Abstract
Galectin-7, a member of the β-galactoside-binding protein family, is primarily expressed in stratified epithelial cells, including keratinocytes. There is information in the literature suggesting a role for this protein in regulation of keratinocyte survival and growth, but the underlying mechanism remains relatively unknown. Moreover, its expression pattern in the epidermis suggests that it is also involved in the regulation of keratinocyte differentiation. Here, we demonstrate that galectin-7 knockdown results in reduced differentiation and increased proliferation of keratinocytes. Using microarray and deep-sequencing analyses, we found that galectin-7 positively and negatively regulates microRNA (miR)-203 and miR-146a expression, respectively. We show that galectin-7 regulates keratinocyte differentiation and proliferation through miR-203 but not miR-146a. A knockdown of either galectin-7 or miR-203 in keratinocytes increases expression of p63, an essential transcription factor involved in skin development. Rescue of miR-203 expression in a galectin-7 knockdown model reduces p63 expression to baseline. Increased galectin-7 expression up-regulates c-Jun N-terminal kinase (JNK) protein levels, which is required for miR-203 expression. Finally, we establish that galectin-7 can be associated with JNK1 and protect it from ubiquitination and degradation. Thus, our data suggest an intracellular function of galectin-7: regulation of keratinocyte proliferation and differentiation through the JNK1-miR-203-p63 pathway.
Keywords: Galectin-7, microRNA, keratinocytes, p63, proliferation, differentiation
INTRODUCTION
Epidermal regeneration and homeostasis are important for maintenance of normal skin function, especially in the wound healing process (Raja et al., 2007). Regeneration of keratinocytes and the establishment of the epidermis are accompanied by keratinocyte migration, proliferation, and differentiation. Proliferating basal-layer keratinocytes are a major source of cells for replenishment of the epidermis (Fuchs and Raghavan, 2002). Aberrant proliferation of keratinocytes is associated with skin diseases, including psoriasis, atopic dermatitis, and contact dermatitis (Jensen et al., 2004; Proksch and Brasch, 2012; Tonel and Conrad, 2009), and it has been linked to neoplastic transformation.
Galectin-7 is a 15-kDa protein belonging to the galectin family of β-galactoside-binding proteins. It is strongly expressed in stratified epithelia, hair follicles, and thymic Hassall’s corpuscles (Madsen et al., 1995; Magnaldo et al., 1998). Its expression is induced by p53 and ultraviolet B (UVB) light but reduced by exogenous retinoic acid. Moreover, its expression is downregulated in SV40-transformed cells and squamous cell carcinoma (SCC) cells (Bernerd et al., 1999; Magnaldo et al., 1995; Polyak et al., 1997). Galectin-7 promotes apoptosis if transfected into HeLa cells, likely via the c-Jun N-terminal kinase (JNK) signaling pathway (Kuwabara et al., 2002; Madsen et al., 1995). Galectin-7 is localized in the nucleus and cytoplasm; these observations are consistent with its involvement in intracellular functions (Bernerd et al., 1999; Gendronneau et al., 2008; Madsen et al., 1995).
Galectin-7 has been shown to contribute to the maintenance of skin homeostasis after skin injury; specifically, keratinocytes in galectin-7 knockout mice displayed a hyperproliferative phenotype in vivo, when the skin was exposed to UVB or was wounded (Gendronneau et al., 2008). However, the underlying mechanism was not elucidated. Moreover, galectin-7 is more weakly expressed in the proliferating basal-layer cells than the in supra-basal layers (Magnaldo et al., 1998), suggesting that it may contribute to the differentiation of keratinocytes, but this possible function has heretofore not been investigated.
SCC is a common type of skin cancer. UV irradiation and p53 mutations are strongly associated with its development, and as mentioned above, both affect galectin-7 expression in healthy keratinocytes. Intracellular galectins are known to contribute to various stages of carcinogenesis, such as apoptosis, tumor cell migration, and metastasis (Liu and Rabinovich, 2005; Vladoiu et al., 2014). Downregulation of galectin-7 in SCCs may play an important role in tumor progression, but the mechanism is still unknown.
In this study, we demonstrated that galectin-7 knockdown results in reduced keratinocyte differentiation and increased keratinocyte proliferation. We then investigated the function of galectin-7 in keratinocytes by first identifying the miRNAs it regulates during characterization of galectin-7 knockdown keratinocyte cell lines. We found that galectin-7 positively regulates miR-203 and controls keratinocyte proliferation and differentiation through this miRNA, which, in turn, downregulates p63. We also established that galectin-7 is associated with and stabilizes JNK, which positively regulates miR-203 expression. We can conclude that galectin-7 controls keratinocyte proliferation and differentiation through the galectin-7-JNK-miR-203-p63 pathway. Our results also provide insights into how galectin-7 may contribute to the pathogenesis of SCC.
