Abstract
Cancer formation is a complex and highly regulated multi-step process which is highly dependent of its environment, from the tissue to the patient. This complexity implies the development of specific treatments adapted to each type of tumor. The initial step of cancer formation requires the transformation of a healthy cell to a cancer cell, a process regulated by multiple intracellular and extracellular stimuli. The further steps, from the anarchic proliferation of cancer cells to form a primary tumor to the migration of cancer cells to distant organs to form metastasis, are also highly dependent of the tumor environment but of intracellular molecules and pathways as well. In this review, we will focus on the regulatory role of reactive oxygen species (ROS) and autophagy levels during the course of cancer development, from cellular transformation to the formation of metastasis. These data will allow us to discuss the potential of this molecule or pathway as putative future therapeutic targets.
Abbreviations: 3-MA, 3-methyladenine; AMPK, AMP-activated protein kinase; ATG, autophagy related gene; ATG8, autophagy related gene 8; BNIP3, BCL-2/adenovirus E1B 19-kDa-interacting protein 3; EMT, epithelial-mesenchymal transition; GABARAP, GABAA receptor-associated protein; GABARAPL1, GABARAP protein-like 1; GEC1, glandular epithelial cell 1; GABARAPL2, GABARAP protein-like 2; HIF-1α, hypoxia-inducible factor-1 α; HMGB1, high-mobility group protein B 1; HSP, heat shock protein; KEAP1, Kelch-like ECH-associated protein 1; LC3, light chain 3; mTORC1, mammalian target of rapamycin complex; NLRP3, NOD-like receptor family, pyrin domain containing 3; NRF2, nuclear factor erythroid 2-related factor 2; PINK1, PTEN induced putative kinase 1; ROS, reactive oxygen species; SQSTM1, sequestosome 1; ULK1, unc-51 like autophagy activating kinase 1
Keywords: ROS, Mitochondria, Antioxidant, Mitophagy, Autophagy, Cancer
Graphical abstract
Highlights
-
•
In cancer cells, ROS are able to regulate the different steps of autophagy pathway.
-
•
During cancer initiation, anti-tumoral autophagy is going through ROS elimination.
-
•
During cancer development, pro-tumoral autophagy is linked to decreased ROS levels.
-
•
Autophagy inhibitor or antioxidant with anti-cancer drug: a new therapeutic approach?
Introduction
Macroautophagy (hereafter called autophagy) is a multi-step process which maintains cellular homeostasis via the degradation and recycling of long-lived proteins, intracellular aggregates as well as damaged organelles. This process requires the intervention of about 40 proteins and leads to the formation of a double membrane structure, called the phagophore, which engulfs part of the cytoplasm as well as organelles, to form an autophagosome. This vesicle ultimately fuses with the lysosome to induce the formation of an autophagolysosome and the degradation and recycling of its content [1]. The induction of autophagy is regulated by several kinase cascades but the main regulator of the induction of the phagophore formation stays the mammalian target of rapamycin complex 1 (mTORC1) kinase which can integrate the different extracellular stresses (for a review, see [2]). Following inhibition of mTORC1, unc-51 like autophagy activating kinase 1 (ULK1)/ATG1 and phosphatidyl inositol kinase 3 (PI3K) are subsequently activated to induce the initiation of the formation of a phagophore. The elongation of the double membrane structure is then activated by two post-translational modifications, similar to those described during ubiquitinylation or SUMOylation, leading to the covalent addition of ATG12 onto ATG5 and ATG8 family members onto phospholipids. The latter is considered to be correlated to the levels of autophagy and therefore to be accepted as a direct marker of intracellular autophagy.
Autophagy is regulated by numerous stresses such as nutrient starvation, hypoxia, ATP/AMP ratio, intracellular ROS levels, bacteria and virus infection or chemical drugs. This cellular pathway, first believed to be non-specific, is now divided in basal or induced autophagy which can be either not selective or selective [3]. The latter requires cargo adapter proteins, such as Sequestosome 1 (SQSTM1)/p62, neighbor of BRCA1 gene 1 (NBR1) or Bcl-2/adenovirus E1B 19-kDa-interacting protein 3 long form (NIX/BNIP3L), to induce the recruitment of cargos, which are the molecules or organelles targeted for degradation, into the autophagosomes. This step also requires an interaction between the cargo adapters and the ATG8 family proteins (LC3, GABARAP, GABARAPL1/GEC1 and GABARAPL2) via two domains present within the cargo adapter proteins: an Ubiquitin Binding domain (UBA) recognizing ubiquitinylated substrates, and a LC3-interaction domain (LIR) recognizing ATG8 proteins [4], [5], [6], [7]. Amongst the different types of selective autophagy, we can cite mitophagy, inducing the degradation of damaged mitochondria, xenophagy, leading to the degradation of extracellular pathogens, and aggrephagy, allowing the degradation of intracellular aggregates [8].
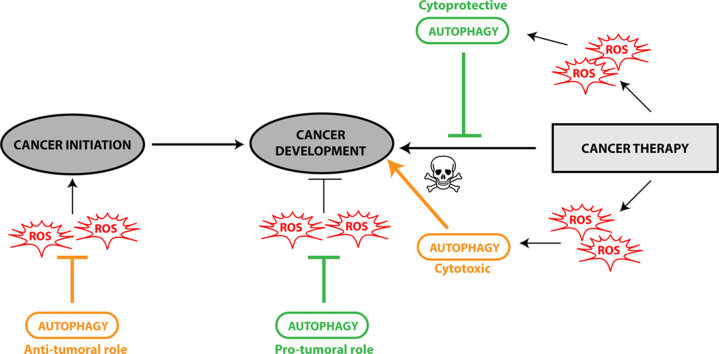
Autophagy has been described to be involved in biological processes such as cellular homeostasis, development and immune response but it has also been demonstrated to be deregulated in different pathologies, in particular neurodegenerative diseases or cancers [9]. If it is now well accepted that autophagy presents a protective role in neurodegenerative diseases, its effect is far more complex and paradoxal in cancers. According to the stage of the tumor as well as the tissue or the cells targeted, the role of autophagy can be positive or negative for the growth of the tumor [10], [11], [12]. For example, autophagy can prevent the transformation of healthy cells into cancerous cells but can also induce resistance to chemotherapeutic treatments. More importantly, this positive or negative effect seems to be directly correlated to autophagy levels induced by different intracellular or extracellular stressors such as ROS accumulation.
In this review, we will describe the link between ROS production and its effect on autophagy during the different stages of the formation of a tumor: cellular transformation, tumor growth, interaction between stroma and cancer cells, induction of metastasis and response to chemical treatments.
Relationship between autophagy and ROS
ROS are a group of molecules including superoxide anion (O2•−), hydroxyl radical (OH•) and hydrogen peroxide (H2O2). These molecules are synthetized within the cells through oxygen metabolism and represent, at low levels, important signaling molecules [13]. Moreover, ROS can also be involved in oxidative stress which is characterized by elevated intracellular levels of ROS leading to proteins, lipids and DNA damage [14]. In healthy cells, mitochondria produce the majority of intracellular ROS through the leaking of electrons from the electron transport chain during oxidative phosphorylation [15], [16]. Intracellular ROS levels can then increase the mitochondrial dysfunction inducing the accumulation of high concentration of ROS, oxidization of proteins, lipids and DNA, redox imbalance and oxidative stress [17], [18]. Moreover, ROS can also play an important role in inflammation by inducing the secretion of pro- or anti-inflammatory molecules regulating the different steps of cancer development (for a detailed review, see [19]).
