Abstract
Objective:
Previous studies have established a relationship between cannabis use and affective problems among adolescents and young adults; however, the direction of these associations remains a topic of debate. The present study sought to examine bidirectional associations between cannabis use and depressive symptoms, specifically testing the validity of two competing hypotheses: the cannabis effect hypothesis, which suggests that cannabis use contributes to the onset of later depressive symptoms; and the self-medication hypothesis, which posits that individuals increase their use of a substance to alleviate distressing psychological symptoms.
Method:
Participants in this study were 264 low-socioeconomic-status males assessed at ages 17, 20, and 22. Cross-lag panel models were fit to test bidirectional associations between cannabis use frequency and depressive symptoms across the transition from adolescence to early adulthood. In addition, analyses were conducted within two high-risk subsamples to examine whether associations between cannabis use frequency (ranging from never used to daily use) and depressive symptoms differed among regular cannabis users (used cannabis more than once per week) or subjects reporting at least mild levels of depressive symptoms.
Results:
Cannabis use and depressive symptoms were concurrently correlated. Cannabis use predicted increases in later depressive symptoms, but only among the mild-depression subsample. Depressive symptoms predicted only slight increases in later cannabis use, among the subsample of regular cannabis users.
Conclusions:
Temporal patterns of cannabis use and depressive symptoms provide evidence for the cannabis effect but limited evidence for the self-medication hypothesis. Adolescents higher in depressive symptoms may be vulnerable to the adverse psychological effects of using cannabis. Results are discussed in terms of implications for basic research, prevention, and intervention.
Cannabis is the most widely used illicit drug in America (Degenhardt et al., 2008), with adolescent and young adult males using at the highest rates (Pujazon-Zazik & Park, 2009). Cannabis use typically begins in late adolescence, with the average age at initiation estimated to be 17.5 years (Substance Abuse and Mental Health Services Administration [SAMHSA], 2013). Researchers have observed a dose-response relationship between cannabis use in adolescence and early adulthood and myriad negative outcomes (e.g., impaired cognition; Bolla et al., 2002), including mental health problems such as psychosis and depression (Degenhardt & Hall, 2006; Troisi et al., 1998). Recent cross-sectional and longitudinal studies of adolescents have consistently linked cannabis use with affective problems (Brook et al., 2002; Patton et al., 2002; Troisi et al., 1998). Despite this established covariation, researchers disagree on the nature of this association (Degenhardt et al., 2003). Several theories have been proposed in an attempt to explain the link between substance use and mental illness in general, and cannabis use and depression in particular.
One theory, referred to in the present study as the cannabis effect, suggests that persistent and heavy cannabis use precipitates the development of depression. Researchers have postulated several pathways by which cannabis use could lead to later depression. From a neurological perspective, white matter abnormalities associated with prolonged, heavy cannabis use may offer an explanation for this direction of effects (Matochik et al., 2005). Specifically, a cross-sectional study conducted by Medina and colleagues (2007) found frontal lobe white matter abnormalities coupled with regular cannabis use to have additive and interactive effects in predicting depressive symptoms among adolescents. Other researchers have proposed that using cannabis can precipitate depressive symptoms through psychosocial failure. For example, Marmorstein and Iacono (2011) found education failure, unemployment, and legal troubles to partially mediate the relationship between a cannabis use disorder (as defined by the Diagnostic and Statistical Manual of Mental Disorders, Third Edition [DSM-III]; American Psychiatric Association, 1980) in adolescence and a diagnosis of major depression in early adulthood.
Validity for the cannabis effect is supported by several longitudinal studies that suggest that the emergence of depressive symptoms follows the onset of regular cannabis use (Bovasso, 2001; Brook et al., 2002; Patton et al, 2002). A prospective study of 1,601 Australian adolescents found that among females, but not males, daily cannabis users in adolescence were five times more likely to experience a depressive or anxious state in early adulthood than nonusers, whereas weekly users were twice as likely, controlling for previous depression and anxiety (Patton et al., 2002). Additional analyses revealed no significant relationship between depression and anxiety in adolescence and later cannabis use, suggesting that heavy cannabis use in adolescence may increase the risk of psychological distress in early adulthood, but not the other way around.
Alternatively, the self-medication hypothesis suggests that individuals experiencing symptoms of a mental illness begin using a substance to alleviate the distressing symptoms (Khantzian, 1985). Consistent with this perspective, cannabis users commonly report that their greatest motives for using cannabis are relief from affective symptoms, including depression and anxiety (Bottorff et al., 2009), and many cannabis users report immediate euphoric effects after consuming the drug (Lukas et al., 1995). Nevertheless, longitudinal support for the self-medication hypothesis has been mixed (Bardone et al., 1998; Miller-Johnson et al., 1998). Miller-Johnson and colleagues (1998) followed a group of low-socioeconomic-status (SES) African American adolescents (N = 304) from sixth through tenth grade and found that sixth-grade depressive symptoms did not significantly predict cannabis use in either eighth or tenth grade. However, these adolescents were likely not followed long enough to thoroughly examine associations between early depressive symptoms and later cannabis use, based on national averages that suggest cannabis use typically begins around age 17.5 (SAMHSA, 2013). Repetto and colleagues (2008) did find depressive symptoms to predict increases in cannabis use 1 year later among at-risk ninth-grade African American males, accounting for grade point average, SES, previous cannabis use, and other substance use. It should be noted that the reported euphoric effects of using cannabis last about 1 hour (Lukas et al., 1995), and therefore, the reinforcement pattern delineated in the self-medication hypothesis may be better observed in a more immediate fashion, rather than longitudinally at intervals several years apart. Still, a longitudinal approach can be valuable for elucidating larger patterns of depression in predicting cannabis use over time.
It is possible that neither hypothesis on its own sufficiently explains the relationship between the two variables. Instead, the covariation between cannabis use and depressive symptoms may be better characterized and explained bidirectionally, with each variable reciprocally exacerbating the other over time. Pacek and colleagues (2013) did find some evidence of a bidirectional relationship between cannabis use and depressive symptoms in a sample of adults in which a baseline cannabis use disorder predicted major depression 3 years later and baseline major depression predicted a later cannabis use disorder, controlling for baseline diagnoses of depression and cannabis use disorders, respectively.
Last, a common-factors perspective suggests that cannabis use and depressive symptoms may not directly influence the development of one another, but rather share common underlying risk factors. From a genetics perspective, the “reward deficiency syndrome” model posits that a variant form of the A1 allele for the dopamine D2 receptor makes certain individuals less susceptible to everyday rewards, leading to feelings of dysphoria and higher levels of sensation seeking, including substance use (Blum et al., 1996). In this way, both depression (Forbes & Dahl, 2012) and substance use (Koob & Le Moal, 2001) could co-occur through their common association with disrupted reward function. Alternatively, psychosocial factors such as parental psychological dysfunction and child conduct problems have been found to predict both cannabis use (Sitnick et al., 2014) and depressive symptoms (Zoccolillo, 1992), potentially serving as a common vulnerability.
Although there is a solid foundation of research on the covariation between cannabis use and depressive symptoms, there is room for advancement, particularly between adolescence and emerging adulthood. Previously considered part of adulthood, emerging adulthood (ages 18–25) has been recognized as a unique period of development characterized by rapid transitions (Arnett, 2000) and continuing brain development (Steinberg, 2005). Decisions made during this period can have persisting consequences for long-term future outcomes. Based on the associations between early and frequent cannabis use and depressive symptoms in adolescence with poor psychosocial development in young adulthood (Fergusson & Boden, 2008; Kandel & Davies, 1986), coupled with the frequent co-occurrence of cannabis use and depressive symptoms seen among adolescents and young adults (Brook et al., 2002; Troisi et al., 1998), it is important to increase our understanding of how each type of problem behavior influences (or fails to influence) the other across these periods of developmental transition. Research of this nature could help elucidate how these problem behaviors influence and affect the course of one another from adolescence to emerging adulthood.
