Abstract
Libman–Sacks endocarditis, first discovered in 1924, is a cardiac manifestation of systemic lupus erythematosus (SLE). Valvular involvement has been associated with SLE and antiphospholipid syndrome (APS). Mitral valve, especially its posterior leaflet, is most commonly involved.
We report a case of a 34 year old woman with antiphospholipid antibody syndrome and SLE, who presented with mitral valve regurgitation. The patient underwent a prosthetic mitral valve replacement, with no followup complications.
We suggest mechanical valve replacement employment in the management of mitral regurgitation in Libman–Sacks endocarditis, in view of the recent medical literature and our own case report.
Keywords: Endocarditis, Mitral valve repair, Mitral valve replacement
Introduction
Libman–Sacks endocarditis, first described in 1924, is the most characteristic cardiac manifestation of systemic lupus erythematosus (SLE), although the association between Libman–Sacks endocarditis and antiphospholipid syndrome (APS) was first discovered in 1985 [1], [2]. Mitral valve, especially its posterior leaflet, is most frequently affected [3]. Transthoracic and transesophageal echocardiography have shown that valvular involvement is greater in SLE patients who have antiphospholipid antibodies and severely symptomatic individuals may require surgery [4].
Case report
We report a case of a 34-year-old woman, with a known diagnosis of APS and SLE for 10 years, who presented with a 6-month history of progressing dyspnea. Other comorbidities included hypertension and hypothyroidism. Examination revealed tachycardia and a pan-systolic murmur.
A transesophageal echocardiogram showed a severely dilated left atrium with normal left ventricular systolic function, severe mitral regurgitation with sessile vegetations on the posterior leaflet, a left ventricular end-diastolic diameter of 60 mm, and an ejection fraction of 60%. Mild tricuspid regurgitation was also reported. The patient was scheduled for valve replacement.
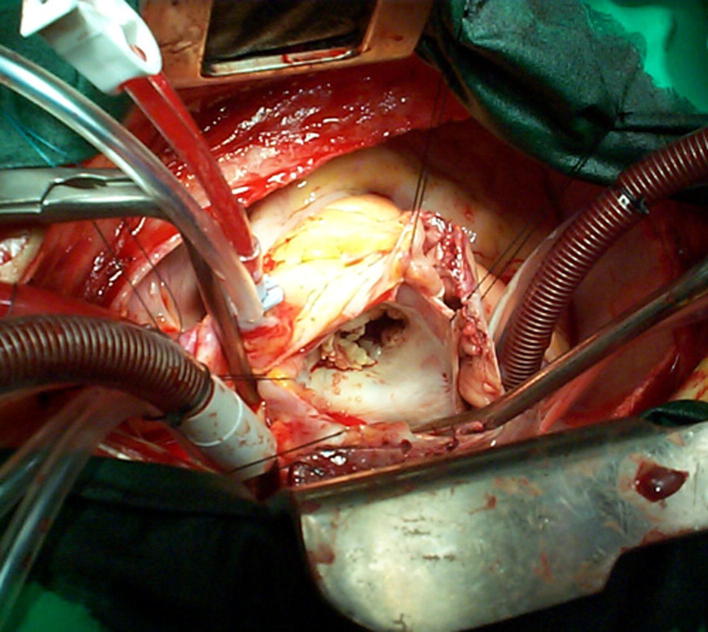
In the operating room, the mitral valve was exposed with a superior septal approach (Fig. 1). The anterior leaflet was fully excised, while the posterior leaflet with its vegetations was partially excised. A 29 mm carbomedics mechanical valve was placed.
Figure 1.
Mitral valve of the patient showing vegetations.
Histopathological examination revealed architectural distortion of the valve leaflets with myxoid degenerative changes, characteristic of Libman–Sacks endocarditis.
No intraoperative or postoperative complications were encountered. A 6-day postoperative chest radiograph showed proper position and an echocardiogram revealed normal function of the prosthesis. After 7 days the patient was discharged on long-term anticoagulation. Follow-up after more than 1 year showed no complications, including any thromboembolic events.
Discussion
Libman–Sacks endocarditis is characterized by mulberry-like clusters of verrucous lesions on the valves, most commonly the mitral valve, followed by the aortic valve. They exhibit heterogeneous echogenicity and no independent movement and can lead to complications including valve regurgitation, stenosis, infective endocarditis, and thromboembolic events [4].
Histopathology reveals inflammation with cell proliferation, degeneration, mononuclear infiltrates, and fibrin deposits [3], [4]. The natural history of the disease suggests progression over time with deterioration of the lesions [4], [5].
