Abstract
Objective:
Taking a step beyond prior alcohol research on pregnancy trimesters, we produced pregnancy month-specific drinking estimates for women in the United States in order to shed light on time variations of alcohol drinking during pregnancy, as might be determined by alcohol dependence. We posited that (a) pregnancy might prompt cessation of drinking soon after pregnancy status is discovered, a finding obscured in trimester-specific estimates, and (b) a possible alcohol-dependence effect on drinking persistence among pregnant women might be observed via the monthly approach.
Method:
Data are from the 2002–2011 National Surveys on Drug Use and Health (Restricted-Data Analysis System [R-DAS]), with large nationally representative samples of U.S. civilians, including 12- to 44-year-old females stratified by pregnancy status and month of pregnancy, and with assessment of recent alcohol dependence as well as heavy episodic drinking (HED).
Results:
Pregnancy’s possibly protective constraints on drinking can be seen as early as Month 2. We observed considerable variability of drinking prevalence (%) before Trimester 1 ended, with no appreciable variation across Months 4–9. A possible alcohol-dependence effect on drinking persistence is seen when the contrast is made in relation to expected values for pregnant women without alcohol dependence.
Conclusions:
We detected a possibly ameliorative pregnancy effect on alcohol use and HED, with variation in drinking prevalence across the months of the first trimester. Alcohol dependence might be affecting drinking persistence among pregnant women, but this effect cannot account for the drinking persistence observed here.
In contrast to the more typical trimester-by-trimester view of alcohol drinking in studies of pregnant women, the current investigation presents month-by-month estimates. Drawing on the strengths of the U.S. National Surveys on Drug Use and Health (NSDUH; Substance Abuse and Mental Health Services Administration [SAMHSA], 2012), we hypothesized that there might be an ameliorative effect of pregnancy on drinking. This ameliorative effect is possibly seen within the first pregnancy months soon after a woman discovers she is pregnant, and hence not captured in the trimester-wise view. We present month-specific estimates of heavy episodic drinking (HED), here defined as drinking five or more drinks on the same occasion on at least 1 day in the past 30 days. We also present estimates for recently active alcohol dependence syndrome (defined as alcohol dependence present within 1 year of the assessment date), which might help account for why some mothers do not stop drinking when they become pregnant.
In most past U.S.-based research, alcohol use before and throughout pregnancy generally has been assessed retrospectively via survey questions administered in the later trimesters or postpartum. This approach can yield measurement problems, not the least of which is a memory recall or reporting error (Cheng et al., 2011; Floyd et al., 1999; Grant et al., 2009; Zhao et al., 2012). This study’s data constrain this threat via questions that are focused specifically on the 30 days before assessment, with sampling that encompasses all U.S. community-dwelling women, and with a measurement approach such that women are asked whether they are pregnant and the month of pregnancy (insofar as it is known), within the context of the NSDUH general health interview module. It is worth mentioning that some studies suggested that retrospective assessment of alcohol drinking may be at least as effective as an antenatal report in identifying risk drinking related to child outcomes and may also avoid some factors that contribute to denial and maternal underreporting during pregnancy (Alvik et al., 2006; Hannigan et al., 2010).
As such, this work is novel, in part because of its nationally representative sample and its monthly approach. In the only two published national survey reports on drinking in pregnancy, the research teams produced trimester-specific estimates but did not report the month-specific values estimated for this project (Muhuri & Gfroerer, 2009; SAMHSA, 2009). Searching for prior nationally representative estimates, we found the National Birth Defects Prevention Study (NBDPS), from which Ethen and colleagues (2009) derived the following month-specific drinking prevalence estimates for Trimester 1 only (Month 1: 22.5%, Month 2: 8.5%, and Month 3: 5.5%), which suggested dramatic drinking reduction between Month 1 and Month 2 of pregnancy. However, on close inspection, we learned that these NBDPS estimates actually are based on women in only 8 of the 50 states. Moreover, the NBDPS alcohol questions sometimes were asked after the pregnancy outcome was known, with recall intervals as long as 9 months (Yoon et al., 2001). In contrast, the NSDUH sampling frame includes all 50 states plus the District of Columbia, and the assessment is concurrent with the pregnancy, with retrospection over no more than the 30 days before assessment (i.e., during the months of pregnancy). That is, the NSDUH approach sets the maximum length for drinking recall at 30 days (the 30-day interval just before the date of survey assessment). There is no need for the pregnant women to think back over intervals of time longer than the 30-day window covered in these NSDUH assessments.
