Abstract
Objective:
This study examined the acquisition of conditioning between novel stimuli and single doses of alcohol in social drinkers. Environmental stimuli present during the consumption of alcohol or other drugs come to elicit conditioned responses that subsequently increase drug seeking. However, relatively few studies have examined the process of acquisition of these conditioned drug responses in human subjects.
Method:
We used a procedure previously developed to study acquisition of conditioned responses to a methamphetamine-associated cue. In the present study we applied the paradigm to alcohol, pairing de novo neutral cues with alcohol in social drinkers (N = 36). We obtained measures of self-report, behavioral preference, emotional reactivity (assessed using facial electromyography), and attention to specific cues paired with administration of 0.6 g/kg 95% absolute alcohol or placebo.
Results:
After conditioning, participants showed an increase in attention toward the alcohol-paired cue, and this increase was associated with ratings of liking the alcohol-containing beverage during the conditioning sessions. In contrast to our previous findings with methamphetamine, the alcohol-paired cue did not elicit changes in emotional reactivity (measured by facial electromyography) or behavioral preference.
Conclusions:
This study extends our previous findings with a stimulant drug to alcohol and highlights possible similarities and differences in conditioning with different classes of drugs. Conditioning with alcohol was less robust than with methamphetamine, but in both cases the conditioning that did occur was related to positive subjective drug response.
Drug-associated cues are thought to play a crucial role in the acquisition of, maintenance of, and relapse to substance use disorders (Wikler, 1965). The formation of associations between cues and drugs is largely based on the premise of classical conditioning, during which initially neutral cues that are repeatedly paired with drugs acquire conditioned incentive properties (Robinson & Berridge, 1993; Stewart et al., 1984). This idea has been widely supported by findings with preclinical animal models demonstrating both the acquisition and expression of classically conditioned responses (Di Ciano & Everitt, 2004; Weiss, 2005). Studies with humans, however, have focused primarily on the expression of responses to generic drug cues in established users (e.g., Carter & Tiffany, 1999; Drobes & Tiffany, 1997; Robbins et al., 1997), with little information regarding the acquisition of these associations. As such, we have scarce information regarding the formation of these associations in humans, including the role of subjective drug effects, how quickly the associations form, and which specific response modalities become conditioned.
Preclinical studies with animal models have shown that cues associated with drug administration can come to elicit conditioned responding and drug-seeking behavior (Crombag et al., 2008; Shaham et al., 2003). Drug-associated cues can provoke drug-seeking behaviors in the absence of the drug and even after prolonged periods of abstinence (Grimm et al., 2001). Interestingly, animals vary in their responses to conditioned drug cues in ways that suggest variation in vulnerability to develop dependence-like behaviors (Flagel et al., 2008, 2009). Although these animal models have given us insight regarding both the acquisition and expression of conditioned responses to drug-associated cues, systematic, empirical evidence of drug conditioning in humans is sparse.
To date, most research on drug cue reactivity in humans comes from studies with substance-dependent populations (e.g., Robbins et al., 1997; Volkow et al., 2006). In these studies, smokers, drinkers, or other drug users view generic drug cues, such as pictures of a pack of cigarettes, a beer bottle, or drug paraphernalia. Responses to the cues are measured using self-report, behavioral, physiological, or imaging techniques. Of note, the magnitude of response to drug cues among abstinent drug users can predict real-world outcomes. For example, in alcohol-dependent patients attempting to abstain from alcohol use, those who exhibit a greater bias in attention toward alcohol cues relapse faster than those with a lesser attentional bias to the cues (Garland et al., 2012), and similar findings have been reported with smokers (Waters et al., 2003) and cocaine users (Carpenter et al., 2006). However, the acquisition of these associations took place outside the laboratory and without experimental control, and thus the underlying mechanisms remain unknown. Although many theories of drug abuse and dependence highlight the role of conditioned drug cues in continued substance use (Kalivas & Volkow, 2005; Robinson & Berridge, 1993), responses to these generic drug-related stimuli may be a result of factors other than purely Pavlovian conditioning processes (e.g., signaling of drug availability, expectancies, other memory processes; Schuster & Johanson, 1988). As such, understanding the acquisition of drug-cue associations, and the variation in this process, will help to predict progression to excessive drug or alcohol use as well as relapse.
We recently developed a conditioning paradigm showing that healthy, nondependent humans acquire associations between a stimulant drug (methamphetamine; 20 mg) and a contextual, non–drug-related cue (i.e., picture of an ocean or mountain scene) present during the drug-taking experience (Mayo & de Wit, 2015; Mayo et al., 2013). Within this paradigm, we obtained multiple potential indicators of conditioning to determine variation within and across conditioning modalities. One such measure was behavioral preference for the cue, obtained using a novel task assessing preference for the drug-paired cue (Mayo et al., 2013, see Method, below). Positive and negative affective responses to the cue were measured using facial electromyography (EMG), a method used previously in drug cue studies with smokers (Geier et al., 2000; Waters et al., 2003; Winkler et al., 2001) and drinkers (Mason et al., 2009). Given the rich literature on attentional bias toward drug cues, we also included a measure of attention toward the conditioned cue. Finally, we asked participants to provide ratings of cue “liking” to compare these self-reported outcomes to physiological and behavioral measurements.
Following conditioning with this paradigm, the methamphetamine-paired cue came to elicit a range of conditioned responses, including enhanced behavioral preference, increased positive emotional reactivity (as assessed by facial EMG of the corrugator and zygomatic muscles), and an increase in attention. Participants did not change their self-reported ratings of the cues after conditioning. Interestingly, conditioned responding varied such that those reporting greater positive subjective drug effects (i.e., liking of drug effects) during the conditioning sessions demonstrated the greatest increase in attention toward the methamphetamine cue following conditioning. Given these results, we wanted to extend our findings to conditioning with another common drug of abuse: alcohol. Here, we sought to determine if (a) conditioning would occur with a moderate dose of alcohol and (b) whether individuals who report more positive subjective effects from alcohol during conditioning also develop the most robust conditioned responses.