RESULTS
Galectin-7 knockdown reduces keratin-1 and keratin-10 expression and promotes keratinocyte proliferation
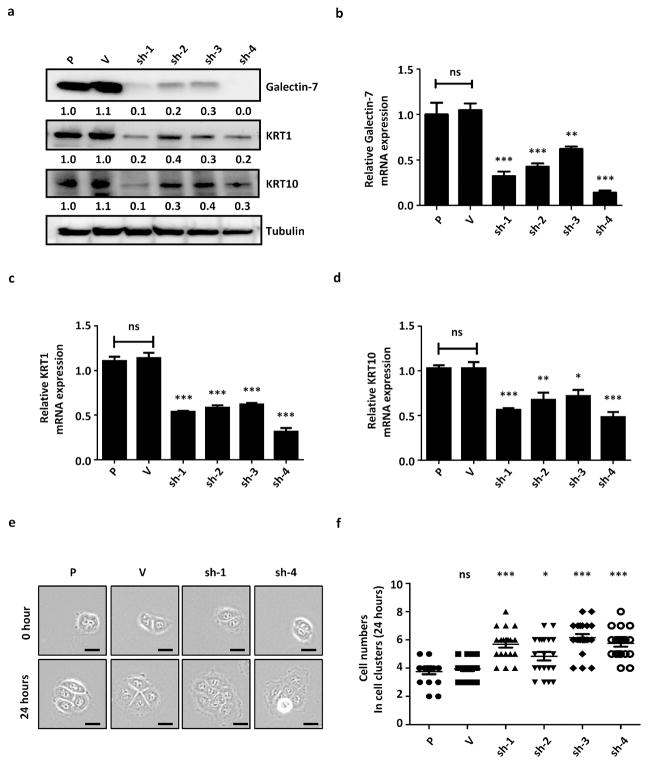
To examine the function of galectin-7 in keratinocytes, we generated galectin-7 knockdown cells from an immortalized cell line, HaCaT, using four small hairpin RNA (shRNA)-encoding lentiviruses. The knockdown of galectin-7 was verified by real-time PCR and immunoblot analysis (Figure 1a and b). In galectin-7 knockdown keratinocytes, the mRNA and protein expression of the differentiation markers keratin-1 and keratin-10 was reduced significantly (Figure 1a, 1c and 1d). The keratinocyte line with the lowest galectin-7 expression level (sh-4) also expressed lower levels of keratin-1 and keratin-10, as verified by immunoblot and real-time PCR analyses (Figure 1a, 1c and 1d). To examine whether galectin-7 regulates keratinocyte differentiation through calcium signaling, we examined calcium-induced differentiation as previously described (Deyrieux and Wilson, 2007). By immunoblot analysis, we found that the reduction of keratin-1 and keratin-10, as well as involucrin, by galectin-7 knockdown was independent of calcium concentration (Supplementary Figure S1a). Here, the vector control also affected the expression levels of involucrin and keratin-1, but the effects of galectin-7 were evident, as compared to control. The fact that other differentiation markers (loricrin, filaggrin) are not affected is consistent with the information in the literature that these markers are differentially regulated and not necessarily all affected as the cells are driven to differentiate (Moravcova et al., 2013; Reichelt and Magin, 2002). In addition, we examined the expression of epithelial-mesenchymal transition (EMT) markers, and found that the protein expression of E-cadherin, N-cadherin, claudin-1, β-catenin, sanil and slug was not affected appreciably and regulated consistently in all knockdown clones (Supplementary Figure S1b). Based on its effect on keratin-1 and keratin-10, we conclude galectin-7 is a positive regulator of keratinocyte differentiation.
Figure 1.
Galectin-7 knockdown keratinocytes exhibit reduced expression of differentiation markers keratin-1 and keratin-10 and a hyperproliferative phenotype. (a) The protein levels of galectin-7, keratin-1 and keratin-10 in knockdown stable cell lines, and (b, c and d) the mRNA levels of galectin-7, keratin-1 and keratin-10 in stable galectin-7 knockdown cells were quantified in triplicated experiments. (e) Time-lapse images of galectin-7 knockdown cell clones, parental (P), and empty-vector control (V). Scale bar = 50μm. (f) The numbers of cells from individual colonies of each group of parental (P), empty-vector control (V), and four galectin-7 knockdown cell clones. All statistical analyses were performed by individually comparing with the HaCaT parental cell line (P), ns: not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
We further examined whether the decrease in keratin-1 and keratin-10 correlated with the cell’s proliferative ability, as suggested by previous studies (Kartasova et al., 1992; Pivarcsi et al., 2001). We quantified the cell proliferation ability of these lines for 24 hours after they reached the G1 phase from the single-cell stage, by long-term time-lapse imaging analysis (Hinchcliffe, 2005). We found that galectin-7 knockdown cells proliferated faster than control cells, as indicated by the higher progeny cell numbers (Figure 1e and f). This was confirmed by examining reconstituted human epidermis (RHE) derived from galectin-7 knockdown cells (Supplementary Figure S1c). In addition, we investigated the role of galectin-7 in normal human primary keratinocytes (HEKn) and obtained results similar to those for HaCaT cells. The galectin-7 knockdown clones (si-1 and si-3) displayed reduced keratin-1 and keratin-10, as well as increased cell proliferation as compared to control clones (siNC). Another clone derived from HEKn treated with galectin-7 siRNA, but with an unaffected galectin-7 level (si-2), also served as the control (Supplementary Figures 2a, b, c and d). Our results suggest that galectin-7 suppresses keratinocyte proliferation and promotes keratinocyte differentiation.
Galectin-7 regulates miRNA expression in keratinocytes
MicroRNAs are crucial regulators of keratinocyte differentiation and proliferation (Yi et al., 2008). Several miRNAs are differentially expressed in lesions in skin diseases (Sonkoly et al., 2007). We studied the miRNA expression by microarray and small-RNA deep-sequencing analyses. Four miRNAs were differentially expressed, namely miR-10b, miR-146a, miR-203, and miR-4270; they were expressed > 4-fold higher or lower in galectin-7 knockdown cells (sh-1) compared to both parental and empty-vector control cells (Table S1). We found 10 miRNAs by means of small-RNA deep-sequencing that had a ≥2-fold differential read ratio, with the cutoff at 1000 reads (Table S2). Only two miRNAs, miR-146a and miR-203, were found to be differentially expressed in galectin-7 knockdown keratinocytes according to both microarray (with a 4-fold change) and deep sequencing analyses (with a reads ratio of >2 or <0.5).
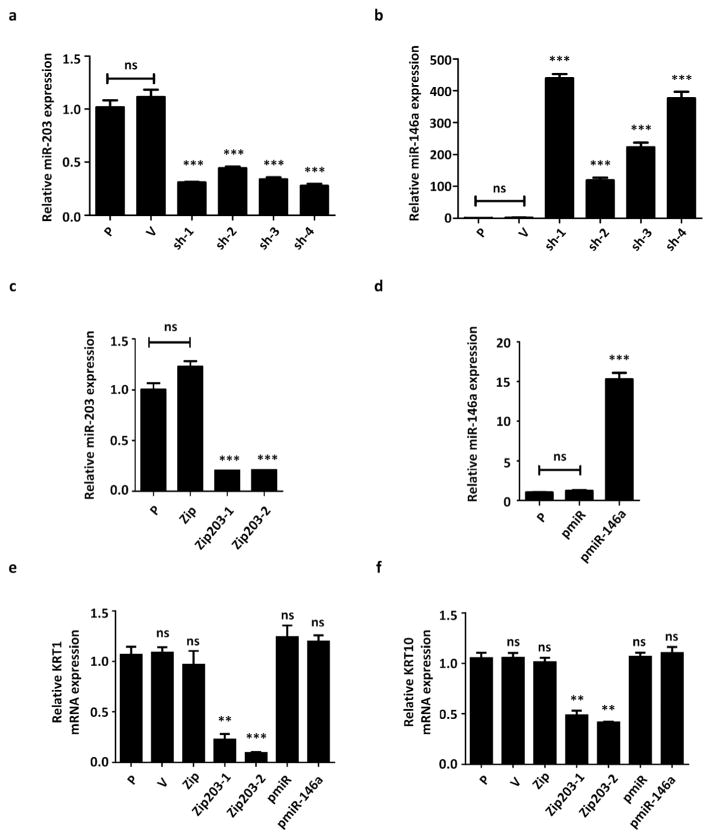
The upregulation of miR-146a and downregulation of miR-203 were further confirmed by real-time PCR in all four galectin-7 knockdown clones (Figure 2a and b). In addition, the regulation of miR-203 and miR-146a expression by galectin-7 was confirmed using HEKn, in which both keratin-1 and keratin-10 were also reduced by galectin-7 knockdown (Supplementary Figure S2a–f).
