Abstract
The enteric nervous system, the largest division of the peripheral nervous system, is derived from vagal neural crest cells that invade and populate the entire length of the gut to form diverse neuronal subtypes. Here, we identify a novel population of neurons within the enteric nervous system of zebrafish larvae that express the transgenic marker ptf1a:GFP within the midgut. Genetic lineage analysis reveals that enteric ptf1a:GFP+ cells are derived from the neural crest and that most ptf1a:GFP+ neurons express the neurotransmitter 5HT, demonstrating that they are serotonergic. This transgenic line, Tg(ptf1a:GFP), provides a novel neuronal marker for a subpopulation of neurons within the enteric nervous system, and highlights the possibility that Ptf1a may act as an important transcription factor for enteric neuron development.
Keywords: serotonergic neuron, ptf1a, enteric nervous system, zebrafish
Introduction
The enteric nervous system (ENS) is comprised of interconnecting ganglia within the myenteric and submucosal plexuses that run along the gut wall (Bornstein et al., 1984). The ENS regulates gastrointestinal motility and, as the largest portion of the peripheral nervous system, is often referred to as the “second brain” (Furness, 2006). The ENS is largely derived from the “vagal” neural crest that arises from the dorsal neural tube in the post-otic region (LeDouarin and Teillet, 1973; Anderson et al., 2006). Vagal neural crest cells migrate away from the neural tube, moving ventrally toward the anterior foregut. After entering the foregut, they change direction and begin migrating caudally such that they eventually populate the entire gut, a migration process that takes days and is the longest of any embryonic cell migration. Once reaching their final destinations, enteric neural crest (ENC) differentiate into a diverse array of enteric neurons and glial cells along the entire length of the gut (Furness, 2006; Sasselli et al., 2012). Because improper neural crest development gives rise to developmental defects such as Hirschsprung’s disease (colonic aganglionosis) (Bergeron et al., 2012), there has been great interest in understanding the migration, specification and differentiation of enteric neural crest.
Although recent studies have identified various neurotransmitter markers of terminally differentiated enteric neuron subtypes within the ENS (Uyttebroek et al. 2010; rev. in Sasselli et al., 2012), much less is known about the transcription factors that mediate terminal differentiation towards a particular neuron lineage. Nonetheless, some experiments have shed light on this issue. For example, a null allele of Ascl1, a basic helix-loop-helix (bHLH) transcription factor, results in a complete loss of all serotonergic neurons from the ENS, demonstrating its requirement in differentiation of this enteric neuron subtype (Blaugrund et al. 1996). Analogously, inactivation of the bHLH transcription factor Hand2 in neural crest cells leads to disrupted ganglia patterning and selective loss of VIP+ neurons, indicating its importance in differentiation of peptidergic enteric neurons in the mouse gut (Hendershot et al. 2007). However, understanding of the transcription factor code responsible for enteric neuron subtype specification and terminal differentiation remains limited. Accordingly, identification of novel factors expressed in the developing ENS will highlight candidate genes that may be involved in these processes.
Pancreas specific transcription factor 1a, ptf1a, is expressed in the developing zebrafish pancreas (Lin et al., 2004; Zecchin et al., 2004), retina (Jusuf and Harris, 2009) and cerebellum (Kani et al., 2010) during embryonic development. Ptf1a plays a critical role in zebrafish ventral pancreas specification and exocrine pancreas development (Lin et al., 2004; Zecchin et al., 2004; Dong et al., 2008), as well as functioning during inhibitory neuron subtype differentiation in the retina (Dullin et al., 2007; Fujitani et al., 2006), cerebellum (Hoshino et al., 2005). Given the essential roles of Ptf1a in the subtype specification of various neuron types within the nervous system, we sought to examine if it was also present within the developing ENS. Here, our results using the transgenic fish line Tg(ptf1a:GFP) reveal that ptf1a is expressed in a subset of neurons in the developing ENS of zebrafish larvae. Thus, these data identify Ptf1a as a novel neuronal marker in the developing ENS.