Method
Overall design
This study was designed to investigate the acquisition of conditioned responses to a novel cue paired with alcohol (or placebo) in social drinkers. Participants underwent a conditioning procedure in which one audiovisual stimulus (cue) was paired with alcohol (ALC; 0.6 g/kg 95% absolute alcohol in juice), and a different cue was paired with a placebo (PBO; 1% absolute alcohol with juice). We measured behavioral preference, self-report ratings, psychophysiological indices of emotional states, and attention for both cues before and after conditioning to assess changes as a function of conditioning (see Mayo & de Wit, 2015). The University of Chicago Biological Science Division Institutional Review Board approved the study.
Participants
Social drinkers (N = 36; Table 1) ages 21–35 years were recruited from the community and screened with a psychiatric interview, electrocardiogram, and physical examination. Inclusion criteria were body mass index of 19 kg/m2–26 kg/m2, high school education, fluency in English, resting blood pressure less than140/90 mmHg, resting heart rate less than 80 beats per minute, and consumption of fewer than four caffeinated drinks per day. Qualifying participants were those who consumed 5–28 standard alcohol drinks per week, with at least 3 or more drinks on one occasion in the past month. Exclusion criteria were current substance use disorder or lifetime substance dependence; regular medication; history of cardiovascular illness; current Axis I disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994); or mood disorder or psychotic symptoms within the past year, as determined by the psychiatric symptoms checklist (Derogatis, 1983). Pregnant or nursing mothers were excluded from participation.
Table 1.
Participant characteristics (N = 36)
| Variable | n |
| Gender | |
| Male | 18 |
| Female | 18 |
|
% (n) or M (SE) |
|
| Race | |
| White | 78% (25) |
| Black/African American | 16% (5) |
| Asian | 6% (2) |
| Other | 13% (4) |
| Age, in years | 25.3 (0.7) |
| Education, in years | 15.7 (0.3) |
| Body mass index, kg/m2 | 22.3 (0.3) |
| Current drug use, within past month | |
| Caffeinated drinks per day | 1.8 (1.6) |
| Cigarettes per week | 2.0 (1.5)a |
| Marijuana use per month | 4.9 (4.3)b |
| Current alcohol use, within past month | |
| Total drinks | 25.3 (4.2) |
| Drinks per week | 11.0 (1.1) |
| Drinking days per week | 3.6 (0.3) |
| Average drinks per drinking day | 3.3 (0.2) |
| Max. amount of drinks on one occasion | 6.8 (0.4) |
| Number of heavy drinking episodes | 3.1 (0.5) |
| Lifetime drug use, ever used—nonmedical use only | |
| Marijuana | 89% (32) |
| Hallucinogens | 31% (10) |
| MDMA | 28% (9) |
| Stimulants | 25% (8) |
| Opiates | 13% (4) |
| Sedatives | 13% (4) |
Notes: “Current drug use” and “current alcohol use” indicate use within the past month; “lifetime drug use” refers to ever using that drug in one’s entire lifetime. Only recreational (i.e., nonmedical) drug use is represented. “Heavy drinking episodes” are defined as 5+ drinks on an occasion for men and 4+ drinks on an occasion for women. Max. = maximum; MDMA = 3,4-methylenedioxymethamphetamine (Ecstasy).
Indicates cigarettes per week for those who smoke (n = 5);
indicates number of marijuana uses per month for those who use marijuana (n = 17).
Alcohol administration
The 0.6 g/kg alcohol dose was prepared in a 12% solution by volume with 95% absolute alcohol and juice. Participants chose either orange or cranberry juice, which was then used for all four drink administration sessions. Placebo drinks included a 1% absolute alcohol taste mask. For men, beverages were prepared in a volume of 450 ml/70 kg and were divided into equal fourths (i.e., 112.5 ml/70 kg). Women received 85% of the dose of alcohol administered to men (i.e., 0.51 g/kg) to adjust for gender differences in body water composition (Frezza et al., 1990; King et al., 2011; Sutker et al., 1983). The drinks were administered over two drinking periods. In the first period, one drink was consumed within 5 minutes, followed by a 5-minute break, then another drink consumed within the following 5 minutes. Fifteen minutes later, subjects consumed the remaining two drinks in the same manner. Beverages were served chilled in clear, lidded cups and consumed through a drinking straw under doubleblind conditions.
Session procedures
Orientation session.
During an orientation session, qualifying participants were informed of study procedures and provided informed consent. They practiced the study tasks and selected their preferred juice (cranberry or orange juice). Participants were told they could receive a stimulant-like drug (e.g., caffeine), sedative-like drug (e.g., antihistamine), alcohol, or placebo at subsequent sessions. They were instructed to abstain from drugs for 48 hours (marijuana for 7 days) and alcohol for 24 hours, but to consume their normal amount of caffeine or nicotine before sessions. Compliance was assessed with breath-alcohol concentration (BrAC) levels (Alco-Sensor III, Intoximeters, St. Louis, MO), urine drug test (ToxCup, Branan Medical Corporation, Irvine, CA) and, for women, pregnancy test (AimStickPBD, hCG professional, Craig Medical Distribution, Vista, CA). Sessions took place in comfortably furnished rooms with a computer, television, and video player. When not completing study procedures, participants were allowed to relax, watch selected movies, or read. Participants were compensated for their participation.
Pretest session (Session 1).
