Abstract
Objective:
Despite growing evidence that chronic marijuana use is associated with cognitive impairment, particularly when use is initiated at an early age, national trends demonstrate significant decreases in the perceived risk of marijuana corresponding with increased use, especially among youth. The current study assessed the impact of marijuana use on executive function and whether patterns of marijuana use, including earlier age at onset, higher frequency, and increased magnitude of use, predict impairment.
Method:
Forty-four chronic, heavy marijuana smokers (37 male, 7 female) and 32 healthy, nonsmoking control participants (20 male, 12 female) recruited from the Greater Boston area completed two assessments of executive function: the Stroop Color Word Test and Wisconsin Card Sorting Test (WCST).
Results:
Marijuana smokers had poorer executive function relative to control participants, a between-group difference that was primarily driven by individuals with early onset of marijuana use (before age 16; n = 21); significance remained even when controlling for frequency and magnitude of use. Further, earlier age at marijuana onset and increased marijuana use predicted poorer neurocognitive performance, and perseverative errors on the WCST significantly predicted marijuana group membership.
Conclusions:
These findings underscore the impact of early onset of marijuana use on executive function impairment independent of increased frequency and magnitude of use. In addition, poorer performance on the WCST may serve as a neuropsychological marker for heavy marijuana users. These results highlight the need for additional research to identify predictors associated with early marijuana use, as exposure to marijuana during a period of developmental vulnerability may result in negative cognitive consequences.
Marijuana remains the most commonly used drug other than alcohol in the United States. Results from the Substance Abuse and Mental Health Services Administration (SAMHSA, 2014) national survey reported that between 2007 and 2013, the number of Americans reporting marijuana use within the past month increased from 14.5 million to 19.8 million. Similarly, heavy marijuana use (marijuana use on 20 or more days in the past month) increased from 5.1 million to 8.1 million people (SAMHSA, 2014). According to the Monitoring the Future study, which surveys drug use among high school students and young adults, 21.2% of high school seniors used marijuana within the past 30 days, and more than a quarter of these—5.8% of seniors overall—reported daily marijuana use (Johnston et al., 2015).
Results from the Monitoring the Future survey also suggest that perceived risk related to marijuana use may be a leading indicator of marijuana use patterns. For the past two decades, perceived risk of marijuana use has substantially declined among 12th graders, dovetailing with increased use in this population. In 2014, 36% of high school seniors viewed regular marijuana use as harmful, compared with 52% of seniors surveyed 5 years earlier (Johnston et al., 2015). Together, these data indicate a trend of decreased perception of risk related to marijuana use coinciding with increased marijuana use among the nation’s most vulnerable population, those who are not yet neurodevelopmentally mature.
Increasing national media coverage and ongoing debates regarding the legalization of medical marijuana often highlight potential benefits, and it is therefore not surprising that national trends demonstrate a strong relationship between decreased perception of risk and increased marijuana use. In light of these shifts in attitude, it is important to examine the impact of chronic marijuana use and to determine if assessing such factors as age at onset, frequency, and magnitude of marijuana use provides an opportunity to predict neurocognitive outcomes.
Numerous studies have reported marijuana-associated impairments in frontal function, most notably during tasks that require executive control, inhibition, and decision making (for review, see Crane et al., 2013; Crean et al., 2011). Further, several investigations have specifically examined the role of age at marijuana onset, with results suggesting that earlier age at marijuana onset is related to impairment on measures of visual scanning (Ehrenreich et al., 1999), verbal IQ (Pope et al., 2003; Solowij et al., 2011), and executive function (Battisti et al., 2010; Fontes et al., 2011; Gruber et al., 2012b). Gruber and colleagues (2012b) found that marijuana smokers performed more poorly than healthy control subjects on tasks of executive function, including the Wisconsin Card Sorting Test (WCST) and Stroop Color Word Test (Stroop). It is interesting to note that when the marijuana group was divided into early- and late-onset groups, results revealed that the significant differences detected between the marijuana and control groups were primarily attributable to the poor performance of the early-onset marijuana smokers. Fontes et al. (2011) also assessed the effect of marijuana onset on these tasks. Results indicated that early-onset marijuana smokers performed more poorly on the WCST and Stroop compared with the control group, and the early-onset marijuana group also tended to show impairment relative to the late-onset marijuana group. Correlation analyses have further supported these findings, indicating that earlier age at marijuana onset and increased frequency (smoking episodes/week) and magnitude of marijuana use (grams smoked/week) are all associated with poorer executive functioning (Gruber et al., 2012b).
In light of these findings, recent studies have attempted to elucidate the role of marijuana use in predicting cognitive function. Battisti and colleagues (2010) found that earlier age at marijuana onset was a significant predictor of reduced accuracy on the interference condition of the Stroop. Earlier age at onset of regular marijuana use as well as increased quantity of marijuana use (cones/bowls smoked per week) have also been shown to significantly predict verbal memory (Solowij et al., 2011). Lisdahl and Price (2012) reported that in a young adult sample, more frequent marijuana use (joints smoked per year) predicted poorer psychomotor speed, sustained attention, and cognitive inhibition. Harvey et al. (2007) noted that more total days of marijuana use predicted poorer executive function and spatial working memory. In addition, Lane and colleagues (2007) used logistic regression analyses in a group of delinquent youth and found increased delinquency and marijuana use predicted increased total errors on the WCST; only marijuana group membership predicted increased perseverative errors.
Given these findings, the current investigation was designed to examine the relationship between executive function and marijuana use variables in well-characterized, chronic marijuana smokers and to assess whether patterns of marijuana use, including age at onset, frequency, and magnitude of marijuana use could predict alterations in executive function. We hypothesized that, consistent with previous studies, marijuana smokers would perform tasks of executive function more poorly than control participants, and that this difference would be driven by individuals with early onset of marijuana use. Further, using regression analyses, we planned to extend previous findings by exploring how patterns of marijuana use may predict executive function. We hypothesized that within the marijuana-smoking group, earlier age at marijuana onset, heavier marijuana use, and more frequent marijuana use would predict poorer executive functioning. We also predicted that task performance could be used as a neuropsychological marker for marijuana group membership.
Method
Participants
Participants were recruited from the Greater Boston area and included 44 chronic, heavy marijuana-smoking participants (37 male, 7 female) aged 18–46 and 32 healthy nonsmoking control participants (20 male, 12 female) aged 18–47. To assess the potential impact of age at onset on cognitive function, marijuana smokers were divided into early-onset (regular marijuana use before age 16; n = 21) and late-onset (regular marijuana use starting at age 16 or older; n = 23) groups. This method of defining early-onset versus late-onset marijuana use has previously been used in several studies (Gruber et al., 2011, 2012a, 2014; Kempel et al., 2003). Before participation, all study procedures, including the voluntary nature of the study, were explained, and participants were required to read and sign an informed consent form approved by the McLean Hospital Institutional Review Board.
All participants received the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P; First et al., 1994), to ensure that individuals did not meet criteria for any Axis I pathology other than marijuana abuse or dependence for the smoking group. Given previous study findings, which have noted a relationship between alcohol and cognitive performance (e.g., Lisdahl et al., 2013), participants were excluded from the study if they reported drinking more than 20 alcoholic beverages per week, met criteria for binge drinking, or met diagnostic criteria for current or past alcohol dependence. In addition, participants were excluded if they reported more than 15 lifetime uses of any category of illicit drugs or recreational use of prescription drugs, or had a positive urine screen for any drug (excluding marijuana for the marijuana group). Exclusion criteria also included head injury with loss of consciousness, neurological disorders, current or previous use of psychotropic medications, and nonnative English speakers (required for the neurocognitive battery). Although a number of participants had completed other studies, which have previously been published (Gruber et al., 2012b), approximately 35% of the participant pool was newly recruited. Further, regression analyses completed for the current study have not previously been reported.