Results
The transgenic line Tg(ptf1a:GFP) labels a subset of ENS neurons during larval stages of zebrafish development
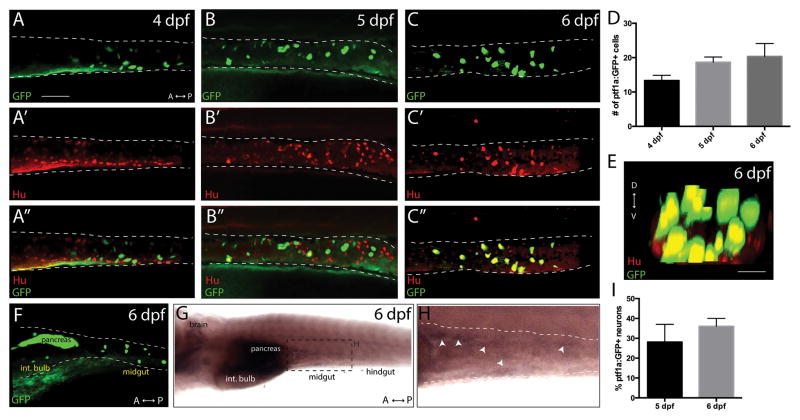
The transgenic line Tg(ptf1a:GFP) previously has been shown to mark cells that express ptf1a in vivo (Godinho et al., 2007; Jusuf and Harris, 2009; Kani et al., 2010). To examine if cells that express ptf1a are present in the developing ENS, ptf1a:GFP+ larvae were examined from 3 to 6 days post fertilization (dpf), the time during which terminal differentiation commences in the developing zebrafish ENS. Whole mount confocal microscopy revealed that ptf1a:GFP+ cells were first present at 4 dpf in the midgut (Fig. 1A), and persisted at 5 dpf and 6 dpf (Fig. 1B,C), with a few ptf1a:GFP+ cells also seen in the intestinal bulb (foregut) by 6 dpf (Fig. 1F). There was an average of 13 ptf1a:GFP+ cells in the midgut at 4 dpf, 19 cells at 4 dpf and 21 cells at 6 dpf (Fig. 1D). GFP+ cells were also present in other regions of the embryo, such as the pancreas (Fig. 1F). In order to detect endogenous ptf1a transcripts in the developing ENS, we performed in whole mount in situ hybridization. Consistent with the distribution of cells observed in the transgenic line, this analysis revealed the presence of ptf1a transcripts in the developing brain, pancreas and the midgut, but not the hindgut, at 6 dpf (Fig. 1G,H).
Figure 1. The transgenic line Tg(ptf1a:GFP) marks a subset of neurons within the larval ENS.
Maximum projection confocal stacks at (A–A″) 4 dpf, (B–B″) 5 dpf and (C–C″) 6 dpf depicting ptf1a:GFP+ and Hu+ cells within the midgut (dashed lines), scale bar: 70 microns, for A–C. (D) Bar graph illustrating total number of ptf1a:GFP+ cells in the gut from 4 dpf to 6 dpf, error bars represent s.e.m., n=6. (E) Maximum intensity confocal projection along the z-axis reveals that ptf1a:GFP+/Hu+ cells co-localize in a concentric pattern along the gut tube at 6 dpf, scale bar: 100 microns. (F) Lateral view of a 6 dpf ptf1a:GFP+ larval fish. (G,H) Ventrolateral view following whole mount in situ hybridization against ptf1a reveals that ptf1a localizes to the brain, pancreas and midgut of 6 dpf larvae. (I) Bar graph illustrating the percentage of total Hu+ neurons that are ptf1a:GFP+ in the midgut at 5 dpf and 6 dpf, error bars represent s.e.m., n= 6. Int. bulb-intestinal bulb.