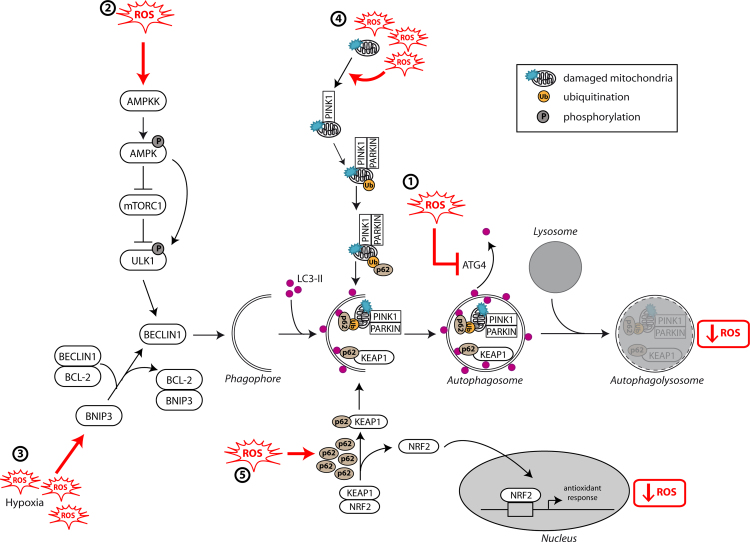
As previously described, ROS levels have been shown to regulate autophagy induction in the cells. In a specific manner, induction of autophagy following nutrient starvation requires the production of H2O2 that oxidizes ATG4, an enzyme involved in ATG8 protein maturation and delipidation. This oxidization modification mainly inactivates the delipidation activity of ATG4 leading to an increased formation of LC3-associated autophagosomes [20] (Fig. 1(1)). In an indirect manner, ROS can regulate autophagy through AMP-activated protein kinase (AMPK). Indeed, oxygen and nutrient deprivation induce AMP:ATP accumulation and activation of AMPK leading to the inhibition of mTORC1 and to autophagy induction [21], [22]. AMPK also regulates autophagy by phosphorylating ULK1/ATG1, activation which is necessary to induce autophagy upon starvation [23], [24], [25]. AMPK is sensitive to oxidative stress and can be phosphorylated by the upstream kinase AMPK kinase (AMPKK) following H2O2 accumulation, leading to its activation and to an indirect induction of autophagy [26] (Fig. 1(2)). ROS can also regulate autophagy through the regulation of transcription factor activity such as NF-κB leading to the induction of autophagy gene expression (BECLIN1/ATG6 or SQSTM1/p62) in cancer cells [27], [28], [29].
Fig. 1.
Relationship between autophagy and ROS. ROS levels regulate autophagy levels by different pathways such as: (1) oxidization of ATG4 leading to accumulation of autophagosomes, (2) activation of the AMPK signaling cascade inducing the initiation of autophagy through the ULK1 complex, (3) disruption of BECLIN1–BCL-2 interaction leading to the initiation of autophagy or (4) alteration of mitochondria homeostasis leading to mitophagy activation. Autophagy inhibits ROS accumulation through (4) the elimination of damaged mitochondria by mitophagy or (5) the degradation of KEAP1 by selective autophagy mediated by SQSTM1/p62 and the expression of NRF2-regulated antioxidant genes.
Autophagy, ROS and tumorigenesis
Recent studies have described a complex role of the autophagy pathway during tumor initiation. On one hand, autophagy protects against the production of ROS in the cells and therefore inhibits their deleterious effect on DNA mutation, which have been extensively described to induce tumorigenesis, defined as the transformation of a normal cell into a cancer cell [30], [31] (Fig. 2). Autophagy is then considered as a tumor suppressor mechanism by mainly preventing ROS accumulation through elimination of damaged mitochondria which are known to be the major source of ROS. This selective autophagy, called mitophagy, is mediated by two different molecular pathways: NIX/BNIP3L and PARKIN (PARK2)/PTEN induced putative kinase 1 (PINK1) [32], [33], [34], [35]. Nix/BNIP3L interacts with GABARAP and GABARAPL1 at the autophagosome and targets mitochondria for degradation [36], [37]. PARKIN/PINK1 allows the selective degradation of damaged and dysfunctional mitochondria in response to mitochondrial membrane depolarization induced by ROS [38] (Fig. 1(4)). It has been previously shown that elimination of damaged mitochondria by autophagy leads to decreased ROS production, thereby limiting tumor-promoting effect of ROS [39]. Consequently, autophagy inhibition, following ATG5 or ATG7 deletion, leads to chronic oxidative stress, accumulation of damaged mitochondria, tissue damage and inflammation which all favor tumor initiation [40], [41], [42]. Moreover, autophagy can regulate oxidative stress through the nuclear factor erythroid 2-related factor 2 (NRF2)/kelch-like ECH-associated protein 1 (KEAP1) and SQSTM1/p62 pathway [43], [44]. SQSTM1/p62, an autophagy substrate and cargo adapter, can interact with and target KEAP1 to the autophagosomes leading to its selective degradation [45] and the release of NRF2 which can then translocate to the nucleus and activate antioxidant-defense genes, such as glutathione peroxidase, superoxide dismutase and thioredoxin [46] (Fig. 1(5)). Upon normal conditions, NRF2 is sequestered by KEAP1 and inactivated by proteasomal degradation. Following oxidative stress, either ubiquitin activity of KEAP1 is inhibited or SQSTM1/p62 is up-regulated and interacts with KEAP1 leading to the inhibition of the degradation of NRF2 and to its activation.
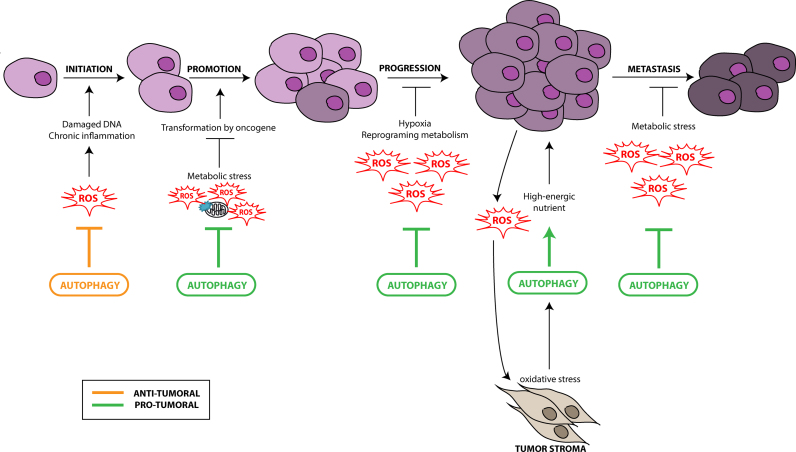
Fig. 2.