At this 2-hour session, we assessed pre-conditioning responses toward the cues with the following outcome measures (see Conditioning Measures for detailed measure description); behavioral preference, ratings of self-reported cue “liking,” emotional reactivity–acquired EMG, and attention assessed via monitoring eye gaze using electrooculography. The cues consisted of an ocean or mountain background image on a computer screen (Figure 1), accompanied by appropriate sounds (waves crashing or birds chirping, respectively). Cues were presented with E-Prime 2.0 (PST Inc., Pittsburgh, PA). The order of the conditioning measures was randomized across subjects but consistent within subjects.
Figure 1.
Overall study design and timeline of conditioning sessions. 1A: Design of the study and cue + alcohol pairings. 1B: Breath alcohol concentration (BrAC) over the course of the first (ALC 1; dotted line) and second (ALC 2; solid line) alcohol sessions. Values are mean change scores from baseline (SE). Arrows indicate drink administration (total of four drinks). The gray shaded area indicates the 30-minute task period, during which the appropriate cue is presented. ALC = alcohol; PBO = placebo; min = minute.
Conditioning sessions (Sessions 2–5).
Four 4-hour conditioning sessions were conducted from 1300 to 1700 hours at least 24 hours apart. At each session, participants first completed compliance tests, pre-drug mood ratings, and cardiovascular measures (blood pressure and heart rate), and then consumed the drinks as described above. Fifteen minutes later, they performed three simple computer tasks for 30 minutes total while the cues (ocean or mountain) were presented as background screens behind a smaller central panel presenting the tasks. One background screen (ocean or mountain) was always present during both ALC sessions, whereas the other (mountain or ocean; whichever was not paired with ALC) was present during both PBO sessions, each with corresponding sounds (waves crashing or birds chirping). Tasks were displayed using Presentation software (Neurobehavioral Systems, Inc., Berkeley, CA). Sessions always alternated between ALC and PBO, but the order of session (ALC or PBO first) and cues (ALC-paired: ocean or mountain) were counterbalanced across subjects. Participants also completed standardized mood and drug effect questionnaires, cardiovascular measures (blood pressure, heart rate), and a BrAC reading 5 minutes before and 25, 55, 95, 140, 180, 220 minutes after administration of the first drink. Participants were released at 1700 h if their BrAC was less than .04% and they could pass a field sobriety test.
The aforementioned computer tasks, completed during cue exposure, were included to ensure that subjects attended to the cue on the screen and ensure uniform exposure to the cues. The tasks included a monetary incentive delay task (Knutson et al., 2000, 2001), a prediction error task (Mayo et al., 2013), and a risk-taking task (Lane & Cherek, 2000; as modified in Gilman et al., 2012). Each task lasted approximately 8–10 minutes, for a total task completion time of approximately 30 minutes. To ensure motivation to complete the computer tasks, participants were told they could earn money based on their performance. Each participant earned approximately $25 at each session, and earnings did not differ between ALC and PBO sessions. For detailed information regarding these tasks, see Mayo et al. (2013).
Posttest session (Session 6).
A posttest session was conducted at least 24 hours after the last conditioning session, at the same time of day as the pretest. The procedure was identical to the pretest and provided the primary measures of change in subjective, behavioral, and emotional responses to the cues from before to after conditioning.
Conditioning measures
All conditioning measures were completed at the pretest (Session 1) and posttest (Session 6).
Conditioning Task 1: Behavioral preference measure.
This measure assessed subjects’ preferences for the two study cues (Mayo & de Wit, 2015; Mayo et al., 2013). In this task, subjects viewed each cue (ocean or mountain) combined with images from each of the three computer tasks (from the conditioning sessions). In each trial, they first viewed two separate combinations of cue image and computer task image (i.e., ocean + task 1; mountain + task 1) presented on the screen individually. The pairs of images were then placed side by side, and subjects had to quickly indicate their preferred combination by pressing the corresponding mouse button. They viewed all combinations of cue and task images compared against one another (a total of 15 pairs of images) in a full-factorial design. We assessed preference for task images with the same cue to rule out biases for the tasks at baseline or after conditioning. No task bias was observed, so we collapsed the tasks for analysis. Trials in which the cue (ocean or mountain) was the same (i.e., ocean + task 1; ocean + task 2) gave us no information regarding cue preference and were therefore eliminated, leaving nine trials providing information about preference for the cues (i.e., ocean + task 2; mountain + task 3). The primary outcome measure was the change in number of ALC-paired cue selections (0–9) from before to after conditioning.
Conditioning Task 2: Subjective rating measure.
This measure was used to assess self-reported liking of the cues. In this task, each cue (cue image + sound) was presented individually with a prompt across the top of the image asking, “How much do you LIKE this image?” Participants were told to respond on a scale of 0 (not at all) to 100 (very much) by inputting their response using the keyboard.
Conditioning Task 3: Emotional reactivity measure.
Emotional reactivity was assessed by measuring corrugator and zygomatic reactivity in response to each cue (Drobes & Tiffany, 1997; Geier et al., 2000; Lang et al., 1993). For this task, each cue (with accompanying sound) was presented for 6 seconds, 10 times, in a randomized order. Presentations were separated by a variable intertrial interval (4–5 seconds) during which a fixation cross was presented on the screen. Responses were quantified as mean EMG activity in the corrugator and the zygomatic muscle (individually) during the 6-second presentation minus the mean EMG activity for the 1 second before the cue was presented. EMG was measured over left brow (corrugator) and cheek (zygomatic) with 4 mm Ag/AgCl electrodes and an 8 mm gel-filled Ag/AgCl ground sensor on the forehead. EMG signals were amplified, 10–500 Hz band pass filtered, digitized at 1,000 Hz, 60 Hz band stop filtered, rectified, and integrated over 20 ms using EMG100C amplifiers, and MP150 Data Acquisition System and AcqKnowledge software from Biopac Systems Inc. (Goleta, CA).