Why might month-specific epidemiological data be a useful complement to the currently prevailing estimates from trimester-specific data? We ask for consideration of whether adverse outcomes such as fetal alcohol syndrome (FAS) might be seen in response to drinking in Months 1 or 2, possibly before onset of pregnancy is detected by the drinking mother. Our own best answer to this question is that the timing of alcohol use during pregnancy might well be crucial for adverse pregnancy outcomes as well as developmental disorders, particularly when pre-clinical or clinical research has shown pre-conceptional or epigenetic focal points (Andersen et al., 2012; Maier & West, 2001; Rudeen et al., 2007; van Uitert et al., 2013; Wang et al., 2007). Working in the realm of clinical research, Feldman and colleagues (2012) recently found an increased risk of physical features of FAS when drinking extended into the second half of the first trimester. Here, the phenotype of interest involved clinical features characteristic of FAS: facial anomalies, growth retardation, cognitive impairment, and/or behavioral abnormalities, including smooth philtrum and thin vermillion, as well as an increased risk for reduced birth weight and reduced birth length. In addition, preclinical evidence highlights the potential importance of drinking exposures during Weeks 2–6 of human pregnancy, with potential cell death in the developing fetal brain following in-utero alcohol exposure (Dunty et al., 2001). In a departure from prior trimester-focused research approaches, and by using the more fine-grained monthly approach, we hope that our study will add value to what has been published by others and will encourage future clinical and pre-clinical research on fetal effects in the earliest weeks after conception.
Method
The population under study consists of noninstitutionalized civilian residents of the United States, age 12 years and older, during the interval from 2002 through 2011. Each year’s NSDUH multistage area probability sampling survey approach seeks to achieve samples of designated respondents and to recruit participants so that the resulting population sample distributions, after sampling weights are applied, are balanced with the U.S. decennial census distributions for pertinent variables (e.g., sex, age, ethnic self-identification). Poststratification adjustment factors promote achievement of this goal for study estimates based on the pooled 2002–2011 NSDUH data made available via a recent innovation known as the NSDUH Restricted-Data Analysis System (R-DAS), on which the present epidemiological estimates are based. Generally, NSDUH survey response levels exceed 70% (SAMHSA, 2012). The NSDUH study protocols have been reviewed and approved by cognizant institutional review boards for protection of human subjects in research.
With respect to assessment, the R-DAS version of NSDUH data discloses what each mother reports about her current month of pregnancy via questions such as, “Are you pregnant?” An affirmative answer is followed by this question: “How many months pregnant are you?”
The key response variable in this study is based on the pregnant woman’s answers to NSDUH standardized questions about recent active drinking of alcoholic beverages. The alcohol questions are positioned within an audio computer-assisted self-interview (ACASI), with 10 or more minutes separating alcohol questions from later questions about pregnancy. Examples of the alcohol questions are, “During the past 30 days, on how many days did you drink one or more drinks of an alcoholic beverage?” and “During the past 30 days, how many drinks did you usually have? Count as a drink a can or bottle of beer, a wine cooler or a glass of wine, champagne, or sherry, a shot of liquor or a mixed drink or cocktail.” A HED question is, “During the past 30 days, on how many days did you have five or more drinks on the same occasion? By occasion, we mean at the same time or within a couple of hours of each other.” Recently active alcohol dependence was assessed via an ACASI module of standardized NSDUH items written to tap dependence criteria listed in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994). A positive history of recently active alcohol dependence required fulfillment of three or more dependence criteria (six standard criteria: [a] spending a great deal of time over a period of a month getting, using, or getting over the effects of alcohol; [b] unable to keep set limits on alcohol use or used more often than intended; [c] needed to use alcohol more than before to get desired effects or noticed that using the same amount had less effect than before; [d] unable to cut down or stop using the alcohol every time she tried or wanted; [e] continued to use alcohol even though it was causing problems with emotions, nerves, mental health, or physical problems; and [f] gave up participation in important activities due to alcohol use; and a seventh withdrawal symptom criterion if the respondent had experienced alcohol-specific withdrawal symptoms at one time that lasted for longer than a day after she cut back or stopped using).
