebellum and cortical regions that are typically associated with reading impairment.
1.3. White matter integrity in adults and children with reading impairment
Fractional anisotropy (FA) is a complex measure of white matter structural integrity. It is a composite measure that expresses the proportion of the longest eigenvalue, λ1, to the two shorter eigenvalues, λ2 and λ3, in an ellipsoid (Beaulieu, 2002). In the central nervous system, λ1 represents the water diffusivity parallel to the axonal fibers and is referred to as the axial diffusivity (AD). The water diffusivities perpendicular to the axonal fibers, λ2 and λ3, are averaged and referred to the radial diffusivity (RD) (Basser, 1995; Basser, Pajevic, Pierpaoli, Duda, & Aldroubi, 2000; Song et al., 2002; Xue, van Zijl, Crain, Solaiyappan, & Mori, 1999). Changes in RD in the absence of changes in AD are typically associated with myelin abnormalities (Keller & Just, 2009; Beaulieu, 2002; Song et al., 2002, 2005) while changes in AD in the absence of changes in RD are typically associated with an increase in axon diameter (Keller & Just, 2009; Dougherty et al., 2007).
Studies of white matter integrity in adults and children with dyslexia have generally focused on regions typically associated with the language network and do not involve the cerebellum. In adults, DTI studies have identified differences in FA in the frontotemporal and TP regions important for language comprehension and articulatory processes (Fletcher et al., 2007; Klingberg et al., 2000; Pugh et al., 2010; Steinbrink et al., 2008). DTI studies of children with dyslexia have also implicated bilateral (Deutsch et al., 2005) and left TP regions (Niogi & McCandliss, 2006; Rimrodt, Peterson, Denkla, Kaufmann, & Cutting, 2010) and, more broadly, the left superior longitudinal fasciculus (Carter et al., 2009). Abnormal orientation of fibers (specifically an increase in superior–inferior oriented fibers in its TP projection that ordinarily runs anterior-laterally) in the right superior longitudinal fasciculus (Carter et al.) and higher FA in right frontal regions have also been described (Rimrodt et al., 2010). Taking a slightly different approach, longitudinal studies of children with dyslexia reported that left TP regions were significant predictors of reading outcomes (Hoeft et al., 2011; Meyers et al., 2014) while intensive intervention was associated with increases in FA in the left centrum semiovale (anterior to TP region) (Keller & Just, 2009).
In the only published DTI study which included the cerebellum, Richards et al. (2008) also conducted a whole-brain DTI study of fathers of children in a familial study of dyslexia, 14 with dyslexia and 7 without dyslexia. Typical readers had greater FA in language-related white matter tracts (28 bilateral frontal, temporal, parietal, and occipital areas as defined by Anatomical Automatic Labeling (AAL) atlas boundaries and an additional 7 by DTI atlas boundaries). Notably, 9 regions showed greater FA for the group with dyslexia, including the left and right cerebellum (using the AAL atlas) and middle cerebellar peduncle (using the DTI atlas boundaries). The presence of greater FA raises the question of whether the cerebellum is somehow over-compensating for a weak reading network (Richards et al., 2008).
In an effort to summarize DTI findings in white matter, Vandermosten, Boets, Wouters, and Ghesquiere (2012) conducted an activation likelihood estimation (ALE) analysis resulting in two clusters of significant ALE value: a region near the left TP region and the IF gyrus (Vandermosten, Boets, Wouters et al., 2012). The lack of reported cerebellar findings may reflect the fact that the cerebellum is rarely included in quantitative analyses. These studies are notable because they have continued to advance the notion that myelination, important for rapid conduction of action potentials, could disturb accurate decoding and the transmission of rapidly changing stimuli (Klingberg et al., 2000).
1.4. Rationale
Evidence of differences in white matter between adults and children with dyslexia and typically developing readers clearly demonstrates the importance of a neural networks approach to the study of dyslexia. However, because the cerebellum is rarely included in analysis, very little is known about white matter integrity in cerebellar-cortical tracts and how they relate to impaired reading. Conventional descriptions of cerebellar-cortical connectivity describe a circuit in which motor cortices influence the cerebellum via the pons and where efferent projections from the deep cerebellar nuclei influence regions of the cortex through the ventrolateral nucleus of the thalamus (Schmahmann & Pandya, 2008). Although cerebellar cortical fibers are mostly crossed, a fair number of projections from the neocortex (20–30%) terminate on ipsilateral cerebellum via the pons (Krienen & Buckner, 2009). It has been proposed that these circuits play a critical role in the automatization and optimization of behavior, including cognition and emotion (Schmahmann & Pandya, 2008). This is important because a direct role of the cerebellum in reading is not clearly established; if the cerebellum is involved, it may reflect tracts that originate in the cerebellum and influence cortical regions involved in reading.
Laylock et al. (2008) suggested that the changes in white matter they detected in a postmortem sample in addition to an abnormal ratio of metabolites in the right cerebellar hemisphere may indicate either excessive connectivity or abnormal myelination. As previously mentioned, Richards et al. (2008) found greater FA in the left and right cerebellum in a group of individuals with dyslexia relative to typical readers. This study was limited by lack of regional segmentation but it represents an important area for future research. Finally, Fernandez et al. (2013) found less volume in the anterior lobe of the cerebellum bilaterally in a group of children with dyslexia relative to typical readers. This study aimed to examine the white matter integrity of cerebellar-cortical tracts in children and adolescents with specific deficits in decoding. To that end, we examined ipsilateral and contralateral tracts that originate in the anterior lobe of the cerebellum and connect with cortical regions typically associated with reading: the TP region, OT region, and IF gyrus.
1.5. Hypotheses
Based on the results obtained by Richards et al. (2008) in which greater FA was observed in adults with dyslexia in the cerebellum bilaterally and consistent with our previous findings (Fernandez et al., 2013) demonstrating reduced volume of the anterior cerebellum bilaterally in children with dyslexia, we expected to find (1) greater FA values in the white matter fiber tracts that connect the anterior cerebellum and the TP region bilaterally in the group of children with dyslexia relative to typical readers. This would indicate larger than expected axonal volume, excessive or greater myelination, or both. It should be noted that the development of the anterior lobe of the cerebellum has an inverted U-shaped trajectory with age at peak volume at 13.5 years for girls and 15.7 years for boys (Tiemer et al., 2010), therefore it will be important to consider age in the analysis.
An alternate hypothesis is that only the contralateral white matter between the right anterior lobe of the cerebellum and the left TP region will demonstrate abnormal white matter integrity. This result would be consistent with existing literature implicating both the right anterior lobe of the cerebellum and the left TP region in dyslexia.
Structural differences in the IF gyrus and OT regions have also been associated with dyslexia (Fletcher et al., 2007). The white matter pathways connecting these areas, which respectively contribute to articulatory mapping and graphemic analysis, with the cerebellum have not been adequately investigated. However, given the role of these regions in accurate decoding, we expect to find “abnormal” (either greater or lesser) FA in the white matter connections between the anterior cerebellum and these regions.