The present study sought to confirm previous findings of covariation between cannabis use and depressive symptoms and shed light on the directionality of the association between cannabis use and depressive symptoms over several years in a sample of racially diverse, low SES young men. This sample reflects an understudied population with particular higher risk for both cannabis use and mental health problems (Finlay et al., 2012). We used a three-wave cross-lag panel model that allowed us to test reciprocal, transactional relationships between cannabis use and depressive symptoms at three time points from adolescence to early adulthood. This model provides a novel approach toward studying the relationship between cannabis use and depression and allows us to test for potential bidirectional relationships between the variables. In addition, to examine if patterns of covariation differ among young men with more regular cannabis use or those with elevated levels of depressive symptoms, we examined cross-lag associations within these subgroups separately, something not done in previous studies. To account for possible third-variable explanations, we included family income, alcohol use, tobacco use, race, education, pre-adolescent antisocial behavior, and parental depressive symptoms as covariates in all analyses. In addition, as neurocognitive deficits appear to be salient predictors of depression (Maalouf et al., 2011), we accounted for youths’ preadolescent IQ scores. Time spent incarcerated or on probation was also controlled for to parse out cannabis abstinence due to court-ordered drug tests or other legal restrictions.
Method
Participants
Participants were 264 young men drawn from a larger sample of 310 recruited as part of the Pitt Mother & Child Project, an ongoing longitudinal study of child vulnerability and resilience in low SES families (Shaw et al., 2003). Mother–son dyads were recruited from the Women, Infants, and Children program around the Pittsburgh metropolitan area. The youth were 18 months old at the initial assessment. Among the retained sample of 264 participants, 139 (52.7%) identified as European American, 103 (39.0%) identified as African American, and 22 (8.3%) identified as other. Mean annual family income at the initial assessment was U.S. $12,796 (SD = $7,904). By the age 22 assessment, 87.9% had completed high school or an equivalent General Educational Development (GED) credential, and 45.9% had received some post–high school education. Informed consent was obtained from the youth’s primary caregiver at waves for which the youth was under 18 and from the youth at subsequent waves. Participants were financially compensated.
Procedure
The present study used data gathered from assessments at 11, 12, 17, 20, and 22 years. The 11-, 12-, and 17-year assessments were conducted in the participant’s home. During these home assessments, a primary caregiver (typically the youth’s biological mother) participated. Participants were told that their answers would be kept confidential and, when possible, parents and youth self-administered their questionnaires in separate rooms to maximize privacy. In addition, a Certificate of Confidentiality was obtained from the National Institutes of Health to offer further protection of privacy. The age 20 and 22 assessments took place in the laboratory and were exclusive to the youth. In general, retention rates were high over the 20.5 years of the study, with 252 (81.3%) of the initial 310 subjects participating at age 22.
Measures
Depressive symptoms.
Depressive symptoms were assessed using the 21-item version of the Beck Depression Inventory (BDI; Beck et al., 1988). Target youth self-administered the measure at the age 17, 20, and 22 assessments. Primary caregiver scores were collected at the age 12 assessment and were included as a covariate in the cross-lag models. For each item, participants were asked to indicate which of four statements most closely described the way they were feeling “in the past six months including today.” An example of a cluster of statements is: “I do not feel sad (0)”; “I feel sad (1)”; “I am sad all of the time and can’t snap out of it (2)”; or “I am so sad or unhappy that I can’t stand it (3).” The questionnaire was scored by summing the results from each cluster. Cronbach’s alphas revealed good internal consistency among items (α’s = .873*, .823, .856, and .865 at ages 12*, 17, 20, and 22, respectively; *indicates BDI score from the primary caregiver at age 12).
Cannabis use.
Age 17 cannabis use was measured with a single self-report item from the Self-Report of Delinquency (SRD; Elliott et al., 1985) administered to the target youth. Participants were asked, “In the past year, have you used cannabis?” Response choices were: 0 (never), 1 (once/twice), or 2 (more often).
Cannabis use at ages 20 and 22 was assessed using a single item adapted from the Alcohol and Drug Consumption Questionnaire, a widely adapted measure for alcohol and drug use (Cahalan et al., 1969). Subjects self-reported their cannabis use on the following scale: 0 (have never tried), 1 (no use in the last year), 2 (less than once a month), 3 (once a month), 4 (2–3 times a month), 5 (once a week), 6 (2–3 times a week), 7 (4–6 times a week), or 8 (every day). Good internal consistency was observed between the cannabis use scores at 20 and 22 (α = .796).
Covariates.
In addition to primary caregiver depressive symptoms (see above), several other variables were included as covariates in the cross-lag models.
(a) Demographic information: At the 17-year assessment, parents provided information on income (per month). Target youth ethnicity was dummy coded (0 = European American; 1 = other). Target youth reported their highest level of education completed at age 22.
(b) Youth antisocial behavior: Childhood antisocial behaviors were assessed using a 33-item adaptation of the SRD (Elliot, 1985). The SRD measures the self-reported frequency of antisocial behaviors over the past year (“In the past year, have you taken something from a store without paying for it?”). Response choices were 0 (never), 1 (once/twice), or 2 (more often). A composite score was calculated by summing the responses to 26 items (7 items regarding peer behaviors were disregarded). The composite score demonstrated good internal reliability (α = .783).
(c) Tobacco and alcohol use: Past-year alcohol and tobacco use were also assessed using items from the SRD (Elliott et al., 1985) administered at age 17. Tobacco use was assessed by asking, “Have you secretly smoked a cigarette, smoked a pipe, or chewed tobacco?” An alcohol use score with a range of 0–6 was created by summing responses from three items: “Have you secretly taken a sip from a glass or bottle of [beer] [wine] [liquor]?” There was good internal consistency among the alcohol use items (α = .873). Previous studies have successfully used the SRD to measure adolescent substance use (e.g., Sitnick et al., 2014).
(d) Youth IQ: Youth completed the Block Design and Vocabulary subtests of the Wechsler Intelligence Scale for Children (Wechsler, 1991) at the age 11 in-home assessment. These subtests have a high average correlation with the Full Scale IQ and have high test–retest reliability and internal consistency (Sattler, 1992). Full Scale IQ scores were prorated from these subtest scores per Tellegen and Briggs (1967).
(e) Adult court records: Involvement in the legal system may inhibit an individual’s cannabis use because of a lack of access while incarcerated or because of attempting to avoid illicit activity while on probation. To assess adulthood involvement in the Pennsylvania legal system, we searched the Pennsylvania state public court records website using each subject’s name and birth date. These records were last checked in February 2014. For the present study, we accounted for only individuals who had been sentenced to probation or confinement. These individuals were coded as 1 (n = 22), and individuals without a record of probation or confinement were coded as 0.
Sample reduction and analyses
Individuals missing data at two or more of the three assessment points on both the depressive symptoms and cannabis use assessments were excluded from all analyses (n = 46). The retained sample contained 264 participants. A cannabis-user subsample (n = 111) was created with individuals who reported using cannabis 2–3 times a week or more frequently at either (or both) age 20 or 22. Descriptive analyses revealed that the average cannabis user (anyone who reported ever using cannabis) used cannabis about once per week at ages 20 and 22. Therefore, we chose a cutoff to approximate a sample of individuals using cannabis at above-average levels. This cutoff has previously been used to distinguish heavy cannabis users (Bolla et al., 2002). In addition, a subsample of mild-depression participants (n = 61) was created using subjects who recorded a BDI score of 12 or higher during at least one assessment. According to Beck and colleagues (1988), a BDI score of 10 is indicative of a mild mood disturbance; however, a cutoff score of 12 was chosen for the present study to provide a slightly higher threshold for depression while maintaining sufficient subjects to examine group differences. For each subsample, individuals were included by meeting the defined criteria at one time point in order to maintain sufficient subjects for analysis. Cross-tabulation analyses revealed that 33 individuals met criteria for both the cannabis-user and mild-depression subgroups.
Of the variables used in the current study, attrition analyses revealed that the retained sample of 264 used cannabis at significantly higher rates at age 22, t(245) = -1.24, p < .01; had a higher level of education at age 22, t(275) = -.874, p < .05; and had a higher antisocial behavior score at age 12, t(233) = 2.593, p < .01, relative to the 46 cases without complete cannabis use or depressive symptoms data. Formal attrition analyses were not conducted on the cannabis-user or mild-depression subgroups because of the small number of attrited cases (ns = 3 and 1, respectively).
