Abstract
Recent research has demonstrated top–down effects on meter induction in the auditory modality. However, little is known about these effects in the visual domain, especially without the involvement of motor acts such as tapping. In the present study, we aim to assess whether the projection of meter on auditory beats is also present in the visual domain. We asked 16 musicians to internally project binary (i.e., a strong-weak pattern) and ternary (i.e., a strong-weak-weak pattern) meter onto separate, but analog, visual and auditory isochronous stimuli. Participants were presented with sequences of tones or blinking circular shapes (i.e., flashes) at 2.4 Hz while their electrophysiological responses were recorded. A frequency analysis of the elicited steady-state evoked potentials allowed us to compare the frequencies of the beat (2.4 Hz), its first harmonic (4.8 Hz), the binary subharmonic (1.2 Hz), and the ternary subharmonic (0.8 Hz) within and across modalities. Taking the amplitude spectra into account, we observed an enhancement of the amplitude at 0.8 Hz in the ternary condition for both modalities, suggesting meter induction across modalities. There was an interaction between modality and voltage at 2.4 and 4.8 Hz. Looking at the power spectra, we also observed significant differences from zero in the auditory, but not in the visual, binary condition at 1.2 Hz. These findings suggest that meter processing is modulated by top–down mechanisms that interact with our perception of rhythmic events and that such modulation can also be found in the visual domain. The reported cross-modal effects of meter may shed light on the origins of our timing mechanisms, partially developed in primates and allowing humans to synchronize across modalities accurately.
Keywords: beat perception, meter induction, cross-modal timing mechanisms, music evolution
Introduction
Metrical structure is fundamental for our perception of rhythm in music. It allows humans to process the temporal events of music in an organized manner. Metrical structure is based on two distinct processes: beat extraction and meter induction (Fitch, 2013). The former consists of extracting an isochronous beat from a stream of events. This results in beats appearing as periodic points over time. The latter consists of the hierarchical organization of these periodic beats into sequences of strong and weak patterns. The downbeat (the perceptually prominent beat) usually occurs at a subharmonic frequency of the beat, such as 2:1, 3:1, or other more complex integer ratios. The saliency of the downbeat is usually elicited by variations of loudness, pitch, or timbre in the perceived sound. It can also be generated endogenously via an active top-down process of rhythmic perception (Nozaradan et al., 2011; London, 2012). This endogenous sense of regular alternations of strong and weak patterns is commonly termed as subjective metricization (Keller, 2012). In short, meter induction organizes periodic beats in a hierarchical manner and can be modulated voluntarily.
Recent research has advanced in the identification of neural correlates of beat perception and meter induction. It has been demonstrated that neural activity increases at the frequencies corresponding to the beat and those corresponding to an induced meter (see Nozaradan et al., 2011, 2012). A frequency-tagging approach by means of electroencephalography (EEG) has been used to explore these neural substrates (Nozaradan, 2014; Nozaradan et al., 2015). This method uses periodic properties of the stimuli to induce steady-state evoked potentials (SS-EPs): changes in voltage that are stable in phase and amplitude over time. These neural responses can be elicited via different modalities (Vialatte and Maurice, 2009; Vialatte et al., 2010a; Nozaradan et al., 2012) and are easily recorded with EEG. Once SS-EPs are analyzed in the frequency domain, narrow-band peaks appear at the frequencies corresponding to the external stimuli. In addition to the emergence of these peaks reflecting bottom–up processing, those frequencies representing the beat and the meter are selectively enhanced. Crucially, top–down effects of meter induction are also captured, such as when meter is internally driven and imposed on the stimuli. When binary (march-like) and ternary (waltz-like) meter was mentally projected onto an auditory stimulus, Nozaradan et al. (2011) found an enhancement at the frequencies corresponding to the subharmonics of the beat: f/2 and f/3, respectively. Thus, the increase of neural activity at selected frequencies reflects the voluntarily control of meter induction, that is, the top–down processing that guides attention toward relevant points in the stimulus.
So far, the neural correlates of meter induction have primarily been studied in the auditory modality. There is an open debate about whether meter is restricted to the auditory modality or whether other modalities can access meter. Recent behavioral research has revealed that metrical structure is also apparent in visual stimuli. Lee et al. (2015) presented participants with videos of choreographies marking strong and weak beats at the same time as isochronous sounds. In their first experiment, deviant timbre sounds were presented in different metrical positions. Participants were slower in reacting to deviant sounds placed at the visually inferred strong positions, which suggests visual meter induction and a split of attention between modalities. Although beat and meter have been tested in other modalities, such as visual and tactile, these studies mostly involve motor acts, such as synchronized tapping (reviewed in Repp, 2005; Repp and Su, 2013). For example, it has been found that the act of moving at certain meter leads to listening preferences in infants (Phillips-Silver and Trainor, 2005, 2008). Movement also shapes the internal representation of auditory rhythms by enhancing the EEG signal at the metrical frequency (Chemin et al., 2014). However, it is difficult to disentangle the relative contribution of the motor act itself from metrical effects in these sensorimotor synchronization (SMS) studies.
There are good reasons to believe that beat processing and meter induction may be amodal. Several species have been found to effectively use interval-based and beat-based mechanisms in both the auditory and the visual modalities (Repp, 2005; Repp and Su, 2013; Patel and Iversen, 2014; Ravignani et al., 2014; Merchant et al., 2015). For example, visual rhythm synchronization has been attested in the behavior of some insects, such as fireflies (Buck and Buck, 1968), and in controlled laboratory studies with non-human primates, such as macaques (Zarco et al., 2009). Moreover, the accurate synchronizations observed during dance and music across human cultures (Fitch, 2013) suggests that these timing mechanisms might not only engage the visual modality, but also make use of a non-domain-specific meter.
The possibility that rhythm synchronization engages a domain-general timing mechanism makes it necessary to explore the extent to which we can also identify neural correlates for meter induction across modalities. The present work aims to explore meter induction in the visual modality by comparing the effects of endogenously driven meter projected onto auditory and visual periodic stimuli. We apply a frequency-tagging approach to the SS-EPs elicited during meter induction without the involvement of motor behavior. Our objective is to explore the extent to which meter induction is domain-specific: whether it is tightly constrained to the auditory modality, or whether similar neural correlates can be found across modalities. As visually transmitted meter is observed in some natural settings, such as musicians following a conductor in an orchestra, or dancers synchronizing with each other, our prediction is that we should observe similar metrical effects in both the auditory and the visual modalities.
Materials and Methods
Participants
Sixteen healthy musicians were included in the present study (9 females, 6 left-handers, mean age: 23.38 ± 3.85, age range: 18–35). There were 17 participants in total, but one male was excluded due to an excess of artifacts in the EEG data. All participants had extensive musical experience, starting at 6.31 ± 2.30 years of age, and six of them reported some training in dance. We chose to recruit musicians because it is clear to them what differentiates binary from ternary meter and because they have extensive experience extracting metrical cues from audiovisual sequences of rhythms. No participant reported any history of hearing, visual, motor, or psychiatric disorders, and all participants had normal or corrected-to-normal vision. All participants signed a written consent form and received payment for their participation in the study.
Ethics Statement
All procedures were approved by the ethical committee from the Universitat Pompeu Fabra.
Stimuli
Stimuli consisted of isochronous sequences of either tones (in the auditory condition) or flashes (in the visual condition). They were presented at a frequency of 2.4 Hz (IOI = 416.66 ms). Every sequence lasted for 35 s and was comprised of 84 tones or flashes. All the target frequencies fell within the ecological range of tempo perception and production (Vialatte and Maurice, 2009; Nozaradan et al., 2011). Every event (tone or flash) within the sequence progressively diminished until the next one appeared (see Figure 1), thus marking the onset of the beat with the maximum intensity of sound (in the auditory condition) or with the maximum intensity of light (in the visual condition). Each condition had eight auditory or visual 35-s sequences. After each 35-s sequence, the pitch or the color of the stimuli was changed in order to maintain participants’ attention. Within each 35-s sequence, the stimuli were the same.