The plan for data analysis was organized in relation to standard “explore, analyze/estimate, explore” cycles, in which the first steps involved exploratory data analyses to shed light on univariate distributions. In the initial estimation step, the task was analysis-weighted estimation of month-specific prevalence proportions for alcohol use and HED. Next, we estimated the degree to which recently active alcohol dependence (vs. nondependent drinking) might help account for persistence of drinking during pregnancy. The key inference is that when drinking persists beyond the first month of pregnancy, one of the explanations for drinking persistence is that the mother is affected by alcohol dependence.
The R-DAS is an online analysis system with allowances for analysis-weighted estimation of frequencies and cross-tabulations, as well as Taylor series variances, but no allowance for more advanced statistical methods such as multiple regression (Seedall & Anthony, 2013). The R-DAS does not permit listing of individual cases and does not allow users to directly generate unweighted frequencies, although approximate unweighted cell counts can be derived, as explained elsewhere (Vsevolozhskaya & Anthony, 2014). Accordingly, we estimated prevalence differences for drinking alcohol within 30 days before assessment (with 95% confidence interval [CI]), comparing pregnant women with recent alcohol dependence to pregnant drinkers with no alcohol dependence (Newcombe, 1998).
Results
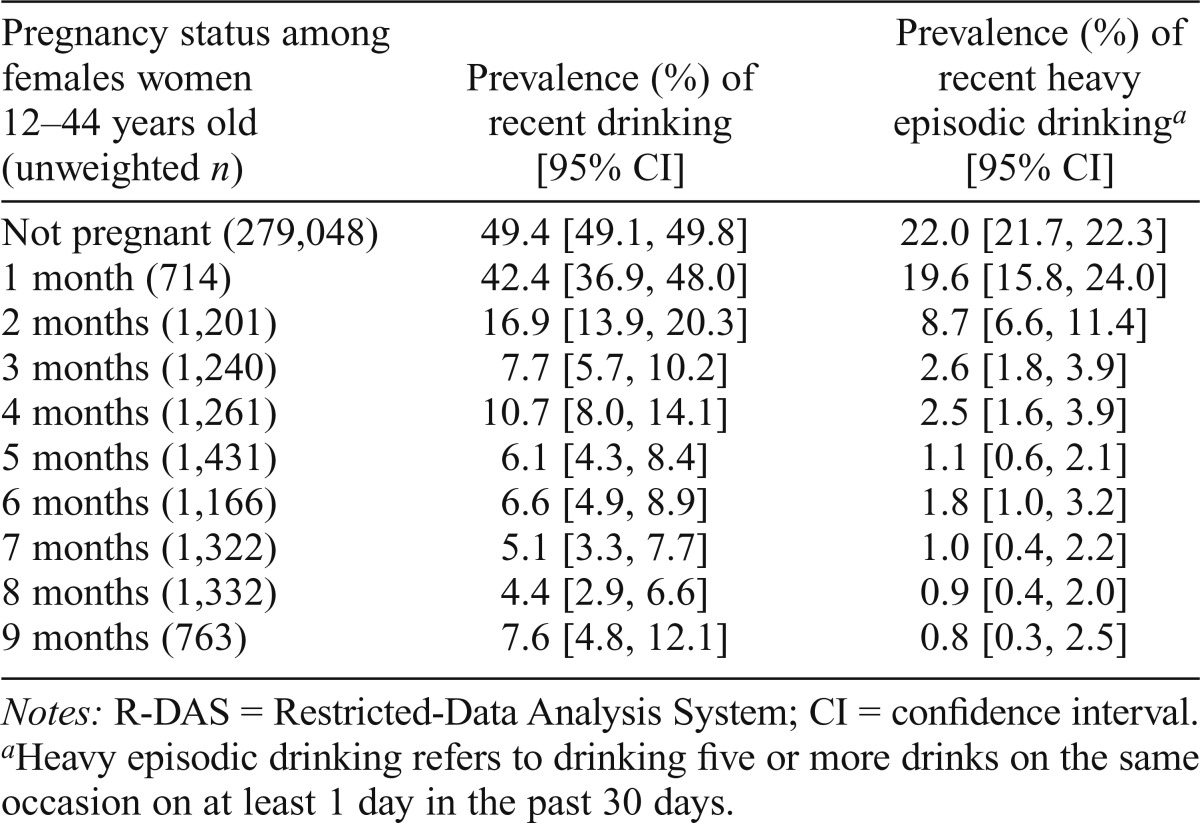
The main estimates of the study are presented in Table 1. The overall unweighted number of pregnant mothers in the sample was 10,430 (weighted percentage = 3.7% of the total sample); 10.4% of them had consumed alcoholic beverages in the month before assessment. The prevalence proportion for alcohol drinking among women in Month 1 of pregnancy was observed to be 42.4% (95% CI [36.9, 48.0]). That is, roughly 4 of every 10 pregnant women had consumed an alcoholic beverage within the 30 days before the date of assessment. HED in Month 1 is less frequent, reflected in an estimate of 19.6%. That is, roughly 2 of every 10 pregnant women in Month 1 had consumed five or more drinks on the same occasion on at least 1 day in the 30 days before the date of assessment. Considerable variability in the estimated prevalence of alcohol use and HED can be seen across the first trimester (Months 1–3).