To qualify for study entry, marijuana smokers were required to have used marijuana a minimum of 2,500 times, report current marijuana use at least 5 times per week, and test positive for urinary cannabinoids. Marijuana smokers were also required to abstain from smoking marijuana at least 12 hours before their study visit to ensure that they were not acutely intoxicated at the time of assessment. All participants provided a urine sample on arrival at the laboratory, and to ensure compliance, marijuana participants were led to believe that researchers could use this sample to detect the last use of marijuana, a method we have successfully used in the past (Gruber & Yurgelun-Todd, 2005; Gruber et al., 2011, 2012a, 2012b, 2014). Further, all urine samples were screened for illicit and prescription drugs including amphetamines, cocaine, tetrahydrocannabinol (THC), methamphetamine, opioids, phencyclidine (PCP), benzodiazepines, tricyclic antidepressants, barbiturates, methadone, Ecstasy (3,4-methylenedioxymethamphetamine, or MDMA), and oxycodone. An aliquot was sent to an outside laboratory for quantification of urinary THC concentration via gas chromatography–mass spectrometry.
Assessments and procedures
Marijuana smokers provided information regarding their history of marijuana use, including age at onset, which was defined as the age at which subjects began using marijuana on a routine, expected, and consistent basis (i.e., first “regular” use). Using this information, duration of regular marijuana use (years) was also calculated. A modified Timeline Followback (TLFB; Sobell et al., 1988) procedure was used to assess frequency (marijuana smoking episodes per week) and magnitude (grams of marijuana used per week) of use, with a specific focus on the previous 7–10 days. Lifetime and current use patterns were also verified using the SCID-P. Information regarding current/lifetime alcohol use was assessed during the SCID-P and TLFB with all subjects reporting the number of days of alcohol use in the past month. Current nicotine use was assessed using the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991).
All participants also completed a battery of clinical rating scales, including the Beck Depression Inventory (Beck et al., 1961), Hamilton Anxiety Scale (Hamilton, 1959), and Profile of Mood States (McNair et al., 1971), providing a profile of current mood state. General intellectual function (IQ) was measured with the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) or an abbreviated version of the Wechsler Adult Intelligence Scale–Revised (Wechsler, 1981). Cognitive performance, specifically executive function, was assessed using the Stroop and the WCST.
The Stroop serves as a measure of cognitive interference, the ability to inhibit prepotent responses in favor of less automatic ones. This task comprised three conditions: color naming, word reading, and interference. The color-naming condition requires participants to name the color of red, blue, and green rectangles. During the word-reading condition, participants read aloud color words that are printed in black ink (red, blue, or green). The interference condition requires participants to inhibit the natural, automatic tendency to read words, and instead name the color of words printed in incongruously colored ink (e.g., the word red printed in blue). Performance is measured by the number of omission errors (no response) and commission errors (incorrect responses) as well as overall task accuracy (MacLeod, 1991).
The WCST is considered a gold-standard measure of executive function; it assesses the ability to shift and maintain set, form abstract concepts, and use feedback. The task consists of 128 response cards (divided into two runs of 64 cards per deck) and 4 stimulus cards. Each card is printed with geometric figures that vary in color, shape, and number. Participants must match each card from their response deck to one of the four stimulus cards but are not informed of the correct sorting rule. Using trial and error as well as examiner feedback (correct or incorrect), participants must determine and maintain the correct sorting rule. After 10 consecutive correct matches (a category), the sorting rule changes without informing the participant, who must then determine the new sorting rule. To ensure acquisition of the rule structure, clarification of the rules is provided to all participants after the completion of the first run (Deck 1) and before beginning the second run (Deck 2; Stuss et al., 1983). Performance is measured by number of categories completed; perseverative errors, which occur when participants continue to sort to an incorrect matching rule despite feedback; and losses of set, which occur when participants have established a sorting rule by completing five correct responses but then place an incorrectly matched card. Superior performance on this task is indicated by maximizing number of categories and minimizing perseveration errors and losses of set (Berg, 1948; Lezak et al., 2004).
Statistical analyses
Two different types of between-group analyses were performed: two-group analyses (controls vs. all marijuana smokers) and three-group analyses (controls vs. early-onset marijuana smokers vs. late-onset marijuana smokers). For the two-group analyses, independent sample t tests were performed when the assumptions for parametric tests were met; otherwise, adjusted t tests were used. For the three-group analyses, one-way analyses of variance (ANOVAs) with Scheffé post hoc tests were used when the assumptions for parametric tests were met; otherwise, Kruskal–Wallis one-way ANOVAs with Mann–Whitney U post hoc pairwise comparisons were used. To more fully assess the relationship between marijuana use variables, univariate regression analyses were conducted to examine whether marijuana use could predict executive function performance. Given the distribution of the data, nonlinear distributions were used for all regression analyses with marijuana use variables (age at onset, smoking episodes/week, and grams used/week); exponential distributions best fit the data. Last, to examine whether specific measures of executive function could accurately discriminate between marijuana users and non-users, binary logistic regressions were performed examining whether group membership (control vs. marijuana) could be predicted by modeling performance during the Stroop and WCST. All analyses were performed using IBM SPSS Statistics for Windows, Version 20 (IBM Corp., Armonk, NY).
Results
Demographics
Groups were well matched for both two-group (Table 1) and three-group (Table 2) analyses, with no significant differences in age, IQ, clinical state, or nicotine use, suggesting that these variables did not contribute significantly to the subsequent analyses.
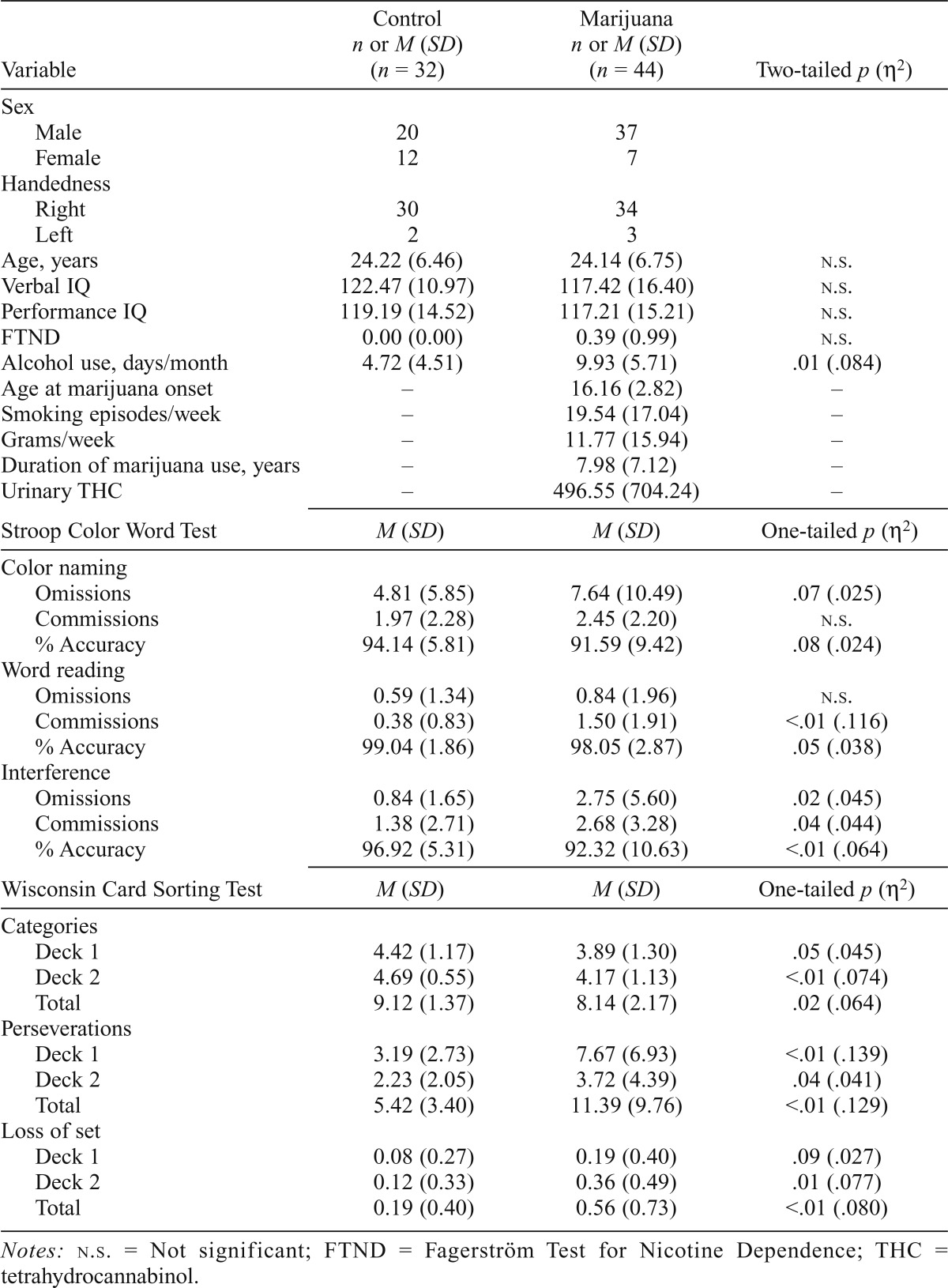
Table 1.