FIGURE 1.
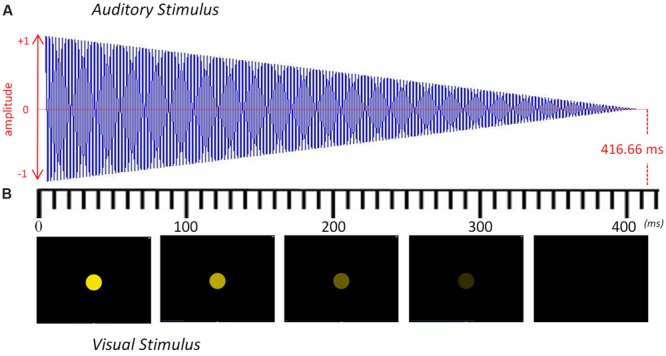
Representation of the auditory and visual stimuli over time. (A) Sound envelope of the auditory stimulus modulated from maximum to minimum volume. (B) Five frames of the visual stimulus depicting the modulation from maximum to minimum luminescence. Each stimulus lasted 416.66 ms and was repeated 84 times to yield a 35-s sequence with a steady beat at 2.4 Hz. The pitch or the color was changed after every 35-s sequence to maintain participants’ attention.
The auditory stimuli were presented at a comfortable hearing level through two speakers placed 70 cm in front of the participant. We converted a pure sinusoidal tone into stereo by using Audacity. These pure tones were raised half a tone in each 35-s sequence, going from an F4 up to a C5. The entire auditory condition, therefore, consisted of eight different sequences of 84 repeated sinusoidal tones: F4 (349.2 Hz), F#4 (370.0 Hz), G4 (392.0 Hz), G#4 (415.3 Hz), A4 (440.0 Hz), A#4 (466.2 Hz), B4 (493.9 Hz) and C5 (523.3 Hz).
The visual stimuli were presented on a computer screen (1280 × 1024 resolution) placed 70 cm in front of the participant. A colored circle was placed at the center of the screen with a black background and had a radius of 25 mm. To create the blinking effect, we progressively diminished its luminescence until it turned completely black (see Figure 1). These flashes changed color after every 35-s sequence of 84 flashes with the following RGB progression: red (255 0 0), orange (255 128 0), yellow (255 255 0), green (128 255 0), turquoise (0 255 128), light blue (0 255 255), dark blue (0 0 255), and violet (128 0 255). The auditory and visual stimuli were created using Matlab (v.2013, The MathWorks) and presented with Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997).
Procedure
Participants were seated in a comfortable armchair in a soundproof room with the keyboard placed on their lap. Our study consisted of three conditions: the passive beat condition (which served as a control), the binary imagery task, and the ternary imagery task. These three conditions were the same for the auditory and visual modalities. They were pseudo-randomly presented to each participant, with the control condition always presented first, and the visual and auditory modalities alternated, forming a total of 16 possible combinations. During the control condition, the participants were passively either looking at the flashes or listening to the sounds. In order to avoid the induction of an involuntary meter in the control condition, the well-known tick-tock effect (Brochard et al., 2003), we reminded the participants at the beginning of each 35-s sequence to perceive each beat individually, that is, as independent from the previous and the following tone or flash. During the binary and ternary tasks, in contrast to the control condition, participants were asked to mentally project a binary structure (strong–weak pattern) or a ternary structure (strong–weak–weak pattern) onto the same perceptual stimuli presented during the control condition. In other words, they were asked to silently project a metrical structure focusing on the subharmonics of the beat: f/2 (1.2 Hz) for the binary meter and f/3 (0.8 Hz) for the ternary meter. Participants were asked to start the meter imagery task as soon as the first stimulus was presented. At the end of each 35-s sequence, they had to report whether the last beat was strong or weak as a way of maintaining their attention and making sure they were focused on the task. Because the participants had to press the space bar to go on to the next block, they were allowed to take a break when needed. Once the study finished, a questionnaire was given to the participant to evaluate and comment on the stimuli and the difficulty of each task. Psychtoolbox was used to run the experiment.
Electrophysiological Recording
The EEG signal was recorded using a BrainAmp amplifier and the BrainVision Analyzer Software package (v.2.0; Brain Products) using an actiCAP with 60 electrodes placed on the scalp according to the International 10/10 system (Fp1, Fp2, AF7, AF3, AF4, AF8, F7, F3, F1, Fz, F2, F4, F8, FT9, FT7, FT8, FT10, FC5, FC3, FC1, FC2, FC4, FC6, C5, C3, C1, Cz, C2, C4, C6, T7, T8, TP9, TP7, TP8, TP10, CP5, CP3, CP1, CPz, CP2, CP4, CP6, P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO9, PO3, POz, PO4, PO10, O1, Oz, O2). Vertical and horizontal eye movements were monitored using two electrodes placed on the infra-orbital ridge and the outer canthus of the right eye. Two additional electrodes were placed on the left and right mastoid. The signals were referenced to the FCz online channel and all electrode impedances were kept below 25 kΩ. The signals were amplified and digitized at a sampling rate of 1000 Hz.
Data Analyses
Preprocessing of the continuous EEG recordings was implemented using BrainVision Analyzer 2.1 (Brain Products GmbH). First, any channel that appeared flat or noisy was interpolated from the surrounding channels via spherical spline interpolation. All the channels were then filtered using a zero-phase Butterworth filter to remove slow drifts in the recordings, with a high pass filter at 0.1 Hz (48 dB/oct) and low pass filter at 10 Hz (time constant 1.591549, 48 dB/oct). Channels with EEG exceeding either ±100 μV at any channel, activity <0.5 μV, or voltage step/sampling >50 μV within intervals of 200 ms, were automatically detected offline. Subsequently, eye blinks and muscular movements were corrected using Ocular Correction ICA. Finally, the filtered EEG data was segmented into epochs of 36 s (corresponding to the 35-s sequences with an extra second in the beginning) for each condition and modality. These files were then exported to Matlab. All further analyses were performed in Matlab and SPSS (version 19, IBM).
For each condition and modality, eight epochs lasting 32.5 s were obtained by removing the first 3.5 s of each sequence. This removal, as justified in Nozaradan et al. (2011), discards the evoked potential related to the stimuli onset and relies on the fact that SS-EPs require several repetitions or cycles to be elicited (Repp, 2005; Vialatte et al., 2010a). In order to enhance the signal-to-noise ratio and attenuate activities that are not phase locked to the auditory and visual stimuli, the EEG epochs for each participant, modality, and condition were averaged across trials. To get the signal’s amplitude (in μV), we applied a fast Fourier transform (FFT), and to get its power (in μV2), we squared the modulus of the FFT. Both frequency spectra ranged from 0 to 500 Hz with a frequency resolution of 0.0305 Hz. The obtained signal is assumed to correspond to the EEG activity induced by the physical stimuli and the meter imagery. However, it may also include residual background noise due to spontaneous activity. Two different signal-to-noise techniques were applied: the subtraction method used in Nozaradan et al. (2011) for the amplitude spectrum (in μV) and a relative measure that compared each individual’s binary and ternary meter values to their own beat condition as a baseline for the power spectrum (converting μV2 into decibels), thus minimizing variation due to group variability.