Autophagy, ROS and cancer. The role of autophagy in cancer depends on the tissue, the stage and the type of tumor. During the early steps of cancer development, autophagy plays a protective role by preventing ROS-induced damages on DNA and protein leading to an inhibition of tumorigenesis. During the late steps of cancer development (promotion, progression and metastasis), autophagy presents a pro-tumoral role through the elimination of ROS-induced metabolic stress and the production of nutrients required for cancer cell survival. During the development of the primary tumor, a strong interaction between stroma cells of the microenvironment and cancer cells has been observed. The cancer cells under hypoxia induce the formation of ROS which can activate autophagy in stroma cells. These cells will then provide high-energic nutrients, such as lactate or ketones, necessary for cancer cell survival and proliferation.
Autophagy also presents a tumor-suppressive role by regulating chronic inflammation which has been described as an important risk of cancer initiation such as in liver cancer [47], [48]. Inflammation is an important process activated by a loss of tissue or cell homeostasis which can be induced by tissue damage, cell death or pathogen infection. The activation of inflammation leads to the release of soluble molecules (cytokines, chemokines, ROS, matrix proteinases or vascular epithelial growth factor, VEGF) by macrophages and mast cells in order to activate the recruitment and infiltration of leukocytes at the site of injury. Several studies have shown that autophagy defects following deletion of ATG16L1, BECLIN1 or LC3B induce an accumulation of damaged mitochondria and mitochondrial ROS leading to an induction of inflammation linked to increased levels of IL-1β and IL-18 [49], [50], [51], [52]. Indeed, ROS generation activate pro-inflammatory factors such as the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome [50], [52], a multiprotein complex responsible of maturation and secretion of these pro-inflammatory cytokines. But, IL-1 has also been demonstrated to be involved in the production of ROS and high levels of IL-1 have been associated with a poor prognosis [53]. Moreover, autophagy also increases the extracellular release of nuclear protein high-mobility group box protein 1 (HMGB1) depending of its redox status [54], [55]. The secreted HMGB1 can then be recognized by different membrane receptors such as toll-like receptors (TLR2, 4 and 9) or macrophage-1 antigen (Mac-1) leading to the acceleration of the pro-inflammatory response dependent on cytokines and type-I interferon (IFN) and later to tumor progression as well as metastasis [56]. Defects of autophagy therefore lead to an inflammatory environment which contributes to cancer initiation. However, the interplay between inflammatory cytokines and cancer remains unclear given the pro- and anti-tumoral effect of these molecules depending on the tumor context.
During later stages of tumor initiation, autophagy is required for cell transformation by the RAS oncogene in order to promote cell tolerance to stress (Fig. 2). Indeed, autophagy inhibition reduces transformation, proliferation of mouse embryonic fibroblasts (MEF) transformed with HRAS and MDA-MB-231 breast cancer cells presenting ectopic KRAS expression [57]. In these models, autophagy is necessary for energy production during transformation thanks to its role in glucose uptake and glycolytic flux. Moreover, other studies have shown that immortalized baby mouse kidney (iBMK), MCF-10A and pancreatic ductal adenocarcinoma (PDAC) cell lines described to express oncogenic RAS present high levels of basal autophagy and that inhibition of autophagy following ATG5 or ATG7 deletion prevent RAS-induced growth and proliferation [58], [59], [60]. This observation could be explained by the fact that mitochondrial respiration is necessary for RAS-induced tumorigenesis and that active autophagy is necessary to maintain cellular homeostasis [61]. Indeed, impairment of autophagy in these different models is associated with decreased cell survival, accumulation of damage mitochondria and oxidative stress suggesting that activation of oncogenic RAS induces autophagy to sustain metabolic needs and lead to an “addiction” of cells to autophagy [58], [59], [62], [63], [64]. Moreover, deficiency in SQSTM1/p62 has been shown to reduce tumorigenicity and increase ROS levels following RAS activation [58], [65], [66].
Similar results were obtained in a different model of tumorigenesis. Inhibition of autophagy by FAK family-interacting protein of 200 kDa (FIP200) deletion in an in vitro breast cancer model driven by the polyoma virus middle T (PyMT) oncogene leads to decreased tumor initiation and progression associated with an increased number of mitochondria presenting an abnormal morphology [67], confirming the pro-tumorigenic role of autophagy.
Taken together, these studies confirm the complex and paradoxal role of autophagy in cancer initiation. Indeed, autophagy can act as a tumor suppressive mechanism during early stages of cancer development through the prevention of inflammation and genome instability [41], [42] but can also induce cancer cell survival during transformation-induced metabolic stress [68], [69].
Autophagy, ROS, tumor progression and metastasis
During in vivo tumor formation, autophagy has been shown to be necessary for the cancer cells to survive under hypoxic stress before the vascularization of the tumor [30]. Even if the mechanism is still unclear, many studies suggest a role of autophagy in the regulation of cancer cell metabolism allowing them to meet requirements for rapid proliferation.
High levels of autophagy are indeed observed in hypoxic regions of tumors and autophagy has been described to be activated by hypoxia (lack of adequate oxygen supply) and ischemia (glucose deprivation and hypoxia) to promote survival of cancer cells [41], [42], [64] (Fig. 2). Hypoxia induces ROS production leading to the stabilization of hypoxia-inducible factor-1α (HIF-1α) [70]. HIF-1α, a key regulator of oxygen homeostasis, induces mitophagy through expression of Bcl-2/adenovirus E1B 19-kDa-interacting protein 3 (BNIP3) allowing the cells to survive during prolonged hypoxia by preventing increased levels of ROS [71]. Indeed, BNIP3 is an HIF-1α target gene and disrupts the interaction between BECLIN1/ATG6 and BCL-2, inducing its release and autophagy induction [72] (Fig. 1(3)). Disruption of autophagy by BECLIN1, ATG5 or ATG7 knockdown leads to hypoxia-induced cell death. BNIP3-induced autophagy is necessary to prevent the increase in ROS production during hypoxia [73] and therefore represents a survival adaptive mechanism [69], [74]. Autophagy can also be induced, independently of HIF-1α, by different pathways such as AMPK and Unfolded Protein Response (UPR) during hypoxia [75], [76]. Moreover, cancer cells require higher bioenergetic needs than normal cells leading to a reprograming of metabolism. This includes the recycling of intracellular components to increase the production of ATP and tricarboxylic acid (TCA) cycle intermediates which in return leads to accumulation of ROS due to increased oxidative phosphorylation [10], [77], [78].
Tumor progression and aggressiveness are characterized by metastasis, epithelial–mesenchymal transition (EMT) and angiogenesis. Metastasis is a multi-step process which allows cancer cells to migrate to distant organ sites [79], [80]. EMT is the first step of metastasis and is characterized by the loss of epithelial properties as well as the acquirement of mesenchymal properties leading to increased cell mobility [81]. Previous studies have described a pro-metastasis role of autophagy (Fig. 2). For example, inhibition of autophagy by FIP200 deletion leads to a decrease in metastatic potential associated with an accumulation of damaged mitochondria which could lead to increased level of ROS [67]. Moreover, increased autophagy in human cancer is associated with metastasis and poor prognosis for patients with melanoma and breast cancer [82], [83]. Interestingly, autophagy also promotes resistance to anoikis (detachment-induced cell death) and invasion [84], [85], two processes necessary for colonization of other organs. Indeed, detachment of cells from the primary tumor induces autophagy which in return provides resistance to anoikis [57], [86], [87] which is necessary for the metastasis of hepatocellular carcinoma (HCC) and mammary epithelial cells (MEC) [88], [89]. During detachment from the extracellular matrix (ECM), epithelial cells present modifications of their metabolism, including a reduction of ATP production and increased ROS production [90], which will lead to AMPK-induced autophagy in response to metabolic stress [23] or to protein kinase like endoplasmic reticulum kinase (PERK)-induced autophagy in response to oxidative stress [91]. Inhibition of autophagy by knock-down of ATG12 or deficiency in SQSTM1/p62 also leads to a decrease in invasion and migration phenotypes correlated with metabolism defects in glioma and glioblastoma cells [92], [93]. Similarly, autophagy increases HCC invasion abilities through activation of EMT [94]. Moreover, inhibition of autophagy in pancreatic ductal adenocarcinoma (PDAC) leads to tumor regression of xenografts and orthotopic models and mice prolonged survival [59].