Figure 2.
miR-146a and miR-203 are differentially expressed in galectin-7 knockdown keratinocytes, whereas miR-203 knockdown cells show reduced expression of keratin-1 and keratin-10. The amounts of miR-203 (a, c) and miR-146a (b, d) in four stable galectin-7 knockdown cell clones and two control cell clones (a, b), stable miR-203 knockdown (c) and miR-146a overexpression (d) cells were quantified and compared with their vector controls and parental control. (e and f) The mRNA levels of keratin-1 and keratin-10 in two stable miR-203 knockdown cell clones and one stable miR-146a-overexpressing cell clone. All experiments were done at lease in triplicate and statistical analyses were performed by individually comparing with the HaCaT parental cell line (P), ns: not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Reduced miR-203 expression in galectin-7-knockdown keratinocytes affects cell differentiation and proliferation
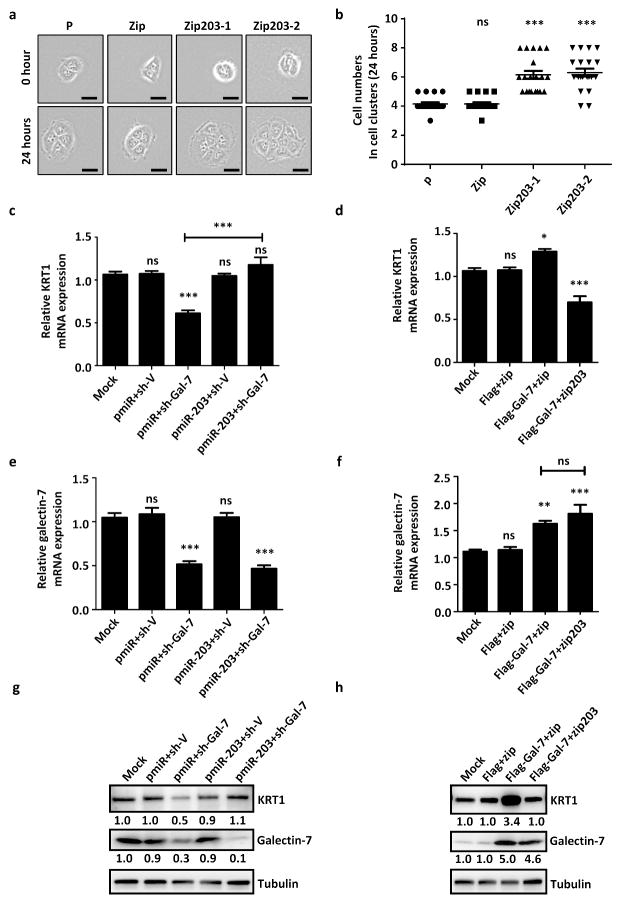
To determine the role of galectin-7-controlled miRNAs in keratinocyte proliferation and differentiation, we generated stable miR-203 knockdown and miR-146a-overexpressing HaCaT cell lines. The effective knockdown and overexpression of these miRNAs were confirmed by real-time PCR (Figure 2c and d). Two miR-203 knockdown lines, but not miR-146a overexpressing lines, showed reduced expression of keratin-1 and keratin-10 (Figure 2e and f). By means of time-lapse cell proliferation analysis, we demonstrated that miR-203 knockdown cells exhibited a hyper-proliferative phenotype similar to galectin-7 knockdown cells (Figure 3a and b).
Figure 3.
Ectopic expression of miR-203 can restore the galectin-7 knockdown phenotype of keratin-1 expression. (a) The proliferated miR-203 knockdown clones were monitored using time-lapse microscopy. Scale bar = 50μm. (b) The proliferation rate of miR-203 knockdown clones was quantified by counting the number of progeny cells 24 h after the first round of division (n = 20). (c – h) HaCaT cells were transiently transfected with indicated plasmids and their corresponding control plasmids. (c–f) Real-time PCR; (g and h) Immunoblot analysis are presented. All experiments were performed at least in triplicate, and the statistical analyses were performed by individually comparing with HaCaT parental cells (P), ns: not significant, ***P < 0.001.
To examine whether the galectin-7 control of keratinocyte differentiation is dependent on miR-203 expression, we ectopically expressed miR-203 in galectin-7 knockdown cells. This action restored keratin-1 expression (Figure 3c). Furthermore, overexpression of galectin-7 increased the expression of keratin-1 and this effect was reversed by knocking down miR-203 (Figure 3d). Our data suggest that miR-203 acts downstream of galectin-7 to regulate keratin-1 expression. In each case, galectin-7 levels were measured by real-time PCR and immunoblot analyses (Figures 3e–h). Overexpression of miR-203 in HaCaT cells had no significant effect on keratin-1 expression (Figure 3c and g). This result could be due to the abundance of endogenous miR-203 in keratinocytes, which could make the effect of the exogenous (transfected) miRNA relatively weak. Moreover, the increase in keratin-1 expression induced by ectopic expression of galectin-7 could be reversed by knocking down miR-203 (Figures 3d and h). Finally, the expression level of miR-203 in galectin-7 knockdown cells can be restored by overexpression of miR-203 and overexpression of galectin-7 can induce miR-203 expression (Supplementary Figures 3a and 3b). Taken together, these data suggest that galectin-7 and miR-203 act on the same pathway to regulate keratinocyte differentiation. However, in view of the finding described above that overexpression of miR-203 in HaCaT cells had no significant effect on keratin-1 expression, we cannot exclude the possibility that galectin-7 may need to engage other pathways to regulate keratin-1 expression (see Discussion).