2. Methods
2.1. Participants
Participants in the present study were recruited from two large-scale reading intervention studies (Vaughn, Cirino et al., 2010; Vaughn, Wanzek et al., 2010) that began in 2005 and extended through 2010. All students and their parents provided informed consent in accordance with the rules and regulations of the Committees for the Protection of Human Subjects (CPHS) of the University of Houston and the University of Texas Health Science Center in Houston. In the first study sample, typical and struggling readers in grade 5–7 were randomly selected to participate in the reading intervention study during grades 6–8 of the following year. Students were excluded from participation in the study if: (a) they were enrolled in a special education life skills class; (b) their State-Developed Alternative Assessment (SDAA) Reading performance levels were equivalent to a 3rd grade reading level or lower; (c) they presented a significant sensory disability; or (d) were not taught primarily in English. These children were assessed on a variety of reading measures pre- and post-intervention. Of these children, 21 were included in the present sample. In the second study sample, children with poor reading skills were identified in the first grade. Their oral reading progress was monitored every 2 weeks for 8 weeks to ensure their at-risk status and children who continually failed a reading screener went on to participate in an intervention program, 15 of which were included in the present study (Denton et al., 2011).
A third study sample of third graders with math and reading difficulties recruited for a math intervention study which began in 2003 yielded 6 participants in the group of children with dyslexia (Fuchs et al., 2007). Additionally, a study of children with spina bifida (Juranek, Dennis, Cirino, El-Messidi, & Fletcher, 2010) that began in 2005 provided 14 of the typically developing children. Selection criteria and imaging protocols were deliberately similar across all these studies to build a larger sample for neuroimaging analyses even as technological advances and improved scanner capabilities were developed.
Only right-handed children were included in the present study. The children completed a structural MRI at baseline prior to intervention as part of their participation in the functional imaging study (e.g. magnetoencephalography). From these children, two groups were formed. Children in the group with decoding impairment scored below the 26th percentile on a measure of single word decoding. Most of these children also had comprehension and fluency deficits. Typically developing children did not show impairment on reading measures and had no history of reading difficulties. The 25th percentile was selected because it has been commonly employed in studies of people with learning disabilities (Fletcher et al., 1994). All children had a composite IQ score of at least 70 on the Kaufman Brief Intelligence Test-2nd edition (KBIT-2) (Kaufman & Kaufman, 2004) to be included in the study.
2.2. Measures
2.2.1. Decoding
The Woodcock–Johnson III Letter-Word Identification (LWI) was used to assess word reading accuracy. This measure has been widely used in studies of dyslexia (e.g., Fletcher et al., 1994) and has excellent reliability (LWI r = 0.918) (Woodcock, McGrew, & Mather, 2001). A subset of participants from the math study received the Wide Range Achievement Test, Third Edition (WRAT-3). Alternate form correlations (.92) of Reading, a word decoding test, support the reliability of the measure (Wilkinson, 1993).
2.2.2. Fluency
A composite score combining the Sight Word Efficiency (SWE) and Phonemic Decoding Efficiency (PDE) subtests from the Test of Word Reading Efficiency (TOWRE) was utilized to assess real and pseudoword reading ability. Excellent reliability is also reported for these measures (SWE r = .91, PDE r = .90, Total WRE r = .93) (Torgensen, Wagner, & Raschotte, 1999). A portion of the group of typical readers with archival data did not receive a reading fluency measure.
2.2.3. Comprehension
All participants received the Passage Comprehension measure from the Woodcock–Johnson III Tests of Achievement (r = .88). This measure was used to screen for reading comprehension issues among participants in the typical developing group and to characterize the group of students with dyslexia (Woodcock et al., 2001).
2.2.4. Intelligence
The KBIT-2, an individually administered intellectual function measure, was used primarily to preclude intellectual disability. Because all assessments occurred in the schools as part of the intervention studies, assessment time was limited. The Matrices subtest was administered pre-intervention and Verbal Knowledge subtest was administered post-intervention and prorated for the verbal domain score. The reliability of the Verbal (r = 0.90) and Nonverbal (r = 0.86) scores is high among children and adolescents aged 4–18 and excellent for the IQ Composite among ages 10–18 (r = 0.93) (Kaufman & Kaufman, 2004). The subgroup of typically developing children with archival data received the Stanford-Binet Intelligence Scales-4; reliabilities range from .94 to .96 for children under age 17 (Becker, 2003).
2.3. MRI acquisition
T1-weighted MR images were collected on a 3T Philips Intera system with an 8 channel SENSE (Sensitivity Encoding) technology head coil. Images were acquired in the coronal plane with voxel dimensions of .9375 × .9375 × 1 mm and a matrix of 256 × 256. Repetition time was either 8.5 ms or 8.6 ms; echo time was either 3.9 ms or 4.0 ms; flip angle was 6.0°. For DTI acquisition, a single-shot spin-echo diffusion sensitized echo-planar imaging (EPI) sequence was implemented using a balanced Icosa21 encoding scheme with 21 uniformly distributed orientations. The following DTI parameters were utilized: TR = 6100 ms; TE = 84 ms; 44 slices total; square FOV = 24 cm2; acquisition matrix = 256 × 256; slice thickness = 3 mm; b-value = 1000 s/mm2. A single non-diffusion weighted or “low b” image with a b-value = 0 s/mm2 was acquired as an anatomical reference volume.
2.4. Image processing procedures
To analyze structural connectivity between cortical and cerebellar regions involved in reading we used probabilistic tractography (Behrens et al., 2003). Our processing pipeline can be summarized as the following sequence of steps:
2.4.1. Parcellation of T1-weighted images
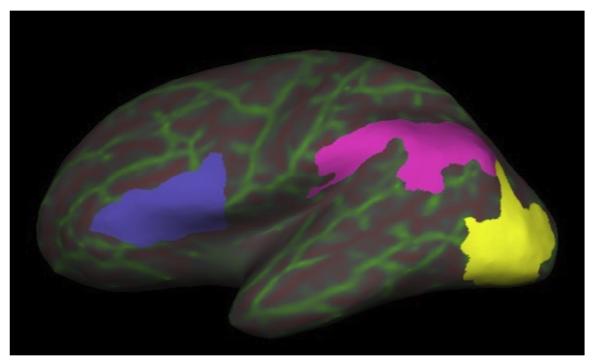
The Lonestar cluster at TACC (Texas Advanced Computing Center) was utilized to perform resource-intensive computations involved with the recon-all processing stream included in FreeSurfer software version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/). Briefly, T1-images were skull-stripped and brain tissue was segmented into gray or white matter and CSF, and then parcellated into cortical regions of interest according to the Desikan (Fischl et al., 2004) and Destrieux (Destrieux, Fischl, Dale, & Halgren, 2010) atlases. Cortical masks for angular gyrus, sulcus intermedius primus and supramarginal gyrus were merged together to create a single TP mask in each hemisphere. Masks for inferior occipital gyrus and sulcus and middle occipital sulcus and lunatus sulcus were merged to create a single OT mask in each hemisphere. Masks for pars opercularis and pars triangularis were merged to create a single IF mask in each hemisphere (Fig. 1).
Fig. 1.
Cortical regions of interest. Left hemisphere inflated surface view is displayed using Tksurfer in Freesurfer v5.3.0. The green and red lines are indices of curvature overlaid on the inflated surface and are helpful for referencing gyri (green) and sulci (red). Each cortical gray matter label used in the probabilistic tractography analyses are color-coded overlays on the inflated surface. Blue = inferior frontal gyrus;pink = temporoparietal; yellow = occipitotemporal.