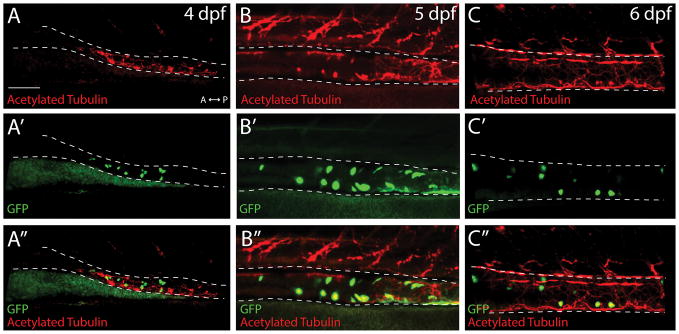
To assess if the ptf1a:GFP+ cells observed in the gut were neurons, we performed double whole mount immunochemistry against GFP and pan-neuronal markers, Hu or acetylated tubulin. At 4 dpf, confocal images revealed that a minority of ptf1a:GFP+ cells co-localized with Hu (Fig. 1A′,A″), or with acetylated tubulin, as seen in both static confocal images and a 3D rendering movie (Fig. 2A′,A″; Supporting Information Movie 1). However, at 5 and 6 dpf, a majority of ptf1a:GFP+ cells co-localized with Hu (Fig. 1B′,B″-C′,C″; Supporting Information Movie 2) and acetylated tubulin (Fig. 2B′,B″-C′,C″), revealing their neuronal identity. At 6 dpf, analysis of confocal stack projections along the z-axis revealed that the ptf1a:GFP+ cells were located in a concentric pattern along the outer layer of the gut tube, co-localizing with Hu (Fig. 1E). These data indicate that ptf1a:GFP+ cells are present within the gut prior to their terminal differentiation into neurons at 4 dpf, and that its expression is maintained in differentiated neurons at 5 and 6 dpf. At 5 dpf, quantification revealed that an average of 28% of Hu+ neurons were ptf1a:GFP+ in the midgut, while at 6 dpf 36% were ptf1a:GFP+ (Fig. 1I), demonstrating that ptf1a is not expressed in all enteric neurons, but rather in a subset of neurons.
Figure 2. ptf1a:GFP+ cells co-localize with the neuronal marker acetylated tubulin in the gut.
Maximum projection confocal stacks at (A–A″) 4 dpf, (B–B″) 5 dpf and (C–C″) 6 dpf depicting ptf1a:GFP+ and Acetylated tubulin+ cells within the midgut (dashed lines), scale bar: 70 microns.
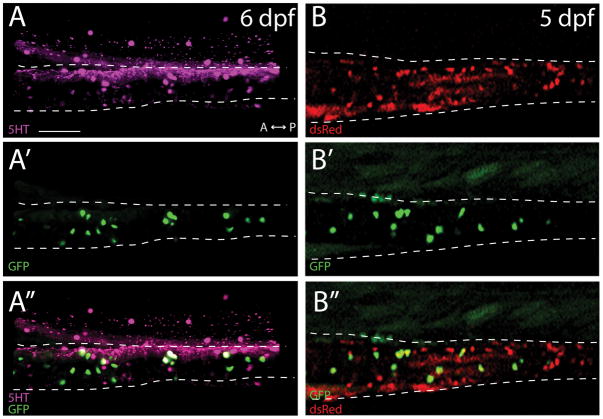
Previously, it has been shown that ~30% of midgut neurons contain the neurotransmitter serotonin (5HT) at 5 dpf (Uyttebroek et al., 2010). In order to determine whether ptf1a:GFP+ neurons in the midgut were also 5HT+, we performed double immunostaining of ptf1a:GFP larvae with antibodies against GFP and 5HT. At 5 dpf, an average of 97% of ptf1a:GFP+ cells were 5HT+ (Fig. 3A–A″), indicating that the ptf1a:GFP+ neuron population are largely serotoninergic neurons within the larval midgut.
Figure 3. ptf1a:GFP cells are largely serotonergic enteric neurons that are derived from the neural crest.