Increased ΔNp63 expression in galectin-7 knockdown cells can be restored by ectopic miR-203 expression
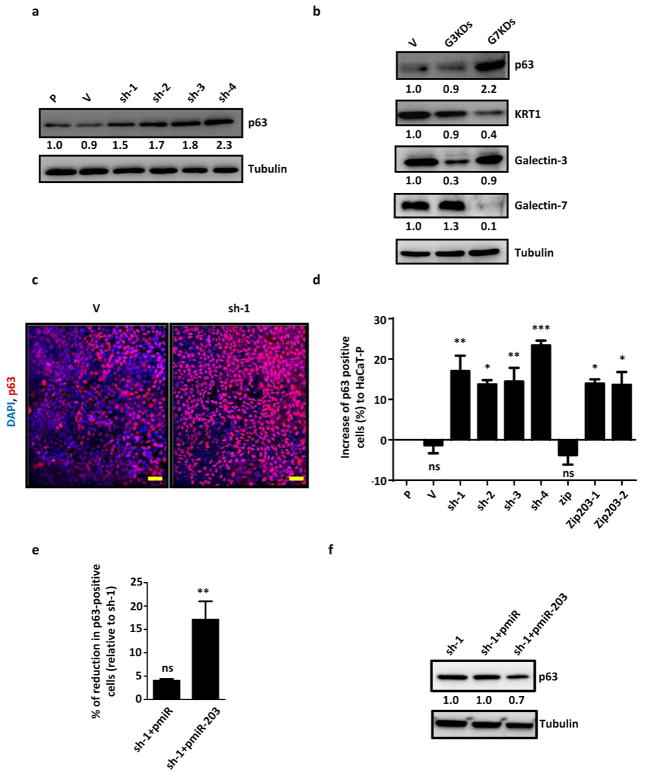
To determine how galectin-7 regulates keratinocyte proliferation and differentiation through miR-203, we examined the expression of a known miR-203 target, ΔNp63, in galectin-7 knockdown cells. This target protein belongs to the p53 family, and it is an essential transcription regulator in skin development (Yang et al., 1999). In stable galectin-7 knockdown cells, we observed increased expression of the ΔNp63 protein (Figure 4a). A transient knockdown of galectin-7, but not galectin-3, resulted in increased expression of ΔNp63, along with decreased expression of keratin-1 (Figure 4b). Consistently, siRNA-mediated knockdown of galectin-7 in HEKn cells resulted in increased expression of ΔNp63 (Supplementary Figure 2b).
Figure 4.
Galectin-7 regulates ΔNp63 expression through miR-203. (a) The p63 protein levels; (b) The galectin-3, galectin-7, p63, and keratin-1 protein levels; (c) Immunofluorescence of p63; and (d) The relative ratio of p63-positive cells in galectin-7 and miR-203 knockdown cells after normalization to parental cells are presented. The statistical analyses were performed in triplicated experiments by individually comparing with the HaCaT parental cells, *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar = 200μm. (e) The relative ratio of p63-positive cells in transfected galectin-7 knockdown cells (sh-1) by indicated plasmids was normalized to sh-1 cells. The statistical analyses were performed by individually comparing with the galectin-7 knockdown cell line (sh-1), ns: not significant, **P < 0.01. (f) The p63 protein levels of transiently transfected HaCaT cells.
Consistent with the above results, immunofluorescence staining revealed that a knockdown of galectin-7 or miR-203 resulted in a significant increase in the percentage of ΔNp63-positive cells (Figure 4c and d). Moreover, miR-203 knockdown cells contained a similar percentage of ΔNp63-positive cells to that of galectin-7 knockdown cells (Figure 4d).
Next, we demonstrated that ectopic expression of miR-203 in galectin-7 knockdown cells (sh-1) could reduce the percentage of ΔNp63-positive cells and the protein expression levels of ΔNp63 (Figure 4e and f). Thus, we verified that p63 is a target of miR-203 and is regulated by the galectin-7-miR-203 signaling pathway.
JNK1 is required for miR-203 expression in keratinocytes
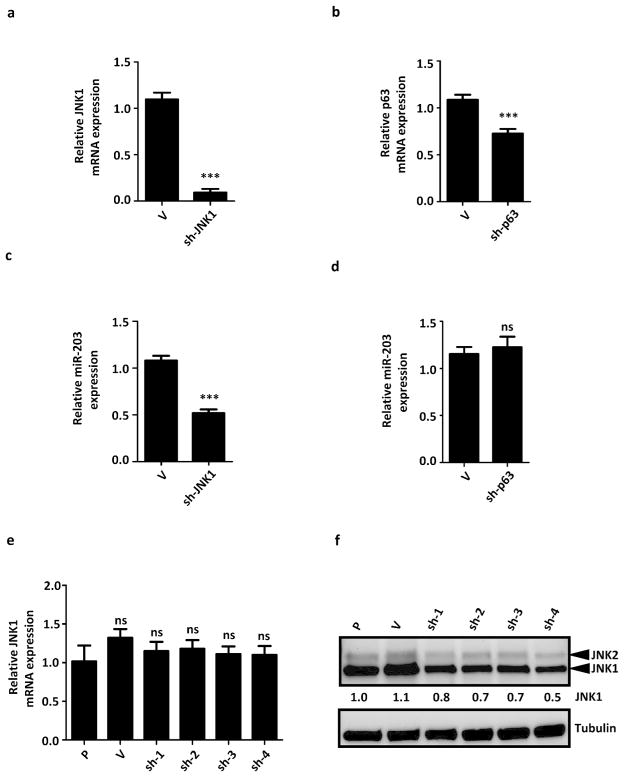
Previous reports suggested that miR-203 expression is regulated by p53 and mitogen-activated protein kinase (MAPK) (McKenna et al., 2010; Melar-New and Laimins, 2010). Differentiation inducing stimulations (TPA and calcium) can also regulate miR-203 expression through JunB and c-Jun in complex with activator protein 1 (AP-1) (Sonkoly et al., 2010). JNK is an upstream regulator of c-Jun and may participate in regulating miR-203. We thus hypothesized that JNK1 signaling is required for miR-203 expression in HaCaT cells. An shRNA-mediated knockdown of JNK1, but not p63, resulted in a reduction in miR-203 expression, suggesting that miR-203 is regulated by JNK1 (Figures 5a–d). By real-time PCR analysis of galectin-7 knockdown cell lines, we found no difference in JNK1 mRNA levels, as compared to control (Figure 5e). However, by immunoblot analysis, we found that the protein levels of JNK1 were reduced in galectin-7 knockdown HaCaT (Figure 5f) and HEKn cells (Supplementary Figure 2b). Our results suggest that galectin-7 directly or indirectly regulates JNK1 protein stability.