A cerebellar parcellation procedure described by Juranek (2010) and utilized by Fernandez (2013) was used to mask the anterior lobe of the cerebellum as defined by lobules I–V, a region bounded by the most posterior point of the fourth ventricle, corpus medullare, and primary fissure (Fernandez et al., 2013; Juranek et al., 2010; Pierson et al., 2002; Schmahmann, Doyon, Toga, Petrides, & Evans, 2000).
2.4.2. Transformation of T1-weighted image and parcellations into diffusion space
The linear transformation tool (FLIRT) from FMRIB’s Software Library (FSL) version 5.0.1. (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Smith et al., 2004; Woolrich et al., 2009) was used to co-register the T1-weighted volume with the same individual’s b = 0 s/mm2 non-diffusion weighted volume using a 12 degree of freedom affine transformation matrix. The inverse of this transformation matrix was used to bring the parcellation labels into diffusion space.
2.4.3. DTI metrics
Diffusion tensor data was processed using FSL version 5.0.1 (Jenkinson et al., 2012; Smith et al., 2004; Woolrich et al., 2009). Images underwent a quality assurance protocol which evaluated motion and corrected for eddy current distortions. The non-diffusion weighted volume (b = 0 s/mm2) was skull-stripped to create a brain mask using the brain extraction toolbox (Smith, 2002). Tensors were reconstructed using DTIFIT to generate fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) maps (Behrens, Barker et al., 2003).
2.4.4. Probabilistic tractography
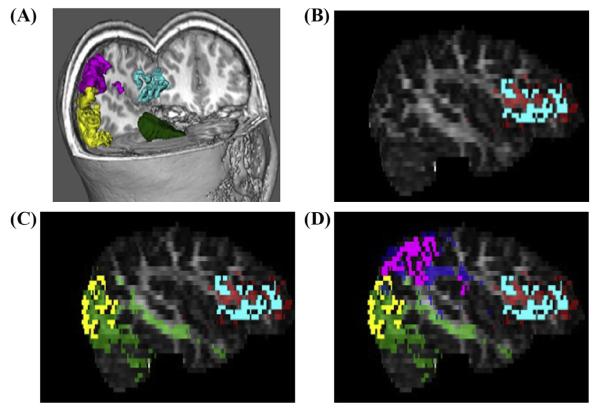
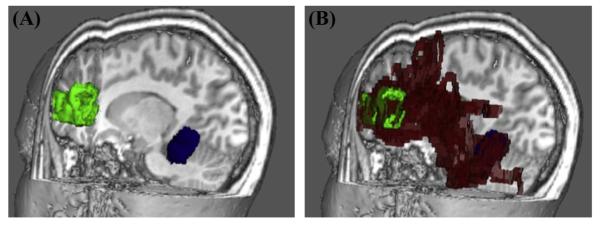
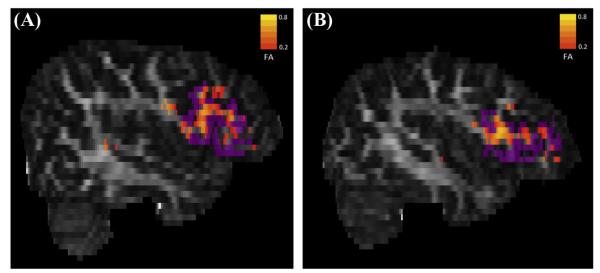
To ensure that cerebellar-cortical tractography occurred only within brain tissue, the FA map underwent erosion with a three-dimensional kernel of 3 × 3 × 3 voxels. As demonstrated in Fig. 2, a total of twelve probabilistic tractography outputs were created using FSL’s PROBTRACKX (Behrens, Barker et al., 2003). Six of these outputs were ipsilateral to the cerebellar seed mask. The first three ipsilateral tracks were seeded from the left anterior cerebellum (LAC) to the left TP, left OT, and left IF. The second set of ipsilateral tracks was seeded in the right anterior cerebellum (RAC) to homologous cortical masks in the right hemisphere. The other six probabilistic tractography outputs were contralateral to the cerebellar seed mask. While three of these were seeded from the LAC to TP, OT, and IF in the right hemisphere, the other three were seeded from the RAC to TP, OT, and IF in the left hemisphere. Fig. 3 demonstrates a 3D display showing trajectory of ProbtrackX output between RAC and left IF in high resolution T1-space for a representative study participant. Five thousand streamlines were generated from each voxel in the seed area. We used a curvature threshold of 0.2 (approximately 80 degrees) for stopping streamline trajectories (Behrens et al., 2003). Furthermore, tracking was restricted to only white matter by thresholding voxels with FA values between 0.2 and 1.0. The final tracts were binarized before obtaining mean FA, AD, and RD values for each tract. Fig. 4 illustrates an FA scalar map (heat scale) demonstrating FA values in a pathway resulting from probabilistic tractography between RAC and left IF in a poor reader (A) and a typical reader (B). The entire process of computations for steps 2–4 was fully automated through the use of in-house developed bash scripts executing FSL commands. Computations were performed on a local Linux Red Hat 6 workstation.
Fig. 2.
Example of merging multi-model imaging data for probabilistic tractography. (A) Cortical gray matter used in tractography are displayed in FSL’s 3D viewer in a color-coded fashion (green = RAC mask; yellow = right OT mask; pink = right TP mask; blue = right IFG mask). (B) Cortical GM mask of left IFG from Freesurfer is co-registered with DTI ProbtrackX output for evaluating structural connectivity between RAC and Left IFG (red). (C) Cortical GM mask of left OT mask from Freesurfer is co-registered with DTI ProbtrackX output for evaluating structural connectivity between RAC and left OT (green). (D) Cortical GM mask of left TP mask from Freesurfer is co-registered with DTI ProbtrackX output for evaluating structural connectivity between RAC and left TP (blue).
Fig. 3.
Example of pathway generated by ProbtrackX output. 3D display showing trajectory of ProbtrackX output (red) between RAC (blue seed mask) and LIF (green target mask) in high resolution T1-space for a representative study participant.
Fig. 4.
FA scalar maps of a poor reader and typical reader. FA scalar map (heat scale) demonstrating FA values in pathway resulting from probabilistic tractography between RAC (seed mask) and LIF (purple target mask) in a poor reader (A) and a typical reader (B).
3. Results
Statistical analyses were conducted on Statistical Analysis Software (SAS) 9.3.
3.1. Demographic data
The participants ranged in age from 6 to 15 years. Table 1 presents information about group demographics, while Table 2 contains summaries of the behavioral data. The groups differed significantly in age, t(1, 54) = 3.29, p = 0.0018; the group of children with dyslexia was two years older than the typical readers. There were no differences in sex, χ2(1) = 0.2968, p = 0.59, or ethnicity χ2(3) = 2.25, p = 0.5228. Females represented approximately half of the sample in each group (D = 48%, TR = 56%). The majority of participants in each group were of Black or Hispanic racial/ethnic background by self-report. Race and ethnicity were well balanced between the groups and accurately reflected the racial/ethnic breakdown of the communities from which the samples were obtained (e.g. greater Houston metropolitan area).