Maximum projection confocal stack of the midgut (A–A″) reveal that ptf1a:GFP+ cells co-localize with the neurotransmitter 5HT at 6 dpf. (B–B″) Images of live triple transgenic larval fish, ptf1a:GFP;-4725sox10:Cre;elf1a:loxp-GFP-loxp-dsRedpA, shows that ptf1a:GFP+ cells are neural crest derived. Scale bar: 70 microns.
ptf1a:GFP+ cells in the developing ENS are derived from the neural crest
ptf1a is expressed in the developing pancreas, where it plays key roles in regulating pancreas formation (Lin et al., 2004). On the other hand, in the brain and retina, it is expressed in differentiating neuron subtypes (Dullin et al., 2007; Kani et al., 2010) during neurogenesis and retinogenesis, respectively. To date, ptf1a expression has not been described in neural crest derived structures. To ascertain whether the ptf1a:GFP+ neurons observed in the gut were neural crest derived, we crossed Tg(ptf1a:GFP) fish with the Tg(-4725sox10:Cre;elf1a:loxp-GFP-loxp-dsRedpA) line, which permanently labels neural crest cells and all of their derivatives by ubiquitous expression of the dsRed fluorescent protein (Rodrigues et al., 2012), allowing for lineage analysis in vivo. In live embryos at 5 dpf, 100% of ptf1a:GFP+ cells in the gut were also positive for dsRed, indicating that they were indeed derived from the neural crest cells (Fig. 3B–B″).
Discussion
In this study we report the presence of a novel population of ptf1a:GFP+ neurons located within the midgut of the developing ENS of zebrafish larvae, a majority of which are serotonergic. This is the first report describing ptf1a expression within the ENS in any organism. This line can be used as an in vivo marker of differentiating and terminally differentiated neurons, thus serving as a useful tool for developmental studies of the ENS.
During zebrafish ENS differentiation, enteric progenitors migrate caudally along the gut tube, within the gut mesenchyme, fully populating the gut by 3 dpf. Subsequently, these enteric progenitors undergo extensive proliferation in order to generate thousands of enteric neurons in the appropriate proportions and in precise locations within the adult zebrafish gut (Uyttebroek et al., 2010). That we did not detect ptf1a:GFP+ cells prior to 4 dpf indicates that it is activated after the initial neural crest invasion of the gut, suggesting a possible later role during enteric neuroblast proliferation and/or terminal differentiation. In support of this idea, we found that ptf1a:GFP+ cells in the gut co-localized with the neuronal markers Hu and acetylated tubulin, as well as largely co-localizing with the neurotransmitter serotonin, 5HT. During zebrafish ENS development, serotonergic neurons encompass between ~25–30% of total neurons in the larval gut by 5 dpf (Uyttebroek et al., 2010). In adult fish, these proportions are maintained in the proximal gut and midgut, while in the hindgut the proportion reduces sharply to ~10% of total neurons (Uyttebroek et al., 2010). Interestingly, within the developing Xenopus retina, Ptf1a regulates the balance of inner nuclear layer neuron subtypes, specifically controlling the number of GABAergic interneurons born during retinogenesis (Dullin et al., 2007). Ectopic expression of ptf1a was sufficient to increase the number of 5HT+ neurons, while Ptf1a loss decreased the number of 5HT+ neurons within the retina (Dullin et al., 2007), suggesting an important role for Ptf1a in the allocation or differentiation of serotonergic neuron subtypes. In conjunction with these previous studies, our discovery of the presence of ptf1a:GFP+/5HT+ cells in the gut suggests that Ptf1a may play similar roles. Future studies aimed at further investigating the role of Ptf1a in development of enteric neuron subtypes, as well as its functional regulation within the ENS, will provide novel insight into the mechanisms underlying neurogenesis within the peripheral nervous system.