Figure 5.
JNK1 is required for miR-203 expression and down-regulated at the protein level in galectin-7 knockdown cells. (a and b) The knockdown of JNK1 and p63 mRNA levels. (c and d) Expression of miR-203 in HaCaT cells with a transient knockdown of either JNK1 or p63. (e) The relative mRNA levels of JNK1 in galectin-7 knockdown cells. (f) The protein levels of JNK1 in galectin-7 knockdown cells. The relative fold changes of JNK1, p63, and miR-203 in JNK1 and p63 knockdown HaCaT cells were normalized to the empty-vector control. The statistical analyses were performed by individually comparing with the HaCaT parental cell line (P), ns: not significant, ***P < 0.001.
Galectin-7 co-localizes with JNK1 and protects it from ubiquitination
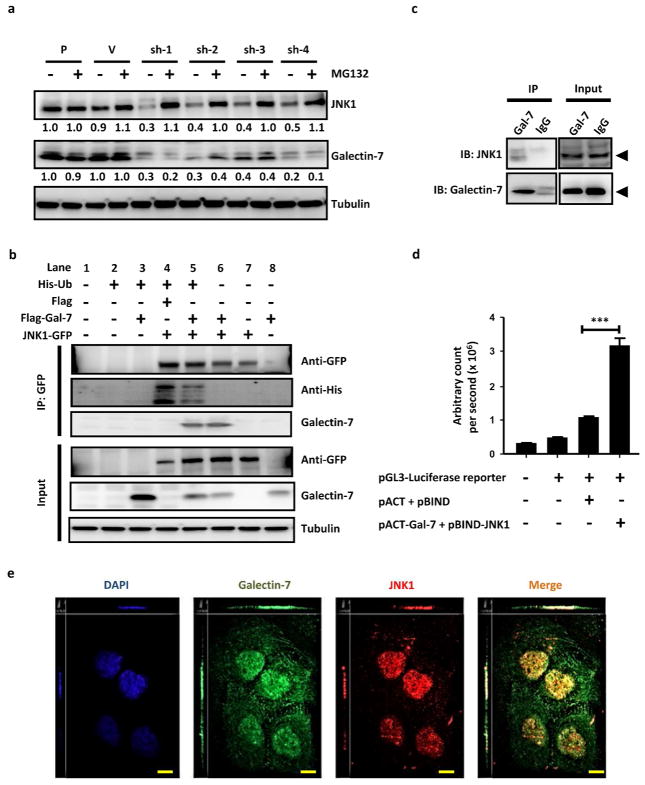
Other studies have shown that the JNK1 protein is regulated during cell cycle progression through an ubiquitin-dependent proteasome pathway (Gutierrez et al., 2010). To examine whether galectin-7 regulates JNK1 degradation and ubiquitination in HaCaT cells through the proteasome degradation pathway, we first determined whether the reduced JNK1 expression in galectin-7 knockdown cells was due to proteasome degradation, by applying the proteasome inhibitor MG-132. MG-132 treatment of galectin-7 knockdown cells restored the JNK1 protein levels (Figure 6a), suggesting that the decrease in galectin-7 enhances the degradation of JNK1 by the proteasome.
Figure 6.
Galectin-7 interacts with JNK1 and inhibits its ubiquitination and degradation. (a) Immunoblot analysis of JNK1, galectin-7, and tubulin in galectin-7 knockdown cells treated with or without MG-132. (b) HeLa cells containing ectopically expressed JNK1-GFP were treated with MG-132, and the ubiquitination of immunoprecipitated JNK1-GFP was detected by using an anti-his antibody to detect his-tag-ubiquitin. Lanes 1–3 served as control. (c) Co-immunoprecipitation of JNK1 with galectin-7 from HaCaT cells was performed by using an anti-galectin-7 antibody. (d) The interaction of JNK1 and galectin-7 as demonstrated by a mammalian two-hybrid system. The statistical analysis was performed by comparing between vector controls (pACT+pBIND) with pACT-Gal-7+pBIND-JNK1 co-transfected with pGL3-Luciferase reporter. ***P < 0.001. (e) Immunofluorescence of galectin-7 and JNK1 was performed using confocal microscopy with Z-stack sectioning. Scale bar = 10μm.
Next, we analyzed JNK1 immunoprecipitated from HeLa cells, which had been co-transfected with histidine-tagged-ubiquitin and galectin-7 and subsequently treated with MG-132. We found that JNK1 was ubiquitinated, and overexpression of galectin-7 reduced the extent of ubiquitination (Figure 6b, lanes 4 and 5). These data are also consistent with our immunostaining results using antibodies against galectin-7, JNK1, and ubiquitin, quantified by high-content image analysis. The percentage of co-localization of JNK1 with ubiquitin signals increased from 30% in the parental and empty-vector control cells to 50% in galectin-7 knockdown cells (Supplementary Figure S4a and b).
Galectin-7 interacts with JNK1
To determine whether galectin-7 can directly interact with JNK1 and affect its ubiquitination, we performed co-immunoprecipitation, mammalian two-hybrid, and confocal-microscopy assays. We found that ectopically expressed JNK1 in HeLa cells could be co-immunoprecipitated with galectin-7 (Figure 6b, lanes 5 and 6). Co-immunoprecipitation of JNK1 and galectin-7 was also demonstrated in HaCaT cells (Figure 6c). A mammalian two-hybrid system using the fusion proteins JNK-1Gal4DB and galectin-7-Vp16 confirmed the JNK1-galectin-7 interaction (Figure 6d). In addition, confocal immunofluorescence staining showed that JNK1 was mainly located in the nucleus, where it co-localized with galectin-7. The threshold Pearson’s correlation coefficient of nuclear galectin-7 and JNK1, as determined by the method used by (Barlow et al., 2010), was 0.655 ± 0.09 (mean ± SD, n = 4; Figure 6e).
DISCUSSION
Although galectins are expressed in intracellular compartments, and some have been shown to perform important intracellular functions (Liu et al., 2002; Vladoiu et al., 2014), other studies have mostly dealt with the extracellular functions of galectins. Galectin-7 is known to reside in both the nucleus and cytoplasm of keratinocytes (Inagaki et al., 2008; Kuwabara et al., 2002) and was shown to bind to Bcl-2 intracellularly (Villeneuve et al., 2011). In the present study, we uncovered an intracellular function of galectin-7 in keratinocytes: regulation of cell proliferation and differentiation by maintaining homeostasis of miRNA signaling pathways.