Conditioning Task 4: Attention measure.
The attention measure consisted of a modified visual probe task using the two visual cues (i.e., Mayo & de Wit, 2015; Wardle et al., 2012). During this task, each trial began with a 1-second fixation cross, followed by presentation of the two study cues on the right and left of the screen, for 2 seconds. Both cues then disappeared and were replaced by gray rectangles of the same size, one of which contained a white circle or square visual probe. Subjects were instructed to classify the probe as a circle or square as quickly as possible by pressing a key. After a response, or 10 seconds with no response, a variable intertrial interval (750–1,250 ms) began. Probe shape, location, and cue location were counterbalanced across trials. The 40 trials were presented in random, counterbalanced order.
The outcome of interest was relative attention toward the ALC-paired cue, quantified in two ways: (a) initial orienting attention, or the direction of the first gaze when the cues appeared and (b) sustained attention, calculated as the average amount of dwell time or “sustained attention” directed toward each cue during a 2-second presentation time. Gaze was quantified using electrooculography with 4 mm Ag/AgCl electrodes attached 1.5 cm from the outer canthus of each eye, and data were treated similar to EMG data. Trained raters discarded trials in which gaze was not centrally fixated before the trial, initial fixation was <100 ms after picture onset (reflecting anticipatory eye movements), or noise obscured eye movements.
Subjective measures of alcohol effects
Subjective effects of the drug were assessed during conditioning sessions (Sessions 2-5) using two self-report measures: the Drug Effects Questionnaire (DEQ; Johanson & Uhlenhuth, 1980; Morean et al., 2013) and the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993). The DEQ contains ratings of “feel” drug effects, “like” effects, “dislike” effects, feel “high,” and “want more” drug. The BAES measures stimulant- and sedative-like effects of alcohol. The stimulant-like effects subscale is the composite of the following items: Elated, Energized, Excited, Stimulated, Talkative, Up, and Vigorous. The sedative-like subscale includes [difficulty to] Concentrate, Down, Inactive, Sedated, Slow Thoughts, Heavy Head, and Sluggish, each rated on an 11-point scale from not at all (0) to extremely (10).
Physiological measures of alcohol effects
BrAC readings (Alco-Sensor III; Intoximeters, St. Louis, MO) were obtained during the conditioning sessions (Sessions 2–5) at regular intervals. Heart rate and blood pressure were measured at the same intervals using a portable monitor (LifeSource Model UA-787).
Statistical analysis
Direct effects of alcohol.
The subjective and physiological effects of alcohol during the conditioning sessions were assessed using a repeated-measures analysis of variance (RM-ANOVA), with time (baseline, 6 time points after drink administration) and treatment (ALC, PBO) as within-subjects factors. Differences at individual time points were evaluated using Bonferroni’s post hoc testing. Similar RM-ANOVA testing with time and session (first vs. second session) was used to test for differences between the two ALC sessions, as well as the two PBO sessions.
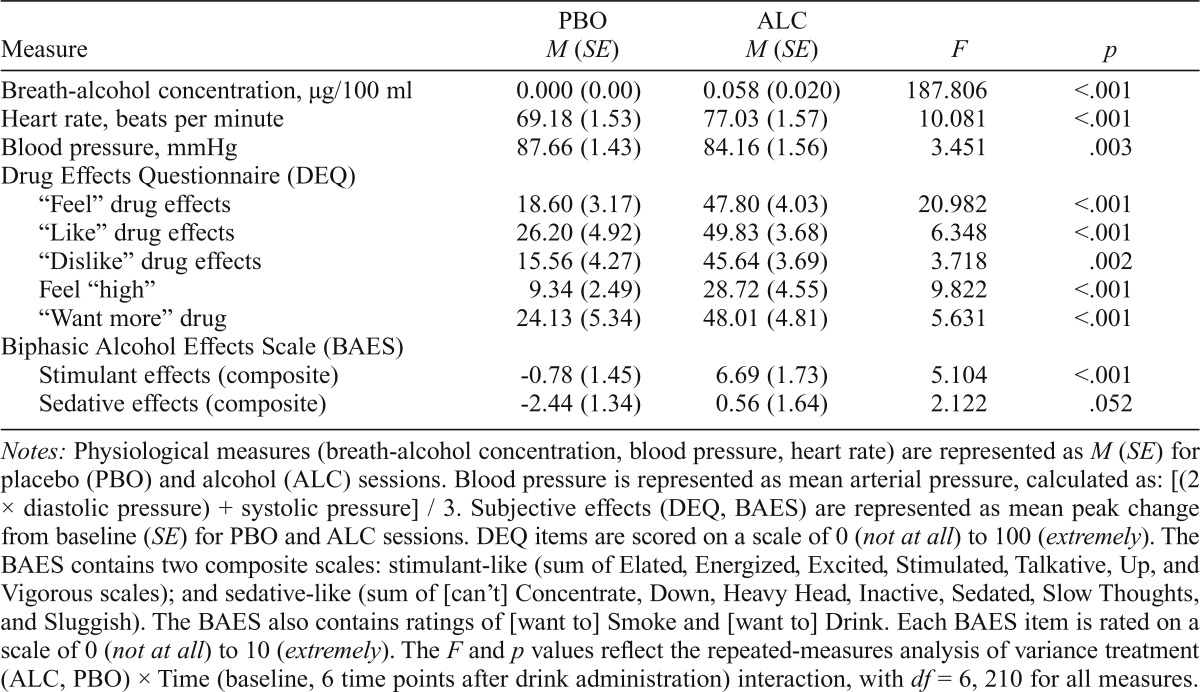
To explore correlations among subjective drug effects and conditioning measures, peak change scores from baseline for PBO and ALC sessions (average of two PBO sessions, two ALC sessions) were also calculated (Table 2).