However, the role of autophagy in metastasis is still a double-edged sword [95] and autophagy can also inhibit autophagy independently of ROS. Autophagy can inhibit metastasis by activating the release of HMGB1 which induces an anti-tumor immune response [95]. Autophagy may also regulate metastasis by inhibiting tumor necrosis and immune cell infiltration necessary for this metastasis [64], [96], [97]. Similarly, autophagy can inhibit EMT by degrading SNAIL and TWIST, two major regulators of this process, leading to decreased EMT and invasion phenotypes [98]. We can hypothesize that autophagy might also inhibit EMT through elimination of ROS and inhibition of inflammation. In fact, EMT can be induced by IL-1, IL-6 or TGFβ cytokines which regulate SNAIL or TWIST. ROS can also induce HIF-1α and lysil oxidase (LOX) expression leading to decreased levels of E-Cadherin associated with an activation of EMT and cancer cell migration [99].
Angiogenesis is a key step during tumor growth and metastasis [100] but the relationship between angiogenesis and autophagy is poorly understood. Indeed, several studies have shown that angiogenesis inhibitors induce autophagy and apoptosis in endothelial cells, independently of nutrient or hypoxia stresses [101], [102]. On the contrary, treatment of human aortic endothelial cells (HAEC) with chemerin, an angiogenesis stimulator, increases ROS production in association with an upregulation of autophagy genes [103].
Angiogenesis has also been described to be regulated by inflammation. Indeed, hypoxic cancer cells present elevated levels of HIF-1α which lead to the expression of angiogenic factors such as VEGF which can also be released by activated immune cells (e.g. macrophages) during inflammation. Therefore, autophagy would allow cell survival under hypoxia and starvation in the tumor core while inflammation would lead to the induction of angiogenesis, two processes regulated by ROS accumulation.
Autophagy, ROS and stroma–tumor relationship
Tumor stroma is a compartment which includes different cell types such as myofibroblasts/cancer-associated fibroblasts (CAFs), immune cells, endothelial and adipocyte cells. These cells are in constant interaction with tumor cells and can regulate tumor cell proliferation, tumor growth, progression and metastasis [104]. Tumor stroma is characterized by the presence of different stress factor such as hypoxia, high level of ROS, high metabolic stress and lack of growth factors which can induce autophagy in several tumor compartments and especially in CAFs [105], [106], [107], [108], [109], [110]. Recent studies highlighted a discrepancy in the autophagic activities between tumor and stroma cells and a model called "the autophagic tumor stroma model of cancer metabolism” has been proposed by the authors [111], [112], [113], [114], [115] (Fig. 2). In this model, cancer cells can induce an oxidative stress mimicking hypoxia in CAFs and therefore increasing ROS production. This stress activates the transcription factors HIF-1α and NF-κB leading to the induction of autophagy and mitophagy in the microenvironment surrounding the tumor. The increase of autophagy in tumor stroma allows cells to survive against senescence and induce the secretion of recycled and high-energetic nutrients, such as ketone and lactate, which will feed cancer cells during tumor growth and metastasis [116], [117], [118], [119]. Moreover, ROS production in fibroblasts increases DNA damage, genomic instability and stemness in cancer cells, mechanisms essential for tumor progression.
Therefore, contrary to the initial “Warburg effect” dogma describing that cancer cells rely mainly on glycolysis during tumor growth, cancer cells are now described to present a decreased autophagy flux, a high oxidative phosphorylation and a high ROS production. On the other hand, while the stroma cells rely on a high autophagic activity which is believed to recycle the nutrients necessary in order to ‘feed’ the adjacent proliferating cancer cells. This model has been confirmed by a genome wide transcriptional analysis which demonstrated that more than 95 mitochondria-associated genes were up-regulated in human breast cancer cells [120]. Among these genes, 40 were involved in mitochondrial translation of proteins of the oxidative phosphorylation chain. More interestingly, these genes were not expressed, or only at very low levels, in the adjacent stroma cells and some of these markers were associated with a poor clinical outcome. The authors conclude that the tumor cells should be seen as a “parasite undergoing anabolic reprogramming to accentuate its mitochondrial power”.
These two models can be combined to describe the oxidative phosphorylation active/non-autophagic cancer cells to be ‘fed’ in nutrients by the glycolytic/autophagic stroma cells.
Autophagy, ROS and cancer therapy
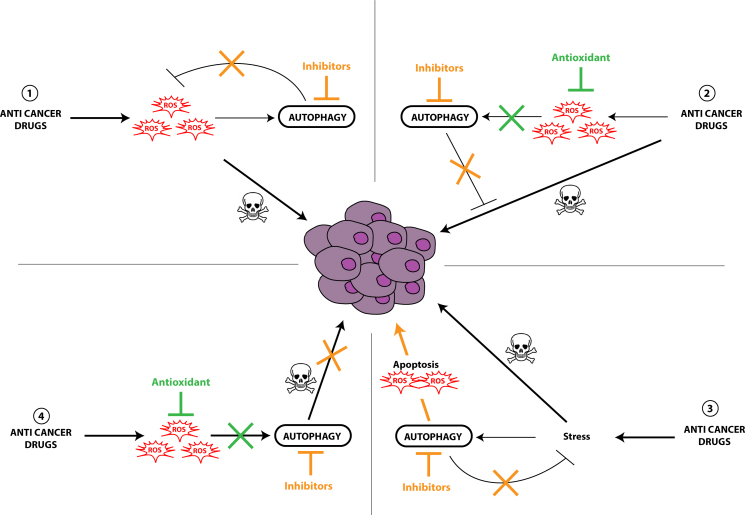
According to the recent paradigm, autophagy has been shown to be induced by and to protect against cellular stress induced by numerous drugs used in cancer treatment. These observations led to the development of several clinical trials involving the addition of an inhibitor of the autophagy flux, such as hydroxychloroquine, together with a drug already in use in cancer treatment in order to potentiate its effect (for a review, see [121]). More interestingly, various anti-cancer treatments have been shown to activate ROS-induced autophagy which in turn leads to either the development of cell drug resistance or to the induction of apoptosis or both. Regarding the complexity of cellular effects observed during cancer therapy, several models have been proposed, each involving an important role of ROS as well as autophagy or apoptosis. The first model describes the use of anticancer drugs inducing the accumulation of ROS leading to cell death. Nevertheless, the accumulation of ROS also increased cytoprotective autophagy levels leading to cancer cell drug resistance and cancer cell survival. In this particular case, the use of autophagy inhibitors restored the sensitivity to the treatment (Fig. 3(1)). One example is the use of Ciclopiroxolamine (CPX) in human rhabdomyosarcoma (Rh30 and RD) cells which induces ROS-induced cytoprotective autophagy. The authors demonstrated the activation of the Mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinases 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 MAP kinase (p38 MAPK) signaling cascade by CPX and ROS but only JNK has been shown to be directly involved in autophagy induction [122]. In the second model, Honokiol, a drug currently in development for the treatment of prostate cancer, induces autophagy in vitro and in tumors in mice models inducing cell drug resistance. The inhibition of drug-induced autophagy by 3-methyladenine (3-MA)or ATG5 siRNA led to increased cancer cell death via apoptosis [123](Fig. 3(2)). Moreover, the authors showed that the use of antioxidants (N-acetylcysteine, catalase, superoxide dismutase) in their models inhibits autophagy levels demonstrating the direct involvement of ROS in the induction of autophagy. This model can