Comparison of control participants and marijuana smokers
| Variable | Control n or M (SD) (n = 32) | Marijuana n or M (SD) (n = 44) | Two-tailed p (η2) |
| Sex | |||
| Male | 20 | 37 | |
| Female | 12 | 7 | |
| Handedness | |||
| Right | 30 | 34 | |
| Left | 2 | 3 | |
| Age, years | 24.22 (6.46) | 24.14(6.75) | n.s. |
| Verbal IQ | 122.47 (10.97) | 117.42 (16.40) | n.s. |
| Performance IQ | 119.19 (14.52) | 117.21 (15.21) | n.s. |
| FTND | 0.00 (0.00) | 0.39 (0.99) | n.s. |
| Alcohol use, days/month | 4.72 (4.51) | 9.93 (5.71) | .01 (.084) |
| Age at marijuana onset | – | 16.16 (2.82) | – |
| Smoking episodes/week | – | 19.54 (17.04) | – |
| Grams/week | – | 11.77 (15.94) | – |
| Duration of marijuana use, years | – | 7.98 (7.12) | – |
| Urinary THC | – | 496.55 (704.24) | – |
| Stroop Color Word Test | M (SD) | M (SD) | One-tailed p (η2) |
| Color naming | |||
| Omissions | 4.81 (5.85) | 7.64 (10.49) | .07 (.025) |
| Commissions | 1.97 (2.28) | 2.45 (2.20) | n.s. |
| % Accuracy | 94.14 (5.81) | 91.59 (9.42) | .08 (.024) |
| Word reading | |||
| Omissions | 0.59 (1.34) | 0.84 (1.96) | n.s. |
| Commissions | 0.38 (0.83) | 1.50 (1.91) | <.01 (.116) |
| % Accuracy | 99.04 (1.86) | 98.05 (2.87) | .05 (.038) |
| Interference | |||
| Omissions | 0.84 (1.65) | 2.75 (5.60) | .02 (.045) |
| Commissions | 1.38 (2.71) | 2.68 (3.28) | .04 (.044) |
| % Accuracy | 96.92 (5.31) | 92.32 (10.63) | <.01 (.064) |
| Wisconsin Card Sorting Test | M (SD) | M (SD) | One-tailed p (η2) |
| Categories | |||
| Deck 1 | 4.42 (1.17) | 3.89 (1.30) | .05 (.045) |
| Deck 2 | 4.69 (0.55) | 4.17 (1.13) | <.01 (.074) |
| Total | 9.12 (1.37) | 8.14 (2.17) | .02 (.064) |
| Perseverations | |||
| Deck 1 | 3.19 (2.73) | 7.67 (6.93) | <.01 (.139) |
| Deck 2 | 2.23 (2.05) | 3.72 (4.39) | .04 (.041) |
| Total | 5.42 (3.40) | 11.39 (9.76) | <.01 (.129) |
| Loss of set | |||
| Deck 1 | 0.08 (0.27) | 0.19 (0.40) | .09 (.027) |
| Deck 2 | 0.12 (0.33) | 0.36 (0.49) | .01 (.077) |
| Total | 0.19 (0.40) | 0.56 (0.73) | <.01 (.080) |
Notes: n.s. = Not significant; FTND = Fagerström Test for Nicotine Dependence; THC = tetrahydrocannabinol.
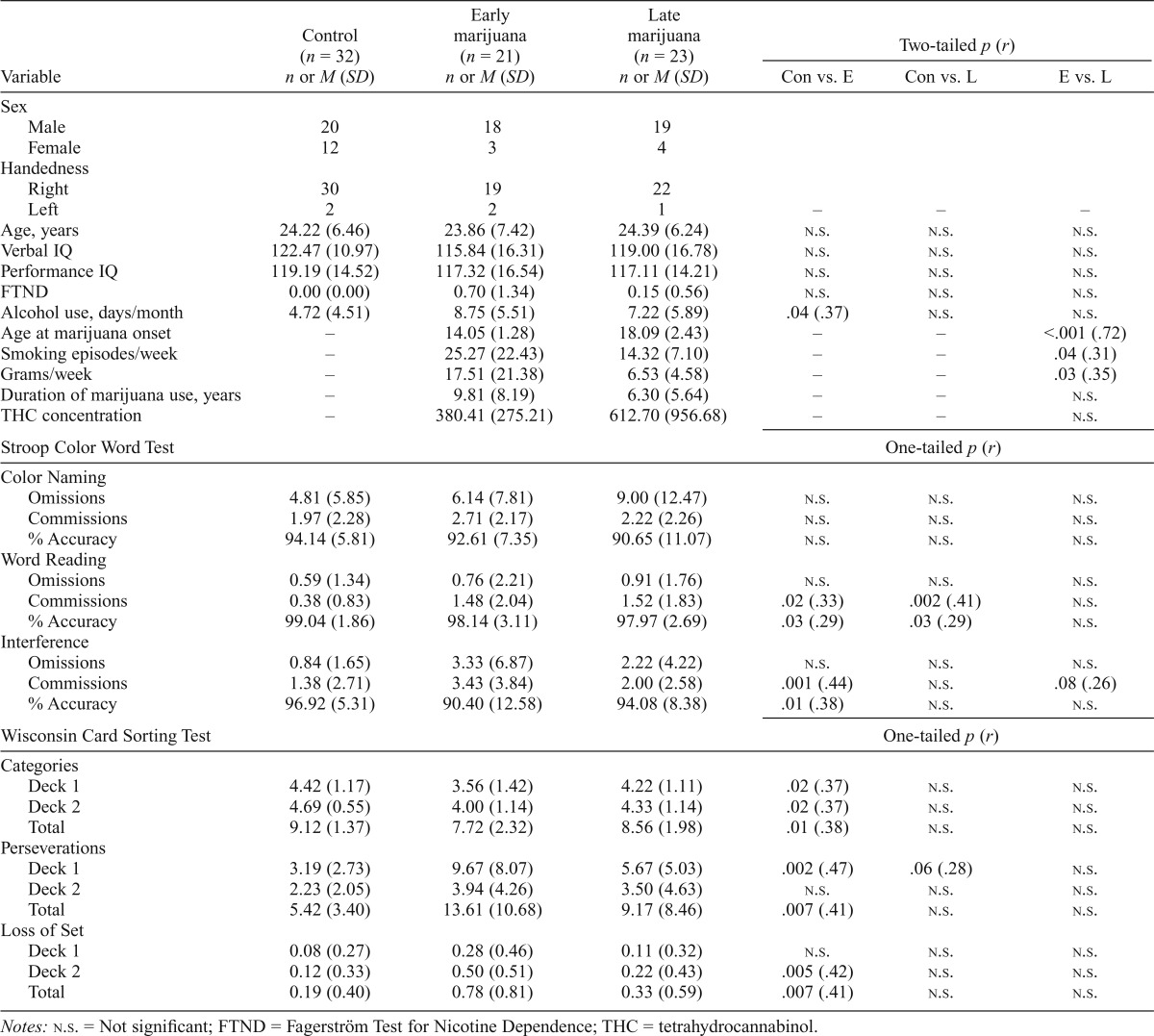
Table 2.