Table 2.
Effects of alcohol
| Measure | PBO M (SE) | ALC M (SE) | F | P |
| Breath-alcohol concentration, µg/100 ml | 0.000 (0.00) | 0.058 (0.020) | 187.806 | <.001 |
| Heart rate, beats per minute | 69.18 (1.53) | 77.03 (1.57) | 10.081 | <.001 |
| Blood pressure, mmHg | 87.66 (1.43) | 84.16 (1.56) | 3.451 | .003 |
| Drug Effects Questionnaire (DEQ) | ||||
| “Feel” drug effects | 18.60 (3.17) | 47.80 (4.03) | 20.982 | <.001 |
| “Like” drug effects | 26.20 (4.92) | 49.83 (3.68) | 6.348 | <.001 |
| “Dislike” drug effects | 15.56 (4.27) | 45.64 (3.69) | 3.718 | .002 |
| Feel “high” | 9.34 (2.49) | 28.72 (4.55) | 9.822 | <.001 |
| “Want more” drug | 24.13 (5.34) | 48.01 (4.81) | 5.631 | <.001 |
| Biphasic Alcohol Effects Scale (BAES) | ||||
| Stimulant effects (composite) | -0.78 (1.45) | 6.69 (1.73) | 5.104 | <.001 |
| Sedative effects (composite) | -2.44 (1.34) | 0.56 (1.64) | 2.122 | .052 |
Notes: Physiological measures (breath-alcohol concentration, blood pressure, heart rate) are represented as M (SE) for placebo (PBO) and alcohol (ALC) sessions. Blood pressure is represented as mean arterial pressure, calculated as: [(2 × diastolic pressure) + systolic pressure] / 3. Subjective effects (DEQ, BAES) are represented as mean peak change from baseline (SE) for PBO and ALC sessions. DEQ items are scored on a scale of 0 (not at all) to 100 (extremely). The BAES contains two composite scales: stimulant-like (sum of Elated, Energized, Excited, Stimulated, Talkative, Up, and Vigorous scales); and sedative-like (sum of [can’t] Concentrate, Down, Heavy Head, Inactive, Sedated, Slow Thoughts, and Sluggish). The BAES also contains ratings of [want to] Smoke and [want to] Drink. Each BAES item is rated on a scale of 0 (not at all) to 10 (extremely). The F and p values reflect the repeated-measures analysis of variance treatment (ALC, PBO) × Time (baseline, 6 time points after drink administration) interaction, with df= 6, 210 for all measures.
Effects of conditioning.
The primary outcome measures were the change in response to the study cues from before to after conditioning on the measures of behavioral preference, subjective liking, emotional reactivity, and attention. Behavioral preference was analyzed as the change in the number of ALC-paired cue selections from before to after conditioning, using a paired t test. Subjective liking, emotional reactivity (corrugator reactivity, zygomatic reactivity), and attention (initial attention, sustained attention) were analyzed using RM-ANOVA with phase (pre- and post-conditioning) and cue (ALC-, PBO-paired) as within-subjects factors using IBM SPSS Statistics 23 (Armonk, NY).
Results
Direct effects of alcohol during conditioning sessions
BrACs reached the expected level (M = .058, SE = .020); effect of alcohol F(1, 35) = 333.518, p < .0001, ηp2 = .907. There was no difference in BrACs between the first and second alcohol sessions, F(1, 35) = 0.090,p = .766, ηp2 = .003. Post hoc testing revealed that BrAC was significantly elevated at the time of cue presentation (Figure 1). Although blood pressure decreased throughout all sessions, this effect was greater following administration of alcohol, Treatment × Time interaction: F(6,210) = 3.451,p = .003, ηp2 = .090). Heart rate was increased by alcohol administration, Treatment × Time: F(6, 210) = 10.081,p < .0001, ηp2 = .224. Follow-up post hoc tests determined that both blood pressure and heart rate were affected by alcohol during the period of cue presentation.
Alcohol increased DEQ ratings of “feel” drug effects, “like” drug effects, “dislike” drug effects, feel “high,” and “want more,” and it increased BAES stimulant-like scale and marginally decreased BAES sedation scale (Table 2). Post hoc analysis revealed that these effects peaked during the time of cue presentation. Overall, there was no relationship between subjective response to alcohol and drinking habits. However, we did find that participants who reported consuming more drinks per week also reported greater ratings of DEQ “want more” drug (Pearson’s r = .463; p = .004).
Conditioning measures
Behavioral preference for the ALC-associated cue did not change from pretest session (mean preference = 5.06, SE = 0.37) to posttest session (mean preference = 4.86, SE = 0.37); t(35) = 0.53, p = 0.60, Cohen’s d = 0.177. Conditioning with alcohol did not increase self-reported ratings of the cues. Subjects rated “liking” of the ALC cue lower overall, but this occurred with both pre- and post-measures and was not influenced by the conditioning procedure, main effect of cue: F(1, 35) = 5.10, p = .03, ηp2 = .127.
For the emotional reactivity analysis, one participant was eliminated because of equipment malfunction, resulting in n = 35. Conditioning sessions did not significantly affect corrugator reactivity, Cue x Phase: F(1, 34) = 1.709, p = .200, ηp2 = .48 (Figure 2). Overall, conditioning sessions produced no change in zygomatic reactivity to the cues, Cue × Phase interaction: F(1, 34) = 0.155, p = 0.696, ηp2 = .005 (Figure 2). However, further analysis of this measure showed that women exhibited enhanced zygomatic reactivity in response to the ALC cue after conditioning, whereas men showed decreased reactivity, Cue × Phase × Sex: F(1, 34) = 8.72, p = .006, ηp2 = .209. There were no sex differences in corrugator reactivity or any other conditioning measures.