also explain the effects observed with drugs targeting mitochondria, such as Mitoquinone in MDA-MB-231 cells [124] or Quercetin in human glioblastoma cells (U373MG) [125]. The alteration of mitochondria homeostasis would increase ROS levels but also activate autophagy, the latter leading to cell survival by degrading damaged mitochondria. Inhibiting autophagy levels, by 3-MA, chloroquine or ATG7 knockdown, will restore the effect of the compound through induction of ROS-induced apoptosis. A third model has been described for the use of 3-bromopyruvate (3BrPA), an inhibitor of hexokinase II involved in glycolysis, in breast cancer cells (MDA-MB-231) or artemisinin, an anti-malaria drug, in human lung cancer cells (A549). The authors proposed that increased ROS-induced apoptosis was the consequence of the combined treatment of 3BrPA and chloroquine and that ROS accumulation was not the cause of the induction of autophagy [126] (Fig. 3(3)). In this model, the addition of autophagy inhibitors together with the anticancer drug would increase cell death but an antioxidant would not. A fourth model has been described in breast cancer cell lines using carnosol, a polyphenol, which induced early ROS-induced autophagy and late apoptosis leading to cell death [127]. The authors showed that autophagy and cell death were correlated to mitochondrial damage as well as increased ROS levels. In this model, the authors demonstrated the tight link between autophagy and apoptosis and that autophagy inhibitors and antioxidants would inhibit cancer cell death (Fig. 1(4)). Similar data were obtained with psoralidin or Resveratrol which increased ROS accumulation and autophagy levels leading to human lung cancer (A549) and human colon cancer (HT-29, COLO 201) cell death, respectively. These effects were blocked by the addition of 3-MA or antioxidants [128], [129].
Fig. 3.
Interplay between ROS and autophagy in the regulation of therapy efficiency. Anti-cancer treatments can increase ROS-induced autophagy leading to cell drug resistance (1–3). Given these observations, inhibitors of autophagy (1–3) or antioxidants (2) might be used to potentiate or restore the cytotoxicity effect of the drug. Regarding the fourth model, ROS accumulation induces cytotoxic autophagy and apoptosis therefore the use of autophagy inhibitors or antioxidants would inhibit the effect of the drug (4). These four models, involving ROS, autophagy and apoptosis, point out the difficulty to find the most relevant combination of autophagy inhibitors or antioxidants together with anti-cancer drugs in cancer therapy and the necessity to first characterize and understand the cancer cell response to cancer therapies.
Altogether, these studies highlight the complexity of choosing the right treatment for a specific patient or a specific tumor. This observation led researchers to adapt their protocols to the genetic or biochemical background of each patient, a procedure called theranostics or personalized medicine. This approach is based on the development of new diagnostic protocols, targeting new specific biomarkers, whose results would lead to the use of a specific targeted therapy. In regards of the interplay of ROS and autophagy, the first difficulty will be to find easy and rapid protocols to quantify both ROS and autophagy levels in cellulo, in situ and in vivo. Regarding ROS levels, some protocols have already been set up to quantify in vivo levels. We can cite the use of fluorescent probes regulated by ROS such as Dichlorofluorescein diacetate (DCF-DA), Dihydrorhodamine 123 (DHR) or Amplex Ultrared, a highly specific fluorogenic substrate used to detect H2O2 in human skeletal muscle [130], [131], or the use of spin probes, modified by ROS, and detected in human tissues by Electron Paramagnetic Resonance (EPR) [132]. A second protocol would consist of the detection of biological molecules modified in vivo by ROS and then considered as biomarkers. For example, we can cite the 15-F2t-IsoP(8-Iso-PGF2α) which gives a major urinary metabolite (2,3-Dinor-5,6-Dihydro-15-F2t-IsoP(F2-IsoP-M)) following oxidization [133]. A third, but indirect possibility would be to quantify mitochondrial activity, which is described as a major cause of ROS accumulation in tissues, by using fluorescent probes. For example, 18F-fluorobenzyl triphenylphosphonium cation (18F-FBnTP) has been used as a PET (Positron emission tomography) imaging probe to quantify mitochondrial membrane potential defects in vivo in breast or prostate cancers [134]. The major problem will be to accurately quantify autophagy levels in cancer and stroma cells. Indeed, LC3B levels have been described as an accurate marker of the number of autophagosomes in cells but we also know that an accumulation of these intracellular vesicles can be linked to an increase of autophagy induction or an inhibition of autophagosome degradation by the lysosomes. To our knowledge, no other autophagy marker is currently available in order to discriminate between an increase or a decrease of overall autophagy flux in vivo [135]. Therefore, before using the pair autophagy/ROS as new cancer biomarkers to choose the adequate therapy protocol, research will have to develop new diagnostic tests to quantify these markers in vivo.
Conclusions
In this review, we summarized the current data showing the involvement of ROS signaling and autophagy during the course of cancer initiation, progression as well as response to cancer therapy but more importantly, we pointed out the complex interplay between these two cellular signal and mechanism. Taken together, these data particularly demonstrate that the effects of ROS and autophagy are different according to the stage of the development of the tumor. Precautions will have to be taken before considering the choice of a particular treatment since we showed that, according to the drug used, ROS or autophagy can favor or inhibit the applied treatment. Therefore a potential new way of cancer treatment might be to include antioxidants or autophagy inhibitors to inhibit cytoprotective ROS-induced autophagy during the course of treatment. But, in some cases, adding antioxidants or autophagy inhibitors might also decrease ROS or autophagy-induced cell death. It is therefore now clear that, in the future, in order to choose the more efficient combination of drugs for each treatment, an extensive knowledge of the cellular events occurring in each particular tumor category (tissue, cells, stage, autophagy levels, ROS levels) would be necessary to tightly regulate the balance between ROS accumulation and ROS-induced autophagy or apoptosis and induce cancer cell death and tumor regression in vivo. The future of cancer treatment will require the development of personalized medicine, called theranostic, and the development of ‘one specific treatment for one specific tumor in one patient'.
Acknowledgment
Laura Poillet is supported by a fellowship of the (12V131). This work is supported by funding from the Ligue contre le Cancer, région Grand-Est (Conférence de Coordination Interrégionale du Grand Est, CCIR-GE 2013, SP/SW n°214-2014).