For the amplitude spectrum, noise was removed by subtracting the averaged amplitude of the two surrounding non-adjacent frequency bins, ranging from -0.15 to -0.09 Hz and from 0.09 to 0.15 Hz, at each frequency bin from 0.5 to 5 Hz. For the power spectrum, we assume that the beat condition serves as a baseline, since the subjects were passively listening to the stimuli, and that their EEG activity corresponds to the processing of beat without any top–down projection of meter. We took the power spectrum of each meter imagery condition for each participant and divided it by their own control condition. Subsequently, we converted these values into dB by taking the log10 of each value and multiplying by 10. Following this procedure, we do not need to compare the selected frequency bins among conditions and modalities against the control, but instead check whether the obtained values at each frequency of interest differ from zero. This procedure makes the comparison between modalities more reliable, as the effect of meter is relative to the control condition of each modality.
In order to correct for spectral leakage from our target frequencies in the amplitude spectrum, we averaged every target frequency bin ±0.0305 Hz, the three frequency bins centered on the target frequencies, as Nozaradan et al. (2011) did. However, to compare between the peaks, we only took the values from every target frequency bin. We did not apply this technique for spectral leakage to the original power spectrum because, after using the baseline correction method, the induced activity was centered very concisely in a single bin. The mean of all electrodes across each individual’s scalp was calculated for each condition and modality at each target frequency, revealing multiple peaks in the data. For the amplitude spectra, the values for each target frequency (0.8, 1.2, 2.4, 4.8 Hz) were separately submitted to two-way repeated measures ANOVAs with the factors Modality (auditory and visual) and Condition (beat, binary, ternary). When one of the ANOVA factors was significant, post hoc pairwise comparisons were performed with Fischer’s LSD and Bonferroni. Size effects were expressed using the partial η2. For the power spectra, one sample t-tests were used to see if the values at each target frequency significantly differed from zero. The significance level was set at p < 0.05 for all statistical analyses.
The present approach does not deal with topological effects because our hypothesis does not predict any region of interest for a domain-general meter induction, although certain correlations could appear (such as occipital areas showing a strong connection to the visual modality of the stimuli). This is the reason why the means from every electrode were averaged across the scalp, thereby excluding selection biases.
Results
Amplitude Spectra
In Figure 2, the mean of all participants’ amplitudes (red line) is plotted over each individual’s amplitude spectra (gray lines) at the target frequencies (0.8, 1.2, 2.4, 4.8 Hz). A clear peak appears at the frequency of the stimuli (2.4 Hz) in all the conditions (beat, binary, ternary). Similarly, a peak is observed for the first harmonic (4.8 Hz) in all three conditions. However, there seem to be differences across conditions regarding the sub-harmonics. The peak at 1.2 Hz only appears in the auditory binary condition, while the peak at 0.8 Hz is found in both auditory and visual ternary conditions. Furthermore, there is a larger peak at the beat frequency for all three auditory conditions compared to their visual analogs, whereas the inverse effect occurs at the frequency of the first harmonic, depicting a larger peak for all three visual conditions.
FIGURE 2.
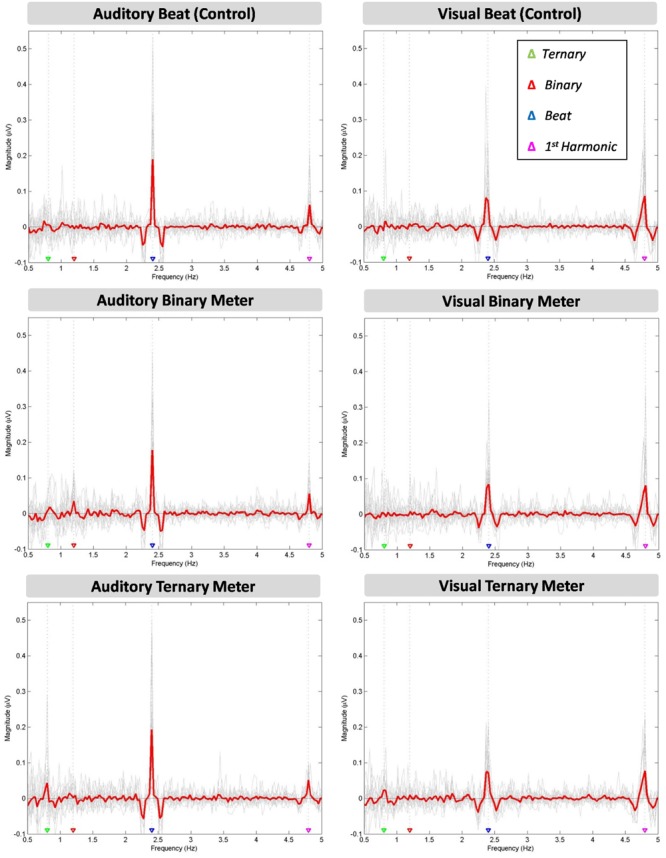
Six amplitude spectra depicting the amplitude (μV) of the averaged EEG signal at each frequency between 0.5 and 5 Hz. The auditory (left column) and visual (right column) modalities are split into three conditions: the beat control (first row), the binary meter imagery task (second row), and the ternary meter imagery task (third row). The mean of all participants’ amplitudes (gray lines) is depicted in red for each condition and modality. The frequencies of the ternary meter, binary meter, beat, and its first harmonic are signaled with gray dotted lines and colored triangles over the abscissa: 0.8 Hz (green), 1.2 Hz (red), 2.4 Hz (blue) and 4.8 Hz (magenta).
A two-way repeated measures ANOVA [Modality (auditory and visual) × Condition (beat, binary, ternary)] was applied to each frequency of interest (0.8, 1.2, 2.4, 4.8 Hz) separately. Table 1 reports the values obtained from ANOVAS for Modality, Condition, and their interaction. For the frequency of the first harmonic of the beat (4.8 Hz), there was a main effect of Modality: F(1,15) = 14.945, η2 = 0.499, p = 0.002. For the ternary subharmonic of the beat (f/3 = 0.8 Hz), a main effect of Condition was observed [F(2,30) = 5.018, η2 = 0.251, p = 0.013]. However, for the binary subharmonic of the beat (f/2 = 1.2 Hz), no main effect of Condition was found [F(2,30) = 1.599, η2 = 0.096, p = 0.219]. Post hoc pairwise comparisons (see Table 2) using a Bonferroni adjusted alpha indicated that (i) the averaged amplitudes of the ternary condition were greater than those of the beat control at 0.8 Hz (MD = 0.454, p = 0.046), and (ii) the averaged amplitudes of the visual modality were greater than those of the auditory modality at 4.8 Hz (MD = 1.091, p = 0.002). Interestingly, less restrictive post hoc pairwise comparisons using Fischer’s Least Significant Difference (LSD) showed that the averaged amplitudes of the ternary condition were also greater than those of the binary condition at 0.8 Hz (MD = 0.434, p = 0.022).
Table 1.