Descriptive statistics and intercorrelations were calculated between all variables. A three-wave cross-lagged panel model was fit using Mplus Version 6.11 (Muthén & Muthén, 1998–2011) to test all hypotheses. Autoregressive effects were examined to determine the stability of both cannabis use and depressive symptoms across the three assessment points. Cross-sectional relationships between cannabis use and depressive symptoms were estimated at each age to test the correlations between cannabis use and depressive symptoms within each time point. Regression paths were fit to determine the reciprocal and transactional effects that cannabis use and depressive symptoms have on one another between ages 17 and 20, 20 and 22 (controlling for age 17 levels), and 17 and 22. R-square values were assessed to determine the model effect on each outcome variable. As previously noted, the following covariates were included in all cross-lag models: family income (age 17), tobacco and alcohol use (age 17), ethnicity (age 22), education (age 22), child antisocial behavior (age 12), parental depressive symptoms (age 12), youth IQ (age 11), and adulthood incarceration or probation status. Model fit was assessed for all cross-lag models per recommendations by Hooper and colleagues (2008) using the following fit indices: model chi-square, root mean square error of approximation (<.10 indicates acceptable fit), standardized root mean square residual (<.08 indicates acceptable fit), and comparative fit index (>.90 indicates acceptable fit). Power analyses revealed that the model for the full sample was sufficiently powered, but the models for both subsamples were slightly underpowered (Preacher & Coffman, 2006).
Results
Descriptive statistics and intercorrelations
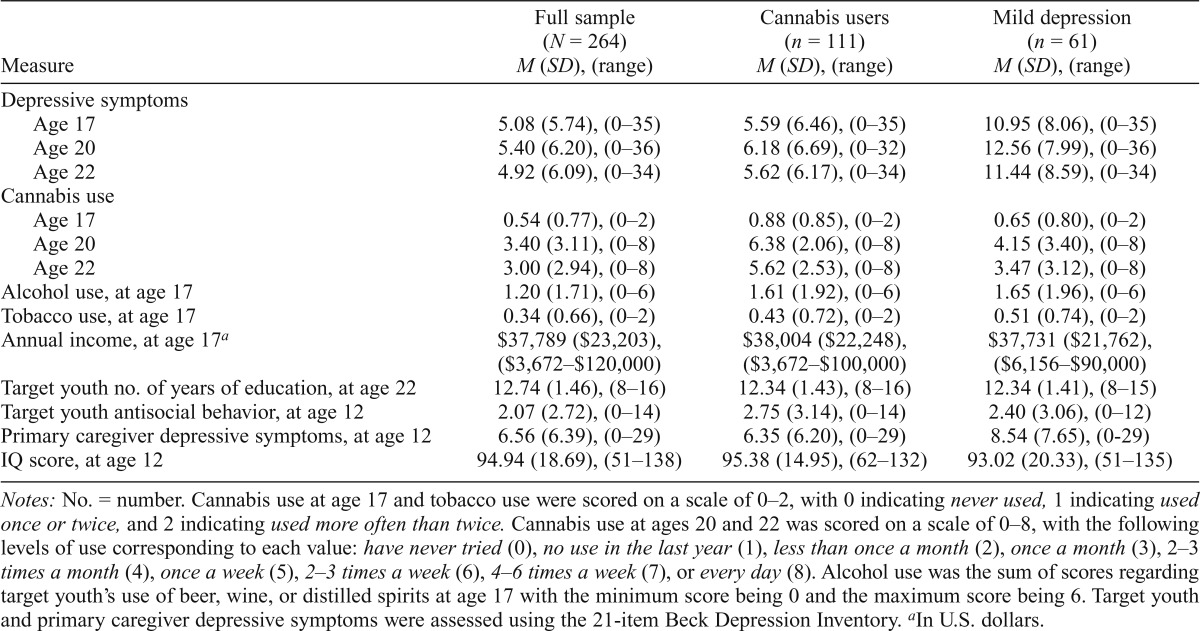
All descriptive and correlational analyses were conducted using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY). Descriptive analyses of cannabis use revealed that 79.5% of participants reported using cannabis at least once in their lives. Past-year use was reported by 33.8%, 55.0%, and 48.5% of subjects at ages 17, 20, and 22, respectively. Daily use was reported by 16.7% and 12.1% of subjects at ages 20 and 22, respectively. Mean BDI scores for all ages were relatively low, but in accordance with norms for adolescent and young adult males. For all descriptive data, see Table 1. Variables with skew greater than 1 were transformed for analyses using log transformations per Tabachnick and Fidell (2007).
Table 1.
Mean data for depressive Symptoms, cannabis use, alcohol use, income, and primary caregiver education
| Measure | Full sample (N = 264) M (SD), (range) | Cannabis users (n = 111) M (SD), (range) | Mild depression (n = 61) M (SD), (range) |
| Depressive symptoms | |||
| Age 17 | 5.08 (5.74), (0–35) | 5.59 (6.46), (0–35) | 10.95 (8.06), (0–35) |
| Age 20 | 5.40 (6.20), (0–36) | 6.18 (6.69), (0–32) | 12.56 (7.99), (0–36) |
| Age 22 | 4.92 (6.09), (0–34) | 5.62 (6.17), (0–34) | 11.44 (8.59), (0–34) |
| Cannabis use | |||
| Age 17 | 0.54 (0.77), (0–2) | 0.88 (0.85), (0–2) | 0.65 (0.80), (0–2) |
| Age 20 | 3.40 (3.11), (0–8) | 6.38 (2.06), (0–8) | 4.15 (3.40), (0–8) |
| Age 22 | 3.00 (2.94), (0–8) | 5.62 (2.53), (0–8) | 3.47 (3.12), (0–8) |
| Alcohol use, at age 17 | 1.20 (1.71), (0–6) | 1.61 (1.92), (0–6) | 1.65 (1.96), (0–6) |
| Tobacco use, at age 17 | 0.34 (0.66), (0–2) | 0.43 (0.72), (0–2) | 0.51 (0.74), (0–2) |
| Annual income, at age 17a | $37,789 ($23,203),($3,672–$120,000) | $38,004 ($22,248),($3,672–$100,000) | $37,731 ($21,762),($6,156–$90,000) |
| Target youth no. of years of education, at age 22 | 12.74(1.46), (8–16) | 12.34 (1.43), (8–16) | 12.34(1.41), (8–15) |
| Target youth antisocial behavior, at age 12 | 2.07 (2.72), (0–14) | 2.75 (3.14), (0–14) | 2.40 (3.06), (0–12) |
| Primary caregiver depressive symptoms, at age 12 | 6.56 (6.39), (0–29) | 6.35 (6.20), (0–29) | 8.54 (7.65), (0–29) |
| IQ score, at age 12 | 94.94 (18.69), (51–138) | 95.38 (14.95), (62–132) | 93.02 (20.33), (51–135) |
Notes: No. = number. Cannabis use at age 17 and tobacco use were scored on a scale of 0–2, with 0 indicating never used, 1 indicating used once or twice, and 2 indicating used more often than twice. Cannabis use at ages 20 and 22 was scored on a scale of 0–8, with the following levels of use corresponding to each value: have never tried (0), no use in the last year (1), less than once a month (2), once a month (3), 2–3 times a month (4), once a week (5), 2–3 times a week (6), 4–6 times a week (7), or every day (8). Alcohol use was the sum of scores regarding target youth’s use of beer, wine, or distilled spirits at age 17 with the minimum score being 0 and the maximum score being 6. Target youth and primary caregiver depressive symptoms were assessed using the 21-item Beck Depression Inventory.
In U.S. dollars.