Table 1.
Estimated analysis-weighted prevalence of drinking among nonpregnant and pregnant females ages 12–44 years, by pregnancy month. Data for the United States based on the R-DAS online analysis system of the National Surveys on Drug Use and Health, 2002–2011.
| Pregnancy status among females women 12–44 years old (unweighted n) | Prevalence (%) of recent drinking [95% CI] | Prevalence (%) of recent heavy episodic drinkinga [95% CI] |
| Not pregnant (279,048) | 49.4 [49.1, 49.8] | 22.0 [21.7, 22.3] |
| 1 month (714) | 42.4 [36.9, 48.0] | 19.6 [15.8, 24.0] |
| 2 months (1,201) | 16.9 [13.9, 20.3] | 8.7 [6.6, 11.4] |
| 3 months (1,240) | 7.7 [5.7, 10.2] | 2.6 [1.8, 3.9] |
| 4 months (1,261) | 10.7 [8.0, 14.1] | 2.5 [1.6, 3.9] |
| 5 months (1,431) | 6.1 [4.3, 8.4] | 1.1 [0.6, 2.1] |
| 6 months (1,166) | 6.6 [4.9, 8.9] | 1.8 [1.0, 3.2] |
| 7 months (1,322) | 5.1 [3.3, 7.7] | 1.0 [0.4, 2.2] |
| 8 months (1,332) | 4.4 [2.9, 6.6] | 0.9 [0.4, 2.0] |
| 9 months (763) | 7.6 [4.8, 12.1] | 0.8 [0.3, 2.5] |
Notes: R-DAS = Restricted-Data Analysis System; CI = confidence interval.
Heavy episodic drinking refers to drinking five or more drinks on the same occasion on at least 1 day in the past 30 days.
Evidence of a possible ameliorative effect of pregnancy on alcohol use and HED can be seen by Month 2, with the Month 1 estimate of 42% taken as an expected value. By comparison, for women in Month 2 of pregnancy, only 17% consumed alcohol.
HED showed a marked reduction within Trimester 1. As noted, the Month 1 value is 19.6%. By Month 3, fewer than 3% of the pregnant women qualified as heavy episodic drinkers. Estimated HED prevalence estimates for women assessed in pregnancy Months 4–8 were relatively stable.