Table 1.
Demographics.
| Dyslexia (n = 29) | Typical readers (n = 27) | t/χ2 | df | p | |
|---|---|---|---|---|---|
| Age range [M(SD)] | 7–15 [12.10 (2.47)] | 7–15 [10.07 (2.11)] | 3.29 | 54 | 0.0018 |
| Gender (% female) | 48 | 56 | 0.30 | 1 | 0.5859 |
| Race/Ethnicity | 2.25 | 3 | 0.5228 | ||
| White (Non-Hispanic) | 1 | 4 | |||
| Hispanic | 12 | 10 | |||
| Black | 15 | 12 | |||
| Other | 1 | 1 |
M = Mean, SD = Standard Deviation.
Table 2.
IQ and reading characteristics.
| Dyslexia [SS (SD)] | Typical readers [SS (SD)] | t | df | p | |
|---|---|---|---|---|---|
| Full-scale IQ | 87.53 (9.8) | 102.18 (11.40) | −5.12 | 53 | 0.000 |
| Verbal IQ | 76.66 (18.62) | 102.04 (13.04) | −5.87 | 54 | 0.000 |
| Nonverbal IQ | 91.01 (14.97) | 103.52 (16.41) | −3.72 | 53 | 0.000 |
| Decoding | 79.62 (9.5) | 108.74 (14.5) | −8.94 | 54 | 0.000 |
| Comprehension | 80.39 (12.1) | 105.59 (11.6) | −7.50 | 48 | 0.000 |
| Reading fluency | 85.30 (13.0) | 99.30 (7.6) | −3.53 | 34 | 0.001 |
SS = Standard Score, SD = Standard Deviation.
As Table 2 shows, on average, the group of readers with dyslexia performed over one and a half standard deviations below the typical reader group on standardized measures of decoding and reading comprehension and one standard deviation below the typical readers on a standardized measure of reading fluency. Group differences in IQ (full-scale and index scores) were observed and were expected; however, research has demonstrated that children with reading difficulties with higher IQ scores have similar cognitive (Stuebing, Barth, Molfese, Weiss, & Fletcher, 2009; Stuebing, Fletcher, LeDoux, Lyon, & Shaywitz, 2002) and functional neuroimaging (Simos, Sideridis, Kasselimis, & Mouzaki, 2013; Tanaka et al., 2011) characteristics to those with lower IQ. In addition, matching or covarying for IQ differences is generally inappropriate because IQ is related to reading, creating artificial groups of typical and atypical readers and generally causing regression to the mean and violating the assumptions of covariance analysis (Campbell & Erlebacher, 1979; Dennis et al., 2009).
3.2. Probabilistic diffusion tractography
3.2.1. Hypotheses 1 and 2: Between-group differences in cerebellar-cortical pathways
Summary statistics organized by group and by tract for each of the DTI metrics (FA, RD, and AD) are provided in Table 3. A mixed-effects model with a single between-subjects variable (group) and four within-subjects variables: age at MRI, seed region (LAC, RAC), terminal hemisphere (left, right), and terminal cortical region (TP, OT, IF) were utilized to analyze tract structure and interactions with age. This procedure was run separately for each DTI metric (e.g. FA, RD, AD).
Table 3.
Summary of diffusion tensor imaging values by group.
| Seed region | Projection area | Volume |
Fractional Anisotropy (FA) |
Radial Diffusivity (RD) |
Axial Diffusivity (AD) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dyslexia |
Typical readers |
Dyslexia |
Typical readers |
Dyslexia |
Typical readers |
Dyslexia |
Typical readers |
|||||||||||||||
| Mean | SD | Mean | SD | Diff. | Mean | SD | Mean | SD | Diff. | Mean | SD | Mean | SD | Diff. | Mean | SD | Mean | SD | Diff. | |||
| LAC | Left | TP | 31,905 | 18,169 | 23,978 | 10,136 | 7927 | 0.429 | 0.010 | 0.417 | 0.011 | 0.012 | 0.599 | 0.017 | 0.614 | 0.017 | −0.015 | 1.208 | 0.024 | 1.216 | 0.034 | −0.008 |
| OT | 43,077 | 18,434 | 36,275 | 17,696 | 6803 | 0.419 | 0.014 | 0.411 | 0.009 | 0.008 | 0.616 | 0.019 | 0.628 | 0.014 | −0.013 | 1.226 | 0.033 | 1.233 | 0.031 | −0.007 | ||
| IFG | 30,825 | 21,537 | 19,945 | 7989 | 10,880 | 0.427 | 0.014 | 0.420 | 0.011 | 0.007 | 0.597 | 0.019 | 0.613 | 0.017 | −0.016 | 1.196 | 0.022 | 1.216 | 0.038 | −0.020 | ||
| Right | TP | 26,436 | 19,196 | 18,198 | 6408 | 8238 | 0.446 | 0.014 | 0.431 | 0.014 | 0.015 | 0.594 | 0.018 | 0.609 | 0.016 | −0.015 | 1.244 | 0.028 | 1.241 | 0.034 | 0.003 | |
| OT | 30,611 | 19,210 | 26,333 | 8903 | 4279 | 0.441 | 0.017 | 0.430 | 0.014 | 0.011 | 0.607 | 0.021 | 0.615 | 0.014 | −0.008 | 1.262 | 0.032 | 1.253 | 0.035 | 0.008 | ||
| IFG | 27,854 | 17,722 | 15,691 | 8054 | 12,163 | 0.442 | 0.014 | 0.430 | 0.014 | 0.011 | 0.591 | 0.015 | 0.610 | 0.017 | −0.019 | 1.227 | 0.034 | 1.240 | 0.035 | −0.013 | ||
| RAC | Left | TP | 27,654 | 14,014 | 22,939 | 8691 | 4714 | 0.446 | 0.013 | 0.433 | 0.012 | 0.013 | 0.598 | 0.017 | 0.612 | 0.015 | −0.015 | 1.250 | 0.027 | 1.251 | 0.030 | −0.001 |
| OT | 33,249 | 13,390 | 30,316 | 10,354 | 2933 | 0.441 | 0.015 | 0.428 | 0.013 | 0.013 | 0.608 | 0.021 | 0.620 | 0.013 | −0.012 | 1.267 | 0.032 | 1.260 | 0.035 | 0.007 | ||
| IFG | 27,856 | 15,398 | 20,663 | 8750 | 7193 | 0.441 | 0.015 | 0.431 | 0.011 | 0.010 | 0.593 | 0.022 | 0.610 | 0.020 | −0.017 | 1.229 | 0.026 | 1.241 | 0.038 | −0.012 | ||
| Right | TP | 30,147 | 16,371 | 23,863 | 7178 | 6283 | 0.425 | 0.011 | 0.417 | 0.013 | 0.008 | 0.596 | 0.017 | 0.610 | 0.014 | −0.014 | 1.191 | 0.026 | 1.207 | 0.038 | −0.016 | |
| OT | 39,256 | 17,219 | 37,994 | 11,089 | 1262 | 0.416 | 0.010 | 0.412 | 0.010 | 0.004 | 0.613 | 0.019 | 0.622 | 0.012 | −0.009 | 1.210 | 0.024 | 1.222 | 0.024 | −0.011 | ||
| IFG | 31,556 | 15,466 | 21,321 | 7833 | 10,235 | 0.421 | 0.012 | 0.417 | 0.012 | 0.004 | 0.598 | 0.015 | 0.614 | 0.017 | −0.016 | 1.187 | 0.026 | 1.213 | 0.039 | −0.026 | ||
LAC = Left Anterior Cerebellum, RAC = Right Anterior Cerebellum.