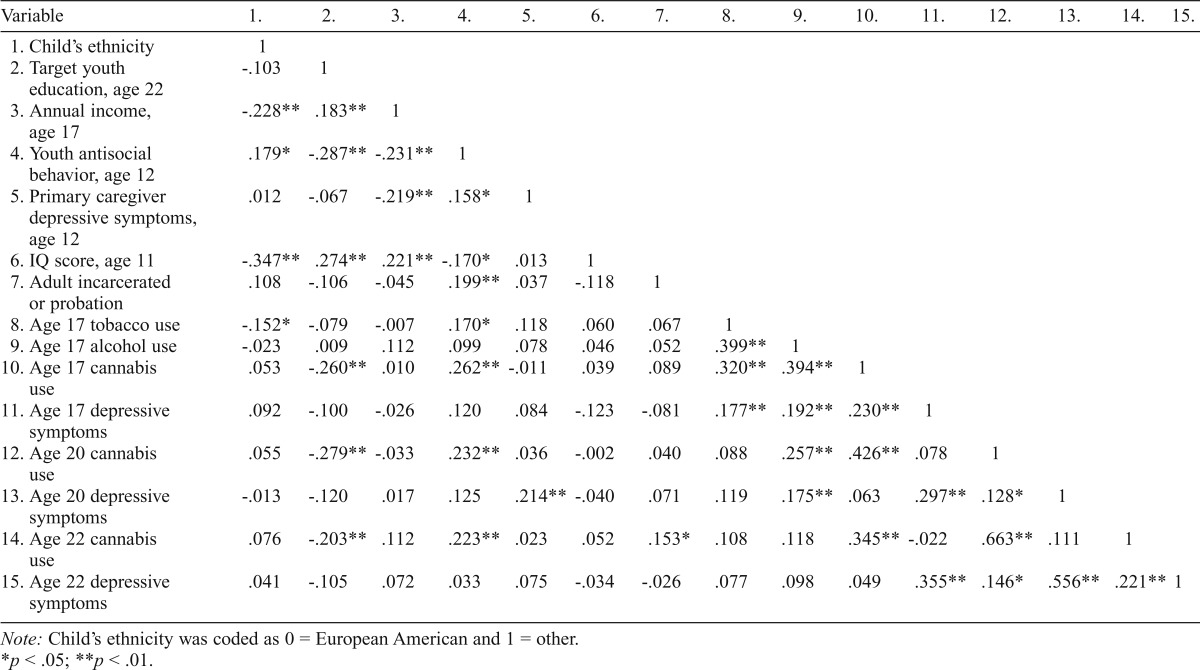
In terms of intercorrelations, a small correlation was observed between cannabis use and depressive symptoms at ages 17, 20, and 22. Alcohol use at age 17 was significantly correlated with both cannabis use and depressive symptoms at age 17 and at age 20, but it was nonsignificantly correlated with either variable at age 22. Education level at age 22 was negatively correlated with cannabis use at ages 17, 20, and 22; however, IQ scores were not correlated with either depressive symptoms or cannabis use at any age. See Table 2 for all intercorrelational data.
Table 2.
Correlational data for the study sample
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. |
| 1. Child’s ethnicity | 1 | ||||||||||||||
| 2. Target youth education, age 22 | -.103 | 1 | |||||||||||||
| 3. Annual income, age 17 | -.228** | .183** | 1 | ||||||||||||
| 4. Youth antisocial behavior, age 12 | .179* | -.287** | -.231** | 1 | |||||||||||
| 5. Primary caregiver depressive symptoms, age 12 | .012 | -.067 | -.219** | .158* | 1 | ||||||||||
| 6. IQ score, age 11 | -.347** | .274** | .221** | -.170* | .013 | 1 | |||||||||
| 7. Adult incarcerated or probation | .108 | -.106 | -.045 | .199** | .037 | -.118 | 1 | ||||||||
| 8. Age 17 tobacco use | -.152* | -.079 | -.007 | .170* | .118 | .060 | .067 | 1 | |||||||
| 9. Age 17 alcohol use | -.023 | .009 | .112 | .099 | .078 | .046 | .052 | .399** | 1 | ||||||
| 10. Age 17 cannabis use | .053 | -.260** | .010 | .262** | -.011 | .039 | .089 | .320** | .394** | 1 | |||||
| 11. Age 17 depressive symptoms | .092 | -.100 | -.026 | .120 | .084 | -.123 | -.081 | .177** | .192** | .230** | 1 | ||||
| 12. Age 20 cannabis use | .055 | -.279** | -.033 | .232** | .036 | -.002 | .040 | .088 | .257** | .426** | .078 | 1 | |||
| 13. Age 20 depressive symptoms | -.013 | -.120 | .017 | .125 | .214** | -.040 | .071 | .119 | .175** | .063 | .297** | .128* | 1 | ||
| 14. Age 22 cannabis use | .076 | -.203** | .112 | .223** | .023 | .052 | .153* | .108 | .118 | .345** | -.022 | .663** | .111 | 1 | |
| 15. Age 22 depressive symptoms | .041 | -.105 | .072 | .033 | .075 | -.034 | -.026 | .077 | .098 | .049 | .355** | .146* | .556** | .221** | 1 |
Note: Child’s ethnicity was coded as 0 = European American and 1 = other.
p < .05;
p < .01.
Cross-lag model of cannabis use and depressive symptoms
Full sample.
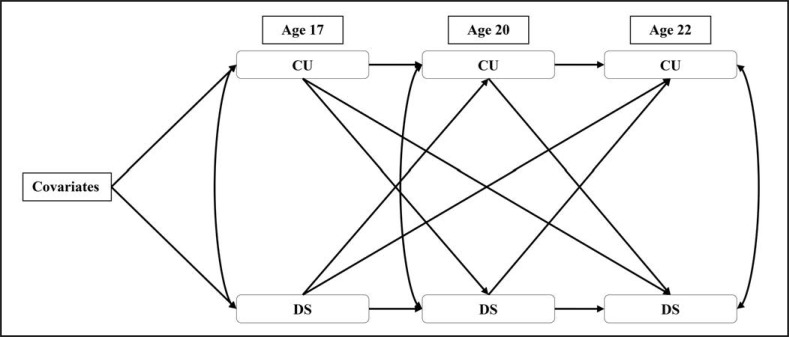
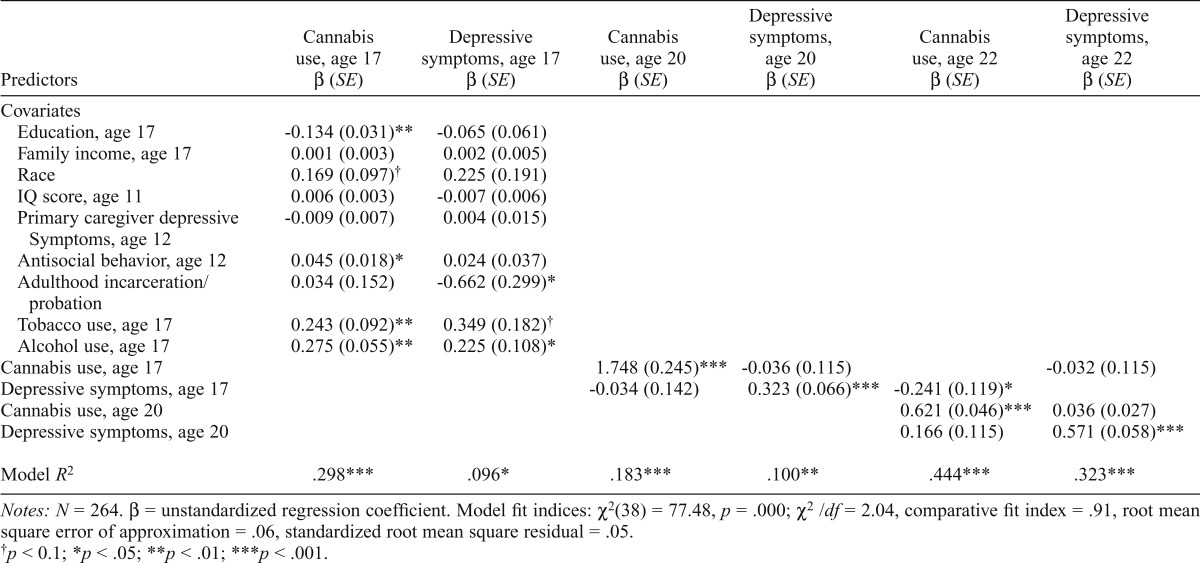
A cross-lag model of cannabis use and depressive symptoms was fit to test all study hypotheses (Figure 1). This model represented a good fit to the data. Cannabis use at age 17 significantly predicted cannabis use at age 20. In turn, cannabis use at age 20 significantly predicted cannabis use at age 22. In addition, depressive symptoms at age 17 predicted depressive symptoms at age 20, and depressive symptoms at age 20 predicted depressive symptoms at age 22. Concurrently, cannabis use and depressive symptoms were correlated at ages 17, 20, and 22 (rs = .128–.182, ps < .05). No significant, positive cross-lag pathways were observed in this model, but age 17 depressive symptoms negatively predicted age 22 cannabis use. See Table 3 for all model results and fit indices.
Figure 1.
Cross-lag model of cannabis use and depressive Symptoms. Note: Covariates include family income at age 17, target youth alcohol and tobacco use at age 17, target youth race, target youth educational attainment at age 22, parental depressive symptoms at the age 12 assessment, target youth antisocial behavior at age 12, IQ score at 11, and incidence of probation or incarceration in adulthood. CU = cannabis use; DS = depressive symptoms.
Table 3.