By analyzing cell clones ectopically expressing galectin-7 and miR-203, we found evidence that galectin-7 participates in the regulation of miR-203, and gained some insight into the mechanisms involved. Overexpression of galectin-7 had no significant effects on the biosynthesis of miR-203 (data not shown). Therefore, we believe that galectin-7 regulates miR-203 at the transcriptional level. The results of chromatin immunoprecipitation and real-time PCR experiments did not show direct binding of galectin-7 to the miR-203 promoter (data not shown). It is likely that galectin-7 regulates expression of miRNAs via proteins crucial for the biosynthesis of the specific miRNAs. Indeed, we identified one such protein, JNK1, which is down-regulated at the protein level, but not at the mRNA level, in galectin-7 knockdown cells. We previously found that overexpression of galectin-7 resulted in enhanced JNK1 phosphorylation (Kuwabara et al., 2002), without affecting the levels of JNK1. The difference might reflect varying functions of galectin-7 in different cell types. Moreover, knockdown of JNK1 reduced miR-203 expression. Thus, it appears that galectin-7 regulates miR-203 expression by controlling the turnover of JNK1.
It has been reported that JNK1 and JNK2 are degraded by the large multimeric anaphase-promoting complex with the coactivator protein Cdh1 during cell cycle progression, through an ubiquitination-dependent pathway (Gutierrez et al., 2010). We found that galectin-7 suppresses JNK1 ubiquitination, and the degradation of JNK1 is sensitive to the proteasome inhibitor MG-132. However, these results may not totally exclude the possibility that galectin-7 may also participate in other protein degradation systems such as autophagy. In addition, as observed for both galectin-1 and galectin-3 (Inagaki et al., 2008; Vyakarnam et al., 1997; Yu et al., 2002), galectin-7 can shuttle between the nucleus and cytoplasm, although whether this phenomenon is relevant to the regulation of JNK expression remains to be clarified.
With regard to the role of miR-203 in mediating galectin-7’s regulation of keratinocyte differentiation, we demonstrated 1) ectopic expression of miR-203 can restore the expression level of keratin-1 in galectin-7 knockdown cells, and 2) the induction of keratin-1 expression by ectopically expressed galectin-7 is suppressed by miR-203 knockdown. These data support that galectin-7 regulates keratin-1 expression through the miR-203 pathway. However, we need to address our finding that ectopic expression of miR-203 in HaCaT cells had no effect on keratin-1 expression. This could be due to both the high abundance of miR-203 and limited availability of target p63 mRNA in keratinocytes. Thus, miR-203 knockdown produced more effects than its overexpression. This general phenomenon has been reported in other studies (e.g.,(Arvey et al., 2010)). Nevertheless, ectopic expression of galectin-7 is known to induce expression of a number of genes, as reported in our previous studies (Kuwabara et al., 2002). Galectin-7 may promote keratinocyte differentiation through one or more of these other genes.
In the studies of galectin-7 knockout mice subjected to stressful conditions including UVB irradiation or tail injury, (Gendronneau et al., 2008) concluded that galectin-7 suppresses keratinocyte proliferation and survival. There, an increase of ki67-positive cells was observed in the epidermis of galetin-7-deficient mice, compared to wild-type mice. Thus, galectin-7 deficiency results in a hyper-proliferative phenotype when keratinocytes are subjected to stress. However, the underlying mechanism was not elucidated. In our studies, we provided evidence that galectin-7 negatively regulates keratinocyte proliferation which is consistent with the findings described above. This is based on single cell proliferation assay and p63 expression analyses in homeostasis condition. Moreover, we established the pathway through which galectin-7 regulates keratinocyte proliferation.
Our study sheds some light on the relationship between galectin-7 and SCC, which is known to be associated with UV irradiation. Galectin-7 is a p53-inducible gene (Polyak et al., 1997), and it can be induced by UV irradiation. In addition, galectin-7 is down-regulated in SCC (Magnaldo et al., 1998). Both p53 and ΔNp63 are transcription factors participating in the regulation of keratinocyte apoptosis, proliferation, and differentiation (Polyak et al., 1997). In SCC, the p53 family of proteins (p53, p63, and p73) has distinct but interconnected functions; p53 is often mutated, but the ΔNp63 isoform is overexpressed (Missero and Antonini, 2014). Moreover, in healthy keratinocytes, SCC cell lines, and SCC, ΔNp63 α trans-activates PIR2/Rnf14B and thereby regulates epithelial cell proliferation. Unlike p53, which is a tumor suppressor, the major isoform of p63, ΔNp63α, promotes proliferation of healthy keratinocytes and expression of the SCC-suppressing gene Notch1 (Yugawa et al., 2010).
Galectin-7 may also be linked to SCC through miR-203, which is downregulated in SCC (Dziunycz et al., 2010), but induced by UV irradiation (Syed et al., 2013). In addition, the expression of ΔNp63 is regulated by miR-203 (Yi et al., 2008). Thus, our data suggest that downregulation of galectin-7 in keratinocytes (caused by mutations in p53) may contribute to the pathogenesis of SCC, because the expression of ΔNp63 is increased, resulting in enhanced cell proliferation. Moreover, our study shows a link between p53 and ΔNp63 through the galectin-7-JNK1-miR-203 pathway.
It should be noted that galectin-7 may have opposite functions in different types of cancer (St-Pierre et al., 2012). Ectopic expression of galectin-7 in a colon carcinoma cell line was shown to suppress tumor growth (Ueda et al., 2004). On the other hand, galectin-7 is overexpressed in aggressive mouse lymphoma cells, and its expression level correlates with cancer progression (Moisan et al., 2003). In breast cancer, overexpression of galectin-7 enhances spontaneous metastasis (Demers et al., 2010). These variable roles of galectin-7 are possibly due to the fact galectin-7 expression is differentially regulated in various cancers, and this protein may target dissimilar pathways in different cell types.
In summary, we examined the function of galectin-7 in keratinocytes and uncovered a regulatory pathway—involving miR-203 and p63—that controls cell proliferation and differentiation. Furthermore, we demonstrate that galectin-7 regulates miR-203 expression through a direct interaction with JNK1, and that this interaction protects JNK1 from ubiquitination and degradation. In addition, our data suggest that galectin-7 may be involved in the pathogenesis of skin cancers such as SCC.