TP = Temporoparietal, OT = Occipitotemporal, IFG = Inferior Frontal Gyrus.
Diff. = Difference between mean dyslexia and typical readers.
3.2.2. Fractional anisotropy
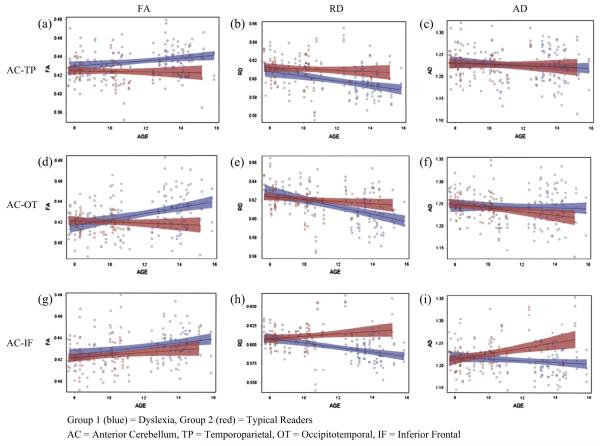
The five-way and four-way interactions were not significant, F < 1. A statistically significant three-way interaction of age, group, and region F(2, 571) = 6.43, p = 0.0017, was observed. Seed region and terminal hemisphere data was collapsed to address the relation between group and age among the three regions of interest, which are illustrated in Fig. 5. On visual inspection of the figure, the difference between FA in children with dyslexia and typical readers is greater for the older children in each of the three groups of tracts.
Fig. 5.
Interactions between age and DTI metrics in anterior cerebellar tracts by terminal cortical region.
To follow-up the three way interaction of group, age, and region, age by group analyses were conducted within region with collapsed data for seed region and terminal hemisphere. A significant interaction between age and group was revealed in the group of tracts terminating in the OT region, F(1, 168) = 7.75, p = 0.006. Inspection of Fig. 5d reveals that younger children with dyslexia have lower FA in white matter tracts terminating in the OT region relative to typical readers, while older children have greater FA. Age interactions were not significant in the other two groups of white matter pathways.
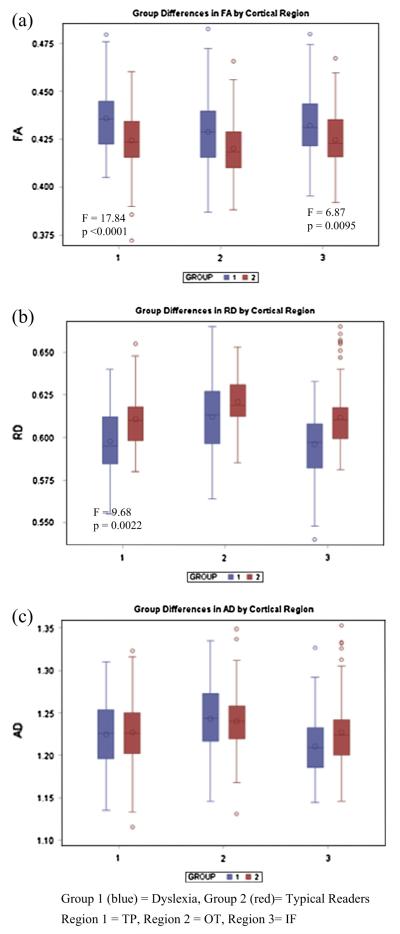
Main effects of group were observed in the group of tracts terminating in the TP region, F(1, 168) = 17.84, p = 0.0001, and in those terminating in the IF gyrus, F(1, 168) = 6.87, p = 0.0095. The main effects of group are illustrated for each region in Fig. 6a.
Fig. 6.
(a–c) Group differences in DTI metrics of cerebellar tracts by terminal cortical region.
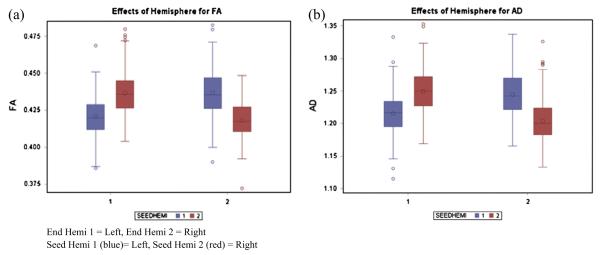
The analysis also demonstrated an interaction between seed region and terminal hemisphere, F(1, 571) = 6.98, p = 0.0085. This interaction reflected typical tract organization, with tracts crossing the midline displaying higher FA than tracts terminating in the hemisphere ipsilateral to the seed region of the left or right anterior cerebellum. The interaction is illustrated in Fig. 7a.
Fig. 7.
(a and b) Hemisphere effects for FA and AD.
3.2.3. Radial diffusivity
Radial diffusivity was examined using a mixed-effects model with the same structure described above for FA. The five-way interaction was not significant, F < 1. However, a statistically significant four-way interaction between age, group, terminal hemisphere, and terminal region was observed, F(2, 571) = 3.21, p = 0.041. Additionally, there was a significant interaction between age, group, and seed region, F(1, 571) = 10.88, p = 0.001.
To examine the four-way interaction, seed region data was collapsed, and the interaction of age, group, and terminal hemisphere were analyzed by region. No significant interactions were observed in the TP tracts. Only a main effect of age was observed in the OT tracts. In contrast, the age, group, and terminal hemisphere interaction was statistically significant for the tracts terminating in the IF gyrus, F(1, 164) = 4.15, p = 0.043. An analysis of age and group by hemisphere in the IF gyrus revealed a statistically significant interaction between age and group in the left hemisphere, F(1, 55) = 6.51, p = 0.0129, and significant main effect of age in the right hemisphere, F(1, 55) = 4.13, p = 0.047.
In the tracts terminating in the left IF gyrus, younger children with dyslexia had higher RD than typical readers, while the older children had lower RD. Because no interactions were present in the analysis of the tracts terminating in the TP region, an examination of interactions between age and group was conducted by hemisphere, which revealed no significant interactions. Age was removed from the model to examine group and hemisphere interactions. Only a main effect of group, in which the children with dyslexia had reduced overall FA relative to the typical readers, was observed, F(1, 166) = 6.75, p = 0.0102 (illustrated in Fig. 6b).
Further analysis of the three-way interaction between seed region, group, and age, revealed a significant interaction between age and group for the tracts coming from the RAC, F(1, 280) = 5.04, p = 0.0256. In the tracts coming from the LAC, only a main effect of age was observed, F(1, 280) = 4.13, p = 0.043. A pattern similar to the one previously described was observed in the RAC in which the younger children with dyslexia have greater RD which decreases with age, while the typical readers have greater RD which decreases with age. This results in group differences that are most apparent in the older children with children with dyslexia having decreased RD relative to the typical readers.