Cross-lag model for full sample
| Predictors | Cannabis use, age 17 β (SE) | Depressive symptoms, age 17 β (SE) | Cannabis use, age 20 β (SE) | Depressive symptoms, age 20 β (SE) | Cannabis use, age 22 β (SE) | Depressive symptoms, age 22 β (SE) |
| Covariates | ||||||
| Education, age 17 | -0.134(0.031)** | -0.065 (0.061) | ||||
| Family income, age 17 | 0.001 (0.003) | 0.002 (0.005) | ||||
| Race | 0.169 (0.097)† | 0.225 (0.191) | ||||
| IQ score, age 11 | 0.006 (0.003) | -0.007 (0.006) | ||||
| Primary caregiver depressive | -0.009 (0.007) | 0.004 (0.015) | ||||
| Symptoms, age 12 | ||||||
| Antisocial behavior, age 12 | 0.045 (0.018)* | 0.024 (0.037) | ||||
| Adulthood incarceration/probation | 0.034 (0.152) | -0.662 (0.299)* | ||||
| Tobacco use, age 17 | 0.243 (0.092)** | 0.349 (0.182)† | ||||
| Alcohol use, age 17 | 0.275 (0.055)** | 0.225 (0.108)* | ||||
| Cannabis use, age 17 | 1.748 (0.245)*** | -0.036 (0.115) | -0.032 (0.115) | |||
| Depressive symptoms, age 17 | -0.034 (0.142) | 0.323 (0.066)*** | -0.241 (0.119)* | |||
| Cannabis use, age 20 | 0.621 (0.046)*** | 0.036 (0.027) | ||||
| Depressive symptoms, age 20 | 0.166 (0.115) | 0.571 (0.058)*** | ||||
| Model R2 | .298*** | .096* | .183*** | .100** | .444*** | .323*** |
Notes: N = 264. β = unstandardized regression coefficient. Model fit indices: χ2(38) = 77.48, p = .000; χ2/df = 2.04, comparative fit index = .91, root mean square error of approximation = .06, standardized root mean square residual = .05.
p < 0.1;
p < .05;
p < .01;
p < .001.
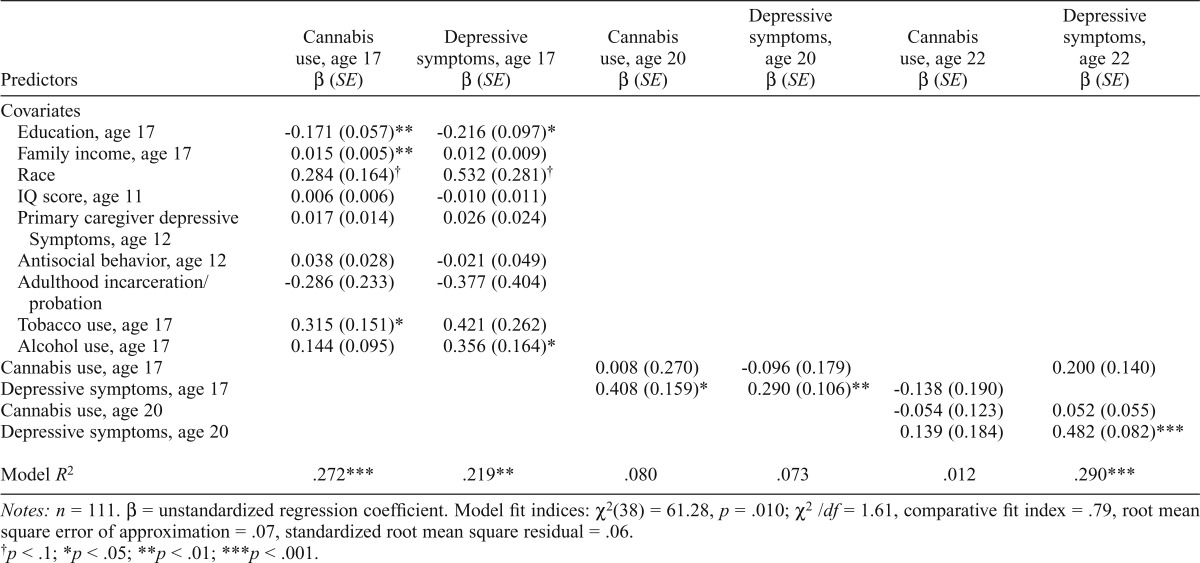
Cannabis-user subsample.
For the cannabis-user sub-sample, the cross-lag model represented an acceptable fit to the data. Cannabis use at age 17 did not predict cannabis use at age 20. Likewise, cannabis use at age 20 did not predict cannabis use at age 22. Age 17 depressive symptoms significantly predicted depressive symptoms at age 20. Depressive symptoms at age 20 positively predicted depressive symptoms at age 22. Depressive symptoms and cannabis use were significantly correlated at age 17 (r = .283, p < .01). Depressive symptoms at age 17 predicted increases in cannabis use at age 20. See Table 4 for all model results and fit indices.
Table 4.
Cross-lag model for cannabis-user subsample
| Predictors | Cannabis use, age 17 β (SE) | Depressive symptoms, age 17 β (SE) | Cannabis use, age 20 β (SE) | Depressive symptoms, age 20 β (SE) | Cannabis use, age 22 β (SE) | Depressive symptoms, age 22 β (SE) |
| Covariates | ||||||
| Education, age 17 | -0.171 (0.057)** | -0.216 (0.097)* | ||||
| Family income, age 17 | 0.015 (0.005)** | 0.012 (0.009) | ||||
| Race | 0.284 (0.164)† | 0.532 (0.281)† | ||||
| IQ score, age 11 | 0.006 (0.006) | -0.010 (0.011) | ||||
| Primary caregiver depressive | 0.017 (0.014) | 0.026 (0.024) | ||||
| Symptoms, age 12 | ||||||
| Antisocial behavior, age 12 | 0.038 (0.028) | -0.021 (0.049) | ||||
| Adulthood incarceration/probation | -0.286 (0.233) | -0.377 (0.404) | ||||
| Tobacco use, age 17 | 0.315 (0.151)* | 0.421 (0.262) | ||||
| Alcohol use, age 17 | 0.144 (0.095) | 0.356 (0.164)* | ||||
| Cannabis use, age 17 | 0.008 (0.270) | -0.096 (0.179) | 0.200 (0.140) | |||
| Depressive symptoms, age 17 | 0.408 (0.159)* | 0.290 (0.106)** | -0.138 (0.190) | |||
| Cannabis use, age 20 | -0.054 (0.123) | 0.052 (0.055) | ||||
| Depressive symptoms, age 20 | 0.139 (0.184) | 0.482 (0.082)*** | ||||
| Model R2 | .272*** | .219** | .080 | .073 | .012 | .290*** |
Notes: n = 111. β = unstandardized regression coefficient. Model fit indices: χ2(38) = 61.28, p = .010; χ2/df = 1.61, comparative fit index = .79, root mean square error of approximation = .07, standardized root mean square residual = .06.
p < .1;
p < .05;
p < .01;
p < .001.
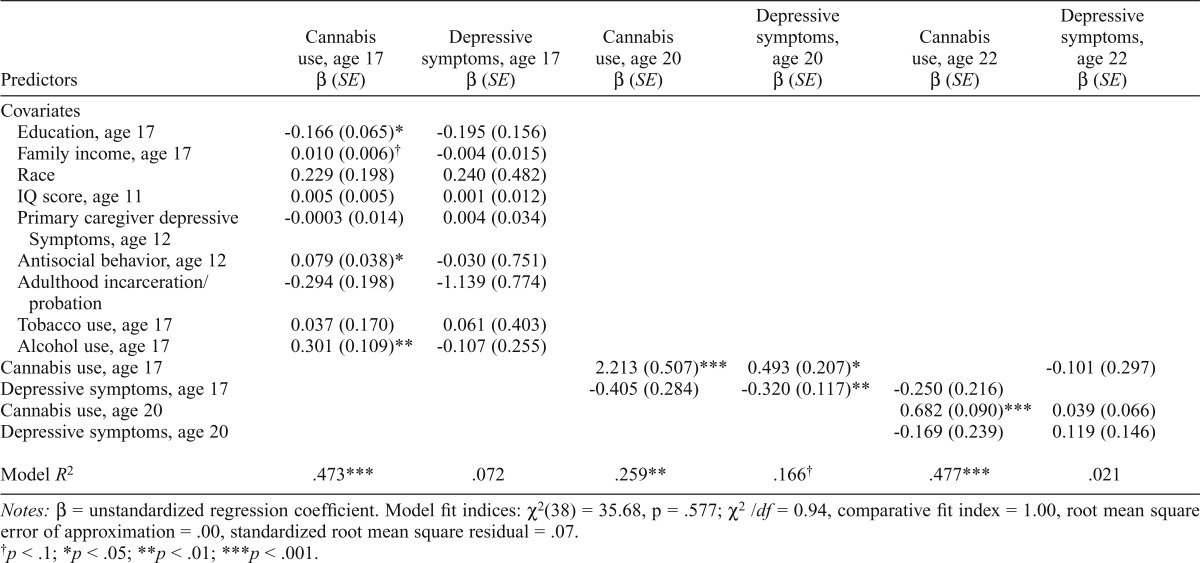
Mild-depression subsample.