Comparison of control (Con) participants, early (E) marijuana smokers, and late (L) marijuana smokers
| Variable | Control (n = 32) n or M (SD) | Early marijuana (n = 21) n or M (SD) | Late marijuana (n = 23) n or M (SD) | Two-tailed p (r) |
||
| Con vs. E | Con vs. L | E vs. L | ||||
| Sex | ||||||
| Male | 20 | 18 | 19 | |||
| Female | 12 | 3 | 4 | |||
| Handedness | ||||||
| Right | 30 | 19 | 22 | |||
| Left | 2 | 2 | 1 | – | – | – |
| Age, years | 24.22 (6.46) | 23.86 (7.42) | 24.39 (6.24) | n.s. | n.s. | n.s. |
| Verbal IQ | 122.47 (10.97) | 115.84 (16.31) | 119.00 (16.78) | n.s. | n.s. | n.s. |
| Performance IQ | 119.19 (14.52) | 117.32 (16.54) | 117.11 (14.21) | n.s. | n.s. | n.s. |
| FTND | 0.00 (0.00) | 0.70 (1.34) | 0.15 (0.56) | n.s. | n.s. | n.s. |
| Alcohol use, days/month | 4.72 (4.51) | 8.75 (5.51) | 7.22 (5.89) | .04 (.37) | n.s. | n.s. |
| Age at marijuana onset | – | 14.05 (1.28) | 18.09 (2.43) | – | – | <.001 (.72) |
| Smoking episodes/week | – | 25.27 (22.43) | 14.32 (7.10) | – | – | .04 (.31) |
| Grams/week | – | 17.51 (21.38) | 6.53 (4.58) | – | – | .03 (.35) |
| Duration of marijuana use, years | – | 9.81 (8.19) | 6.30 (5.64) | – | – | n.s. |
| THC concentration | – | 380.41 (275.21) | 612.70 (956.68) | – | – | n.s. |
| Stroop Color Word Test | One-tailed p (r) | |||||
| Color Naming | ||||||
| Omissions | 4.81 (5.85) | 6.14 (7.81) | 9.00 (12.47) | n.s. | n.s. | n.s. |
| Commissions | 1.97 (2.28) | 2.71 (2.17) | 2.22 (2.26) | n.s. | n.s. | n.s. |
| % Accuracy | 94.14 (5.81) | 92.61 (7.35) | 90.65 (11.07) | n.s. | n.s. | n.s. |
| Word Reading | ||||||
| Omissions | 0.59 (1.34) | 0.76 (2.21) | 0.91 (1.76) | n.s. | n.s. | n.s. |
| Commissions | 0.38 (0.83) | 1.48 (2.04) | 1.52 (1.83) | .02 (.33) | .002 (.41) | n.s. |
| % Accuracy | 99.04 (1.86) | 98.14 (3.11) | 97.97 (2.69) | .03 (.29) | .03 (.29) | n.s. |
| Interference | ||||||
| Omissions | 0.84 (1.65) | 3.33 (6.87) | 2.22 (4.22) | n.s. | n.s. | n.s. |
| Commissions | 1.38 (2.71) | 3.43 (3.84) | 2.00 (2.58) | .001 (.44) | n.s. | .08 (.26) |
| % Accuracy | 96.92 (5.31) | 90.40 (12.58) | 94.08 (8.38) | .01 (.38) | n.s. | n.s. |
| Wisconsin Card Sorting Test | One-tailed p (r) | |||||
| Categories | ||||||
| Deck 1 | 4.42 (1.17) | 3.56 (1.42) | 4.22 (1.11) | .02 (.37) | n.s. | n.s. |
| Deck 2 | 4.69 (0.55) | 4.00 (1.14) | 4.33 (1.14) | .02 (.37) | n.s. | n.s. |
| Total | 9.12 (1.37) | 7.72 (2.32) | 8.56 (1.98) | .01 (.38) | n.s. | n.s. |
| Perseverations | ||||||
| Deck 1 | 3.19 (2.73) | 9.67 (8.07) | 5.67 (5.03) | .002 (.47) | .06 (.28) | n.s. |
| Deck 2 | 2.23 (2.05) | 3.94 (4.26) | 3.50 (4.63) | n.s. | n.s. | n.s. |
| Total | 5.42 (3.40) | 13.61 (10.68) | 9.17 (8.46) | .007 (.41) | n.s. | n.s. |
| Loss of Set | ||||||
| Deck 1 | 0.08 (0.27) | 0.28 (0.46) | 0.11 (0.32) | n.s. | n.s. | n.s. |
| Deck 2 | 0.12 (0.33) | 0.50 (0.51) | 0.22 (0.43) | .005 (.42) | n.s. | n.s. |
| Total | 0.19 (0.40) | 0.78 (0.81) | 0.33 (0.59) | .007 (.41) | n.s. | n.s. |
Notes: n.s. = Not significant; FTND = Fagerström Test for Nicotine Dependence; THC = tetrahydrocannabinol.
Current alcohol use differed significantly between groups, with control participants reporting fewer days of alcohol use per month than the entire marijuana group, F(1, 70) = 6.44, p = .01. The three-group analysis was also significant, F(2, 69) = 3.67, p = .03; control participants reported fewer days of use per month than the early-onset marijuana participants (p = .03), whereas the late-onset marijuana group did not differ compared with the control and early-onset marijuana groups. It is important to note that the average days of alcohol use per month reported was in the low to moderate range for all groups (Table 1); the early-onset marijuana group reported the most days of alcohol use per month (M = 8.75, SD = 5.51), which does not reflect heavy use. However, given the significant difference between the groups, two-tailed Pearson correlation analyses were run to assess the impact of alcohol use on dependent variables. No correlations were significant except for a positive correlation with Stroop Word Reading commission errors, r(70) = .253, p = .032. Between-group analyses for this variable were therefore completed with and without covarying for alcohol use; results were significant either way, suggesting that alcohol use did not contribute significantly to study results.
With regard to marijuana use variables, the early-onset marijuana group reported significantly higher frequency and magnitude of use than the late-onset group (Table 2); specifically, the early-onset group reported more smoking episodes, t(23.65) = 2.14, p = .04, as well as more grams of marijuana used per week, t(21.67) = 2.31, p = .03. The groups did not differ significantly in duration of marijuana use (years) or urinary THC concentration.
Two-group analyses: Comparison of control participants and marijuana smokers
Marijuana smokers performed significantly worse on both the Stroop and the WCST relative to control participants (Table 1). During the Stroop interference condition, marijuana smokers made significantly more commission (p = .04) and omission errors (p = .02), resulting in significantly lower percent accuracy (p < .01). During the WCST, marijuana smokers achieved fewer categories on Deck 1 (p = .05), Deck 2 (p < .01), and the total (Decks 1 and 2 combined; p = .02) than did control participants. Marijuana smokers also made significantly more perseverative errors on Deck 1 (p < .01), Deck 2 (p = .04), and total (p < .01), and exhibited more losses of set on Deck 2 (p = .01) and total (p < .01) relative to the control participants.