Results of the four two-way repeated measures ANOVAs applied to the averaged amplitudes (3 bins) of each frequency of interest: 0.8, 1.2, 2.4, and 4.8 Hz.
| df | F | p | η2 | |
|---|---|---|---|---|
| 0.8 Hz | ||||
| Modality | 1, 15 | 1.890 | 0.189 | 0.112 |
| Condition | 2, 30 | 5.018 | 0.013∗ | 0.251 |
| Modality ∗ Condition | 2, 30 | 0.206 | 0.815 | 0.014 |
| 1.2 Hz | ||||
| Modality | 1, 15 | 2.468 | 0.137 | 0.141 |
| Condition | 2, 30 | 1.599 | 0.219 | 0.096 |
| Modality ∗ Condition | 2, 30 | 1.876 | 0.191 | 0.111 |
| 2.4 Hz | ||||
| Modality | 1, 15 | 1.662 | 0.217 | 0.100 |
| Condition | 2, 30 | 0.214 | 0.809 | 0.014 |
| Modality ∗ Condition1 | 1.343, 20.138 | 0.497 | 0.542 | 0.032 |
| 4.8 Hz | ||||
| Modality | 1, 15 | 14.945 | 0.002∗∗ | 0.499 |
| Condition | 2, 30 | 0.564 | 0.575 | 0.036 |
| Modality ∗ Condition | 2, 30 | 0.309 | 0.736 | 0.020 |
The two levels of Modality (auditory and visual) and Condition (beat control, binary-meter, and ternary-meter) are shown accompanied by their interactions. For each level and interaction, degrees of freedom, F-statistics, p-values, and size effects are reported. 1A Greenhouse–Geisser correction was applied to correct for violations of sphericity. ∗p < 0.05, ∗∗p < 0.01.
Table 2.
Post hoc pairwise comparisons from the two-way repeated measures ANOVAs of the averaged amplitudes (3 bins) with and without adjustments for multiple comparisons.
| Mean difference | Standard error | Significance |
||||
|---|---|---|---|---|---|---|
| Fischer’s LSD | Bonferroni | |||||
| 0.8 Hz | Condition | Condition | ||||
| Bin. | Beat | 0.020 | 0.150 | 0.897 | 1 | |
| Tern. | Beat | 0.454 | 0.166 | 0.015∗ | 0.046∗ | |
| Tern. | Bin. | 0.434 | 0.169 | 0.022∗ | 0.065 | |
| 4.8 Hz | Modality | Modality | ||||
| Aud. | Vis. | -1.091 | 0.282 | 0.002∗∗ | 0.002∗∗ | |
For the frequencies 0.8, 2.4, and 4.8 Hz, the mean differences, standard deviations, and p-values (Fischer’s LSD as non-readjusted alpha, Bonferroni as readjusted alpha) are reported. Significance level was always kept below 0.05. The condition ‘Beat’ stands for the beat control, the condition ‘Bin.’ stands for the binary meter, and the condition ‘Tern.’ stands for the ternary meter. The modality ‘Aud.’ stands for audition, and the modality ‘Vis.’ stands for vision. ∗p < 0.05, ∗∗p < 0.01.
We found modality differences regarding the first harmonic of the beat (4.8 Hz), but not regarding the beat (2.4 Hz), even though the peaks were apparently higher in the auditory modality (see Figure 2). This counterintuitive finding may be related to the way we averaged the amplitudes for each peak, which assumed that a considerable leakage was taking place. However, the amplitude spectra show that the peak for the beat in the auditory modality was sharper and larger, while in the visual modality was wider and shorter.
A second two-way repeated measures ANOVA [Modality (auditory and visual) × Condition (beat, binary, ternary)] was applied to the top-of-the-peak values of each frequency of interest (0.8, 1.2, 2.4, 4.8 Hz) separately, as reported in Table 3. For the frequency of the beat (2.4 Hz), there was a main effect of Modality: F(1,15) = 14.215, η2 = 0.487, p = 0.002. For the ternary subharmonic of the beat (f/3 = 0.8 Hz), a main effect of Condition was observed [F(1.345,20.178) = 5.842, η2 = 0.280, p = 0.018]. For the binary subharmonic of the beat (f/2 = 1.2 Hz), a main effect of Condition [F(1.422,21.334) = 4.609, η2 = 0.235, p = 0.032] and an interaction were attested [F(2,30) = 1.783, η2 = 0.106, p = 0.026]. Post hoc pairwise comparisons (see Table 4) using a Bonferroni adjusted alpha indicated that (i) the peak amplitudes of the ternary condition were higher than those of the beat control at 0.8 Hz (MD = 1.178, p = 0.018), (ii) the peak amplitudes of the binary condition were higher than those of the beat control at 1.2 Hz (MD = 0.655, p = 0.050), and (iii) the peak amplitudes of the auditory modality were higher than those of the visual modality at 2.4 Hz (MD = 3.592, p = 0.002). Less restrictive post hoc pairwise comparisons using Fischer’s LSD showed that the peak amplitudes of the binary condition were significantly higher than those of the beat control at 1.2 Hz (MD = 0.655, p = 0.017).
Table 3.
Results of the four two-way repeated measures ANOVAs applied to the top-of-the-peak amplitudes (1 bin) at 0.8, 1.2, 2.4, and 4.8 Hz.
| df | F | p | η2 | |
|---|---|---|---|---|
| 0.8 Hz | ||||
| Modality | 1, 15 | 3.269 | 0.091 | 0.179 |
| Condition1 | 1.345, 20.178 | 5.842 | 0.018∗ | 0.280 |
| Modality ∗ Condition1 | 1.445, 21.669 | 0.309 | 0.667 | 0.020 |
| 1.2 Hz | ||||
| Modality | 1, 15 | 0.161 | 0.694 | 0.011 |
| Condition1 | 1.422, 21.334 | 4.609 | 0.032∗ | 0.235 |
| Modality ∗ Condition | 2, 30 | 4.134 | 0.026∗ | 0.216 |
| 2.4 Hz | ||||
| Modality | 1, 15 | 14.215 | 0.002∗∗ | 0.487 |
| Condition | 2, 30 | 0.042 | 0.959 | 0.003 |
| Modality ∗ Condition | 2, 30 | 1.783 | 0.202 | 0.106 |
| 4.8 Hz | ||||
| Modality | 1, 15 | 0.748 | 0.401 | 0.047 |
| Condition | 2, 30 | 0.902 | 0.416 | 0.057 |
| Modality ∗ Condition | 2, 30 | 0.007 | 0.993 | 0.000 |
The two levels of Modality (auditory and visual) and Condition (beat control, binary-meter, and ternary-meter) are shown accompanied by their interactions. For each level and interaction, degrees of freedom, F-statistics, p-values, and size effects are reported. 1A Greenhouse–Geisser correction was applied to correct for violations of sphericity. ∗p < 0.05, ∗∗p < 0.01.
Table 4.
Post hoc pairwise comparisons from the two-way repeated measures ANOVAs of the top-of-the-peak amplitudes (1 bin) with and without adjustments for multiple comparisons.
| Mean difference | Standard error | Significance |
||||
|---|---|---|---|---|---|---|
| Fischer’s LSD | Bonferroni | |||||
| 0.8 Hz | Condition | Condition | ||||
| Bin. | Beat | 0.250 | 0.225 | 0.284 | 0.852 | |
| Tern. | Beat | 1.178 | 0.369 | 0.006∗∗ | 0.018∗ | |
| Tern. | Bin. | 0.929 | 0.458 | 0.061 | 0.182 | |
| 1.2 Hz | ||||||
| Bin. | Beat | 0.655 | 0.243 | 0.017∗ | 0.050∗ | |
| Tern. | Beat | 0.145 | 0.141 | 0.320 | 0.961 | |
| Tern. | Bin. | -0.510 | 0.274 | 0.083 | 0.248 | |
| 2.4 Hz | Modality | Modality | ||||
| Aud. | Vis. | 3.592 | 0.953 | 0.002∗∗ | 0.002∗∗ | |
For the frequencies 0.8, 2.4, and 4.8 Hz, the mean differences, standard deviations, and p-values (Fischer’s LSD as non-readjusted alpha, Bonferroni as readjusted alpha) are reported. Significance level was always kept below 0.05. The condition ‘Beat’ stands for the beat control, the condition ‘Bin.’ stands for the binary meter, and the condition ‘Tern.’ stands for the ternary meter. The modality ‘Aud.’ stands for audition and the modality ‘Vis.’ stands for vision. ∗p < 0.05, ∗∗p < 0.01.