The cross-lag model for the mild-depression subsample represented an excellent fit to the data. Age 17 cannabis use was significantly predictive of cannabis use at age 20. In turn, cannabis use at age 20 significantly predicted cannabis use at age 22. Depressive symptoms at age 17 negatively predicted depressive symptoms at age 20, but depressive symptoms at age 20 did not significantly predict depressive symptoms at age 22. Cannabis use was positively correlated with concurrent depressive symptoms at ages 17 and 22 (rs = .255–.285, ps < .05). With regard to the cross-lag pathways, cannabis use at age 17 predicted increases in depressive symptoms at age 20. See Table 5 for all model results and fit indices.
Table 5.
Cross-lag model for mild-depression subsample
| Predictors | Cannabis use, age 17 β (SE) | Depressive symptoms, age 17 β (SE) | Cannabis use, age 20 β (SE) | Depressive symptoms, age 20 β (SE) | Cannabis use, age 22 β (SE) | Depressive symptoms, age 22 β (SE) |
| Covariates | ||||||
| Education, age 17 | -0.166 (0.065)* | -0.195 (0.156) | ||||
| Family income, age 17 | 0.010 (0.006)† | -0.004 (0.015) | ||||
| Race | 0.229 (0.198) | 0.240 (0.482) | ||||
| IQ score, age 11 | 0.005 (0.005) | 0.001 (0.012) | ||||
| Primary caregiver depressive | -0.0003 (0.014) | 0.004 (0.034) | ||||
| Symptoms, age 12 | ||||||
| Antisocial behavior, age 12 | 0.079 (0.038)* | -0.030 (0.751) | ||||
| Adulthood incarceration probation | -0.294 (0.198) | -1.139 (0.774) | ||||
| Tobacco use, age 17 | 0.037 (0.170) | 0.061 (0.403) | ||||
| Alcohol use, age 17 | 0.301 (0.109)** | -0.107 (0.255) | ||||
| Cannabis use, age 17 | 2.213 (0.507)*** | 0.493 (0.207)* | -0.101 (0.297) | |||
| Depressive symptoms, age 17 | -0.405 (0.284) | -0.320 (0.117)** | -0.250 (0.216) | |||
| Cannabis use, age 20 | 0.682 (0.090)*** | 0.039 (0.066) | ||||
| Depressive symptoms, age 20 | -0.169 (0.239) | 0.119 (0.146) | ||||
| Model R2 | .473*** | .072 | .259** | .166† | .477*** | .021 |
Notes: β = unstandardized regression coefficient. Model fit indices: χ2(38) = 35.68, p = .577; χ2/df = 0.94, comparative fit index = 1.00, root mean square error of approximation = .00, standardized root mean square residual = .07.
p < .1;
p < .05;
p < .01;
p < .001.
Discussion
Building on previous studies suggesting that cannabis use and depressive symptoms frequently co-occur, particularly among adolescents and young adults (Brook et al., 2002; Patton et al.; 2002, Troisi et al., 1998), the present study examined reciprocal relationships between cannabis use and depressive symptoms across the transitional period between adolescence and early adulthood to further our understanding of the relationship over time. Specifically, the study tested the validity of two competing hypotheses, the cannabis effect and the self-medication hypothesis, neither of which was strongly supported among the present sample. Consistent with previous findings, cannabis use was cross-sectionally correlated with depressive symptoms (Troisi et al., 1998). Also consistent with extant research, modest support was found for claims that cannabis use leads to later depression (Bovasso, 2001; Brook et al., 2002; Patton et al., 2002), but not the other way around.
Compared with estimations from the Monitoring the Future survey (Johnston et al., 2014), rates of cannabis use in the current study appear to be higher (i.e., estimated 34.5% past-year use among young men ages 19–30 vs. 59.9% observed at age 20). Rates of use, however, were comparable to those seen in another high-risk sample, which is consistent with the epidemiology of cannabis use in male, low-SES, urban populations (Flom et al., 2001). In addition, depressive symptoms in this sample were lower than those previously observed in adolescent and young adult males (Marcotte et al., 1999; O’Hara et al., 1998). This finding was surprising given the risk status of the participants in the present study, as adolescents living in low-SES households have been found to have a greater likelihood of developing depressive symptoms compared with more affluent peers (Jackson & Goodman, 2011).
As expected, both cannabis use and depressive symptoms were modestly stable from ages 17 to 22, consistent with findings from previous research on adolescent males transitioning into adulthood (Hankin et al., 1998; Perkonigg et al., 2008). Paradoxically, within the cannabis-user and mild-depression subsamples, less consistent stability was evident for cannabis use and depressive symptoms, respectively, which may be accounted for by regression to the mean, as those with above-average cannabis use and depression scores would be expected to show decreases relative to the rest of the sample (Nielsen et al., 2007). The nonsignificant autoregressive paths may have also been a product of the selection criteria for each subgroup. Selecting individuals at the upper levels of each construct limits the distribution, and therefore, the instability observed may be explained by small variance in means.
Concurrently, but not longitudinally, cannabis use was positively associated with depressive symptoms even after controlling for several covariates, which is in line with previous research (Troisi et al., 1998). Clinicians should account for the co-occurrence observed when treating patients with depressive or cannabis use disorders, as comorbid cannabis dependence and depression have been found to result in greater likelihood of relapse among adolescents seeking treatment for cannabis dependence (White et al., 2004). That the associations between cannabis use and depressive symptoms were slightly attenuated after accounting for several covariates (i.e., income, education) suggests that potential third-variables may underlie the concurrent covariation between cannabis use and depressive symptoms, which is consistent with a common-factors perspective.
Consistent with a pattern supporting the cannabis effect (Brook et al., 2002; Chen et al., 2002; Marmorstein et al., 2010), a positive association was observed between cannabis use in adolescence and depressive symptoms in early adulthood among the mild-depression subsample. Marmorstein and colleagues (2010) found similar results among adolescent girls who were at a high risk for depression. Specifically, cannabis initiation was linked to greater increases in later depressive symptoms. Combined with the results from the present study, those results suggest that individuals at risk for elevated levels of depressive symptoms may be more vulnerable to deleterious psychological effects of using cannabis. Although the nonexperimental nature of these studies cannot show that cannabis causes an increase in depressive symptoms, and the current findings do not rule out the possibility that both cannabis use and later depressive symptoms are accounted for by some other genetic and/or environmental risk factor (e.g., serotonin transporter gene, neighborhood adversity), that similar results were found over time during similar age periods among both males and females across multiple studies does suggest that adolescent cannabis use may not be a completely innocuous activity.
Contrary to expectations, higher levels of depressive symptoms at age 17 were related to lower levels of cannabis use at 22, among the full sample, whereas depressive symptoms at 17 predicted increases in cannabis use in the cannabis-user subgroup, but the effect was very small. Combined with results from previous studies, it would appear that adolescents do not use cannabis to self-medicate their symptoms of depression (Green & Ritter, 2000; Henry et al., 1993). However, these findings may be a product of study design. As previously stated, euphoric effects of using cannabis dissipate fairly quickly (Lukas et al., 1995); therefore, self-medication patterns may be better observed in short-term prospective studies rather than studies with assessment points several years apart.
Limitations and conclusions
The present study was not without limitations. Data on cannabis use and depressive symptoms were collected by self-reports, and although the autoregressive effects of each variable were accounted for in cross-lagged analyses, the findings are still subject to potential response bias, which could have resulted in either overreporting or underreporting of depressive symptoms or cannabis use (Welte & Russell, 1993). Although measures were taken to abate potential biases (i.e., using separate rooms, allowing subjects to self-administer), the presence of a primary caregiver at the age 17 assessment may have caused some individuals to under-report their use of illicit substances. That the alcohol and tobacco questions on the SRD asked solely about “secretive” use may have also influenced reporting of these substances; some youth use tobacco or alcohol openly at 17 and may not necessarily report using these substances secretly. The use of a different measure for cannabis use at age 17 than at ages 20 and 22 makes it difficult to interpret stability over time, and the instruments used at 17 limited the variability of cannabis use in all analyses. Ideally, self-reports of cannabis use would have been based on several, more detailed items using the same scale to obtain a richer assessment of frequency and intensity, and would be coupled with a drug screening that could confirm or refute the self-report. Still, the results from the present study provide important insight into the direction of the pathways, particularly among two very high-risk populations.