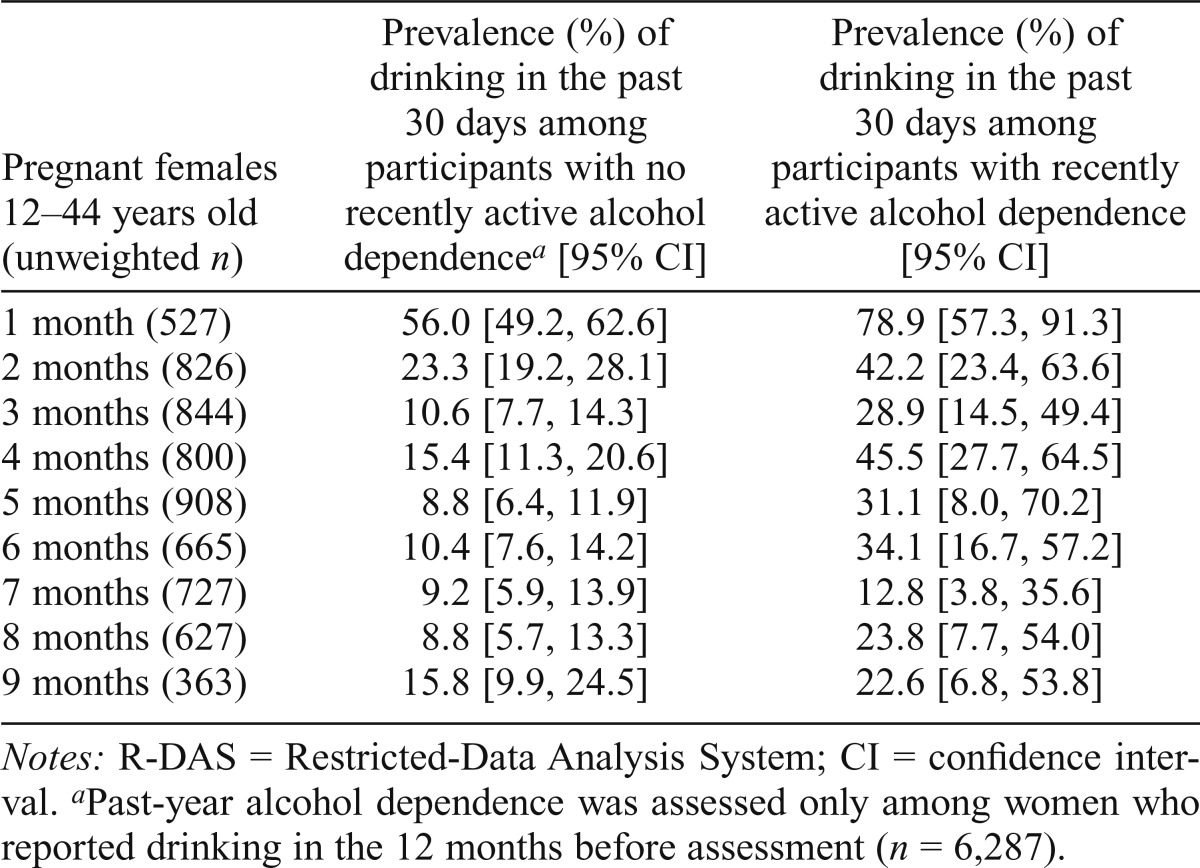
Table 2 displays month-specific drinking prevalence estimates for pregnant women in relation to alcohol dependence status. These estimates convey the degree to which recently active alcohol dependence might be accounting for persistence of drinking during pregnancy. As expected, alcohol dependence case status is associated with recently active drinking during pregnancy, month by month, and the estimated prevalence of alcohol drinking during the 30 days before assessment is greater among pregnant women with recently active alcohol dependence (i.e., alcohol dependence active within the 1 year before assessment) versus pregnant drinkers with no such alcohol dependence.
Table 2.
Estimated analysis-weighted prevalence of drinking by recently active alcohol dependence status among pregnant females ages 12–44 years, by pregnancy month. Data for the United States based on the R-DAS online analysis system of the National Surveys on Drug Use and Health, 2002–2011.
| Pregnant females 12–44 years old (unweighted n) | Prevalence (%) of drinking in the past 30 days among participants with no recently active alcohol dependencea [95% CI] | Prevalence (%) of drinking in the past 30 days among participants with recently active alcohol dependence [95% CI] |
| 1 month (527) | 56.0 [49.2, 62.6] | 78.9 [57.3, 91.3] |
| 2 months (826) | 23.3 [19.2, 28.1] | 42.2 [23.4, 63.6] |
| 3 months (844) | 10.6 [7.7, 14.3] | 28.9 [14.5, 49.4] |
| 4 months (800) | 15.4 [11.3, 20.6] | 45.5 [27.7, 64.5] |
| 5 months (908) | 8.8 [6.4, 11.9] | 31.1 [8.0, 70.2] |
| 6 months (665) | 10.4 [7.6, 14.2] | 34.1 [16.7, 57.2] |
| 7 months (727) | 9.2 [5.9, 13.9] | 12.8 [3.8, 35.6] |
| 8 months (627) | 8.8 [5.7, 13.3] | 23.8 [7.7, 54.0] |
| 9 months (363) | 15.8 [9.9, 24.5] | 22.6 [6.8, 53.8] |
Notes: R-DAS = Restricted-Data Analysis System; CI = confidence interval.