Figure 2.
Responses to alcohol-(ALC) and placebo-(PBO) paired cues before and after conditioning. Following conditioning, the ALC-paired cue elicited a bias in initial orienting but did not elicit change in emotional reactivity or sustained attention. 2A: Corrugator reactivity (mean activation ± SE) in response to the ALC and PBO cues did not change following conditioning. 2B: Zygomatic reactivity (mean activation ± SE) in response to the ALC and PBO cues was not affected by conditioning. 2C: Initial attention (number of first gazes ± SE) toward the ALC-paired cue were increased after conditioning. 2D: Sustained attention (mean dwell time ± SE) toward the ALC and PBO cues was not significantly changed following conditioning. **p <.01.
On the attention task, only data with valid gazes in at least 50% of trials were included in the analysis, which excluded 7 participants (n = 29). Sustained attention toward the ALC-paired cue was not affected by the conditioning paradigm, Cue × Time: F(1, 28) = 2.038, p = .164, ηp2 = .068 (Figure 2). However, there was a significant increase in initial orienting, or the number of first gazes, toward the ALC-paired cue after conditioning, Cue × Phase: F(1, 28) = 7.238, p = .012, ηp2 = .205 (Figure 2). This change in initial orienting was correlated with subjects’ ratings of response to the alcohol during the conditioning sessions: Participants who reported greater ratings of “like” drug effects on the DEQ (calculated as [mean change from baseline at ALC sessions] – [mean change from baseline at PBO sessions]) showed the greatest increase in initial gazes toward the ALC cue (Pearson’s r = .373; p = .042; Figure 3). There was no relationship between habitual drinking patterns and any measure of conditioning.
Figure 3.
Subjective response to alcohol predicts responses to an alcohol-paired cue. Subjects demonstrating greater ratings of liking alcohol effects (Drug Effects Questionnaire [DEQ] “Like drug effects” mean peak change score ± SE) also demonstrated the greatest increase in initial gazes toward the alcohol-paired cue after conditioning (Pearson’s r = .373, p = .042).
Discussion
The results presented here demonstrate early acquisition of conditioned responding after two administrations of alcohol and placebo, each paired with distinctive cues, in nondependent drinkers. Following conditioning, the cue paired with alcohol elicited increased attention, as measured by initial orienting responses. Furthermore, this increase in attention was associated with positive subjective responses to alcohol. However, conditioning was not detected with outcome measures of behavioral preference or emotional reactivity (as measured by zygomatic or corrugator reactivity). These results contrast with our previous findings with methamphetamine (Mayo & de Wit, 2015), in which conditioning produced responses according to measures of behavioral preference, emotional reactivity, and attention. Thus, at this dose of alcohol and in this sample of participants, conditioning with alcohol was less robust than with a stimulant drug.
Our most notable finding is that two pairings of ALC and the cue increased participants’ attention toward the ALC-associated cue, as measured by initial gazes toward the ALC cue at posttest. This finding is consistent with our previous study with methamphetamine (Mayo & de Wit, 2015) and with a previous study of alcohol conditioning with social drinkers wherein a de novo cue came to elicit enhanced gazes (Field & Duka, 2002). Notably, Field and Duka (2002) demonstrated that a cue paired with an even lower dose of alcohol than that used here (0.2 g/kg of 90% absolute alcohol) increased attention, without increasing other conditioned responses (i.e., self-report measures). Taken together, these findings suggest that attention toward conditioned drug-related cues is a highly sensitive and reliable indicator of drug conditioning.
We also saw a relationship between subjective drug response and conditioned attention. That is, individuals who reported “liking” the effects of alcohol most also showed the greatest increases in initial orienting toward the ALC cue following conditioning. This finding is similar to our methamphetamine study, in which positive subjective responses to methamphetamine predicted increases in (sustained) attention (Mayo & de Wit, 2015). These findings are intuitively logical, suggesting that the extent of conditioned attention is an indicator of the reward value of the drug to the individual. Clinically, attentional bias to drug cues in dependent populations predicts time to relapse in both alcohol- and stimulant-dependent populations (Carpenter et al., 2006; Garland et al., 2012). Whether the attentional bias to drug cues is also related to the reward value of the drug in experienced users has yet to be determined. Furthermore, it not explicitly clear how this measure of conditioned attention compares to reports of attentional biases toward drug cues in established drug users (i.e., whether these are related or distinct phenomena). As such, caution should be taken when comparing this study to those assessing attentional bias in dependent populations.
In the present study, conditioning was evident on the measure of attention but did not have an effect on initial measures of behavioral preference, emotional reactivity, or self-reported cue “liking.” This is in contrast to our previous study with methamphetamine, in which we observed conditioning with measures of both emotional reactivity and behavioral preference. There are several possible reasons for the different findings. First, the participants’ experience with the study drugs differed across the studies. Only 30% of participants in the methamphetamine study had ever tried a stimulant drug, whereas in the present study, all the participants were required to have regular exposure to alcohol. This being the case, our findings would suggest that conditioning may be more robust during initial drug use experiences (e.g., during our methamphetamine study for non–stimulant using participants) than experiences with an often-used substance (e.g., ALC in the present study sample). Furthermore, participants in the current study likely had exposure to alcohol outside the laboratory during their participation in the study. This exposure to alcohol (the unconditioned stimulus in this conditioning paradigm) in the absence of our cues (or conditioned stimulus) could prevent effective conditioning within this paradigm. Regular drinking experiences outside the laboratory may be accompanied by occasion setters that facilitate conditioning in these real-world settings but were absent in our laboratory conditions (Holland, 1983).