Three-group analyses: Comparison of control participants, early-onset marijuana smokers, and late-onset marijuana smokers
Three-group analyses confirmed that between-group differences noted in the two-group analyses were primarily driven by the early-onset marijuana smokers (Table 2). For the Stroop interference condition, significant between-group differences were noted for both commission errors and task accuracy; Mann–Whitney U post hoc tests indicated that the early-onset marijuana group made significantly more commission errors (p < .01) and had significantly lower percent accuracy (p = .01) compared with the control group. No significant between-group differences were noted between the early- and late-onset marijuana groups; however, the early group demonstrated a trend for increased commission errors compared with the late group (p = .08). It is notable that the late-onset marijuana group was not significantly different from the control group during the interference condition of this task.
Similarly, significant between-group differences on the WCST emerged for categories achieved, perseveration errors, and losses of set. Mann–Whitney U post hoc tests indicated that the early-onset marijuana group achieved fewer categories on Deck 1 (p = .02), Deck 2 (p = .02), and total (p = .01); made significantly more perseverative errors on Deck 1 (p < .01) and total (p = .01); and had more losses of set on Deck 2 (p = .01) and total (p = .01) relative to the control group. Similar to the Stroop analyses, no significant differences were noted for the late-onset marijuana group relative to either the early-onset group or the control group.
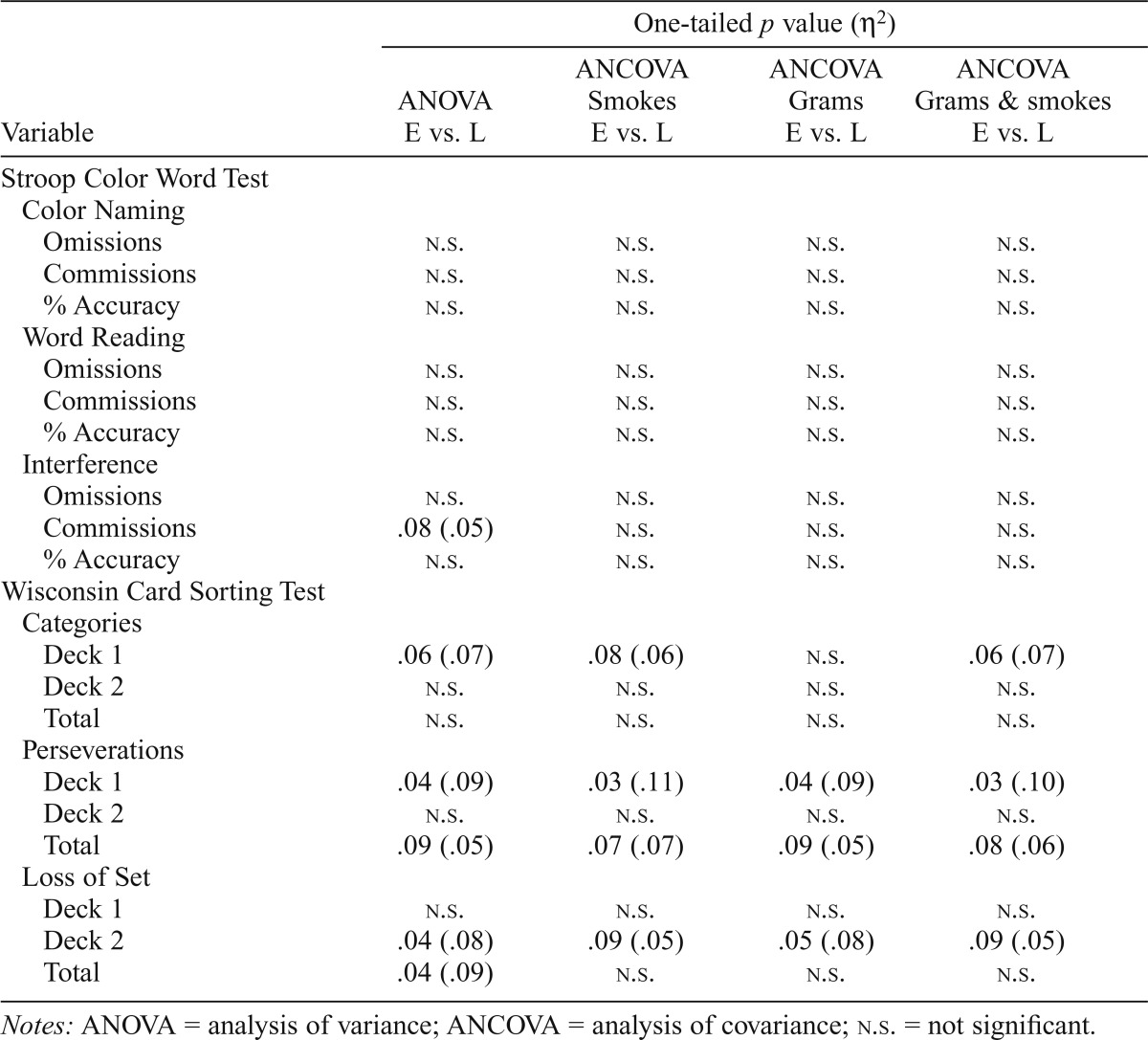
Two-group analyses of covariance: Comparison of early- and late-onset marijuana smokers, controlling for significant differences in marijuana use
Direct comparisons of the early- and late-onset groups using two-group ANOVAs (Table 3) indicated that the early-onset group made significantly more perseverative errors on Deck 1 of the WCST (p = .04) and more losses of set on the WCST during Deck 2 (p = .04) and total (p = .04). A trend emerged for the early-onset group to achieve fewer categories during Deck 1 (p = .06) and to make more total perseverative errors on the WCST (p = .09). As the early- and late-onset marijuana smokers reported significantly more marijuana smoking episodes per week and grams of marijuana used per week, separate analyses of covariance (ANCOVAs) were also performed, controlling for these significant differences individually as well as combined (Table 3). After we controlled for marijuana use variables, results indicated that increased WCST perseverations during Deck 1 within the early-onset marijuana group remained significant (p = .03); differences in categories achieved during Deck 1, total perseverations and losses of set on Deck 2 approached significance (p = .06, p = .08, and p = .09, respectively).
Table 3.
Comparison of early (E) and late (L) marijuana smokers controlling for smoking episodes and grams used per week
| Variable | One-tailed p value (η2) |
|||
| ANOVA E vs. L | ANCOVA Smokes E vs. L | ANCOVA Grams E vs. L | ANCOVA Grams & smokes E vs. L | |
| Stroop Color Word Test | ||||
| Color Naming | ||||
| Omissions | n.s. | n.s. | n.s. | n.s. |
| Commissions | n.s. | n.s. | n.s. | n.s. |
| % Accuracy | n.s. | n.s. | n.s. | n.s. |
| Word Reading | ||||
| Omissions | n.s. | n.s. | n.s. | n.s. |
| Commissions | n.s. | n.s. | n.s. | n.s. |
| % Accuracy | n.s. | n.s. | n.s. | n.s. |
| Interference | ||||
| Omissions | n.s. | n.s. | n.s. | n.s. |
| Commissions | .08 (.05) | n.s. | n.s. | n.s. |
| % Accuracy | n.s. | n.s. | n.s. | n.s. |
| Wisconsin Card Sorting Test | ||||
| Categories | ||||
| Deck 1 | .06 (.07) | .08 (.06) | n.s. | .06 (.07) |
| Deck 2 | n.s. | n.s. | n.s. | n.s. |
| Total | n.s. | n.s. | n.s. | n.s. |
| Perseverations | ||||
| Deck 1 | .04 (.09) | .03 (.11) | .04 (.09) | .03 (.10) |
| Deck 2 | n.s. | n.s. | n.s. | n.s. |
| Total | .09 (.05) | .07 (.07) | .09 (.05) | .08 (.06) |
| Loss of Set | ||||
| Deck 1 | n.s. | n.s. | n.s. | n.s. |
| Deck 2 | .04 (.08) | .09 (.05) | .05 (.08) | .09 (.05) |
| Total | .04 (.09) | n.s. | n.s. | n.s. |
Notes: ANOVA = analysis of variance; ANCOVA = analysis of covariance; n.s. = not significant.