Past-year alcohol dependence was assessed only among women who reported drinking in the 12 months before assessment (n = 6,287).
We made an ex post facto comparison of pregnant drinkers with a history of alcohol dependence versus an expected value based on pregnant drinkers without a history of alcohol dependence and found excess drinking prevalence among the pregnant drinkers with a history of alcohol dependence (i.e., prevalence difference [PD] = 22.4%; p < .05; results are not shown in tables). We also found an excess HED prevalence among the pregnant drinkers with a history of alcohol dependence (i.e., PD = 19.3%; p < .05; results are not shown in tables).
Table 2 also discloses considerable drinking persistence in the absence of alcohol dependence. When we made an ex post facto contrast of pregnant drinkers in Months 2–8 versus an expected value based on nonpregnant drinkers, we found excess alcohol dependence prevalence among the pregnant drinkers (i.e., PD = 4.6; 95% CI [1.6, 8.5]; results are not shown in tables). Despite a relatively large overall sample size, the unweighted numbers of pregnant women, month-by-month, provided little statistical power and precision to estimate an alcohol-dependence effect on drinking among pregnant drinkers month-by-month.
Discussion
Several findings from this research are noteworthy. First, we discovered a potential ameliorative effect of pregnancy on alcohol use, observable soon after a woman learns she is pregnant (i.e., as early as Month 2, and well before the end of Trimester 1). As such, some alcohol research opportunities will be missed unless a month-by-month measurement approach is substituted for the more traditional trimester-by-trimester approach. Second, our estimates show noteworthy variability of drinking prevalence across pregnancy months of Trimester 1. Third, an excess 30-day drinking prevalence and HED prevalence were found among pregnant drinkers with recently active alcohol dependence.
Before detailed review and discussion of this evidence, several limitations of the study merit attention, including reliance on self-report measures with no toxicological assays. Of greater importance might be the lack of a longitudinal design with reassessment of women followed month by month during pregnancy. Instead, the NSDUH offers cross-sectional snapshots of women assessed month by month during pregnancy. As discussed by others, misclassification error almost certainly is present, including differential misclassification to the extent that pregnant women might be less likely to disclose drinking, even in the context of an ACASI approach (Kesmodel & Olsen, 2001).
We also must note that NSDUH assessments do not cover the timing of alcohol cessation or counseling during pregnancy. In addition, the cross-sectional sample includes women who have discovered that they are in the first pregnancy month. For these women, the answers to the NSDUH questions on drinking in the prior 30 days might be in reference to drinking that occurred before conception. Accordingly, the actual prevalence of drinking during the first month of gestation might well be deflated, considering that roughly half of U.S. pregnancies are unplanned (Finer & Zolna, 2014). As such, drinking prevalence in Month 1 sometimes is not much different from drinking prevalence for nonpregnant women of childbearing age. Finally, more detailed analyses of the NSDUH data sets will become possible once the “month of pregnancy” variable is yoked with individual-level variables such as age of the mother and age at first alcohol use in regression models. At present, the RDAS version of the NSDUH data sets permits contingency table analyses but not multiple regression analyses. (There are NSDUH “SDA” data sets for multiple regression analyses, but these data sets do not include information about the specific month of the pregnancy.)
The study’s findings are of interest because the data come from nationally representative samples with standardized assessment protocols of alcohol use, month by month. In this study, there was no requirement for long-term recall, as is typical in retrospective reports over the entire span of pregnancy. Moreover, it is important to note that these monthly NSDUH drinking prevalence estimates are somewhat larger than corresponding estimates from the NBDPS, possibly because of differences in the sampling frames or coverage, or because of measurement differences. As mentioned in the introduction, the long-term recall was required for the NBDPS postpartum assessment. In contrast, the NSDUH approach identified women month by month during pregnancy, with drinking recall over the span of no more than 30 days.