References
- 1.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annual Review of Genetics. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. 19653858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlop E.A., Tee A.R. mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Seminars in Cell and Developmental Biology. 2014;36C:121–129. doi: 10.1016/j.semcdb.2014.08.006. 25158238 [DOI] [PubMed] [Google Scholar]
- 3.Weidberg H., Shvets E., Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Annual Review of Biochemistry. 2011;80:125–156. doi: 10.1146/annurev-biochem-052709-094552. 21548784 [DOI] [PubMed] [Google Scholar]
- 4.Pankiv S. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. Journal of Biological Chemistry. 2007;282(33):24131–24145. doi: 10.1074/jbc.M702824200. 17580304 [DOI] [PubMed] [Google Scholar]
- 5.Kirkin V. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Molecular Cell. 2009;33(4):505–516. doi: 10.1016/j.molcel.2009.01.020. 19250911 [DOI] [PubMed] [Google Scholar]
- 6.Rozenknop A. Characterization of the interaction of GABARAPL-1 with the LIR motif of NBR1. Journal of Molecular Biology. 2011;410(3):477–487. doi: 10.1016/j.jmb.2011.05.003. 21620860 [DOI] [PubMed] [Google Scholar]
- 7.Novak I. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Reports. 2010;11(1):45–51. doi: 10.1038/embor.2009.256. 20010802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemasters J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Research. 2005;8(1):3–5. doi: 10.1089/rej.2005.8.3. 15798367 [DOI] [PubMed] [Google Scholar]
- 9.Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. New England Journal of Medicine. 2013;368(19):1845–1846. doi: 10.1056/NEJMc1303158. 23656658 [DOI] [PubMed] [Google Scholar]
- 10.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nature Reviews Cancer. 2012;12(6):401–410. doi: 10.1038/nrc3262. 22534666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ávalos Y. Tumor suppression and promotion by autophagy. BioMed Research International. 2014;2014:603980. doi: 10.1155/2014/603980. 25328887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenific C.M., Debnath J. Cellular and metabolic functions for autophagy in cancer cells. Trends in Cell Biology. 2014 doi: 10.1016/j.tcb.2014.09.001. 25278333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gough D.R., Cotter T.G. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death and Disease. 2011;2:e213. doi: 10.1038/cddis.2011.96. 21975295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross C.E. Oxygen radicals and human disease. Annals of Internal Medicine. 1987;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. 3307585 [DOI] [PubMed] [Google Scholar]
- 15.St-Pierre J. Topology of superoxide production from different sites in the mitochondrial electron transport chain. Journal of Biological Chemistry. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. 12237311 [DOI] [PubMed] [Google Scholar]
- 16.Murphy M.P. How mitochondria produce reactive oxygen species. Biochemistry Journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. 19061483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poli G. Oxidative stress and cell signalling. Current Med. Chem. 2004;11(9):1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 18.Fruehauf J.P., Meyskens F.L., Jr. Reactive oxygen species: a breath of life or death? Clinical Cancer Research. 2007;13(3):789–794. doi: 10.1158/1078-0432.CCR-06-2082. 17289868 [DOI] [PubMed] [Google Scholar]
- 19.Vendramini-Costa D.B., Carvalho J.E. Molecular link mechanisms between inflammation and cancer. Current Pharmaceutical Design. 2012;18(26):3831–3852. doi: 10.2174/138161212802083707. 22632748 [DOI] [PubMed] [Google Scholar]
- 20.Scherz-Shouval R. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO Journal. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoki K., Zhu T., Guan K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 22.Gwinn D.M. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. 18439900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology. 2011;13(2):132–141. doi: 10.1038/ncb2152. 21258367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan D.F. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. 21205641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan D. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7(6):643–644. doi: 10.4161/auto.7.6.15123. 21460621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi S.L. The regulation of AMP-activated protein kinase by H(2)O(2) Biochemistry Biophysics Research Communication. 2001;287(1):92–97. doi: 10.1006/bbrc.2001.5544. 11549258 [DOI] [PubMed] [Google Scholar]
- 27.Chen Y. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death and Differentiation. 2008;15(1):171–182. doi: 10.1038/sj.cdd.4402233. 17917680 [DOI] [PubMed] [Google Scholar]
- 28.Djavaheri-Mergny M. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. Journal of Biological Chemistry. 2006;281(41):30373–30382. doi: 10.1074/jbc.M602097200. 16857678 [DOI] [PubMed] [Google Scholar]
- 29.Boyer-Guittaut M. The role of GABARAPL1/GEC1 in autophagic flux and mitochondrial quality control in MDA-MB-436 breast cancer cells. Autophagy. 2014;10(6):986–1003. doi: 10.4161/auto.28390. 24879149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debnath J. The multifaceted roles of autophagy in tumors-implications for breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2011;16(3):173–187. doi: 10.1007/s10911-011-9223-3. 21779879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morselli E. Anti- and pro-tumor functions of autophagy. Biochimica et Biophysica Acta. 2009;1793(9):1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. 19371598 [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Ney P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death and Differentiation. 2009;16(7):939–946. doi: 10.1038/cdd.2009.16. 19229244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak I. Mitophagy: a complex mechanism of mitochondrial removal. Antioxidants and Redox Signaling. 2012;17(5):794–802. doi: 10.1089/ars.2011.4407. 22077334 [DOI] [PubMed] [Google Scholar]
- 34.Feng D. Molecular signaling toward mitophagy and its physiological significance. Experimental Cell Research. 2013;319(12):1697–1705. doi: 10.1016/j.yexcr.2013.03.034. 23603281 [DOI] [PubMed] [Google Scholar]
- 35.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nature Reviews Molecular Cell Biology. 2011;12(1):9–14. doi: 10.1038/nrm3028. 21179058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweers R.L. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19500–19505. doi: 10.1073/pnas.0708818104. 18048346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandoval H. Essential role for nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232–235. doi: 10.1038/nature07006. 18454133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuda N. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. Journal of Cell Biology. 2010;189(2):211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morselli E. Oncosuppressive functions of autophagy. Antioxidants and Redox Signaling. 2011;14(11):2251–2269. doi: 10.1089/ars.2010.3478. 20712403 [DOI] [PubMed] [Google Scholar]
- 40.Takamura A. Autophagy-deficient mice develop multiple liver tumors. Genes & Development. 2011;25(8):795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathew R. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes & Development. 2007;21(11):1367–1381. doi: 10.1101/gad.1545107. 17510285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karantza-Wadsworth V. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes & Development. 2007;21(13):1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komatsu M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature Cell Biology. 2010;12(3):213–223. doi: 10.1038/ncb2021. 20173742 [DOI] [PubMed] [Google Scholar]
- 44.Lau A. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Molecular and Cellular Biology. 2010;30(13):3275–3285. doi: 10.1128/MCB.00248-10. 20421418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taguchi K. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(34):13561–13566. doi: 10.1073/pnas.1121572109. 22872865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villeneuve N.F., Lau A., Zhang D.D. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxidants and Redox Signaling. 2010;13(11):1699–1712. doi: 10.1089/ars.2010.3211. 20486766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakurai T. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14(2):156–165. doi: 10.1016/j.ccr.2008.06.016. 18691550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenner C. Decoding cell death signals in liver inflammation. Journal of Hepatology. 2013;59(3):583–594. doi: 10.1016/j.jhep.2013.03.033. 23567086 [DOI] [PubMed] [Google Scholar]