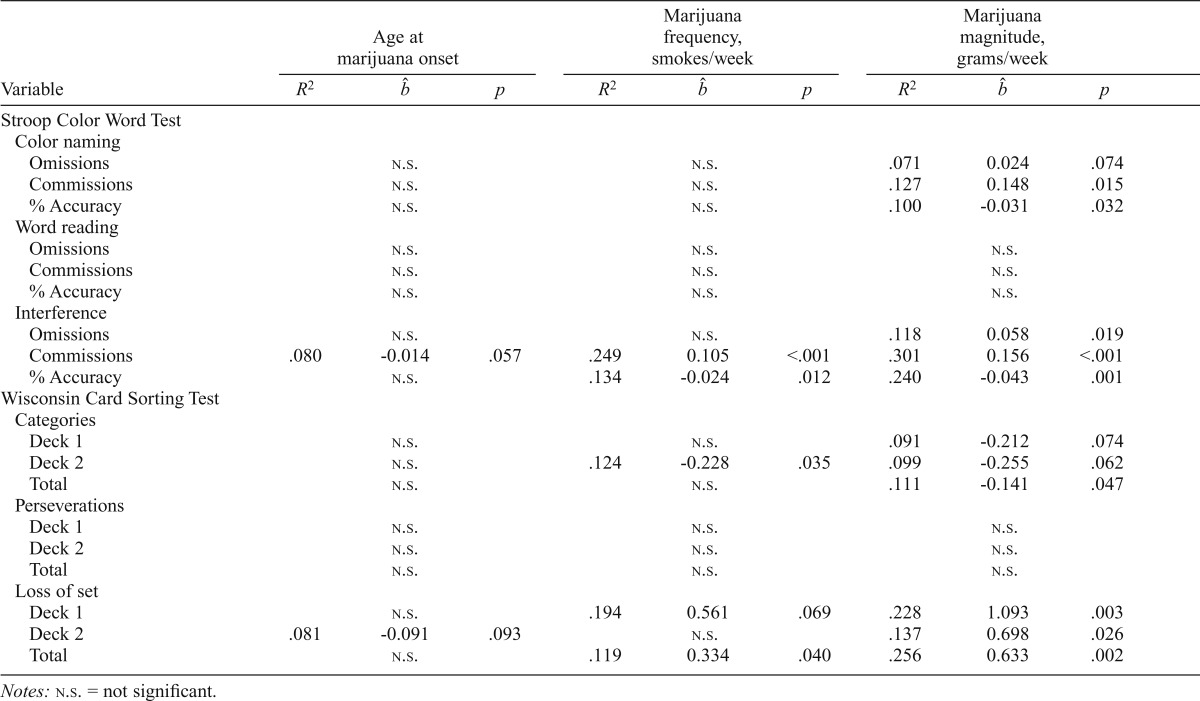
Marijuana use predicts performance: Univariate regression analyses
Among the marijuana smokers, marijuana use characteristics (age at onset, frequency and magnitude of use), which differed significantly between the early- and late-onset marijuana groups, all predicted executive function performance (Table 4). During the interference condition of the Stroop, earlier age at marijuana onset and increased marijuana use predicted increased errors and lower performance accuracy; specifically, higher numbers of smoking episodes per week predicted increased commission errors (p < .01) as well as decreased performance accuracy (p = .01). Higher grams of marijuana per week predicted increased commission errors (p < .01), increased omission errors (p = .02), and lower performance accuracy (p < .01). In addition, earlier age at marijuana onset was related to increased commission errors but did not reach significance (p = .06).
Table 4.
Univariate regressions with marijuana demographics
| Variable | Age at marijuana onset |
Marijuana frequency, smokes/week |
Marijuana magnitude, grams/week |
||||||
| R2 | b | p | R2 | b | p | R2 | b | p | |
| Stroop Color Word Test | |||||||||
| Color naming | |||||||||
| Omissions | n.s. | n.s. | .071 | 0.024 | .074 | ||||
| Commissions | n.s. | n.s. | .127 | 0.148 | .015 | ||||
| % Accuracy | n.s. | n.s. | .100 | -0.031 | .032 | ||||
| Word reading | |||||||||
| Omissions | n.s. | n.s. | n.s. | ||||||
| Commissions | n.s. | n.s. | n.s. | ||||||
| % Accuracy | n.s. | n.s. | n.s. | ||||||
| Interference | |||||||||
| Omissions | n.s. | n.s. | .118 | 0.058 | .019 | ||||
| Commissions | .080 | -0.014 | .057 | .249 | 0.105 | <.001 | .301 | 0.156 | <.001 |
| % Accuracy | n.s. | .134 | -0.024 | .012 | .240 | -0.043 | .001 | ||
| Wisconsin Card Sorting Test | |||||||||
| Categories | |||||||||
| Deck 1 | n.s. | n.s. | .091 | -0.212 | .074 | ||||
| Deck 2 | n.s. | .124 | -0.228 | .035 | .099 | -0.255 | .062 | ||
| Total | n.s. | n.s. | .111 | -0.141 | .047 | ||||
| Perseverations | |||||||||
| Deck 1 | n.s. | n.s. | n.s. | ||||||
| Deck 2 | n.s. | n.s. | n.s. | ||||||
| Total | n.s. | n.s. | n.s. | ||||||
| Loss of set | |||||||||
| Deck 1 | n.s. | .194 | 0.561 | .069 | .228 | 1.093 | .003 | ||
| Deck 2 | .081 | -0.091 | .093 | n.s. | .137 | 0.698 | .026 | ||
| Total | n.s. | .119 | 0.334 | .040 | .256 | 0.633 | .002 | ||
Notes: n.s. = not significant.
During the WCST, higher numbers of smoking episodes per week predicted fewer categories achieved on Deck 2 (p = .04) and increased total losses of set (p = .04); a trend was also detected for higher smoking episodes per week to predict increased losses of set on Deck 1 (p = .07). Higher grams of marijuana used per week was predictive of fewer total categories achieved (p = .05); performance on Deck 1 and Deck 2, individually, only yielded trends (p = .07 and p = .06, respectively). Higher grams per week was also predictive of increased losses of set on Deck 1 (p < .01), Deck 2 (p = .03), and total (p < .01). In addition, a trend was detected for earlier age at marijuana onset to predict increased losses of set on Deck 2 (p = .09).