These results might be due to a similar top–down meter effect for both modalities in the ternary-meter condition enhancing the ternary subharmonic of the beat (f/3), the downbeat of the ternary meter. Unfortunately, this enhancement was not consistently found for the binary subharmonic (f/2) because the averaged values of both auditory and visual binary-meter conditions at 1.2 Hz might not be significantly greater than those of other conditions. In fact, the interaction reported by the second ANOVA (comparing each top-of-the-peak value) at 1.2 Hz points to the absence of the binary-meter effect in one modality. Furthermore, both binary and ternary metrical effects could also be affected by the variability across participants in projecting meter or in unconsciously grouping the beat during the control condition, despite our instructions. In order to control for inter-individual variability (see the gray lines of Figure 2), we explored our data using a different signal-to-noise method, namely using each individual’s beat condition as a baseline in order to obtain a relative measure that takes that person’s variability into account.
Power Spectra
The power spectra for each modality and condition were obtained by taking the modulus squared of the amplitudes resulting from the FFT. Here, we used the control (beat) condition as a baseline to normalize and convert the amplitudes from the metrical conditions (binary, ternary) into decibels. This procedure consisted of applying the following operation: 10 log10
 . This method gives us the opportunity to see relative distances from zero as differences between conditions with respect to the control. When positive, the values indicate more power for the metrical condition, whereas when negative, they indicate more power for the beat baseline. Figure 3 shows all the relativized power spectra displaying the activity of all the electrodes at each frequency.
. This method gives us the opportunity to see relative distances from zero as differences between conditions with respect to the control. When positive, the values indicate more power for the metrical condition, whereas when negative, they indicate more power for the beat baseline. Figure 3 shows all the relativized power spectra displaying the activity of all the electrodes at each frequency.
FIGURE 3.
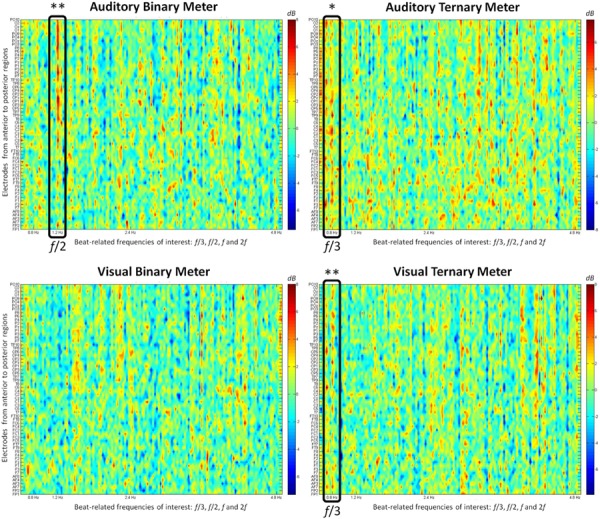
The relative value (from -7 to 8 dB) obtained after subtracting the power spectrum (μV2/Hz) of the control condition from each metrical condition is shown at each electrode. The frequencies of interest (0.8, 1.2, 2.4, and 4.8 Hz) appear on the frequency axis (from 0.6 to 5 Hz). First row: auditory conditions. Second row: visual conditions. First column: binary meter task. Second column: ternary meter task. Black rectangles frame the relevant frequencies when their values are significantly distinct from zero. The electrodes are ordered from anterior to posterior regions, with odd and even numbers representing the right and left hemispheres, respectively. ∗ p < 0.05, ∗∗ p < 0.01.
To summarize the differences between conditions and modalities, Figure 4 depicts the mean of all participants’ electrodes contrasting each condition in both the visual and the auditory modality. The frequencies of interest are marked with a vertical line to make it easier to see the meter-induced peaks at the subharmonics of the beat. Similar to the findings in the amplitude spectra, there are larger peaks at 0.8 Hz for the ternary condition in both modalities, but there is a peak at 1.2 Hz for the binary condition in the auditory modality only. In contrast with our first analyses, no differences arise between modalities at the frequency of the beat (2.4 Hz) in the power spectra. This is due to the use of the beat condition as a baseline. Given that beat is similarly induced in all conditions, dividing the two conditions will result in a relative value close to zero.
FIGURE 4.
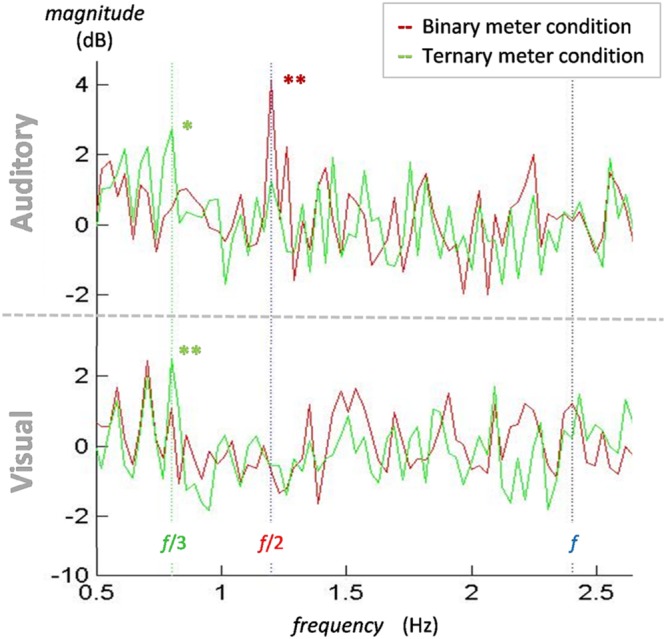
Normalized power spectra of the binary and ternary conditions for the mean of all participants and electrodes in both the auditory and the visual modalities. In both auditory (top) and visual (bottom) modalities, a peak for the ternary condition (green line) appears at 0.8 Hz (green dotted line). A peak for the binary condition (red line) at 1.2 Hz (red dotted line) is only observed in the auditory modality. There is no contrasting peak at 2.4 Hz (blue dotted line) in either modality. ∗p < 0.05, ∗∗p < 0.01.
One-sample t-tests were applied to the spectrum of each target frequency to examine whether the values significantly differed from zero. At 0.8 Hz, the values of the ternary conditions were significantly different from zero in the auditory [t(15) = 2.778, p = 0.014] and the visual [t(15) = 3.692, p = 0.002] modalities. The same occurred at 1.2 Hz for the values of the binary condition in the auditory modality [t(15) = 3.489, p = 0.003]. These findings show that the peaks appearing in both ternary conditions at 0.8 Hz and the auditory binary condition at 1.2 Hz are significantly different from zero and thus larger than the control condition. However, no effects for the visual binary meter were attested.
Discussion
Our work is among the first studies to compare meter induction between two different modalities, audition and vision, without requiring synchronized, overt movement. Although SMS studies have also explored the degree to which participants can reliably extract the pulse from visual rhythms (Patel et al., 2005; Repp, 2005; Nozaradan et al., 2012; Repp and Su, 2013; Iversen et al., 2015), there is not much information to date regarding meter induction in other modalities which do not involve motor acts. Here, we examined whether visually induced beats can be organized in endogenously driven hierarchies. To this end, each participant was asked to internally project binary and ternary meter onto the isochronous stimuli. Their recorded EEG data was converted into the frequency domain, resulting in amplitude and power spectra. We observed an effect of ternary meter induction in both the auditory and the visual modalities, as well as an effect of binary meter induction in the auditory modality. This suggests that some degree of meter induction can also be observed in the visual modality.
