Abstract
Oxidative stress-induced lipid peroxidation has been associated with human physiology and diseases including cancer. Overwhelming data suggest that reactive lipid mediators generated from this process, such as 4-hydroxynonenal (4-HNE), are biomarkers for oxidative stress and important players for mediating a number of signaling pathways. The biological effects of 4-HNE are primarily due to covalent modification of important biomolecules including proteins, DNA, and phospholipids containing amino group. In this review, we summarize recent progress on the role of 4-HNE in pathogenesis of cancer and focus on the involvement of mitochondria: generation of 4-HNE from oxidation of mitochondria-specific phospholipid cardiolipin; covalent modification of mitochondrial proteins, lipids, and DNA; potential therapeutic strategies for targeting mitochondrial ROS generation, lipid peroxidation, and 4-HNE.
Keywords: Lipid peroxidation, 4-HNE, Mitochondria, Cancer, Free radicals, Cardiolipin
Abbreviations: 4-HNE, 4-hydroxy-2-alkenals; 4-ONE, 4-oxo-2-nonenal; 8-oxo-dG, 8-oxoguanine; AA, arachidonic acid; ADH, alcohol dehydrogenases; AKRs, aldo-keto reductases; ALDH, aldehyde dehydrogenases; ALDH2, aldehyde dehydrogenase 2 family(mitochondrial); As2O3, arsenic trioxide; ATP5B, ATP synthase subunit β; CL, cardiolipin; DHA, docosahexaenoic acid; DHN, 1,4-dihydroxy-2-nonene; DOX, doxorubicin; EAA-CL, epoxyalcohol-aldehyde-cardiolipin; ETC, electron transport chain; FapyA, 4,6-diamino-5-formamidopyrimidine; FapyG, 2,6-diamino-4-hydroxy-5-formamidopyrimidine; GSH, glutathione; GS-HNE, glutathionyl-HNE; GSTs, glutathione-S-transferases; HADHA, trifunctional enzyme subunit α; HNA, 4-hydroxy-2-nonenoic acid; HODE, hydroxyoctadecadienoic acid; HpODE, hydroperoxyoctadecadienoic acid; IMM, inner membrane of mitochondria; KODE, keto-octadecadienoic acid; L4CL, tetralinoleoyl cardiolipin; LA, linoleic acid; LPO, lipid peroxidation; mtDNA, mitochondrial DNA; NDUFS2, NADH dehydrogenase [ubiquinone] iron–sulfur protein 2; OMM, outer membrane of mitochondria; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; PUFAs, polyunsaturated fatty acids; ROS, reactive oxygen species; SDHA, succinate dehydrogenase [ubiquinone] flavoprotein subunit; UADT, upper aero digestive track; UCPs, mitochondrial uncoupling proteins
Introduction
Reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide, hydroxyl radicals, singlet oxygen, and lipid peroxyl radicals, are ubiquitous and considered as byproducts of aerobic life [1]. Most of these chemically reactive molecules are short-lived and react with surrounding molecules at the site of formation while some of the more stable molecules diffuse and cause damages far away from their sites of generation. Overproduction of these ROS, termed oxidative stress, may provoke oxidation of polyunsaturated fatty acids (PUFAs) in cellular membranes through free radical chain reactions and form lipid hydroperoxides as primary products [2]; some of these primary oxidation products may decompose and lead to the formation of reactive lipid electrophiles. Among these lipid peroxidation (LPO) products, 4-hydroxy-2-nonenals (4-HNE) represents one of the most bioactive and well-studied lipid alkenals [3]. 4-HNE can modulate a number of signaling processes mainly through forming covalent adducts with nucleophilic functional groups in proteins, nucleic acids, and membrane lipids. These properties have been extensively summarized in some excellent reviews [4], [5], [6], [7], [8], [9], [10].