Severity of depressive symptoms was relatively low at all ages, which limited both the sample size and the severity of the cut point used for the mild-depression subsample. Likewise, our subsample selection criteria are a limitation as individuals were included in the mild-depression or cannabis-user subsample based on elevated scores at only one time point, and these subsamples may only tangentially approximate a clinical population. Future studies seeking to replicate these results in clinical populations may want to include only individuals meeting DSM criteria rather than elevated symptoms counts of cannabis use frequency, because extant studies have found a consistent link between cannabis use disorders and affective disorders (Bovasso, 2001; Marmorstein & Iacono, 2011; Pacek et al., 2013). However, the goals of the present study were not to examine clinical populations but rather to shed some light on higher-risk subpopulations of a community sample. Therefore, the cutoff points were appropriate for our goals, as individuals in the mild-depression and cannabis-user subsamples had higher symptom counts/cannabis use frequency compared with the full sample. In addition, the subsamples were slightly underpowered, highlighting the value of replicating our findings with a larger sample size.
That the sample included only male adolescents from an urban community also limits the generalizability of the findings. Future studies examining transactional covariation between cannabis use and depressive symptoms should include samples of female adolescents based on their higher rates of depressive symptoms during adolescence and beyond (Essau et al., 2010; Weissman et al., 1993) as well as research suggesting a strong relationship between affective problems and cannabis use in females (Marmorstein et al., 2010; Patton et al., 2002). Last, more frequent assessments of both cannabis use and depressive symptoms would have provided a more fine-grained picture of the development of each construct over time. The 3-year gap between the age 17 and age 20 assessments made it especially difficult to interpret the directionality between the variables or to conclude that self-medication effects were present; hence, the possibility of unobserved reciprocal effects cannot be discounted. Future longitudinal research examining directionality between substance use and mental health problems, especially over periods of transition such as adolescence into adulthood, should use more frequent assessments to provide a clearer picture of development (Dishion & Skaggs, 2000; Hussong et al., 2013).
Despite these limitations, this study offers several contributions to the existing body of literature on adolescent depression and cannabis use. Currently, there are very few studies examining the psychological implications of using cannabis in racially diverse samples of at-risk youth. This study replicated previous findings, strengthening the established claim that a positive concurrent relationship between cannabis use and depressive symptoms exists, particularly among low-income populations of young adult males. In addition, consistent with previous literature (Marmorstein et al., 2010), among individuals with elevated levels of depressive symptoms, a pattern supporting the cannabis effect was found. This highlights the need for intervention programs targeting depressed adolescents to emphasize the importance of finding alternative coping resources to prevent co-occurring cannabis use, because cannabis use could exacerbate the long-term clinical course of depression. Prevention efforts aimed at cannabis use or the delay of initiation could have positive consequences for mental health, because adolescence is a vulnerable time for brain development in circuits relative to affective functioning. Last, the high prevalence of cannabis use in this sample emphasizes the importance of intervention programs to coincide with the developmental transition from adolescence to adulthood.
Footnotes
This research was supported by National Institute on Drug Abuse Grants DA25630 and DA26222 (to Daniel S. Shawand Erika E. Forbes). The authors have no potential or competing conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: Author; 1980. [Google Scholar]
- Arnett J. J. Emerging adulthood. A theory of development from the late teens through the twenties. The American Psychologist. 2000;55:469–480. doi:10.1037/0003-066X.55.5.469. [PubMed] [Google Scholar]
- Bardone A. M., Moffitt T. E., Caspi A., Dickson N., Stanton W. R., Silva P. A. Adult physical health outcomes of adolescent girls with conduct disorder, depression, and anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:594–601. doi: 10.1097/00004583-199806000-00009. doi:10.1097/00004583-199806000-00009. [DOI] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Carbin M. G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. doi:10.1016/0272-7358(88)90050-5. [Google Scholar]
- Blum K., Sheridan P. J., Wood R. C., Braverman E. R., Chen T. J., Cull J. G., Comings D. E. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K. I., Brown K., Eldreth D., Tate K., Cadet J. L. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. doi:10.1212/01.WNL.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bottorff J. L., Johnson J. L., Moffat B. M., Mulvogue T. Relief-oriented use of marijuana by teens. Substance Abuse Treatment, Prevention, and Policy. 2009;4:7. doi: 10.1186/1747-597X-4-7. doi:10.1186/1747-597X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovasso G. B. Cannabis abuse as a risk factor for depressive symptoms. American Journal of Psychiatry. 2001;158:2033–2037. doi: 10.1176/appi.ajp.158.12.2033. doi:10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- Brook D. W., Brook J. S., Zhang C., Cohen P., Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Archives of General Psychiatry. 2002;59:1039–1044. doi: 10.1001/archpsyc.59.11.1039. doi:10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- Cahalan D., Cisin I., Crossley H. American drinking practices. New Brunswick, NJ: Center of Alcohol Studies, Rutgers University; 1969. [Google Scholar]
- Chen C.-Y., Wagner F. A., Anthony J. C. Marijuana use and the risk of major depressive episode: Epidemiological evidence from the United States National Comorbidity Survey. Social Psychiatry and Psychiatric Epidemiology. 2002;37:199–206. doi: 10.1007/s00127-002-0541-z. doi:10.1007/s00127-002-0541-z. [DOI] [PubMed] [Google Scholar]
- Degenhardt L., Chiu W.-T., Sampson N., Kessler R. C., Anthony J. C., Angermeyer M., Wells J. E. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO World Mental Health Surveys. PLoS Medicine. 2008;5(7):e141. doi: 10.1371/journal.pmed.0050141. doi:10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L., Hall W. Is cannabis use a contributory cause of psychosis? Canadian Journal of Psychiatry. 2006;51:556–565. doi: 10.1177/070674370605100903. [DOI] [PubMed] [Google Scholar]
- Degenhardt L., Hall W., Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. doi:10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Dishion T. J., Skaggs N. M. An ecological analysis of monthly “bursts” in early adolescent substance use. Applied Developmental Science. 2000;4:89–97. doi:10.1207/S1532480XADS0402_4. [Google Scholar]
- Elliott D. S., Huizinga D., Ageton S. S. Explaining delinquency and drug use. Thousand Oaks, CA: Sage; 1985. [Google Scholar]
- Essau C. A., Lewinsohn P. M., Seeley J. R., Sasagawa S. Gender differences in the developmental course of depression. Journal of Affective Disorders. 2010;127:185–190. doi: 10.1016/j.jad.2010.05.016. doi:10.1016/j.jad.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D. M., Boden J. M. Cannabis use and later life outcomes. Addiction. 2008;103:969–976. doi: 10.1111/j.1360-0443.2008.02221.x. discussion 977–978. doi:10.1111/j.1360-0443.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- Finlay A. K., White H. R., Mun E. Y., Cronley C. C., Lee C. Racial differences in trajectories of heavy drinking and regular marijuana use from ages 13 to 24 among African-American and White males. Drug and Alcohol Dependence. 2012;121:118–123. doi: 10.1016/j.drugalcdep.2011.08.020. doi:10.1016/j.drugalcdep.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom P. L., Friedman S. R., Kottiri B. J., Neaigus A., Curtis R. Recalled adolescent peer norms towards drug use in young adulthood in a low-income, minority urban neighborhood. Journal of Drug Issues. 2001;31:425–443. doi:10.1177/002204260103100204. [Google Scholar]
- Forbes E. E., Dahl R. E. Research review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. doi:10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. E., Ritter C. Marijuana use and depression. Journal of Health and Social Behavior. 2000;41:40–49. doi:10.2307/2676359. [PubMed] [Google Scholar]