3.2.4. Axial diffusivity
A similar model to the one previously described was applied to the analysis of axial diffusivity. The five-way interaction was not significant, F < 1. A significant interaction between seed region and end hemisphere was observed, F(1, 571) = 4.29, p = 0.0389. On visual inspection, the pattern was identical to that observed for FA in which the tracts that cross the midline demonstrated greater AD relative to ipsilateral tracts. Again, this pattern likely represents typical development.
A statistically significant interaction between age, group, and region was observed, F(2, 571) = 15.95, p = 0.001. In follow-up analysis in which seed region and hemisphere data were collapsed, examining group and age interactions by region, no significant main effects or interactions were observed in the tracts terminating in the TP region or OT region. A significant interaction was observed in the tracts terminating in the IF gyrus, F(1, 168) = 5.81, p = 0.017, illustrated in Fig. 5i. The pattern is similar to the one described for RD; the older children with dyslexia demonstrate lower AD, while the older typical readers have greater AD. For all the tracts described with this pattern, the gap continues to widen with age.
4. Discussion
This study was motivated by our previous study showing volumetric reductions in the anterior cerebellum bilaterally in children with dyslexia (Fernandez et al., 2013) and by the cerebellar theory of dyslexia, which has received mixed support. It was not clear why reductions in the anterior region per se would be related to poor word reading, unless there was aberrant connectivity to cortical areas involved in this region. We hypothesized aberrant white matter integrity between the cerebellum and TP regions and possibly other regions in the reading network.
4.1. Fractional anisotropy
The first hypothesis was supported. Greater FA values were apparent in the white matter fiber tracts that project to the TP region bilaterally in the group of children with dyslexia relative to typical readers. These results are not entirely surprising given the abundance of functional neuroimaging research, implicating the TP circuit in reading and writing disorders (Pugh et al., 2001). Although this finding is consistent with Richards et al. (2008), who found greater FA in this region in adults with dyslexia, the mechanism by which FA, a measure of microstructural integrity, would be greater in children with dyslexia is unclear. Reading requires a careful orchestration of motor, cognitive, and sensory process to produce accurate and fluent word reading, a process that becomes fairly automatic for most children. An increase in FA in poor readers may indicate greater reliance on the regulatory functions of the cerebellum to integrate and automate these skills.
Additionally, bilateral findings are somewhat unexpected given the leftward lateralization findings in the literature (Niogi & McCandliss, 2006; Rimrodt et al., 2010), but this may reflect the use of only 21 directions for DTI acquisition.
Evidence was also found in support of the second hypothesis. Group differences were found in the other two groups of tracts of interest originating in the anterior cerebellum: those terminating in the OT region, a region hypothesized to underlie fluency in word recognition, and the IF gyrus, which has been associated with articulatory processes involved in decoding and reading silently (Pugh et al., 2001). In the OT tracts, younger children with dyslexia had decreased FA, which might be expected given that decreased FA is usually associated with the presence of some sort of functional deficit in clinical research. However, greater FA was observed in the older children with dyslexia while lower FA was observed for the older typical readers, giving the appearance of an increasing gap between groups with age. The difference in FA between the two groups in the tracts terminating in the IF gyrus was more direct and greater FA was observed despite age in the group of children with dyslexia.
As a measure of white matter integrity, higher FA can indicate increased myelination, more dense axonal packing, or both. However, this finding is rarely reported in clinical populations (Alexander et al., 2011). The nature of these findings is somewhat unclear, especially in terms of how these types of differences might relate to impaired reading functions. Greater FA has been linked to disability progression in multiple sclerosis (Harrison et al., 2013), a propensity for auditory hallucinations in schizophrenia (Shergill et al., 2007), and crossed cerebro-cerebellar diaschisis in supratentorial brain tumors (Patay et al., 2014). Because this is the first study of its kind, direct comparisons cannot be made. The technology needed to examine white matter microstructures closely enough to determine what axonal differences lead to greater FA in some disorders is currently unavailable and represents an opportunity for growth in the field.
4.2. Radial diffusivity
RD typically has an inverse relation to FA, in the absence of changes to AD. We found this to be true in the present study; in the TP and OT tracts, the greater FA observed in the children with dyslexia was driven by smaller RD relative to typical readers rather than by lesser AD. In all tracts, RD was smaller for the older children with dyslexia relative to older typical readers. These findings are notable, because RD typically decreases with age in healthy children (Faria et al., 2010). The mechanism by which children with dyslexia would demonstrate greater reductions in RD than typically developing children is unclear. As with increased FA, reductions in RD, a measure inversely related with myelination, may indicate an over-reliance on cerebellar processes that adjust and automatize the various cortical functions necessary for accurate and fluent word reading.
This pattern is consistent with previous research involving the corpus callosum, demonstrating greater FA that is driven by lesser RD, including Hasan et al. (2012) who found similar patterns in the CC5 region of the corpus callosum. Similarly, Keller and Just (2009) found increases in RD in struggling readers (and not typical readers) in response to intervention. Although these studies examined the corpus callosum, the body of work suggests that when it comes to reading, increased myelination, rather than smaller axonal diameter or crossing fibers which are indicated by changes in AD, may be the microstructural mechanism of change.
4.3. Axial diffusivity
With regard to AD, no significant group differences were found in the tracts originating in the anterior cerebellum and terminating in the TP or OT region. An interaction similar to the one described above for RD was found in the tracts terminating in the IF gyrus. Notably, the group of tracts terminating in the IF gyrus was the only set to have significant group effects or interactions in all three metrics (FA, RD, and AD). It has been suggested that the IF region is more heavily used by readers with dyslexia, perhaps in compensation for their failure to develop the posterior (TP and OT) reading system adequately (Pugh et al., 2001).
4.4. Hemisphere effects
Significant hemisphere effects were found for the overall sample without regard for group structure, such that cortical target masks contralateral to the seed mask in the cerebellum had greater FA than ipsilateral tracts. Although group differences were not identified with regard to hemisphere, this finding is relevant because it describes white matter tracts that have never been examined at the regional level of cerebellar anatomy in reading impaired children relative to typical readers, and illustrates similar patterns of organization in both groups.
Additionally, the absence of an interaction between group and cerebellar seed region is not surprising given the bilateral reduction in volume observed in our previous research (Fernandez et al., 2013). However, given that language dominance typically lateralizes to the left, one may have expected greater group differences in the tracts terminating in the left hemisphere, and this was not the case.
5. Limitations and future directions
Current research is limited by the fact that quantitative magnetic resonance imaging including DTI utilizes measurements in the order of millimeters whereas axonal diameters range between 1 and 20 μm and the thickness of a healthy myelin sheath is approximately 1 μm. As a result, DTI lacks the sensitivity to detect the specific microstructural differences of interest (e.g., fiber intersections within the cerebellar folia) (Alexander et al., 2011). More sophisticated techniques, such as diffusion spectrum imaging may help bridge that gap (Weeden et al., 2008).