We evaluated the SS-EPs elicited by the beat frequency and its natural first harmonic in both modalities. The emergence of a periodic entrainment at the frequency of the beat’s first harmonic (2f = 4.8 Hz) illustrates a natural preference for integer harmonics that tend to appear involuntarily when a periodic stimulus is presented. In fact, this entrainment may be related to a predisposition to subdivide the beat into integer harmonics, like duplets (1:2) or triplets (1:3) of eighth notes in music. We observed different amplitudes at the frequency of the beat and its first harmonic depending on the stimuli’s modality. There was a significantly higher peak at 2.4 Hz in the auditory modality than in the visual modality. Inversely, there was a significantly greater peak at 4.8 Hz in the visual modality than in the auditory modality. Since our visual stimuli did not abruptly appear and disappear at 4.8 Hz, but progressively vanished (see Figure 1), there is no reason to believe that the stimulus’ offset reinforced the first harmonic in the visual domain. This difference across modalities could be related to the comfortable frequencies that have been found for vision and audition with synchronized tapping (Repp, 2003). While the upper inter-onset interval (IOI) would be the same for both modalities (around 1800 ms), the lower IOI seems to be just above 460 ms for visual flashes and 160 ms for auditory beats (Repp, 2005). These modality-specific boundaries for SMS could reflect the neural demands of extracting and keeping the beat in each modality, possibly working on different frequencies. Importantly, the difference across modalities could also be due to the fact that musicians are well-trained to deal with auditory beats, and may be able to accurately synchronize with the frequency of the auditory stimuli, as shown by the sharper peak at the auditory beat (Figure 2). In contrast, since musicians may be less used to visual beats, their synchronization with the frequency of the visual stimuli may become less accurate and more distributed around 2.4 Hz, as shown by the wider, rounder peak (Figure 2). In this case, experience may play an important role in determining the differences between modalities.
A key finding in the present study is the emergence of periodic responses at the subharmonics f/2 and f/3 of the beat. These effects reflect the voluntary metrical interpretation of the beat in the binary and the ternary conditions. Our results suggest that neural entrainment in both modalities not only occurs for the beat, but also for the subharmonic f/3 during the ternary condition. This finding supports top–down effects of meter induction in the visual modality. However, we did not observe any effect of the binary meter in the visual modality. This lack of effect in the binary condition suggests that there is no automatic conversion between the visual and the auditory modalities. If there were such a conversion, we should observe an effect of binary and ternary meter on both modalities (see Guttman et al., 2005; McAuley and Henry, 2010). Nevertheless, the fact that meter induction is apparent in the visual modality for the ternary, but not for the binary, meter condition suggests that meter induction applies to the visual cues independent of any auditory conversion. To confirm this idea, future research testing visual meter induction in deaf people is needed, as a visual-to-auditory conversion would not be expected in this case. Furthermore, some participants reported in the questionnaire that, instead of “counting” the pulse or thinking metrical patterns in the visual conditions, they made use of visual cues to project the meter onto the flashes, such as imagining the downbeat brighter or slightly displaced toward one side of the screen. The alteration of these visual features to project the metrical structure may also provide evidence against an auditory-to-visual conversion.
The fact that we did not find evidence of meter induction in the visual binary condition could be due to different cognitive demands across the subharmonics of the beat. A magnetoencephalographic study conducted by Fujioka et al. (2010) revealed differences in internally inducing binary and ternary meter in the auditory domain. Distinct time courses of auditory evoked responses on distributed networks were found for binary and ternary meter. The authors also observed that the contrast between strong and weak beats was only present for the ternary meter. Thus, differences between projecting binary and ternary meter could have interacted with the modality we used to induce the beat. Although more research is needed to clarify the differences between binary and ternary meter in terms of cognitive demands, the results we observed from the visual ternary condition support the idea that meter induction can apply beyond the auditory modality.
There is a feature in our visual stimuli that could have contributed to the lack of an effect in the binary condition. We used colored flickering flashes that suddenly appeared and gradually vanished to promote a beat. Stimuli such as flashes have been used extensively to create SS-EPs (Krause et al., 2010; Vialatte et al., 2010b) and even seem to be perceived with binary meter by deaf individuals (Iversen et al., 2015). However, recent studies suggest it is easier to observe SMS in the visual domain using geometrical and spatial features (Hove et al., 2010), as well as stimuli displaying natural and biological motion (Hove et al., 2013b; Su, 2014a,b). Showing a bouncing ball on a screen, Gan et al. (2015) obtained slightly better results on visual synchronization at IOIs from 500 to 900 ms compared to using an auditory metronome. Similarly, Iversen et al. (2015) reported equally accurate performance by deaf and hearing individuals when they had to synchronize with a ball bouncing at an IOI of 600 ms. In light of these findings, we can speculate that using motion cues could have aided the neural synchronization with the beat and perhaps facilitated the projection of the binary meter in the visual modality, whose frequency actually fell within the above-mentioned IOI ranges.
It has been proposed that meter consists of a cyclical fluctuation of attention over isochronous events to yield expectancies and predictions of incoming beats (Jones and Boltz, 1989; Large and Jones, 1999). A mirroring oscillatory network could explain the neuronal engagement to the external stimuli, either auditory or visual, such as the non-linear oscillator network proposed by the Resonance Theory (Large, 2008, 2010; Large et al., 2015). In the visual domain, these predictions would be fundamental to allow for dance synchrony and other coordinated social activities, such as sports. Studies using neuroimaging techniques provide further support for cross-modal meter. For instance, Hove et al. (2013a) found that the basal ganglia activity was more associated with SMS stability than with modality features. Musical meter has also been found to elicit cross-modal attention effects in the caudate nucleus (Trost et al., 2014). Coupled with our findings on the ternary visual meter, the general picture appears to be that of an amodal timing mechanism for beat and meter, allowing humans to deal with temporal information across modalities.
Comparative cognition studies also point toward the idea that timing mechanisms are more detached from a single modality than previously believed. Trained rhesus monkeys (macaques) showed an accurate performance of a synchronization-continuation task with both visual and auditory metronome at different tempos (Zarco et al., 2009), but their tapping behavior was clearly biased toward visual cues (Merchant and Honing, 2013). In addition, Japanese macaques were found to synchronize limb movements when facing each other, suggesting social coordination through visual imitation (Nagasaka et al., 2013). In our closest relatives, the chimpanzees, synchronization has been tested in the auditory domain (Hattori et al., 2013). However, likely by using both visual and auditory information, a bonobo intermittently displayed entrainment and phase matching to distinct isochronous sounds when interactively drumming with a human drummer (Large and Gray, 2015). Rhythmic behaviors, such as the drumming found in wild gorillas and chimps, seem to be tied to social functions (Ravignani et al., 2013). These findings suggest that primates may have evolved social interactive behaviors (i.e., the ancestors of our music and dance) by gradually tuning the neurodynamics of their timing mechanisms toward a more precise visuo- and audio-motor coupling so as to improve social learning, group coordination, and cohesion. Among primates, only humans are complex vocal learners, an ability that requires tighter auditory-motor connections (Petkov and Jarvis, 2012; Patel and Iversen, 2014; Merchant et al., 2015) than those found in the motor cortico-basal ganglia-thalamo-cortical circuit of primates (Merchant and Honing, 2013). We propose that the advantages of our evolved beat-based timing mechanism are not restricted to the auditory modality. Instead, even though the auditory modality has been specialized for the rhythms of speech and music through cultural experience, these same mechanisms may also be available to process rhythms in other modalities, allowing for sign language, dance, and sports.