Cognitive performance predicts group membership: Logistic regression analyses
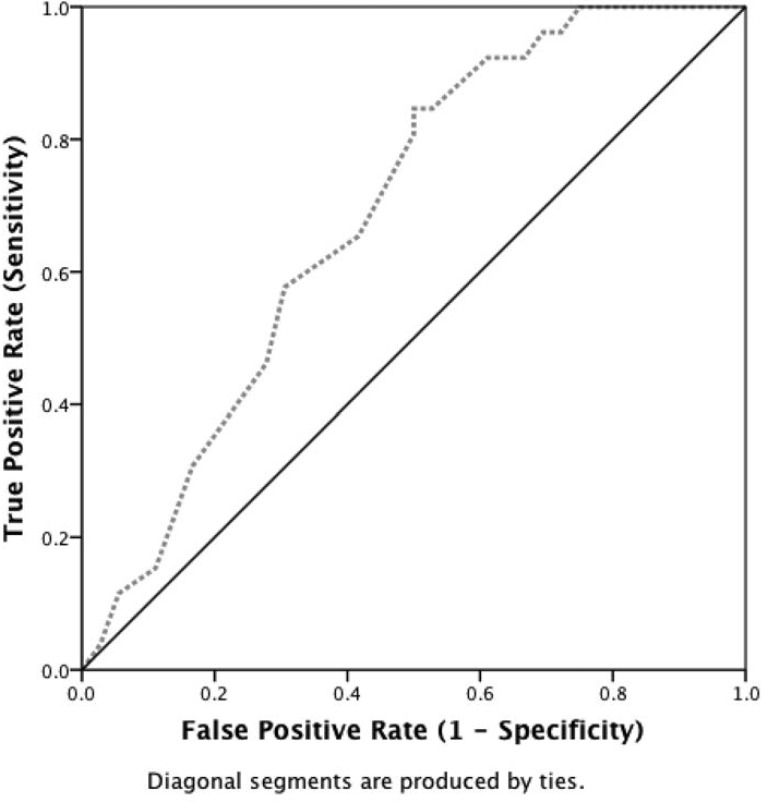
Measures of cognitive performance were also predictive of group membership. The continuous predictor variables in the original model included Stroop interference percent accuracy, as well as WCST total categories, perseverations, and losses of set. Preliminary analysis of this model indicated that WCST total perseverations was the primary contributor; therefore, a more parsimonious model was established using only this predictor. Under the null model for frequency of group membership, the frequency of correct group placement was 58.1% (control = 0%, marijuana = 100%). Adding WCST total perseverations to the model increased the accuracy of the model to 64.5% (control = 57.5%, marijuana = 69.4%); the model was able to correctly identify control participants 57.5% of the time and marijuana smokers 69.4% of the time. A Likelihood Ratio Test indicated that this improvement was statistically significant, χ2(1) = 10.37, p < .001, and the Nagelkerke pseudo R2 indicated that the model including total WCST perseverations accounted for 20.7% of the group variance. The betas for this model demonstrated that when total perseverations equaled 0, the odds of marijuana group membership were .452 to 1 (p = .097), and for every 1-unit increase in total perseverations, the odds of marijuana group membership increased by 1.160 to 1 (p = .014). The receiver operating characteristic curve indicated that WCST total perseverations alone was a moderate predictor of group membership (area under the curve = .688, p = .012; Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curve predicting control/marijuana group membership using Wisconsin Card Sorting Test (WCST) total perseverations. The ROC curve of the predictive value of the binary logistic regression model indicated that total WCST perseverations alone was a moderate predictor of group membership (area under the curve = .688, p = .012). The betas for this model indicated that when total perseverations equaled 0, the odds of marijuana group membership were .452 to 1 (p = .097), and for every 1-unit increase in total perseverations, the odds of marijuana group membership increased by 1.160 to 1 (p = .014).
Discussion
As hypothesized, findings revealed that marijuana smokers exhibited poorer performance on the Stroop and WCST, tasks of executive function, relative to non–marijuana-smoking control subjects. Importantly, these between-group differences were primarily attributable to the early-onset marijuana smokers, because the early-onset group performed significantly worse than control participants on the Stroop interference condition (increased commission errors and lower percent accuracy) as well as on the WCST (fewer categories, more perseverative errors, and more losses of set). Conversely, the late-onset marijuana smokers’ performance was similar to the control participants’ performance, and no statistically significant differences were observed between these two groups on any measure of executive function.
In the current study, early-onset smokers reported significantly higher levels of marijuana use relative to late-onset smokers; the early-onset group smoked 1.75 times more frequently and used greater than 2.5 more grams per week. Even after covarying for marijuana use, the early-onset group still demonstrated impaired performance, particularly on WCST perseverations, compared with the late-onset group, suggesting that age at marijuana onset uniquely contributes to executive function deficits beyond the impact of increased frequency and magnitude of use. Univariate regression analyses also indicated that patterns of marijuana use may be able to predict cognitive impairment. Specifically, earlier age at marijuana onset, increased frequency of use, and higher magnitude of use were all associated with increased impairment on measures of executive functioning. Further, logistic regression analyses indicated that increased perseverative errors on the WCST was a significant predictor of marijuana group membership, suggesting that poorer performance on this task may be a neuropsychological marker for chronic, heavy marijuana use.
These results confirm and extend previous research. Chronic, heavy marijuana smokers have previously demonstrated impaired performance on both the Stroop and WCST relative to control participants (Battisti et al., 2010; Fontes et al., 2011; Gruber et al., 2012b; Lane et al., 2007), and prior work has noted that early-onset marijuana smokers accounted for the between-group differences across multiple cognitive domains (Ehrenreich et al., 1999; Fontes et al., 2011; Gruber et al., 2012b; Pope et al., 2003). Those findings, combined with results from the current study, highlight the importance of examining age at onset of marijuana use. Our results suggest that the task performance of late-onset marijuana smokers is more comparable to that of healthy controls; therefore, inclusion of both early- and late-onset smokers into a single marijuana-using sample could significantly cloud analyses. For example, studies of marijuana smokers predominantly comprising individuals with late-onset marijuana use may fail to detect significant differences relative to healthy control samples. To address this issue, future studies should assess and document marijuana use variables, including age at onset, as well as frequency and magnitude of marijuana use.
In the present study, univariate regression analyses indicated that earlier age at marijuana onset, increased frequency of use, and higher magnitude of use each predicted poorer executive functioning. Our results complement previous research using both correlation and regression analyses to address the role of marijuana use variables. Earlier age at marijuana onset has previously been shown to correlate with impaired performance on the Stroop and WCST tasks (Battisti et al., 2010; Gruber et al., 2012b), whereas increased frequency and magnitude of marijuana use have been shown to be related to impairment in psychomotor speed, sustained attention, and cognitive inhibition (Lisdahl & Price, 2012), as well as verbal memory (Solowij et al., 2011) and executive function (Gruber et al., 2012b; Harvey et al., 2007). In addition, this study incorporated logistic regression techniques and demonstrated that increased total perseverative errors on the WCST is a moderately accurate predictor of marijuana smokers, suggesting that this may be a clinical characteristic of chronic, heavy marijuana use.
Limitations and future directions
Although study findings are compelling, several limitations must be noted. The current study includes data from chronic, heavy marijuana users, with most individuals reporting daily or near-daily use. Accordingly, results may not be generalizable to those who use marijuana less frequently. However, the increasing number of daily marijuana users within the United States was calculated to be greater than 8.1 million people in 2013 (SAMSHA, 2014) and is likely to continue to rise, given relaxed attitudes toward marijuana; therefore, research efforts must examine chronic, heavy marijuana smokers. This population is also most likely to suffer from the effects associated with regular, heavy use, as regular marijuana users who report use more than once one a week have been shown to exhibit impaired memory and executive function compared with less frequent marijuana users (Harvey et al., 2007).
In addition, the current study’s inclusion criteria required all subjects to refrain from using marijuana for 12 hours before completing study procedures; a similar time frame of abstinence has been used in other studies (Battisti et al., 2010; Gruber & Yurgelun Todd, 2005; Gruber et al., 2011, 2012a, 2012b, 2014; Harvey et al., 2007; Solowij et al., 2011). This approach was designed to prevent impairment resulting from acute intoxication. Further, a longer period of marijuana abstinence was not utilized because studies using longer periods of abstinence (e.g. 7 days of abstinence [Lisdahl & Price, 2012] and 28 days of abstinence [Pope et al., 2003]) are more likely assessing cognitive function related to cessation of marijuana use. Instead, the 12-hour abstinence period was implemented to accurately reflect the impact of regular use on cognitive function in a naturalistic sample of current marijuana smokers. In the current study, a negative urine assay could not be used to ensure abstinence, given the brevity of the abstinence period; instead, all participants were led to believe that marijuana use within the last 12 hours could be detected from their urine samples. We therefore cannot disregard the possibility that some participants may have used marijuana within 12 hours of their study visit. Any participant who appeared vaguely intoxicated or reported use within this time frame was rescheduled for a later date. In addition, given the length of study visit, participants’ ability to perform all tasks, and participants’ appearance and demeanor, it is reasonable to conclude that no participants were acutely intoxicated during study procedures.