Mitochondria are vital for cellular bioenergetics and regarded as the major cellular site for ROS production [11]. Our previous works demonstrated that mitochondria are also an important site for 4-HNE formation [12], [13]. Furthermore, it has been well documented that oxidative stress and ROS generation are intimately associated with cancer [14], [15]. Accumulating data suggest that mitochondrial macromolecular adducts from 4-HNE are involved in the initiation and progression of cancer [16], [17]. In this review, we summarize the recent progress on understanding the role of 4-HNE in cancer and focus on the involvement of mitochondria: formation of 4-HNE from oxidation of cardiolipin, covalent modification of mitochondrial biomolecules including proteins, DNA and lipids, and therapeutic targeting the mitochondrial pathways induced by 4-HNE in the context of cancer pathogenesis.
Formation and catabolism of 4-HNE in mitochondria
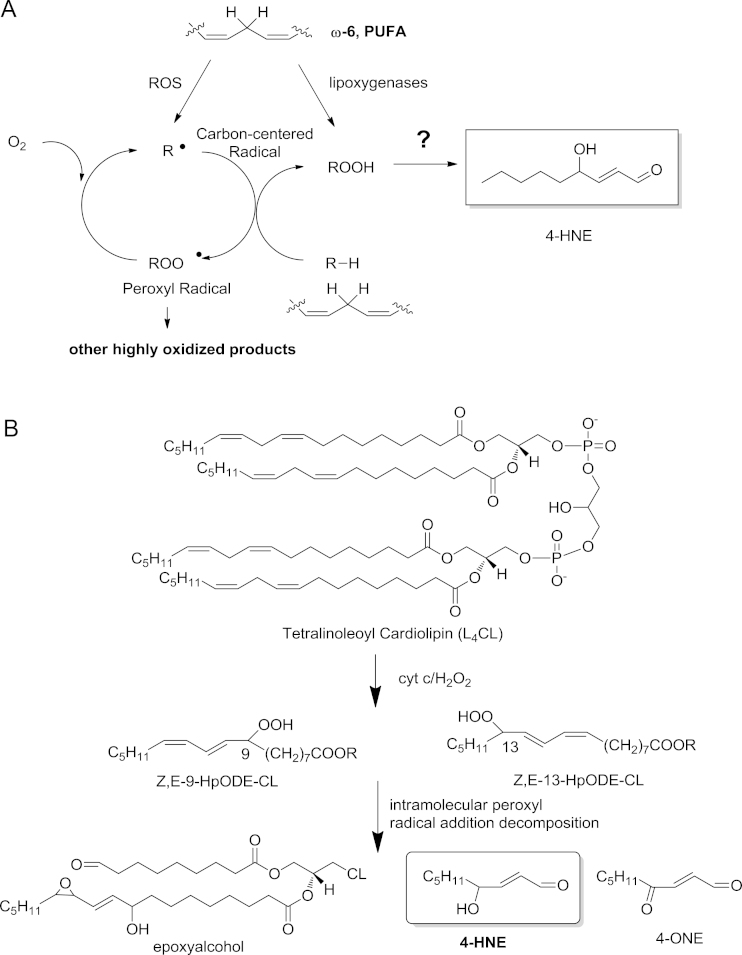
Mitochondrion is the “power house” for most eukaryotic cells, including cancer cells, for its role in ATP production through oxidative phosphorylation. During this process, significant amount of superoxide are produced at complexes I and III of the electron transport chain (ETC). Most of the superoxide is converted to hydrogen peroxide by superoxide dismutases (SOD). One of the main targets of ROS is the membrane lipid bilayers, especially the PUFA chains in lipids. Peroxidation of PUFAs generates an array of primary lipid oxidation products and lipid electrophiles, among which 4-HNE is one of the well-studied active lipid electrophiles [5]. In contrast to its well established biological consequences, chemical mechanisms that led to 4-HNE formation remain to be clearly elucidated [18], [19]. It is generally accepted that 4-HNE is derived from decomposition of hydroperoxide of ω-6 PUFAs at the sn-2 position of glycerophospholipids in cellular membranes (Fig. 1A). Thus phospholipids containing linoleic acid (LA, 18:2, ω-6) and arachidonic acid (AA, 20:4, ω-6) on cytoplasma membranes are considered the major source for 4-HNE production. Our recent work, however, demonstrated that oxidation of mitochondrial phospholipid cardiolipin also led to formation of significant amount of 4-HNE and other oxidation products via a novel chemical mechanism that involved cross-chain peroxyl radical addition and decomposition [20], [21], [22] (Fig. 1B).