The present results suggest that meter induction is domain-independent. A top-down projection of meter, without having any external cue to mark the metrical structure, is available for both the visual and the auditory modalities. This is informative regarding current theories on rhythm evolution and opens interesting questions. If meter induction evolved as a result of purely acoustic rhythm processing, it is then necessary to explain how it emerges in the visual modality. One possibility is that the feeling of meter emerges after linking the kinesthetic and vestibular systems to another perceptual modality, like vision or audition. In fact, the importance of the vestibular system was highlighted by the study of Phillips-Silver and Trainor (2005), which looked at 7-month-old babies’ metrical preferences after being bounced at binary and ternary meter. In addition, this vestibular and kinesthetic feeling of meter could also be the base for the movements found in musicians and dancers, and may point to how they learn to extract meter from distinct modalities. Another possibility, proposed by Fitch (2013), is that the hierarchical component of meter derives from a more general computation that is specialized in building temporal hierarchies. This would explain why meter is present in both music and language and why it could also be applicable to other modalities. If that were the case, the hierarchical organization of beats would be a by-product of our linguistic mind, and the organization of rhythm in music and dance may not be much different from the organization of rhythms in speech and signing, such as those constituting stress and prosody. Finally, it is still an open question whether animals can take advantage of this rhythmic mechanism that is not constrained to the auditory modality. The lack of evidence in the animal kingdom cannot be used as evidence that only humans have meter (Fitch, 2013), as several species show coordinated rhythmic abilities across modalities and deserve to be properly studied (Zarco et al., 2009; Nagasaka et al., 2013; Large and Gray, 2015).
Finally, it is important to consider the extent to which the present findings can be generalized to other populations. We tested musicians, who have been found to show an increased sensitivity to events and physical changes occurring in strong beat positions (Geiser et al., 2010; Repp, 2010; Kung et al., 2011; but see Bouwer et al., 2014). This suggests that musical expertise enhances attention to relevant metrical positions (Grahn and Rowe, 2009; Doelling and Poeppel, 2015). Accordingly, the extensive experience with auditory rhythmic stimuli in the population we tested could contribute to explain the variability in metrical timing observed in the visual domain. Future studies should test non-musicians in order to claim that meter induction is a cross-modal timing mechanism.
Conclusion
The present study tackles the nature of musical meter, fundamental to the organization of events over time in a hierarchical way. We compared meter induction in audition and vision and found a similar effect for projecting ternary meter in both modalities. However, we only found an effect for binary meter in the auditory modality. The fact that ternary meter was successfully projected in both modalities suggests that human rhythmic abilities are more domain-general than previously believed. The existence of meter induction in the visual domain supports the idea of amodal timing mechanisms. These mechanisms seem to be at least partially present in some primates and may have developed in our species. It is the evolution of these amodal timing mechanisms that may have allowed us to master language, music, dance, and other synchronized activities that require a precise timing of actions across modalities.
Author Contributions
AC-M designed and ran the study, analyzed the data, and wrote the article; RdM contributed to the design of the study, analyzing the data, and revising the article; JT designed the study and supervised the analysis of the data and the writing of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Xavier Mayoral for technical support, as well as Albert Compte and Mireia Torralba for helpful and instructive comments.
Footnotes
Funding. This work was supported by the European Research Council (ERC) Starting Grant agreement n.312519 and by the Spanish Ministerio de Economía y Competitividad (MEC) FPI grant BES-2014-070547.
References
- Bouwer F. L., Van Zuijen T. L., Honing H. (2014). Beat processing is pre-attentive for metrically simple rhythms with clear accents: an ERP study. PLoS ONE 9:e97467 10.1371/journal.pone.0097467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The psychophysics toolbox. Spat. Vis. 10 433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Brochard R., Abecasis D., Potter D., Ragot R., Drake C. (2003). The “ticktock” of our internal clock: direct brain evidence of subjective accents in isochronous sequences. Psychol. Sci. 14 362–366. 10.1111/1467-9280.24441 [DOI] [PubMed] [Google Scholar]
- Buck J., Buck E. (1968). Mechanism of rhythmic synchronous flashing of fireflies fireflies of southeast asia may use anticipatory time-measuring in synchronizing their flashing. Science 159 1319–1327. 10.1126/science.159.3821.1319 [DOI] [PubMed] [Google Scholar]
- Chemin B., Mouraux A., Nozaradan S. (2014). Body movement selectively shapes the neural representation of musical rhythms. Psychol. Sci. 25 2147–2159. 10.1177/0956797614551161 [DOI] [PubMed] [Google Scholar]
- Doelling K., Poeppel D. (2015). Cortical entrainment to music and its modulation by expertise. Proc. Natl. Acad. Sci. U.S.A. 112 E6233–E6242. 10.1073/pnas.1508431112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W. T. (2013). Rhythmic cognition in humans and animals: distinguishing meter and pulse perception. Front. Syst. Neurosci. 7:68 10.3389/fnsys.2013.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T., Zendel B. R., Ross B. (2010). Endogenous neuromagnetic activity for mental hierarchy of timing. J. Neurosci. 30 3458–3466. 10.1523/JNEUROSCI.3086-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Huang Y., Zhou L., Qian C., Wu X. (2015). Synchronization to a bouncing ball with a realistic motion trajectory. Sci. Rep. 5 1–9. 10.1038/srep11974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser E., Sandmann P., Jäncke L., Meyer M. (2010). Refinement of metre perception - training increases hierarchical metre processing. Euro. J. Neurosci. 32 1979–1985. 10.1111/j.1460-9568.2010.07462.x [DOI] [PubMed] [Google Scholar]
- Grahn J. A., Rowe J. B. (2009). Feeling the beat: premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 29 7540–7548. 10.1523/JNEUROSCI.2018-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman S. E., Gilroy L. A., Blake R. (2005). Hearing what the eyes see. Psychol. Sci. 16 228–235. 10.1111/j.0956-7976.2005.00808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Tomonaga M., Matsuzawa T. (2013). Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Sci. Rep. 3:1566 10.1038/srep01566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove M. J., Fairhurst M. T., Kotz S. A., Keller P. E. (2013a). Synchronizing with auditory and visual rhythms: an fMRI assessment of modality differences and modality appropriateness. Neuroimage 67 313–321. 10.1016/j.neuroimage.2012.11.032 [DOI] [PubMed] [Google Scholar]
- Hove M. J., Iversen J. R., Zhang A., Repp B. H. (2013b). Synchronization with competing visual and auditory rhythms: bouncing ball meets metronome. Psychol. Res. 77 388–398. 10.1007/s00426-012-0441-0 [DOI] [PubMed] [Google Scholar]
- Hove M. J., Spivey M. J., Krumhansl C. L. (2010). Compatibility of motion facilitates visuomotor synchronization. J. Exp. Psychol. Hum. Percept. Perform. 36 1525–1534. 10.1037/a0019059 [DOI] [PubMed] [Google Scholar]