Another limitation of this study was the dose of alcohol used, as this moderate dose of alcohol (0.6 g/kg) may have been too low to produce robust conditioning, especially given the heterogeneity in drinking patterns within our sample (M = 11.1 drinks per week; SD = 6.3). The dose used here produces modest subjective effects (Holdstock & de Wit, 1998; Table 2) but fails to produce consistent stimulant-like effects that are the hallmark of alcohol reward (King et al., 2011). Interestingly, this same dose (0.6 g/kg) did sustain conditioning using measures of skin conductance and cardiac inter-beat interval (Glautier et al., 1994), and as noted above, a dose of 0.2 g/kg led to an increase in attention (Field & Duka, 2002). However, Field and Duka (2002) also failed to see changes in other measures (i.e., self-report). Thus, the extent of conditioning may depend not only on the dose of drug and the modality of the outcome measure, but also on other factors such as cue salience, temporal spacing of cue and drug, and number of pairings. For instance, we may have seen greater conditioning had our conditioned stimulus predicted the delivery of drug, as opposed to beginning after the onset of the alcohol effects. The impact of these factors and others are elegantly discussed by Rescorla (1988), who appropriately describes the complex nature of conditioning, contrasting it with the “simple” process referred to by others.
Although our results differ from the previous study with methamphetamine (Mayo & de Wit, 2015), they highlight important differences between conditioning with stimulant drugs and alcohol. For example, it has been demonstrated that alcohol can impair perceptual and cognitive functioning (e.g., Tarter et al., 1971). Alcohol intoxication, coupled with the computer tasks participants completed while the cues were being presented during the conditioning sessions, may have impeded conditioning with our visual stimulus. This is supported by the effects of alcohol on attention allocation (Steele & Josephs, 1988). Conversely, methamphetamine, the drug used in the previous study, can enhance learning. Thus, the differences between this and the previous study may be attributable to the differential effects of the pharmacological substances used. Future studies varying the conditioning paradigm (e.g., the attentional load required by computer tasks at conditioning sessions), the drug used as the unconditioned stimulus, or the subject sample (i.e., sedative responders vs. stimulant responders; King et al., 2011) will help resolve some of these questions.
The findings reported here support the idea that nondependent drinkers develop conditioned associations between alcohol and environmental stimuli paired with the alcohol. Participants exhibited an increase in attention toward a stimulus previously paired with a moderate dose of alcohol. The findings extend our previous results (Mayo & de Wit, 2015; Mayo et al., 2013) using this classical conditioning procedure to the study of acquisition of conditioning with alcohol. The conditioned responses with alcohol were evident in fewer domains than the responses observed with methamphetamine, but it is possible that a higher dose of alcohol could lead to stronger conditioning across multiple measures. The results presented here provide an important translational link between preclinical studies, which demonstrate acquisition of drug conditioning, and reports of reactivity to drug-related cues in humans. Future studies investigating individual differences in acquisition and extinction of these cues will advance our ability to treat substance use disorders.
Acknowledgments
The authors are grateful to Francisco Meyer, Nicholas Ruiz, Lauren Walker, and Kristen Kitsch for their assistance in running study sessions, data entry, and data scoring. We also thank the University of Chicago Human Behavioral Pharmacology Lab staff for assisting in subject screening and recruitment.
Footnotes
This research was supported by National Institute on Drug Abuse (NIDA) Grant DA02812, and Leah M. Mayo is supported by NIDA Grant DA32015 (Diversity Supplement).
References
- American Psychiatric Association. Diagnostic and Statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Carpenter K. M., Schreiber E., Church S., McDowell D. Drug Stroop performance: Relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addictive Behaviors. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. doi:10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Carter B. L., Tiffany S. T. Meta-analysis of cue-re-activity in addiction research. Addiction. 1999;94:327–340. doi:10.1046/j.1360-0443.1999.9433273.x. [PubMed] [Google Scholar]
- Crombag H. S., Bossert J. M., Koya E., Shaham Y. Context-induced relapse to drug seeking: A review. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. doi:10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L. SCL-90-R: Manual-II. Towson, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- Di Ciano P., Everitt B. J. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: Implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Supplement 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. doi:10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Drobes D. J., Tiffany S. T. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. doi:10.1037/0021-843X.106.1.15. [DOI] [PubMed] [Google Scholar]
- Field M., Duka T. Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology. 2002;159:325–334. doi: 10.1007/s00213-001-0923-z. doi:10.1007/s00213-001-0923-z. [DOI] [PubMed] [Google Scholar]
- Flagel S. B., Akil H., Robinson T. E. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Supplement 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. doi:10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel S. B., Watson S. J., Akil H., Robinson T. E. Individual differences in the attribution of incentive salience to a reward-related cue: Influence on cocaine sensitization. Behavioural Brain Research. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. doi:10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza M., di Padova C., Pozzato G., Terpin M., Baraona E., Lieber C. S. High blood alcohol levels in women: The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. The New England Journal of Medicine. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. doi:10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Garland E. L., Franken I. H., Howard M. O. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology. 2012;222:17–26. doi: 10.1007/s00213-011-2618-4. doi:10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A., Pauli P., Mucha R. F. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. doi:10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gilman J. M., Smith A. R., Ramchandani V. A., Momenan R, Hommer D. W. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addiction Biology. 2012;17:465–478. doi: 10.1111/j.1369-1600.2011.00383.x. doi:10.1111/j.1369-1600.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glautier S., Drummond C., Remington B. Alcohol as an unconditioned stimulus in human classical conditioning. Psychopharmacology. 1994;116:360–368. doi: 10.1007/BF02245341. doi:10.1007/BF02245341. [DOI] [PubMed] [Google Scholar]