Materials and Methods
Zebrafish maintenance and fish lines
Zebrafish (danio rerio) were maintained at 28.5°C on a 13-hour light/11 hour dark cycle. Animals were treated in accordance with California Institute of Technology IACUC provisions. The Tg(ptf1a:GFP) line (Godinho et al., 2007) and/or Tg(-4725sox10:Cre;elf1a:loxp-GFP-loxp-dsRedpA) line (Rodrigues et al., 2012) was used for all experiments.
in situ hybridization
Hybridizations were performed as previously described (Uribe and Gross, 2010), with the addition of a 20 minute Collagenase type 1A (Sigma C9891) digestion (1 mg/mL) prior to Proteinase K digestion to facilitate penetration of the probe. To generate ptf1a probe, the following primers were used to PCR amplify template containing a T7 polymerase site from pCS2-ptf1a (Zhang et al., 2012), forward 5′ CGACGATGACTTCTTTACGGACC 3′ and reverse 5′ TAATACGACTCACTATAGGTTCCTCGGTGGCAAATGATG 3′, with the T7 site underlined. T7 polymerase was used to generate antisense probe.
Whole mount immunochemistry
Larval fish at 4 dpf, 5 dpf and 6 dpf were fixed overnight at 4°C in 4% PFA, rinsed three times in 1X PBS, incubated in 100% Methanol at −20°C for 1 hour and then rehydrated step-wise into 1X PBS at room temperature. Larvae were then incubated in 100% acetone at −20°C for 11 minutes, rinsed three times in 1X PBS, then digested at room temperature in 10 μg/mL Proteinase K for 45 min. (4 dpf), 55 min. (5 dpf) or for 65 min. (6 dpf). Larvae were then rinsed three times in 1X PBS, fixed for 10 minutes in 4% PFA at room temperature, rinsed 3 times in 1X PBS and then incubated in 5% Donkey serum block diluted in 1X PBS-tween-20, supplemented with 1% DMSO (PBTD), for 3 hours. Embryos were then incubated in either rabbit anti-GFP 1:500 (Life Technologies, A-11122), goat anti-GFP 1:500 (Abcam, ab6673), mouse anti-HuC/D (Hu) 1:200 (Invitrogen), mouse anti-Acetylated tubulin 1:1000 (Sigma, T6793) or rabbit anti-5HT 1:1000 (Immunostar) overnight at 4°C. Embryos were then washed out of primary antibody in 1X PBS-tween-20, then incubated at room temperature in 1:700 secondary antibodies Invitrogen Alexa Fluor Donkey anti-Rabbit 488, anti-Rabbit 647, anti-Goat 488 or Donkey anti-Mouse 594 for 3 hours at room temperature. Embryos were rinsed in 1X PBST and imaged in 75% glycerol/1X PBS on a Zeiss 710 2-photon confocal microscope (Beckman Imaging Center, Caltech).
Live imaging
Embryos positive for both Tg(ptf1a:GFP) and Tg(-4725sox10:Cre;elf1a:loxp-GFP-loxp-dsRedpA) were mounted laterally in 1% low melt agarose dissolved in fish water supplemented with 1X PTU and Tricaine anesthetic, in an imaging chamber. 20–80 micron Z-stacks were acquired using a 20x objective on a Zeiss LSM 710 microscope. Z-stacks were compiled and exported using Imaris Image Analysis software.
Supplementary Material
Acknowledgments
We thank Ryan Anderson (Indiana University School of Medicine) for sharing the Tg(ptf1a:GFP) line and Robert Kelsh (University of Bath) for sharing the Tg(-4725sox10:Cre;elf1a:loxp-GFP-loxp-dsRedpA) line. We thank Crystal Rogers and Stephen Green for helpful discussions, Yuk Fai Leung (Purdue University) for pCS2-ptf1a construct and Martha Henderson and David Mayorga for fish care. Research was supported by grants from NIH DE024157 to M.E.B., from NIH F32 HD080343 to R.A.U. and a Burroughs Wellcome Fund Postdoctoral Enrichment Program Award to R.A.U.