- Iversen J. R., Patel A. D., Nicodemus B., Emmorey K. (2015). Synchronization to auditory and visual rhythms in hearing and deaf individuals. Cognition 134 232–244. 10.1016/j.cognition.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. R., Boltz M. (1989). Dynamic attending and responses to time. Psychol. Rev. 96 459–491. 10.1037/0033-295X.96.3.459 [DOI] [PubMed] [Google Scholar]
- Keller P. (2012). Rhythm and time in music epitomize the temporal dynamics of human communicative behavior: the broad implications of london’s trinity. Empir. Musicol. Rev. 7:17. [Google Scholar]
- Krause V., Pollok B., Schnitzler A. (2010). Perception in action: the impact of sensory information on sensorimotor synchronization in musicians and non-musicians. Acta Psychol. 133 28–37. 10.1016/j.actpsy.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Kung S.-J., Tzeng O. J. L., Hung D. L., Wu D. H. (2011). Dynamic allocation of attention to metrical and grouping accents in rhythmic sequences. Exp. Brain Res. 210 269–282. 10.1007/s00221-011-2630-2 [DOI] [PubMed] [Google Scholar]
- Large E., Gray P. (2015). Spontaneous tempo and rhythmic entrainment in a bonobo (Pan paniscus). J. Comp. Psychol. 129 317–328. 10.1037/com0000011 [DOI] [PubMed] [Google Scholar]
- Large E. W. (2008). “Resonating to musical rhythm: theory and experiment,” in Psychology of Time ed. Grondin S. (Québec: Emerald; ) 189–232. [Google Scholar]
- Large E. W. (2010). “Neurodynamics of music,” in Music Perception. Springer Handbook of Auditory Research eds Jones M., Fay R., Popper A. (London: Springer Science & Business Media; ) 201–258. [Google Scholar]
- Large E. W., Herrera J. A., Velasco M. J. (2015). Neural networks for beat perception in musical rhythm. Front. Syst. Neurosci. 9:159 10.3389/fnsys.2015.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large E. W., Jones M. R. (1999). The dynamics of attending: how people track time-varying events. Psychol. Rev. 106 119–159. 10.1037/0033-295X.106.1.119 [DOI] [Google Scholar]
- Lee K. M., Barrett K. C., Kim Y., Lim Y., Lee K. (2015). Dance and music in “gangnam style”: how dance observation affects meter perception. PLoS ONE 10:e0134725 10.1371/journal.pone.0134725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J. (2012). Hearing in Time: Psychological Aspects of Musical Meter. Oxford: Oxford University Press. [Google Scholar]
- McAuley J. D., Henry M. J. (2010). Modality effects in rhythm processing: auditory encoding of visual rhythms is neither obligatory nor automatic. Attent. Percept. Psychophys. 72 1377–1389. 10.3758/APP.72.5.1377 [DOI] [PubMed] [Google Scholar]
- Merchant H., Grahn J., Trainor L., Rohrmeier M., Fitch W. T. (2015). Finding the beat: a neural perspective across humans and non-human primates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140093 10.1098/rstb.2014.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H., Honing H. (2013). Are non-human primates capable of rhythmic entrainment? Evidence for the gradual audiomotor evolution hypothesis. Front. Neurosci. 7:724 10.3389/fnins.2013.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka Y., Chao Z., Hasegawa N. (2013). Spontaneous synchronization of arm motion between Japanese macaques. Sci. Rep. 3:1151 10.1038/srep01151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S. (2014). Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Trans. R. Soc. Lon. Ser. B Biol. Sci. 369 20130393 10.1098/rstb.2013.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S., Peretz I., Missal M., Mouraux A. (2011). Tagging the neuronal entrainment to beat and meter. J. Neurosci. 31 10234–10240. 10.1523/JNEUROSCI.0411-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S., Peretz I., Mouraux A. (2012). Steady-state evoked potentials as an index of multisensory temporal binding. Neuroimage 60 21–28. 10.1016/j.neuroimage.2011.11.065 [DOI] [PubMed] [Google Scholar]
- Nozaradan S., Zerouali Y., Peretz I., Mouraux A. (2015). Capturing with EEG the neural entrainment and coupling underlying sensorimotor synchronization to the beat. Cereb. Cortex 25 736–747. 10.1093/cercor/bht261 [DOI] [PubMed] [Google Scholar]
- Patel A., Iversen J. (2014). The evolutionary neuroscience of musical beat perception: the Action Simulation for Auditory Prediction (ASAP) hypothesis. Front. Syst. Neurosci. 8:57 10.3389/fnsys.2014.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. D., Iversen J. R., Chen Y., Repp B. H. (2005). The influence of metricality and modality on synchronization with a beat. Exp. Brain Res. 163 226–238. 10.1007/s00221-004-2159-8 [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10 437–442. 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Petkov C., Jarvis E. (2012). Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front. Evolut. Neurosci. 4:12 10.3389/fnevo.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Silver J., Trainor L. (2005). Feeling the beat: movement influences infant rhythm perception. Science 308 1430–1430. 10.1126/science.1110922 [DOI] [PubMed] [Google Scholar]
- Phillips-Silver J., Trainor L. J. (2008). Vestibular influence on auditory metrical interpretation. Brain Cogn. 67 94–102. 10.1016/j.bandc.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Ravignani A., Bowling D. L., Fitch W. T. (2014). Chorusing, synchrony, and the evolutionary functions of rhythm. Front. Psychol. 5:1118 10.3389/fpsyg.2014.01118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravignani A., Gingras B., Asano R., Sonweber R., Matellán V., Fitch T. (2013). “The evolution of rhythmic cognition: new perspectives and technologies in comparative research,” in Proceedings of the 35th Annual Conference of the Cognitive Science Society Berlin. [Google Scholar]
- Repp B. H. (2003). Rate limits in sensorimotor synchronization with auditory and visual sequences: the synchronization threshold and the benefits and costs of interval subdivision. J. Mot. Behav. 35 355–370. 10.1080/00222890309603156 [DOI] [PubMed] [Google Scholar]
- Repp B. H. (2005). Sensorimotor synchronization: a review of the tapping literature. Psychon. Bull. Rev. 12 969–992. 10.3758/BF03206433 [DOI] [PubMed] [Google Scholar]
- Repp B. H. (2010). Sensorimotor synchronization and perception of timing: effects of music training and task experience. Hum. Mov. Sci. 29 200–213. 10.1016/j.humov.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Repp B. H., Su Y.-H. (2013). Sensorimotor synchronization: a review of recent research (2006-2012). Psychon. Bull. Rev. 20 403–452. 10.3758/s13423-012-0371-2 [DOI] [PubMed] [Google Scholar]
- Su Y. H. (2014a). Audiovisual beat induction in complex auditory rhythms: point-light figure movement as an effective visual beat. Acta Psychol. 151 40–50. 10.1016/j.actpsy.2014.05.016 [DOI] [PubMed] [Google Scholar]
- Su Y. H. (2014b). Peak velocity as a cue in audiovisual synchrony perception of rhythmic stimuli. Cognition 131 330–344. 10.1016/j.cognition.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Trost W., Frühholz S., Schön D., Labbé C., Pichon S., Grandjean D., et al. (2014). Getting the beat: entrainment of brain activity by musical rhythm and pleasantness. Neuroimage 103C 55–64. 10.1016/j.neuroimage.2014.09.009 [DOI] [PubMed] [Google Scholar]
- Vialatte F., Maurice M. (2009). “Steady state visual evoked potentials in the delta range (0.5-5 Hz),” in Advances in Neuro-Information Processing eds Köppen M., Kasabov N., Coghill G. (Berlin: Springer; ) 400–407. [Google Scholar]
- Vialatte F.-B., Maurice M., Dauwels J., Cichocki A. (2010a). Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Prog. Neurobiol. 90 418–438. 10.1016/j.pneurobio.2009.11.005 [DOI] [PubMed] [Google Scholar]
- Vialatte F.-B., Maurice M., Tanaka T., Yamaguchi Y., Cichocki A. (2010b). “Analyzing steady state visual evoked potentials using blind source separation,” in Proceedings of the Second APSIPA Annual Summit and Conference Biopolis: 578–582. [Google Scholar]
- Zarco W., Merchant H., Prado L., Mendez J. C. (2009). Subsecond timing in primates: comparison of interval production between human subjects and rhesus monkeys. J. Neurophysiol. 102 3191–3202. 10.1152/jn.00066.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]