MATERIALS AND METHODS
Cell culture and generation of galectin-7 knockdown stable cells
The HaCaT cell line (Boukamp et al., 1988) was cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (GIBCO, MD, USA). Lentivirus-encoded shRNAs against human galectin-7 were obtained from the National RNAi Core Facility (Taiwan). After infection with a shRNA-carrying lentivirus for 3 days, puromycin was used for selection of the transfected cells. Subsequently, four cell clones were cultured in a puromycin-free medium for 2 weeks prior to analysis.
Cell proliferation quantified by time-lapse microcopy
A time-lapse microscope, Leica MDI600B, was used to monitor long-term imaging of cell proliferation (Hinchcliffe, 2005; Nagy et al., 2010). Images of cells were automatically captured every 10 min for three days using a 10× objective lens. Keratinocytes were seeded at the density of 10,000/mL in six-well plates without synchronization, and proliferation was examined. Images of single cells were tracked until they proliferated into the two-cell stage, which is considered to be at the G1 phase. From this point, the two cell clusters were tracked for 24 hours and the number of cells originating from the clusters was quantified at that endpoint. Twenty clusters each from the HaCaT parental, vector control, and galectin-7 knockdown cells were examined. The images were analyzed using MetaMorph software (Mehes et al., 2012).
Generation of miR-203 knockdown and miR-146a stably overexpressing cell clones
To down-regulate miR-203 in HaCaT cells, we used an antisense miR-203 vector (MZIP203-PA-1) and a miRZip scrambled hairpin control vector (MZIP000-PA-1) from System Biosciences (SBI; CA, USA). HaCaT cells were transfected with the pZip control and pZip-203 vectors using the Effectene reagent (Qiagen; MD, USA). Three days after the transfection, transfected cells were enriched by sorting based on the copGFP reporter, followed by puromycin selection. Two pooled miR-203 knockdown cell clones (Zip203-1 and Zip203-2) were generated via independent transfection followed by the selection procedures described above.
We used an miR-146a-overexpressing vector (PMIRH146aPA-2) and a scrambled control hairpin in pCDH-CMV-MCS-EF1-copGFP (CD511B-1) from SBI to generate stable miR-146a-overexpressing and control cell clones, respectively, using a similar protocol as describe above.
Immunofluorescence analysis of p63
Immunofluorescence of p63 was performed on confluent stable galectin-7 and miR-203 knockdown cell clones. To calculate the ratio of p63-positive cells, 4,6-diamidino-2-phenylindole (DAPI) staining was used to visualize and count the cell nuclei. Confocal imaging was conducted under a PerkinElmer Ultraview RS microscope and the Volocity software was used to quantify the number of p63-positive cells. More than 900 cells, randomly selected from different visual fields, were analyzed to determine the percentages of p63-positive cells.
MG-132 treatment
To study the role of proteasome degradation, cells were treated with or without MG-132 (Selleckchem, TX, USA) for 5 h. HeLa cells containing ectopically expressed JNK1-GFP were treated with MG-132, and the ubiquitination of immunoprecipitated JNK1-GFP was detected by immunoblot, using an anti-his antibody to detect his-tag-ubiquitin.
Mammalian two-hybrid analysis
We employed the CheckMate™ Mammalian Two-Hybrid (Promega, CA, USA) system. Galectin-7 and JNK1 were subcloned into the pACT and pBind vector, respectively. These plasmids, along with the reporter pG5luc vector, were co-transfected into 293T cells, and the Dual-Luciferase® Reporter Assay System was used to measure luciferase activity. The data were recorded as counts per second (CPS) using a SpectraMax luminescence microplate reader.
Statistical analysis
Quantitative results were analyzed by using the Prism 6 software (GraphPad Software, CA). One-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used to analyze the differences between the groups and pairs. In addition, an unpaired two-tailed Student’s t-test was used to analyze the differences between pairs of data. P-values < 0.05 denote statistical significance.
Supplementary Material
Acknowledgments
We thank Dr. N. Fusenig (Deutsche Krebsforschungszentrum, Heidelberg, Germany) for kindly providing the HaCaT cell line. This study is supported by Ministry of Science and Technology, Taiwan (MOST 103-3111-Y-001-024) and National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS RO1 AR056343).
Abbreviations
- JNK
c-Jun N-terminal kinase
- SCC
squamous cell carcinoma
Footnotes
CONFLICT OF INTERREST
The authors declare no conflict of interest.
References
- Arvey A, Larsson E, Sander C, et al. Target mRNA abundance dilutes microRNA and siRNA activity. Mol Syst Biol. 2010;6:363. doi: 10.1038/msb.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow AL, Macleod A, Noppen S, et al. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of pearson’s correlation coefficient. Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2010;16:710–24. doi: 10.1017/S143192761009389X. [DOI] [PubMed] [Google Scholar]
- Bernerd F, Sarasin A, Magnaldo T. Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. Proc Natl Acad Sci U S A. 1999;96:11329–34. doi: 10.1073/pnas.96.20.11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers M, Rose AA, Grosset AA, et al. Overexpression of galectin-7, a myoepithelial cell marker, enhances spontaneous metastasis of breast cancer cells. Am J Pathol. 2010;176:3023–31. doi: 10.2353/ajpath.2010.090876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyrieux AF, Wilson VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007;54:77–83. doi: 10.1007/s10616-007-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziunycz P, Iotzova-Weiss G, Eloranta JJ, et al. Squamous cell carcinoma of the skin shows a distinct microRNA profile modulated by UV radiation. J Invest Dermatol. 2010;130:2686–9. doi: 10.1038/jid.2010.169. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Gendronneau G, Sidhu SS, Delacour D, et al. Galectin-7 in the control of epidermal homeostasis after injury. Molecular biology of the cell. 2008;19:5541–9. doi: 10.1091/mbc.E08-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez GJ, Tsuji T, Chen M, et al. Interplay between Cdh1 and JNK activity during the cell cycle. Nat Cell Biol. 2010;12:686–95. doi: 10.1038/ncb2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe EH. Using long-term time-lapse imaging of mammalian cell cycle progression for laboratory instruction and analysis. Cell Biol Educ. 2005;4:284–90. doi: 10.1187/cbe.05-02-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y, Higashi K, Kushida M, et al. Hepatocyte growth factor suppresses profibrogenic signal transduction via nuclear export of Smad3 with galectin-7. Gastroenterology. 2008;134:1180–90. doi: 10.1053/j.gastro.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Jensen JM, Folster-Holst R, Baranowsky A, et al. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J Invest Dermatol. 2004;122:1423–31. doi: 10.1111/j.0022-202X.2004.22621.x. [DOI] [PubMed] [Google Scholar]