The current study was limited by MRI scanner technology at the time of data acquisition. The spatial resolution of the DTI acquisition (3 mm slice thickness) and the angular resolution (21 direction gradient table) were maintained throughout the five year data acquisition period to ensure comparability of subject data collected at the beginning and the end of the period of project performance. While these acquisition parameters are much less sophisticated than current DTI acquisition methods for tractography analyses, to the data acquired was found to appropriately capture the pathways of interest. Greater spatial resolution of voxel dimensions and new encoding schemes that allow for greater angular resolution have been developed, presenting opportunities to improve upon the present research.
Notably, the different tracts were found to have differential associations with age. We found greater FA for the poor readers in white matter tracts connecting the cerebellum with TP and IF regions, and in the OT region FA was greater for the older poor readers, but smaller for the younger ones relative to typical readers. Greater FA in patient samples have been explained with the hypothesis that compensatory mechanisms led them to recruit additional neural resources which contribute to increased myelination or axonal packing. However, because our sample is comprised of children, maturation effects must be considered. FA is known to increase linearly with age in major white matter tracts, while RD is known to decrease with age in healthy children (Faria et al., 2010), and distinct maturational rates between tracts have also been described (Taki et al., 2013). Longitudinal studies could help elucidate the mechanism by which white matter tracts change over time in children with dyslexia as well as potentially describe the effects of intervention on these pathways.
6. Conclusions
Few DTI studies of dyslexia have included the cerebellum in their analyses. Fewer have conducted research with children, and none have examined the cerebellum at a regional level, preventing a direct comparison of our findings to existing research. Microstructural differences in cerebellar-cortical white matter tracts in individuals with dyslexia, as well as the behavioral correlates, are still poorly understood and additional research utilizing innovative technology will be necessary to fully understand the underlying neural mechanisms contributing to impairments in reading. Nevertheless, this study has provided additional evidence for discrete, regionally-bound functions of the cerebellum. An important implication of the present study is that the projections from the anterior cerebellum appear to have a regulatory effect on cortical functions important for reading. These findings complement the current body of research that has previously found evidence of higher-order cognitive functions in the posterior regions of the cerebellum (Schmahmann & Caplan, 2006).
Acknowledgments
This research was supported by grant P50 HD052117 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
References
- Ackermann H. The contribution of the cerebellum to speech and language. Brain and Language. 2013;127(3):315–316. doi: 10.1016/j.bandl.2013.10.006. http://dx.doi.org/10.1016/j.bandl.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity. 2011;1(6) doi: 10.1089/brain.2011.0071. http://dx.doi.org/10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AE, Denton C, Stuebing JM, Cirino PT, Francis DJ, Vaughn S. A test of the cerebellar hypothesis of dyslexia in adequate and inadequate responders to reading intervention. Journal of International Neurological Society. 2010;16:1–11. doi: 10.1017/S1355617710000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Becker KA. History of the Stanford-Binet Intelligence scales: Content and psychometrics (Stanford Binet Intelligence Scales, Fifth Edition Assessment Service Bulletin No.1) Riverside Publishing; Itsaca, IL: 2003. [Google Scholar]
- Behrens TE, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bishop D. Curing dyslexia and attention-deficit hyperactivity disorder by training motor coordination: Miracle or myth? Journal of Pediatrics and Child Health. 2007;43(10):653–655. doi: 10.1111/j.1440-1754.2007.01225.x. http://dx.doi.org/10.1111/j.1440-1754.2007.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, et al. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56:781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Erlebacher A. How regression artifacts in quasi-experimental evaluations can mistakently make compensatory education look harmful. In: Hellmuth J, editor. Compensatory education: A national debate. Vol. III of the disadvantaged child. Brunner/Mazel; New York, NY: 1979. [Google Scholar]
- Carter JC, Lanham DC, Cutting LE, Clements-Stephens AM, Xuejing C, Hadzipasic M, et al. A dual DTI approach to analyzing white matter in children with dyslexia. Psychiatry Research: Neuroimaging. 2009;172:215–219. doi: 10.1016/j.pscychresns.2008.09.005. http://dx.doi.org/10.1016/j.psychresns.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. http://dx.doi.org/10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton CA, Cirino PT, Barth AE, Romain M, Vaughn S, Wexler J, et al. An experimental study of scheduling and duration of “Tier 2” first-grade reading intervention. Journal of Research on Educational Effectiveness. 2011;4(3):208–230. doi: 10.1080/19345747.2010.530127. http://dx.doi.org/10.1080/19345747.2010.530127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. http://dx.doi.org/10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–367. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Eckert M. Neuroanatomical markers for dyslexia: A review of dyslexia structural imaging studies. The Neuroscientist. 2004;10(4):362–371. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thompson J, Berninger VW. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain. 2003;126:482–495. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, et al. Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Faria AV, Zhang J, Oishi K, Li X, Jiang H, Akhter K, et al. Atlas-based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and applications for automated abnormality detection. Neuroimage. 2010;52(2):415–428. doi: 10.1016/j.neuroimage.2010.04.238. http://dx.doi.org/10.1016/j.neuroimage.2010.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez VG, Stuebing KK, Juranek J, Fletcher JM. Volumetric analysis of regional variability in the cerebellum of children with dyslexia. Cerebellum. 2013;12:906–915. doi: 10.1007/s12311-013-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Fuchs LS, Barnes MA. Learning disabilities: From identification to intervention. The Guilford Press; New York, NY: 2007. [Google Scholar]
- Fletcher JM, Shaywitz SE, Shankweiler DP, Katz L, Liberman IY, Stuebing KK, et al. Cognitive profiles of reading disability: Comparisons of discrepancy and low achievement definitions. Journal of Educational Psychology. 1994;86(1):6–23. http://dx.doi.org/10.1037/0022-0663.86.1.6. [Google Scholar]
- Fuchs LS, Seethaler PM, Powell SR, Fuchs D, Hamlett LL, Fletcher JM. Effects of preventative tutoring on the mathematical problem solving of third-grade students with math and reading difficulties. Exceptional Children. 2007;74:155–173. doi: 10.1177/001440290807400202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD. Dyslexia: A new synergy between education and cognitive neuroscience. Science. 2009;325:280–283. doi: 10.1126/science.1171999. http://dx.doi.org/10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Kemper TL. Cytoarchitectronic abnormalities in developmental dyslexia: A case study. Annals of Neurology. 1979;6(2):94–100. doi: 10.1002/ana.410060203. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Menard M, Rosen G. Evidence for aberrant auditory anatomy in developmental dyslexia. Proceedings of the National Academy of Science. 1994 Aug;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D, Whetstone A, Vidal-Jordana M, Chen M, Reich D, Clabresi P. Short term increase in fractional anisotropy paradoxically predicts long term disability progression in multiple sclerosis; 18th Annual Congress of Research in Multiple Sclerosis; Copenhagen, Denmark. Oct, 2013. [Google Scholar]