Before closing, we note that alcohol toxicity can be expected to vary in relation to maternal ethanol dosing and pharmacokinetics. In this regard, HED is noteworthy because it can expose the developing fetus to relatively high blood alcohol concentrations over short periods, with resulting increased risk of adverse pregnancy outcomes (Goodlett & Horn, 2001; Ornoy & Ergaz, 2010). To the extent that adverse effects of alcohol drinking during pregnancy actually vary across months of the same trimester, the monthly approach of this study might stimulate new research. A week-specific approach might prove to be even better than this study’s month-specific approach.
In conclusion, month-specific estimates shed new light on alcohol use in pregnancy and draw attention to a previously undiscovered potential ameliorative effect of pregnancy on drinking persistence, observable as early as Month 2. This phenomenon truly cannot be seen when the traditional trimester approach is used to study drinking by pregnant women. An implication of potential clinical importance pertains to cessation initiatives part and parcel with prenatal care, with benefits seen in relation to the overall health of mothers and their offspring.
If alcohol-attributable health effects on the fetus are found as early as Month 1 of the pregnancy, women who drink might be advised to use pregnancy test kits after every sexual encounter that might give rise to a conception. A package insert with the pregnancy test kit might draw attention to nonlethal and potentially adverse implications for the developing fetus if drinking or other drug use is sustained during the first month of the pregnancy. Of course, the hypothesized beneficial effects of this type of package insert are speculative. The best evidence about this type of patient-oriented intervention will come from future randomized controlled trials on this topic.
Footnotes
This research was completed during Omayma Alshaarawy’s second-year postdoctoral epidemiology fellowship with training program director James C. Anthony. Omayma Alshaarawy’s work was supported by the National Institute on Drug Abuse (T32DA021129), James C. Anthony’s National Institute on Drug Abuse Senior Scientist Award (K05DA015799), and Michigan State University.
References
- Alvik A., Haldorsen T., Groholt B., Lindemann R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcoholism: Clinical and Experimental Research. 2006;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. doi:10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Andersen A. M., Andersen P. K., Olsen J., Grønbæk M., Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. International Journal of Epidemiology. 2012;41:405–413. doi: 10.1093/ije/dyr189. doi:10.1093/ije/dyr189. [DOI] [PubMed] [Google Scholar]
- Cheng D., Kettinger L., Uduhiri K., Hurt L. Alcohol consumption during pregnancy: Prevalence and provider assessment. Obstetrics and Gynecology. 2011;117:212–217. doi: 10.1097/AOG.0b013e3182078569. doi:10.1097/AOG.0b013e3182078569. [DOI] [PubMed] [Google Scholar]
- Dunty W. C., Jr., Chen S. Y., Zucker R. M., Dehart D. B., Sulik K. K. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: Implications for alcohol-related birth defects and neurodevelopmental disorder. Alcoholism: Clinical and Experimental Research. 2001;25:1523–1535. doi:10.1111/j.1530-0277.2001.tb02156.x. [PubMed] [Google Scholar]
- Ethen M. K., Ramadhani T. A., Scheuerle A. E., Canfield M. A., Wyszynski D. F., Druschel C. M., Romitti P. A. the National Birth Defects Prevention Study. Alcohol consumption by women before and during pregnancy. Maternal and Child Health Journal. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. doi:10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H. S., Jones K. L., Lindsay S., Slymen D., Klonoff-Cohen H., Kao K., Chambers C. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: A prospective study. Alcoholism: Clinical and Experimental Research. 2012;36:670–676. doi: 10.1111/j.1530-0277.2011.01664.x. doi:10.1111/j.1530-0277.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- Finer L. B., Zolna M. R. Shifts in intended and unintended pregnancies in the United States, 2001–2008. American Journal of Public Health, 104, Supplement 1. 2014:S43–S48. doi: 10.2105/AJPH.2013.301416. doi:10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. L., Decouflé P., Hungerford D. W. Alcohol use prior to pregnancy recognition. American Journal of Preventive Medicine. 1999;17:101–107. doi: 10.1016/s0749-3797(99)00059-8. doi:10.1016/S0749-3797(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Goodlett C. R., Horn K. H. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Research & Health. 2001;25:175–184. [PMC free article] [PubMed] [Google Scholar]