- 49.Saitoh T., Fujita N., Jang M.H., Uematsu S., Yang B.G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., Tanaka K., Kawai T., Tsujimura T., Takeuchi O., Yoshimori T., Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. 18849965 [DOI] [PubMed] [Google Scholar]
- 50.Bensaad K., Cheung E.C., Vousden K.H. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO Journal. 2009;28(19):3015–3026. doi: 10.1038/emboj.2009.242. 19713938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakahira K. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunology. 2011;12(3):222–230. doi: 10.1038/ni.1980. 21151103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou R. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. 21124315 [DOI] [PubMed] [Google Scholar]
- 53.Roy D., Sarkar S., Felty Q. Levels of IL-1beta control stimulatory/inhibitory growth of cancer cells. Frontiers in Bioscience. 2006;11:889–898. doi: 10.2741/1845. 16146780 [DOI] [PubMed] [Google Scholar]
- 54.Kang R. HMGB1 in cancer: good, bad, or both? Clinical Cancer Research. 2013;19(15):4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. 23723299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang D. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxidants and Redox Signaling. 2011;15(8):2185–2195. doi: 10.1089/ars.2010.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mittal D. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO Journal. 2010;29(13):2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lock R. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Molecular Biology of the Cell. 2011;22(2):165–178. doi: 10.1091/mbc.E10-06-0500. 21119005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo J.Y. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes & Development. 2011;25(5):460–470. doi: 10.1101/gad.2016311. 21317241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S. Pancreatic cancers require autophagy for tumor growth. Genes & Development. 2011;25(7):717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim M.J. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. Journal of Biological Chemistry. 2011;286(15):12924–12932. doi: 10.1074/jbc.M110.138958. 21300795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinberg F. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8788–8793. doi: 10.1073/pnas.1003428107. 20421486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strohecker A.M. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discovery. 2013;3(11):1272–1285. doi: 10.1158/2159-8290.CD-13-0397. 23965987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo J.Y. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes & Development. 2013;27(13):1447–1461. doi: 10.1101/gad.219642.113. 23824538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Degenhardt K. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. 16843265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duran A. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13(4):343–354. doi: 10.1016/j.ccr.2008.02.001. 18394557 [DOI] [PubMed] [Google Scholar]
- 66.Mathew R. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–1075. doi: 10.1016/j.cell.2009.03.048. 19524509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei H. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes & Development. 2011;25(14):1510–1527. doi: 10.1101/gad.2051011. 21764854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris A.L. Hypoxia − a key regulatory factor in tumour growth. Nature Reviews Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. 11902584 [DOI] [PubMed] [Google Scholar]
- 69.Brahimi-Horn M.C., Bellot G., Pouysségur J. Hypoxia and energetic tumour metabolism. Current Opinion in Genetics and Development. 2011;21(1):67–72. doi: 10.1016/j.gde.2010.10.006. 21074987 [DOI] [PubMed] [Google Scholar]
- 70.Chandel N.S. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. Journal of Biological Chemistry. 2000;275(33):25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. Journal of Biological Chemistry. 2008;283(16):10892–10903. doi: 10.1074/jbc.M800102200. 18281291 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Bellot G. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Molecular and Cellular Biology. 2009;29(10):2570–2581. doi: 10.1128/MCB.00166-09. 19273585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rouschop K.M. Autophagy is required during cycling hypoxia to lower production of reactive oxygen species. Radiotherapy and Oncology. 2009;92(3):411–416. doi: 10.1016/j.radonc.2009.06.029. 19616335 [DOI] [PubMed] [Google Scholar]
- 74.Mazure N.M., Pouysségur J. Hypoxia-induced autophagy: cell death or cell survival? Current Opinion in Cell Biology. 2010;22(2):177–180. doi: 10.1016/j.ceb.2009.11.015. 20022734 [DOI] [PubMed] [Google Scholar]
- 75.Papandreou I. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death and Differentiation. 2008;15(10):1572–1581. doi: 10.1038/cdd.2008.84. 18551130 [DOI] [PubMed] [Google Scholar]
- 76.Rouschop K.M. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. Journal of Clinical Investigation. 2010;120(1):127–141. doi: 10.1172/JCI40027. 20038797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabinowitz J.D., White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. doi: 10.1126/science.1193497. 21127245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eng C.H., Abraham R.T. The autophagy conundrum in cancer: influence of tumorigenic metabolic reprogramming. Oncogene. 2011;30(47):4687–4696. doi: 10.1038/onc.2011.220. 21666712 [DOI] [PubMed] [Google Scholar]
- 79.Sethi N., Kang Y. Unravelling the complexity of metastasis – molecular understanding and targeted therapies. Nature Reviews Cancer. 2011;11(10):735–748. doi: 10.1038/nrc3125. 21941285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valastyan S., Weinberg R.A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. 22000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nature Reviews Molecular Cell Biology. 2014;15(3):178–196. doi: 10.1038/nrm3758. 24556840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lazova R., Camp R.L., Klump V., Siddiqui S.F., Amaravadi R.K., Pawelek J.M. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clinical Cancer Research. 2012;18:370–379. doi: 10.1158/1078-0432.CCR-11-1282. 22080440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma X.H. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clinical Cancer Research. 2011;17(10):3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. 21325076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Friedl P., Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. 22118458 [DOI] [PubMed] [Google Scholar]
- 85.Reymond N., d’Agua B.B., Ridley A.J. Crossing the endothelial barrier during metastasis. Nature Reviews Cancer. 2013;13(12):858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 86.Fung C. Induction of autophagy during extracellular matrix detachment promotes cell survival. Biochimica et Biophysica Acta. 2008;19(3):797–806. doi: 10.1091/mbc.E07-10-1092. 18094039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paoli P., Giannoni E., Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochimica et Biophysica Acta. 2013;1833(12):3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. 23830918 [DOI] [PubMed] [Google Scholar]
- 88.Peng Y.F. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy. 2013;9(12):2056–2068. doi: 10.4161/auto.26398. 24157892 [DOI] [PubMed] [Google Scholar]
- 89.Avivar-Valderas A. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene. 2013;32(41):4932–4940. doi: 10.1038/onc.2012.512. 23160380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buchheit C.L., Rayavarapu R.R., Schafer Z.T. The regulation of cancer cell death and metabolism by extracellular matrix attachment. Seminars in Cell and Developmental Biology. 2012;23(4):402–411. doi: 10.1016/j.semcdb.2012.04.007. 22579674 [DOI] [PubMed] [Google Scholar]
- 91.Avivar-Valderas A. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Molecular and Cellular Biology. 2011;31(17):3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macintosh R.L. Inhibition of autophagy impairs tumor cell invasion in an organotypic model. Cell Cycle. 2012;11(10):2022–2029. doi: 10.4161/cc.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galavotti S. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32(6):699–712. doi: 10.1038/onc.2012.111. 22525272 [DOI] [PubMed] [Google Scholar]