The origin of these lesions is very closely related to the presence of antiphospholipid antibodies, hence the association with APS and SLE [3]. Antiphospholipid antibodies interfere with coagulation profiles of the patients affecting the partial thromboplastin time and thrombin time; hence the risk of thromboembolic events. For this, the patients may require long-term anticoagulation [1], [6].
The literature reports a wide range of percentages for valve involvement with SLE, ranging from 11–74%, depending on the diagnostic technique. Transesophageal echocardiography is the best diagnostic modality established so far, being superior to the transthoracic echocardiograph [3], [4].
Treatment options can be broadly divided into medical and surgical. Medical management is reserved for hemodynamically stable patients, where a trial of steroids may be given. However, steroids in themselves may lead to early fibrosis later [3].
There is controversy in literature regarding the use of mitral valve repair versus mitral valve replacement for management of mitral regurgitation in Libman–Sacks endocarditis. Mitral valve replacement may be used for various reasons including lesser mortality, low costs, better ventricular function, a lower need of anticoagulation, and lower complications. Valve replacement offers low recurrence rates compared with repair and may be preferred for more symptomatic and severe cases. It is generally not recommended for women of child-bearing age as they would have to be put on anticoagulation therapy, which is avoided with repair. However, many believe that the patient may be ultimately put on anticoagulants anyway for the underlying disease itself.
A successful case of mitral valve replacement for mitral regurgitation in a patient with SLE was first reported in 1974 by Myerowitz et al. [7]. Hakim et al. [8] reported five such cases with mitral valve regurgitation, of which three underwent prosthetic valve replacement, while two had mitral valve repair. Long-term follow up revealed no recurrence in replacement but one of the two cases of repair relapsed, ultimately requiring valve replacement.
Bouma et al. [4], after a review of literature from 1974 to 2010, preferred mitral valve replacement over repair in most situations.
One issue with valve repair is that when cardiac valvular replacement is done, anticoagulation is mandatory.
Although we are reporting a single case of mitral regurgitation in SLE, in view of the literature and according to our experience, we suggest mitral regurgitation in Libman–Sacks endocarditis to be treated with a mechanical prosthetic mitral valve.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Hojnik M., George J., Ziporen L., Schoenfeld Y. Heart valve involvement (Libman–Sacks endocarditis) in the antiphospholipid syndrome. Circulation. 1996;93:1579–1587. doi: 10.1161/01.cir.93.8.1579. [DOI] [PubMed] [Google Scholar]
- 2.D’Alton J.G., Preston D.N., Bormanis J., Green M.S., Kraag G.R. Multiple transient ischemic attacks, lupus anticoagulant and verrucous endocarditis. Stroke. 1985;16:512–514. doi: 10.1161/01.str.16.3.512. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.L., Naguwa S.M., Cheema G.S., Gershwin M.E. Revisiting Libman–Sacks endocarditis: a historical review and update. Clin Rev Allergy Immunol. 2009;36:126–130. doi: 10.1007/s12016-008-8113-y. [DOI] [PubMed] [Google Scholar]
- 4.Bouma W., Klinkenberg T.J., van der Horst I.C., Wijdh-den Hamer I.J., Erasmus M.E., Bijl M. Mitral valve surgery for mitral regurgitation caused by Libman–Sacks endocarditis: a report of four cases and a systematic review of the literature. J Cardiothorac Surg. 2010;5:13. doi: 10.1186/1749-8090-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyssakis I., Tektonidou M.G., Vasilliou V.A., Samarkos M., Votteas V., Moutsopoulos H.M. Libman–Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med. 2007;120:636–642. doi: 10.1016/j.amjmed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Keeling D., Mackie I., Moore G.W., Greer I.A., Greaves M. British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157:47–58. doi: 10.1111/j.1365-2141.2012.09037.x. [DOI] [PubMed] [Google Scholar]
- 7.Myerowitz P.D., Michaelis L.L., McIntosh C.L. Mitral valve replacement for mitral regurgitation due to Libman–Sacks endocarditis. Report of a case. J Thorac Cardiovasc Surg. 1974;67:869–874. [PubMed] [Google Scholar]
- 8.Hakim J.P., Mehta A., Jain A.C., Murray G.F. Mitral valve replacement and repair. Report of 5 patients with systemic lupus erythematosus. Tex Heart Inst J. 2001;28:47–52. [PMC free article] [PubMed] [Google Scholar]