- Hasan KM, Molfese DL, Walimuni IS, Stuebing KK, Papanicolaou AC, Narayana PA, et al. Diffusion tensor quantification and cognitive correlates of the macrostructure of the corpus callosum in typically developing and dyslexic children. NMR in Biomedicine. 2012;25(11):1263–1270. doi: 10.1002/nbm.2797. http://dx.doi.org/10.1002/nbm.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, et al. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. http://dx.doi.org/10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jenner A, Rosen G, Galaburda A. Neuronal asymmetries in primary visual cortex of dyslexic and nondyslexic brains. Annals of Neurology. 1999;46:189–196. doi: 10.1002/1531-8249(199908)46:2<189::aid-ana8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Juranek J, Dennis M, Cirino PT, El-Messidi L, Fletcher JM. The cerebellum in children with spina bifida and Chiari II malformation: Quantitative volumetrics by region. Cerebellum. 2010;9(2):240–248. doi: 10.1007/s12311-010-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman assessment battery for children. 2nd ed American Guidance Service; Circle Pines, MN: 2004. [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: Remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. http://dx.doi.org/10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY, Hynd GW. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. Journal of Child Neurology. 2008;23(4):368–380. doi: 10.1177/0883073807309235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabreli JD, Moseley ME, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex. 2009;19(10):2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laylock SK, Wilkinson ID, Wallis LI, Darwent G, Wonders SH, Fawcett AJ, et al. Cerebellar volume and cerebellar metabolic characteristics in adults with dyslexia. Annals of the New York Academy of Sciences. 2008;1145:222–236. doi: 10.1196/annals.1416.002. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Given B, Berninger V, Eden G. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129:3329–3342. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzier J, Mohr CM, et al. Anatomical risk factors for phonological dyslexia. Cerebral Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Rosen G, Drislane F, Galaburda A. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proceedings of the National Academy of Science. 1991 Sep;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Petrosini L, Bozzali M, Macaluso E, Caltagirone C, et al. Structural correlates of implicit learning deficits in subjects with developmental dyslexia. New York Annals of the Academy of Sciences. 2008;1145:212–221. doi: 10.1196/annals.1416.010. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, et al. White matter morphometric changes uniquely predict children’s reading acquisition. Psychological Science. 2014:1–14. doi: 10.1177/0956797614544511. http://dx.doi.org/10.1177/09567976614544511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Reaction times and dyslexia. The Quarterly Journal of Experimental Psychology. 1994;47(1):29–48. doi: 10.1080/14640749408401142. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Performance of dyslexic children on cerebellar and cognitive tasks. Journal of Motor Behavior. 1999;31(1):68–78. doi: 10.1080/00222899909601892. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Developmental dyslexia, learning, and the cerebellum. Journal of Neural Transmission (Suppl.) 2005:1–18. doi: 10.1007/3-211-31222-6_2. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Procedural learning difficulties: Reuniting the developmental disorders? Trends in Neuroscience. 2007;30(4):135–141. doi: 10.1016/j.tins.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. http://dx.doi.org/10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Patay Z, Parra C, Hawk H, George A, Li Y, Scoggins M, et al. Quantitative longitudinal evaluation of diaschisis-related cerebellar perfusion and diffusion parameters in patients with supratentorial hemispheric high-grade gliomas after surgery. Cerebellum. 2014 doi: 10.1007/s12311-014-0575-2. http://dx.doi.org/10.1007/s12311-014-0575-2. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. Neuroscience. 2009;10(67) doi: 10.1186/1471-2202-10-67. http://dx.doi.org/10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson R, Corson PW, Sears LL, Alicata D, Magnotta V, O’Leary D, et al. Manual and semiautomated measurement of cerebellar subregions on MR images. Neuroimage. 2002;17:61–76. doi: 10.1006/nimg.2002.1207. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Moore D, Della Porta G, et al. Mapping the word reading circuitry in skilled and disabled readers. In: Cornelissen PL, Hansen PC, Kringelbach ML, Pugh KR, editors. The Neural Basis of Reading. Oxford University Press; New York, NY: 2010. pp. 281–305. [Google Scholar]
- Pugh KR, Mencla WE, Jennera AR, Katz L, Frost SJ, Lee JR, et al. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, et al. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40:1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Reynolds D, Nicolson RI, Hambly H. Evaluation of an exercise-based treatment for children with reading difficulties. Dyslexia: An International Journal of Research and Practice. 2003;9(1):48–71. doi: 10.1002/dys.235. [DOI] [PubMed] [Google Scholar]
- Richards T, Stevenson J, Crouch LC, Johnson K, Maravilla P, Stock R, et al. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. American Journal of Neuroradiology. 2008;29(6):1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Peterson DJ, Denkla MB, Kaufmann WE, Cutting LE. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46:739–749. doi: 10.1016/j.cortex.2009.07.008. http://dx.doi.org/10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D. Cognition, emotion, and the cerebellum. Brain. 2006;129(2):290–292. doi: 10.1093/brain/awh729. http://dx.doi.org/10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI atlas of the human cerebellum. Academic Press; San Diego, CA: 2000. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44(8):1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O’Daly O, Jones DK, Frangou S, et al. A diffusion tensor imaging study of fasciculi in schizophrenia. American Journal of Psychiatry. 2007;164(3):467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Simos PG, Sideridis GD, Kasselimis D, Mouzaki A. Reading fluency estimates of current intellectual function: demographic factors and effects of type of stimuli. Journal of the International Neuropsychological Society. 2013;19(3):355–361. doi: 10.1017/S1355617712001518. http://dx.doi.org/10.1017/S1355617712001518. [DOI] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russel J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubek J, et al. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. http://dx.doi.org/10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Stuebing KK, Barth AE, Molfese PJ, Weiss B, Fletcher JM. IQ is not strongly related to response to reading instruction: A meta-analytic interpretation. Exceptional Children. 2009;76(1):31–51. doi: 10.1177/001440290907600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebing KK, Fletcher JM, LeDoux JM, Lyon GR, Shaywitz SE. Validity of IQ-discrepancy classifications of reading disabilities: A meta-analysis. American Educational Research Journal. 2002;39:469–518. [Google Scholar]
- Taki Y, Thyreau B, Hashizume H, Sassa Y, Takeuchi H, Wu K, et al. Linear and curvilinear corrections of brain white matter volume, fractional anisotropy, and mean diffusivity with age using voxel-based and region-of-interest analyses in 246 healthy children. Human Brain Mapping. 2013;34(8):1842–1856. doi: 10.1002/hbm.22027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Black JM, Hulume C, Stanley LM, Kesler SR, Whitfield-Gabrieli S, et al. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychological Science. 2011;22(11):1422–1451. doi: 10.1177/0956797611419521. http://dx.doi.org/10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemer H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. NeuroImage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgensen JK, Wagner RK, Raschotte CA. Test of word reading efficiency. PRO-ED Publishing, Inc; Austin, TX: 1999. [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Reviews. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vaughn S, Cirino PT, Wanzek J, Wexler J, Fletcher JM, Denton CD, et al. Response to intervention for middle school students with reading difficulties: Effects of a primary and secondary intervention. School Psychology Review. 2010;39(1):3–21. [PMC free article] [PubMed] [Google Scholar]
- Vaughn S, Wanzek J, Wexler J, Barth A, Cirino PT, Fletcher J, et al. The relative effects of group size on reading progress of older students with reading difficulties. Reading and Writing. 2010;23(8):931–956. doi: 10.1007/s11145-009-9183-9. http://dx.doi.org/10.1007/s11145-009-9183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. http://dx.doi.org/10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide range achievement test. 3rd ed Wide Range; Wilmington, DE: 1993. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock–Johnson III tests of achievement. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- Woolrich M, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magnetic Resonance in Medicine. 1999;42(6):1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]