Fig. 1.
Chemical mechanisms for 4-HNE formation from lipid peroxidation. (A) General scheme for the formation of 4-HNE from decomposition of lipid hydroperoxides that can be generated from free radical oxidation of ω-6 PUFA or enzymatic oxidation by lipoxygenases. (B) Lipid electrophiles generated from oxidation of mitochondrial cardiolipin: oxidation of L4CL by the peroxidase activity of cyt c and CL complex in the presence of H2O2 results in the formation of hydroperoxyoctadecadienoic acid (HpODE), 9-HpODE-CL and 13-HpODE-CL. During this process, through intra-molecular peroxyl radical addition and decomposition of an unstable intermediate, several reactive aldehydes are produced including epoxyalcohol-aldehyde-CL (EAA-CL), 4-HNE, and 4-oxo-2-nonenal (4-ONE).
Cardiolipin is a unique class of mitochondrial specific phospholipids that reside almost exclusively in the inner membrane of mitochondria (IMM) and are critical for maintaining the structural integrity of mitochondrial membranes and the function of multiple protein complexes in the ETC [23]. In most mammalian tissues, tetralinoleoylcardiolipin (L4CL) is the major form of cardiolipins, which contains four linoleic acid chains in the same molecule. Incorporation of four LA side chains in L4CL and its association with mitochondria render L4CL to be readily oxidized by ROS and then generate electrophiles through this novel “arm to arm” reaction [20]. We provided further evidence that formation of 4-HNE and other lipid electrophiles from CL played an important role in intrinsic apoptosis in the context of atherosclerosis [13]. This process appears to be involved in cancer. Human kidney cancer tissue showed greater staining for 4-HNE protein adducts both in cytoplasm and mitochondria compared to adjacent tissue [24]. Furthermore, about 30% of the 4-HNE adducted proteins locate in mitochondria and a majority of them are the members of ETC [25], [26].
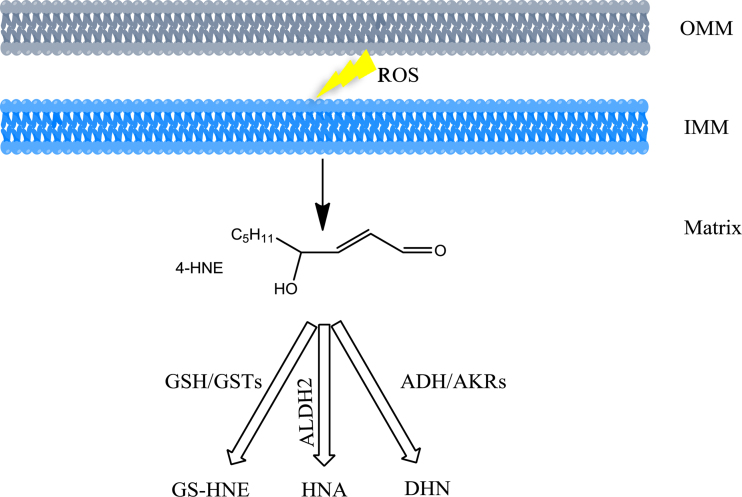
There are three major detoxification pathways to convert 4-HNE to less reactive chemical species and control their steady-state intracellular concentrations (Fig. 2). Firstly, the main catabolic reactions are the formation of adducts with glutathione (GSH), which occurs spontaneously or can be catalyzed by glutathione-S-transferases (GSTs). Secondly, 4-HNE can be reduced to 1,4-dihydroxy-2-nonene (DHN) by aldo-keto reductases (AKRs) or alcohol dehydrogenases (ADH). Thirdly, 4-HNE can be oxidized to 4-hydroxy-2-nonenoic acid (HNA) by aldehyde dehydrogenase (ALDH). Throughout more than 1.5 billion years of obligate endosymbiotic co-evolution, mitochondria have developed a complex system to sense and react to oxidative stress similar to their host. GSH is one of the most important hydrophilic antioxidant in mitochondria for maintaining the redox balance. Similarly, a significant amount of AKRs and ADH is expressed in mitochondria. ALDH2, one member of ALDH family, is exclusively located in mitochondria. It is noteworthy that the superoxide dismutases (SOD) are the well-known antioxidant enzymes that convert superoxide into hydrogen peroxide. Among them, MnSOD resides in mitochondria whereas Cu/ZnSOD is located in cytosol.