- Hankin B. L., Abramson L. Y., Moffitt T. E., Silva P. A., McGee R., Angell K. E. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. doi:10.1037/0021-843X.107.1.128. [DOI] [PubMed] [Google Scholar]
- Henry B., Feehan M., McGee R., Stanton W., Moffitt T. E., Silva P. The importance of conduct problems and depressive symptoms in predicting adolescent substance use. Journal of Abnormal Child Psychology. 1993;21:469–480. doi: 10.1007/BF00916314. doi:10.1007/BF00916314. [DOI] [PubMed] [Google Scholar]
- Hooper D., Coughlan J., Mullen M. R. Structural equation modeling: Guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008;6:53–60. Retrieved from http://www.ejbrm.com/volume6/issue1. [Google Scholar]
- Hussong A. M., Burns A. R., Solis J. M., Rothenberg W. A. Future directions in the developmental science of addictions. Journal of Clinical Child & Adolescent Psychology. 2013;42:863–873. doi: 10.1080/15374416.2013.838772. doi:10.1080/15374416.2013.838772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B., Goodman E. Low social status markers: Do they predict depressive symptoms in adolescence? Race and Social Problems. 2011;3:119–128. doi: 10.1007/s12552-011-9047-1. doi:10.1007/s12552-011-9047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. D., O’Malley P. M., Bachman J. G., Schulenberg J. E., Miech R. A. Monitoring the Future national survey results on drug use, 1975–2013: Volume 2, College students and adults ages 19–55. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Kandel D. B., Davies M. Adult sequelae of adolescent depressive symptoms. Archives of General Psychiatry. 1986;43:255–262. doi: 10.1001/archpsyc.1986.01800030073007. doi:10.1001/archpsyc.1986.01800030073007. [DOI] [PubMed] [Google Scholar]
- Khantzian E. J. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. doi:10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. doi:10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lukas S. E., Mendelson J. H., Benedikt R. Electroencephalographic correlates of marihuana-induced euphoria. Drug and Alcohol Dependence. 1995;37:131–140. doi: 10.1016/0376-8716(94)01067-u. doi:10.1016/0376-8716(94)01067-U. [DOI] [PubMed] [Google Scholar]
- Maalouf F. T., Brent D., Clark L., Tavitian L., McHugh R. M., Sahakian B. J., Phillips M. L. Neurocognitive impairment in adolescent major depressive disorder: State vs. trait illness markers. Journal of Affective Disorders. 2011;133:625–632. doi: 10.1016/j.jad.2011.04.041. doi:10.1016/j.jad.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte D., Alain M., Gosselin M.-J. Gender differences in adolescent depression: Gender-typed characteristics or problem-solving skills deficits? Sex Roles. 1999;41:31–48. doi:10.1023/A:1018833607815. [Google Scholar]
- Marmorstein N. R., Iacono W. G. Explaining associations between cannabis use disorders in adolescence and later major depression: A test of the psychosocial failure model. Addictive Behaviors. 2011;36:773–776. doi: 10.1016/j.addbeh.2011.02.006. doi:10.1016/j.addbeh.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein N. R., White H., Chung T., Hipwell A., Stouthamer-Loeber M., Loeber R. Associations between first use of substances and change in internalizing symptoms among girls: Differences by symptom trajectory and substance use type. Journal of Clinical Child and Adolescent Psychology. 2010;39:545–558. doi: 10.1080/15374416.2010.486325. doi:10.1080/15374416.2010.486325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik J. A., Eldreth D. A., Cadet J.-L., Bolla K. I. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. doi:10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Medina K. L., Nagel B. J., Park A., McQueeny T., Tapert S. F. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. doi:10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Johnson S., Lochman J. E., Coie J. D., Terry R., Hyman C. Comorbidity of conduct and depressive problems at sixth grade: Substance use outcomes across adolescence. Journal of Abnormal Child Psychology. 1998;26:221–232. doi: 10.1023/a:1022676302865. doi:10.1023/A:1022676302865. [DOI] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. Mplus user’s guide. 6th ed. Los Angeles, CA: Author; 1998–2011. [Google Scholar]
- Nielsen T., Karpatschof B., Kreiner S. Regression to the mean effect: When to be concerned and how to correct for it. Nordic Psychology. 2007;59:231–250. doi:10.1027/1901-2276.59.3.231. [Google Scholar]
- O’Hara M. M., Sprinkle S. D., Ricci N. A. Beck Depression Inventory—II: College population study. Psychological Reports. 1998;82:1395–1401. doi: 10.2466/pr0.1998.82.3c.1395. doi:10.2466/pr0.1998.82.3c.1395. [DOI] [PubMed] [Google Scholar]
- Pacek L. R., Martins S. S., Crum R. M. The bidirectional relationships between alcohol, cannabis, co-occurring alcohol and cannabis use disorders with major depressive disorder: results from a national sample. Journal of Affective Disorders. 2013;148:188–195. doi: 10.1016/j.jad.2012.11.059. doi:10.1016/j.jad.2012.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton G. C., Coffey C., Carlin J. B., Degenhardt L., Lynskey M., Hall W. Cannabis use and mental health in young people: Cohort study. BMJ. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. doi:10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkonigg A., Goodwin R. D., Fiedler A., Behrendt S., Beesdo K., Lieb R., Wittchen H. U. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103:439–449. doi: 10.1111/j.1360-0443.2007.02064.x. discussion 450–451. doi:10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Coffman D. L. Computing power and minimum sample size for RMSEA. 2006 [Computer software]. Retrieved from http://quantpsy.org/ [Google Scholar]
- Pujazon-Zazik M., Park M. J. Marijuana: Use among young males and health outcomes. American Journal of Men’s Health. 2009;3:265–274. doi: 10.1177/1557988309340577. doi:10.1177/1557988309340577. [DOI] [PubMed] [Google Scholar]
- Repetto P. B., Zimmerman M. A., Caldwell C. H. A longitudinal study of depressive symptoms and marijuana use in a sample of inner-city African Americans. Journal of Research on Adolescence. 2008;18:421–447. doi:10.1111/j.1532-7795.2008.00566.x. [Google Scholar]
- Sattler J. M. Assessment of children. 3rd ed. San Diego, CA: Jerome M. Sattler, Inc; 1992. [Google Scholar]
- Shaw D. S., Gilliom M., Ingoldsby E. M., Nagin D. S. Trajectories leading to school-age conduct problems. Developmental Psychology. 2003;39:189–200. doi: 10.1037//0012-1649.39.2.189. doi:10.1037/0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Sitnick S. L., Shaw D. S., Hyde L. W. Precursors of adolescent substance use from early childhood and early adolescence: Testing a developmental cascade model. Development and Psychopathology. 2014;26:125–140. doi: 10.1017/S0954579413000539. doi:10.1017/S0954579413000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. doi:10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Author. [Google Scholar]
- Tabachnick B. G., Fidell L. S. Using multivariate statistics. 5th ed. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- Tellegen A., Briggs P. F. Old wine in new skins: Grouping Wechsler subtests into new scales. Journal of Consulting Psychology. 1967;31:499–506. doi: 10.1037/h0024963. doi:10.1037/h0024963. [DOI] [PubMed] [Google Scholar]
- Troisi A., Pasini A., Saracco M., Spalletta G. Psychiatric symptoms in male cannabis users not using other illicit drugs. Addiction. 1998;93:487–492. doi: 10.1046/j.1360-0443.1998.9344874.x. doi:10.1046/j.1360-0443.1998.9344874.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children – Third Edition. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Weissman M. M., Bland R., Joyce P. R., Newman S., Wells J. E., Wittchen H. U. Sex differences in rates of depression: Cross-national perspectives. Journal of Affective Disorders. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. doi:10.1016/0165-0327(93)90025-F. [DOI] [PubMed] [Google Scholar]
- Welte J. W., Russell M. Influence of socially desirable responding in a study of stress and substance abuse. Alcoholism: Clinical and Experimental Research. 1993;17:758–761. doi: 10.1111/j.1530-0277.1993.tb00836.x. doi:10.1111/j.1530-0277.1993.tb00836.x. [DOI] [PubMed] [Google Scholar]
- White A. M., Jordan J. D., Schroeder K. M., Acheson S. K., Georgi B. D., Sauls G., Swartzwelder H. S. Predictors of relapse during treatment and treatment completion among marijuana-dependent adolescents in an intensive outpatient substance abuse program. Substance Abuse. 2004;25:53–59. doi: 10.1300/J465v25n01_08. doi:10.1300/J465v25n01_08. [DOI] [PubMed] [Google Scholar]
- Zoccolillo M. Co-occurrence of conduct disorder and its adult outcomes with depressive and anxiety disorders: A review. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:547–556. doi: 10.1097/00004583-199205000-00024. doi:10.1097/00004583-199205000-00024. [DOI] [PubMed] [Google Scholar]