- Grant T. M., Huggins J. E., Sampson P. D., Ernst C. C., Barr H. M., Streissguth A. P. Alcohol use before and during pregnancy in western Washington, 1989-2004: Implications for the prevention of fetal alcohol spectrum disorders. American Journal of Obstetrics and Gynecology. 2009;200:278.e1–278.e8. doi: 10.1016/j.ajog.2008.09.871. doi:10.1016/j.ajog.2008.09.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan J. H., Chiodo L. M., Sokol R. J., Janisse J., Ager J. W., Greenwald M. K., Delaney-Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44:583–594. doi: 10.1016/j.alcohol.2009.03.003. doi:10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesmodel U., Olsen S. F. Self reported alcohol intake in pregnancy: Comparison between four methods. Journal of Epidemiology and Community Health. 2001;55:738–745. doi: 10.1136/jech.55.10.738. doi:10.1136/jech.55.10.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S. E., West J. R. Drinking patterns and alcohol-related birth defects. Alcohol Research & Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- Muhuri P. K., Gfroerer J. C. Substance use among women: Associations with pregnancy, parenting, and race/ethnicity. Maternal and Child Health Journal. 2009;13:376–385. doi: 10.1007/s10995-008-0375-8. doi:10.1007/s10995-008-0375-8. [DOI] [PubMed] [Google Scholar]
- Newcombe R. G. Interval estimation for the difference between independent proportions: Comparison of eleven methods. Statistics in Medicine. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Ergaz Z. Alcohol abuse in pregnant women: Effects on the fetus and newborn, mode of action and maternal treatment. International Journal of Environmental Research and Public Health. 2010;7:364–379. doi: 10.3390/ijerph7020364. doi:10.3390/ijerph7020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudeen P. K., Cook K., Mengel M. B., Ulione M., Wedding D., Braddock S., Ohlemiller M. Knowledge and attitudes about fetal alcohol syndrome, fetal alcohol spectrum disorders, and alcohol use during pregnancy by occupational therapists in the Midwest. Journal of Allied Health. 2007;36:e203–e220. [PubMed] [Google Scholar]
- Seedall R. B., Anthony J. C. Risk estimates for starting tobacco, alcohol, and other drug use in the United States: Male-female differences and the possibility that ‘limiting time with friends’ is protective. Drug and Alcohol Dependence. 2013;133:751–753. doi: 10.1016/j.drugalcdep.2013.06.035. doi:10.1016/j.drugalcdep.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH Report: Substance use among women during pregnancy and following childbirth. Rockville, MD: Author; 2009. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11–4658; Rockville, MD: 2012. Author. [Google Scholar]
- van Uitert E. M., van der Elst-Otte N., Wilbers J. J., Exalto N., Willemsen S. P., Eilers P. H. C., Steegers-Theunissen R. P. M. Periconception maternal characteristics and embryonic growth trajectories: The Rotterdam Predict study. Human Reproduction. 2013;28:3188–3196. doi: 10.1093/humrep/det375. doi:10.1093/humrep/det375. [DOI] [PubMed] [Google Scholar]
- Vsevolozhskaya O. A., Anthony J. C. Confidence interval estimation in R-DAS. Drug and Alcohol Dependence. 2014;143:95–104. doi: 10.1016/j.drugalcdep.2014.07.017. doi:10.1016/j.drugalcdep.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Gomutputra P., Wolgemuth D. J., Baxi L. Effects of acute alcohol intoxication in the second trimester of pregnancy on development of the murine fetal lung. American Journal of Obstetrics and Gynecology. 2007;197:269.e1–269.e4. doi: 10.1016/j.ajog.2007.06.031. doi:10.1016/j.ajog.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Yoon P. W., Rasmussen S. A., Lynberg M. C., Moore C. A., Anderka M., Carmichael S. L., Edmonds L. D. The National Birth Defects Prevention Study. Public Health Reports, 116, Supplement. 2001;1:32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Ford E. S., Tsai J., Li C., Ahluwalia I. B., Pearson W. S., Croft J. B. Trends in health-related behavioral risk factors among pregnant women in the United States: 2001–2009. Journal of Women's Health. 2012;21:255–263. doi: 10.1089/jwh.2011.2931. doi:10.1089/jwh.2011.2931. [DOI] [PubMed] [Google Scholar]