Furthermore, because the current investigation used a cross-sectional design, it is not possible to determine whether the reduced cognitive performance noted in the early-onset group preceded or is a result of early exposure to marijuana. Work by Squeglia et al. (2014) suggests that compromised inhibitory function during early adolescence before the onset of substance use is related to more frequent and intense use by later adolescence and could therefore be used to help identify those at risk for initiating substance use. Longitudinal studies designed to assess individuals before marijuana use, which then continue to follow subjects over time, are needed to fully address this issue.
Last, it is important to note that issues of collinearity complicate precise interpretation of the data. Although findings suggest that early-onset marijuana use is associated with poorer executive function, individuals in the early-onset marijuana group also reported significantly higher frequency and magnitude of marijuana use relative to late-onset smokers. However, even after covarying for marijuana use, the early-onset group still had relatively impaired executive function compared with the late-onset group. Results from the ANCOVA analyses suggest that although current frequency and magnitude of marijuana use contributes to between-group differences in executive function, these variables cannot account for all of the variance between early- and late-onset smokers. To more fully address the contribution of each of these marijuana use variables, high-powered statistical techniques (e.g., multivariate modeling analyses) should be used in future, larger scale investigations. Such techniques require far larger sample sizes than the current study provides.
Conclusions
Although a growing number of states have enacted legislation to legalize medical and recreational marijuana, mounting evidence suggests that early exposure to the drug is associated with reduced cognitive performance and increased frequency and magnitude of marijuana use in emerging adults. Although frequency and magnitude of marijuana use were predictive of impaired executive function, covariate analyses demonstrated that the differences noted between those with early-onset (regular use before age 16) versus late-onset marijuana use (regular use at age 16 or older) persist, even when increased use is controlled for. These results provide further support for the notion that earlier age at onset of marijuana use is related to poorer cognitive function relative to those who begin to use marijuana later, and suggest that earlier use may be inextricably linked to higher frequency and magnitude of marijuana use. Further, data support the use of neurocognitive assessments (particularly the WCST) to identify potential risk factors for marijuana use. Reduced inhibitory function may ultimately be used as a screening tool for those most likely to initiate substance use and could be used as a target for intervention or treatment strategies. Taken together, the current study findings underscore the need to implement early identification and intervention strategies concerning marijuana use among our nation’s youth, which is likely to have a significant impact on public policy.
Footnotes
Support for this project was provided by National Institute on Drug Abuse grants 5R21-DA021241 and 1R01-DA032646 (awarded to Staci A. Gruber). The authors have no conflicts of interest.
References
- Battisti R. A., Roodenrys S., Johnstone S. J., Pesa N., Hermens D. F., Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. 2010;212:613–624. doi: 10.1007/s00213-010-1988-3. doi:10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Beck A. T., Ward C. H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. doi:10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berg E. A. A simple objective technique for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. doi:10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Crane N. A., Schuster R. M., Fusar-Poli P., Gonzalez R. Effects of cannabis on neurocognitive functioning: Recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review. 2013;23:117–137. doi: 10.1007/s11065-012-9222-1. doi:10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean R. D., Crane N. A., Mason B. J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. doi:10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H., Rinn T., Kunert H. J., Moeller M. R., Poser W., Schilling L., Hoehe M. R. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. doi:10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams B. W. Structured clinical interview for axis I DSM IV disorder, patient edition (SCID-I/P). Version 2.0. New York, NY: Biometric Research Department, NY State Psychiatric Institute; 1994. [Google Scholar]
- Fontes M. A., Bolla K. I., Cunha P. J., Almeida P. P., Jungerman F., Laranjeira R. R., Lacerda A. L. T. Cannabis use before age 15 and subsequent executive functioning. British Journal of Psychiatry. 2011;198:442–447. doi: 10.1192/bjp.bp.110.077479. doi:10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Gruber S. A., Dahlgren M. K., Sagar K. A., Göneng A., Lukas S. E. Worth the wait: Effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2014;231:1455–1465. doi: 10.1007/s00213-013-3326-z. doi:10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S. A., Dahlgren M. K., Sagar K. A., Gönenc A., Killgore W. D. S. Age of onset of marijuana use impacts inhibitory processing. Neuroscience Leiters. 2012a;511:89–94. doi: 10.1016/j.neulet.2012.01.039. doi:10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S. A., Sagar K. A., Dahlgren M. K., Racine M., Lukas S. E. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012b;26:496–506. doi: 10.1037/a0026269. doi:10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S. A., Silveri M. M., Dahlgren M. K., Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology. 2011;19:231–242. doi: 10.1037/a0023034. doi:10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S. A., Yurgelun-Todd D. A. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. doi:10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. doi:10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Harvey M. A., Sellman J. D., Porter R. J., Frampton C. M. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26:309–319. doi: 10.1080/09595230701247772. doi:10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Johnston L. D., O’Malley P. M., Miech R. A., Bachman J. G., Schulenberg J. E. Monitoring the Future National Results on Drug Use: 1975-2014: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- Kempel P., Lampe K., Parnefjord R., Hennig J., Kunert H. J. Auditory-evoked potentials and selective attention: Different ways of information processing in cannabis users and controls. Neuropsychobiology, 48. 2003:95–101. doi: 10.1159/000072884. doi:10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- Lane S. D., Cherek D. R., Tcheremissine O. V, Steinberg J. L., Sharon J. L. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addictive Behaviors, 32. 2007:977–990. doi: 10.1016/j.addbeh.2006.07.007. doi:10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lezak M. D., Howieson D. B., Loring D. W. Neuropsychological assessment. 4th ed. Oxford, England: Oxford University Press; 2004. [Google Scholar]
- Lisdahl K. M., Gilbart E. R., Wright N. E., Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in Psychiatry, 4. 2013:53. doi: 10.3389/fpsyt.2013.00053. doi:10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K. M., Price J. S. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society, 18. 2012:678–688. doi: 10.1017/S1355617712000276. doi:10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C. M. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin, 109. 1991:163–203. doi: 10.1037/0033-2909.109.2.163. doi:10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- McNair D., Lorr M., Droppleman L. Manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Pope H. G., Jr, Gruber A. J., Hudson J. I., Cohane G., Huestis M. A., Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence, 69. 2003:303–310. doi: 10.1016/s0376-8716(02)00334-4. doi:10.1016/S0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B., Leo G. I., Cancilla A. Reliability of a Timeline Method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction, 83. 1988:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. doi:10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Solowij N., Jones K. A., Rozman M. E., Davis S. M., Ciarrochi J., Heaven P. C. L., Yücel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology, 216. 2011:131–144. doi: 10.1007/s00213-011-2203-x. doi:10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Squeglia L. M., Jacobus J., Nguyen-Louie T. T., Tapert S. F. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology, 28. 2014:782–790. doi: 10.1037/neu0000083. doi:10.1037/neu0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D. T., Benson D. F., Weir W. S., Naeser M. A., Lieberman I., Ferrill D. The involvement of orbitofrontal cerebrum in cognitive tasks. Neuropsychologia, 21. 1983:235–248. doi: 10.1016/0028-3932(83)90040-4. doi:10.1016/0028-3932(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary ofNational Findings. Rockville, MD: Author; 2014. (NSDUH Series H-48, HHS Publication No. SMA 14-4863) [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale – Revised. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]