- 94.Li J. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 2013;34(6):1343–1351. doi: 10.1093/carcin/bgt063. 23430956 [DOI] [PubMed] [Google Scholar]
- 95.Kenific C.M., Thorburn A., Debnath J. Autophagy and metastasis: another double-edged sword. Current Opinion in Cell Biology. 2010;22(2):241–245. doi: 10.1016/j.ceb.2009.10.008. 19945838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeNardo D.G., Johansson M., Coussens L.M. Immune cells as mediators of solid tumor metastasis. Cancer and Metastasis Reviews. 2008;27(1):11–18. doi: 10.1007/s10555-007-9100-0. 18066650 [DOI] [PubMed] [Google Scholar]
- 97.Tlsty T.D., Coussens L.M. Tumor stroma and regulation of cancer development. Annual Review of Pathology. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. 18039110 [DOI] [PubMed] [Google Scholar]
- 98.Lv Q., Wang W., Xue J., Hua F., Mu R., Lin H., Yan J., Lv X., Chen X., Hu Z.W. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Research. 2012;72:3238–3250. doi: 10.1158/0008-5472.CAN-11-3832. 22719072 [DOI] [PubMed] [Google Scholar]
- 99.Wang M.C. In vitro synergistic antitumor efficacy of sequentially combined chemotherapy/icotinib in non‑small cell lung cancer cell lines. Oncology Reports. 2015;33(1):239–249. doi: 10.3892/or.2014.3583. 25370413 [DOI] [PubMed] [Google Scholar]
- 100.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. 11001068 [DOI] [PubMed] [Google Scholar]
- 101.Nguyen T.M. Kringle 5 of human plasminogen, an angiogenesis inhibitor, induces both autophagy and apoptotic death in endothelial cells. Blood. 2007;109(11):4793–4802. doi: 10.1182/blood-2006-11-059352. 17272502 [DOI] [PubMed] [Google Scholar]
- 102.Ramakrishnan S. Autophagy and angiogenesis inhibition. Autophagy. 2007;3(5):512–515. doi: 10.4161/auto.4734. 17643071 [DOI] [PubMed] [Google Scholar]
- 103.Shen W. Oxidative stress mediates chemerin-induced autophagy in endothelial cells. Free Radical Biology and Medicine. 2013;55:73–82. doi: 10.1016/j.freeradbiomed.2012.11.011. 23195684 [DOI] [PubMed] [Google Scholar]
- 104.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nature Reviews Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. 16572188 [DOI] [PubMed] [Google Scholar]
- 105.Maes H. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends in Molecular Medicine. 2013;19(7):428–446. doi: 10.1016/j.molmed.2013.04.005. 23714574 [DOI] [PubMed] [Google Scholar]
- 106.Martinez-Outschoorn U.E. The autophagic tumor stroma model of cancer or “battery-operated tumor growth”: a simple solution to the autophagy paradox. Cell Cycle. 2010;9(21):4297–4306. doi: 10.4161/cc.9.21.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martinez-Outschoorn U.E. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9(16):3256–3276. doi: 10.4161/cc.9.16.12553. 20814239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pavlides S. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9(17):3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martinez-Outschoorn U.E. Cytokine production and inflammation drive autophagy in the tumor microenvironment: role of stromal caveolin-1 as a key regulator. Cell Cycle. 2011;10(11):1784–1793. doi: 10.4161/cc.10.11.15674. 21566463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sotgia F. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annual Review of Pathology. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. 22077552 [DOI] [PubMed] [Google Scholar]
- 111.Martinez-Outschoorn U.E. Stromal–epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. International Journal of Biochemistry & Cell Biology. 2011;43(7):1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bensaad K. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. 16839880 [DOI] [PubMed] [Google Scholar]
- 113.Lisanti M.P. Understanding the “lethal” drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biology & Therapy. 2010;10(6):537–542. doi: 10.4161/cbt.10.6.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao X., He Y., Chen H. Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. International Journal of Cancer. 2013;132(1):1–8. doi: 10.1002/ijc.27664. 22684793 [DOI] [PubMed] [Google Scholar]
- 115.Pavlides S. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxidants and Redox Signaling. 2012;16(11):1264–1284. doi: 10.1089/ars.2011.4243. 21883043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martinez-Outschoorn U.E. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9(17):3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bonuccelli G. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9(17):3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Iozzo R.V. Autophagic tumor stroma: a biofuel for cancer growth. Cell Cycle. 2011;10(19):3231–3232. doi: 10.4161/cc.10.19.17124. 21946523 [DOI] [PubMed] [Google Scholar]
- 119.Castello-Cros R. Matrix remodeling stimulates stromal autophagy, “fueling” cancer cell mitochondrial metabolism and metastasis. Cell Cycle. 2011;10(12):2021–2034. doi: 10.4161/cc.10.12.16002. 21646868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sotgia F. Mitochondria “fuel” breast cancer metabolism: fifteen markers of mitochondrial biogenesis label epithelial cancer cells, but are excluded from adjacent stromal cells. Cell Cycle. 2012;11(23):4390–4401. doi: 10.4161/cc.22777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carew J.S., Kelly K.R., Nawrocki S.T. Autophagy as a target for cancer therapy: new developments. Cancer Management and Research. 2012;4:357–365. doi: 10.2147/CMAR.S26133. 23091399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou H. Ciclopirox induces autophagy through reactive oxygen species-mediated activation of JNK signaling pathway. Oncotarget. 2014;5:10140–10150. doi: 10.18632/oncotarget.2471. 25294812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hahm E.R., Sakao K., Singh S.V. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate. 2014;74(12):1209–1221. doi: 10.1002/pros.22837. 25043291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gonzalez Y. Atg7- and Keap1-dependent autophagy protects breast cancer cell lines against mitoquinone-induced oxidative stress. Oncotarget. 2014;5(6):1526–1537. doi: 10.18632/oncotarget.1715. 24681637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim H. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxidative Medicine and Cellular Longevity. 2013;2013:596496. doi: 10.1155/2013/596496. 24379902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Q. Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer. 2014;5(3–4):100–112. doi: 10.18632/genesandcancer.9. 25053988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Al Dhaheri Y. Carnosol induces ROS-Mediated Beclin1-Independent autophagy and apoptosis in triple negative breast cancer. PLOS One. 2014;9(10):e109630. doi: 10.1371/journal.pone.0109630. 25299698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hao W. Psoralidin induces autophagy through ROS generation which inhibits the proliferation of human lung cancer A549 cells. PeerJ. 2014;2:e555. doi: 10.7717/peerj.555. 25250213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miki H. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. International Journal of Oncology. 2012;40(4):1020–1028. doi: 10.3892/ijo.2012.1325. 22218562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Winterbourn C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochimica et Biophysica Acta. 2014;1840(2):730–738. doi: 10.1016/j.bbagen.2013.05.004. 23665586 [DOI] [PubMed] [Google Scholar]
- 131.La Favor J.D., Anderson E.J., Hickner R.C. Novel method for detection of reactive oxygen species in vivo in human skeletal muscle. Physiological Research. 2014;63(3):387–392. doi: 10.33549/physiolres.932587. 24564604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mrakic-Sposta S. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxidative Medicine and Cellular Longevity. 2012;2012:973927. doi: 10.1155/2012/973927. 22900129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roberts L.J., Moore K.P., Zackert W.E., Oates J.A., Morrow J.D. Identification of the major urinary metabolite of the F2-isoprostane 8-iso-prostaglandin F2alpha in humans. Journal of Biological Chemistry. 1996;271:20617–20620. doi: 10.1074/jbc.271.34.20617. 8702808 [DOI] [PubMed] [Google Scholar]
- 134.Smith G., Nguyen Q.D., Aboagye E.O. Translational imaging of apoptosis. Anti-Cancer Agents in Medicinal Chemistry. 2009;9(9):958–967. doi: 10.2174/187152009789377709. 19663785 [DOI] [PubMed] [Google Scholar]
- 135.Klionsky D.J. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]