- Kartasova T, Roop DR, Yuspa SH. Relationship between the expression of differentiation-specific keratins 1 and 10 and cell proliferation in epidermal tumors. Mol Carcinog. 1992;6:18–25. doi: 10.1002/mc.2940060105. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Kuwabara Y, Yang RY, et al. Galectin-7 (PIG1) exhibits pro-apoptotic function through JNK activation and mitochondrial cytochrome c release. The Journal of biological chemistry. 2002;277:3487–97. doi: 10.1074/jbc.M109360200. [DOI] [PubMed] [Google Scholar]
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–73. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nature reviews Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, Flint T, et al. Cloning, expression, and chromosome mapping of human galectin-7. The Journal of biological chemistry. 1995;270:5823–9. doi: 10.1074/jbc.270.11.5823. [DOI] [PubMed] [Google Scholar]
- Magnaldo T, Bernerd F, Darmon M. Galectin-7, a human 14-kDa S-lectin, specifically expressed in keratinocytes and sensitive to retinoic acid. Dev Biol. 1995;168:259–71. doi: 10.1006/dbio.1995.1078. [DOI] [PubMed] [Google Scholar]
- Magnaldo T, Fowlis D, Darmon M. Galectin-7, a marker of all types of stratified epithelia. Differentiation. 1998;63:159–68. doi: 10.1046/j.1432-0436.1998.6330159.x. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, McDade SS, Patel D, et al. MicroRNA 203 expression in keratinocytes is dependent on regulation of p53 levels by E6. J Virol. 2010;84:10644–52. doi: 10.1128/JVI.00703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehes E, Mones E, Nemeth V, et al. Collective motion of cells mediates segregation and pattern formation in co-cultures. PLoS One. 2012;7:e31711. doi: 10.1371/journal.pone.0031711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melar-New M, Laimins LA. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J Virol. 2010;84:5212–21. doi: 10.1128/JVI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C, Antonini D. Crosstalk among p53 family members in cutaneous carcinoma. Experimental dermatology. 2014;23:143–6. doi: 10.1111/exd.12320. [DOI] [PubMed] [Google Scholar]
- Moisan S, Demers M, Mercier J, et al. Upregulation of galectin-7 in murine lymphoma cells is associated with progression toward an aggressive phenotype. Leukemia. 2003;17:751–9. doi: 10.1038/sj.leu.2402870. [DOI] [PubMed] [Google Scholar]
- Moravcova M, Libra A, Dvorakova J, et al. Modulation of keratin 1, 10 and involucrin expression as part of the complex response of the human keratinocyte cell line HaCaT to ultraviolet radiation. Interdiscip Toxicol. 2013;6:203–8. doi: 10.2478/intox-2013-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Pinter G, Kohut G, et al. Time-lapse analysis of cell death in mammalian and fungal cells. DNA Cell Biol. 2010;29:249–59. doi: 10.1089/dna.2009.0980. [DOI] [PubMed] [Google Scholar]
- Pivarcsi A, Szell M, Kemeny L, et al. Serum factors regulate the expression of the proliferation-related genes alpha5 integrin and keratin 1, but not keratin 10, in HaCaT keratinocytes. Arch Dermatol Res. 2001;293:206–13. doi: 10.1007/s004030100217. [DOI] [PubMed] [Google Scholar]
- Polyak K, Xia Y, Zweier JL, et al. A model for p53-induced apoptosis. Nature. 1997;389:300–5. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Proksch E, Brasch J. Abnormal epidermal barrier in the pathogenesis of contact dermatitis. Clin Dermatol. 2012;30:335–44. doi: 10.1016/j.clindermatol.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Raja, Sivamani K, Garcia MS, et al. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–68. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- Reichelt J, Magin TM. Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J Cell Sci. 2002;115:2639–50. doi: 10.1242/jcs.115.13.2639. [DOI] [PubMed] [Google Scholar]
- Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2:e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Wei T, Pavez Lorie E, et al. Protein kinase C-dependent upregulation of miR-203 induces the differentiation of human keratinocytes. J Invest Dermatol. 2010;130:124–34. doi: 10.1038/jid.2009.294. [DOI] [PubMed] [Google Scholar]
- St-Pierre Y, Campion CG, Grosset AA. A distinctive role for galectin-7 in cancer ? Frontiers in bioscience (Landmark edition) 2012;17:438–50. doi: 10.2741/3937. [DOI] [PubMed] [Google Scholar]
- Syed DN, Khan MI, Shabbir M, et al. MicroRNAs in skin response to UV radiation. Current drug targets. 2013;14:1128–34. doi: 10.2174/13894501113149990184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonel G, Conrad C. Interplay between keratinocytes and immune cells--recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009;41:963–8. doi: 10.1016/j.biocel.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Ueda S, Kuwabara I, Liu FT. Suppression of tumor growth by galectin-7 gene transfer. Cancer Res. 2004;64:5672–6. doi: 10.1158/0008-5472.CAN-04-0985. [DOI] [PubMed] [Google Scholar]
- Villeneuve C, Baricault L, Canelle L, et al. Mitochondrial proteomic approach reveals galectin-7 as a novel BCL-2 binding protein in human cells. Molecular biology of the cell. 2011;22:999–1013. doi: 10.1091/mbc.E10-06-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladoiu MC, Labrie M, St-Pierre Y. Intracellular galectins in cancer cells: potential new targets for therapy (Review) Int J Oncol. 2014;44:1001–14. doi: 10.3892/ijo.2014.2267. [DOI] [PubMed] [Google Scholar]
- Vyakarnam A, Dagher SF, Wang JL, et al. Evidence for a role for galectin-1 in pre-mRNA splicing. Molecular and cellular biology. 1997;17:4730–7. doi: 10.1128/mcb.17.8.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–8. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yi R, Poy MN, Stoffel M, et al. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–9. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Finley RL, Jr, Raz A, et al. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. The Journal of biological chemistry. 2002;277:15819–27. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- Yugawa T, Narisawa-Saito M, Yoshimatsu Y, et al. DeltaNp63alpha repression of the Notch1 gene supports the proliferative capacity of normal human keratinocytes and cervical cancer cells. Cancer Res. 2010;70:4034–44. doi: 10.1158/0008-5472.CAN-09-4063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.