- Grimm J. W., Hope B. T., Wise R. A., Shaham Y. Neuroad-aptation: Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. doi:10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L., de Wit H. Individual differences in the biphasic effects of ethanol. Alcoholism: Clinical and Experimental Research. 1998;22:1903–1911. doi:10.1111/j.1530-0277.1998.tb05897.x. [PubMed] [Google Scholar]
- Holland P. C. “Occasion-setting” in Pavlovian feature positive discriminations. In: Commons M. L., Herrnstein R. J., Wagner A. R., editors. Quantitative analyses of behavior: Discrimination processes. Cambridge, MA: Ballinger; 1983. (pp. 9–32) [Google Scholar]
- Johanson C. E., Uhlenhuth E. H. Drug preference and mood in humans: d-amphetamine. Psychopharmacology. 1980;71:275–279. doi: 10.1007/BF00433062. doi:10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., Volkow N. D. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. doi:10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- King A. C., de Wit H., McNamara P. J., Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. doi:10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Adams C. M., Fong G. W., Hommer D.2001, August 15Anticipation of increasing monetary reward selectively recruits nucleus accumbens Journal of Neuroscience, 21(16) RC159. Retrieved from http://www.jneurosci.org/content/21/16/RC159.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. doi:10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Lane S. D., Cherek D. R. Analysis of risk taking in adults with a history of high risk behavior. Drug and Alcohol Dependence. 2000;60:179–187. doi: 10.1016/s0376-8716(99)00155-6. doi:10.1016/S0376-8716(99)00155-6. [DOI] [PubMed] [Google Scholar]
- Lang P. J., Greenwald M. K., Bradley M. M., Hamm A. O. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. doi:10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Martin C. S., Earleywine M., Musty R. E., Perrine M. W., Swift R. M. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. doi:10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mason B. J., Light J. M., Williams L. D., Drobes D. J. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: Effects of gabapentin. Addiction Biology. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. doi:10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L. M., de Wit H. Acquisition of responses to a metham-phetamine-associated cue in healthy humans: Self-report, behavioral, and psychophysiological measures. Neuropsychopharmacology. 2015;40:1734–1741. doi: 10.1038/npp.2015.21. doi:10.1038/npp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L. M., Fraser D., Childs E., Momenan R., Hommer D. W., de Wit H., Heilig M. Conditioned preference to a methamphet-amine-associated contextual cue in humans. Neuropsychopharmacology. 2013;38:921–929. doi: 10.1038/npp.2013.3. doi:10.1038/npp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean M. E., de Wit H., King A. C., Sofuoglu M., Rueger S. Y., O’Malley S. S. The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology. 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. doi:10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R. A. Pavlovian conditioning. It’s not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. doi:10.1037/0003-066X.43.3.151. [DOI] [PubMed] [Google Scholar]
- Robbins S. J., Ehrman R. N., Childress A. R., O’Brien C. P. Relationships among physiological and self-report responses produced by cocaine-related cues. Addictive Behaviors. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. doi:10.1016/S0306-4603(96)00007-X. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. doi:10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Schuster C. R., Johanson C. E. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacology Series. 1988;4:161–175. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Shalev U., Lu L., de Wit H., Stewart J. The rein-statement model of drug relapse: History, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. doi:10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Steele C. M., Josephs R. A. Drinking your troubles away. II: An attention-allocation model of alcohol’s effect on psychological stress. Journal of Abnormal Psychology. 1988;97:196–205. doi: 10.1037//0021-843x.97.2.196. doi:10.1037/0021-843X.97.2.196. [DOI] [PubMed] [Google Scholar]
- Stewart J., de Wit H., Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91:251–268. doi:10.1037/0033-295X.91.2.251. [PubMed] [Google Scholar]
- Sutker P. B., Tabakoff B., Goist K. C., Jr., Randall C. L. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacology, Biochemistry, and Behavior. 1983;18(Supplement 1):349–354. doi: 10.1016/0091-3057(83)90198-3. doi:10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- Tarter R. E., Jones B. M., Simpson C. D., Vega A. Effects of task complexity and practice on performance during acute alcohol intoxication. Perceptual and Motor Skills. 1971;33:307–318. doi: 10.2466/pms.1971.33.1.307. doi:10.2466/pms.1971.33.1.307. [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Wang G.-J., Telang F., Fowler J. S., Logan J., Childress A.-R., Wong C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. doi:10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M. C., Garner M. J., Munafò M. R., de Wit H. Amphetamine as a social drug: Effects of d-amphetamine on social processing and behavior. Psychopharmacology. 2012;223:199–210. doi: 10.1007/s00213-012-2708-y. doi:10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A. J., Shiffman S., Sayette M. A., Paty J. A., Gwaltney C. J., Balabanis M. H. Attentional bias predicts out-come in smoking cessation. Health Psychology. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. doi:10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. doi:10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wikler A.1965Conditioning factors in opiate addiction and relapse Wilner D. I., Kassenbaum G. G.Narcotics 85–100.)New York, NY: McGraw-Hill [Google Scholar]
- Winkler M. H., Wyers P., Much R. F., Stippekohl B., Stark R., Pauli P. Conditioned cues for smoking elicit preparatory responses in healthy smokers. Psychopharmacology (Berlin) 2011;214:781–789. doi: 10.1007/s00213-010-2033-2. doi:10.1007/s00213-010-20332. [DOI] [PMC free article] [PubMed] [Google Scholar]