Abbreviations
- ENS
Enteric Nervous System
- ENC
Enteric Neural Crest
References
- Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006;323:11–25. doi: 10.1007/s00441-005-0047-6. [DOI] [PubMed] [Google Scholar]
- Bergeron KF, Silversides DW, Pilon N. The developmental genetics of Hirschsprung’s disease. Clin Genet. 2013;83(1):15–22. doi: 10.1111/cge.12032. [DOI] [PubMed] [Google Scholar]
- Blaugrund E, Pham TD, Tennyson Vm, Lo I, Sommer L, Anderson DJ, Gershon MD. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Devel. 1996;122:309–320. doi: 10.1242/dev.122.1.309. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB, Lees GM. Electrophysiology and enkephalin immunoreactivity of identified myenteric plexus neurones of guinea-pig small intestine. J Physiol. 1984;351:313–325. doi: 10.1113/jphysiol.1984.sp015247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong PD, Provost E, Leach SD, Stainier DY. Graded levels of Ptf1a differentially regulate endocrine and exocrine fates in the developing pancreas. Genes and Devel. 2008;22(11):1445–1450. doi: 10.1101/gad.1663208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullin JP, Locker M, Robach M, Henningfeld KA, Parain K, Afelik S, Pieler T, Perron M. Ptf1a triggers GABAergic neuronal cell fates in the retina. BMC Dev Biol. 2007;7:110. doi: 10.1186/1471-213X-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, MacDonald RJ, Furukawa T, Fujikado T, Magnuson MA, Xiang M, Wright CV. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Devel. 2006;133:4439–50. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Blackwell Publishing, Inc; 2006. [Google Scholar]
- Godinho L, Williams PR, Claassen Y, Provost E, Leach SD, Kamermans M, Wong RO. Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron. 2007;56(4):597–603. doi: 10.1016/j.neuron.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Sarkar AA, Giovannucci DR, Clouthier DE, Abe M, Howard MJ. Expression of Hand2 is sufficient for neurogenesis and cell type-specific gene expression in the enteric nervous system. Dev Dyn. 2007;236:93–105. doi: 10.1002/dvdy.20989. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, Watanabe M, Bito H, Terashima T, Wright CV, Kawaguchi Y, Nakao K, Nabeshima Y. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–13. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Jusuf PR, Harris WA. Ptf1a is expressed transiently in all types of amacrine cells in the embryonic zebrafish retina. Neural Devel. 2009;4:34. doi: 10.1186/1749-8104-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kani S, Bae YK, Shimizu T, Tanabe K, Satou C, Parsons MJ, Scott E, Higashijima SI, Hibi M. Proneural gene-linked neurogenesis in zebrafish cerebellum. Devel Biol. 2010;343(1–2):1–17. doi: 10.1016/j.ydbio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30(1):31–48. [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Devel Biol. 2004;270(2):474–486. doi: 10.1016/j.ydbio.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Rodrigues FS, Doughton G, Yang B, Kelsh RN. A novel transgenic line using the Cre-lox system to allow permanent lineage labelling of the zebrafish neural crest. Genesis. 2012;50(10):750–757. doi: 10.1002/dvg.22033. [DOI] [PubMed] [Google Scholar]
- Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Uribe RA, Gross JM. Id2a influences neuron and glia formation in the zebrafish retina by modulating retinoblast cell cycle kinetics. Devel. 2010;137(22):3763–3774. doi: 10.1242/dev.050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttebroek L, Shepherd IT, Harrisson F, Hubens G, Blust R, Timmermans JP, Van Nassauw L. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio) J Comp Neurol. 2010;518(21):4419–4438. doi: 10.1002/cne.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecchin E, Mavropoulos A, Devos N, Filippi A, Tiso N, Meyer D, Peers B, Bortolussi M, Argenton F. Evolutionary conserved role of ptf1a in the specification of exocrine pancreatic fates. Devel Biol. 2004;268(1):174–184. doi: 10.1016/j.ydbio.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang Y, Trujillo C, Zhong W, Leung YF. The Expression of irx7 in the Inner Nuclear Layer of Zebrafish Retina Is Essential for a Proper Retinal Development and Lamination. PLoS One. 2012;7(4):e36145. doi: 10.1371/journal.pone.0036145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.