Fig. 2.
Catabolism of 4-HNE in mitochondria. ROS induced lipid peroxidation in IMM and OMM (outer membrane of mitochondria) leads to 4-HNE formation. In matrix, 4-HNE conjugation with GSH produces glutathionyl-HNE (GS-HNE); this process occurs spontaneously or can be catalyzed by GSTs. 4-HNE is reduced to 1,4-dihydroxy-2-nonene (DHN) catalyzed ADH or AKRs. ALDH2 catalyzes the oxidation of 4-HNE to form 4-hydroxy-2-nonenoic acid (HNA).
4-HNE mitochondrial protein adducts in cancer
As mentioned previously, proteins, DNA and membrane lipids are the primary targets for 4-HNE attack. It was estimated that 1–8% of the 4-HNE formed in cells will modify proteins [27], among which, about 30% 4-HNE target proteins locate in mitochondria [25], [26]. Elevated status of oxidative stress has been associated with a majority of cancer types and thus 4-HNE is believed to be a major player that contributes to the mutagenic and carcinogenic effects of lipid peroxidation. Formation of 4-HNE protein adducts in renal and colon cancer tissues has been related to the growth and progression of kidney and colon cancer [4]. Increased levels of 4-HNE have been shown to be associated with liver cancer initiation in animal models and humans [28]. A recent study demonstrated that RLIP76 regulated pancreatic tumorigenesis through maintaining cellular levels of 4-HNE, GS-HNE and GS-DHN [29]. Furthermore, modulation of oxidative stress can be utilized for therapeutic purpose in cancer prevention and treatment [30]. Doxorubicin (DOX) is one of the most effective anticancer drugs and oxidative stress is a primary mechanism of DOX-induced toxicity to cancer cells. However, the cardiac side effects limit its clinical use. Using a redox proteomics method, Zhao et al. [25] identified several HNE-modified mitochondrial proteins in cardiac mitochondria from DOX-treated mice. Most of the proteins are related to mitochondrial energy metabolism, such ATP synthase subunit β (ATP5B), succinate dehydrogenase [ubiquinone] flavoprotein subunit (SDHA) and NADH dehydrogenase [ubiquinone] iron–sulfur protein 2 (NDUFS2) in ETC, and trifunctional enzyme subunit α (HADHA) in citric acid cycle. The enzymatic activities of these proteins were significantly reduced in DOX-treated mice. Treatment of an SOD mimetic averted the production of DOX-induced HNE-protein adducts and mitochondrial dysfunction. These results imply that robust 4-HNE formation can induce cancer cell death through affecting mitochondrial function. Nevertheless, mild mitochondrial function impairment is a major contributing factor in caner. Studies from Anotonio and Paolo showed that partial inhibition of ETC complexes improved tumor cell migration in vitro and in vivo, and the metastasis can be prevented with a mitochondria-targeted superoxide scavenger mitoTEMPO [31], [32]. It should be noted that cardiolipin interacts with a number of IMM proteins including the ETC complexes and this interaction is required for optimal activity of complex I (NADH ubiquinone oxidoreductase), complex III (ubiquinone cytochrome c oxidoreductase), complex IV (cytochrome c oxidase) and complex V (ATP synthase). Furthermore, any alteration in the cardiolipin structure, content and composition may lead to mitochondrial dysfunction [33], [34], [35]. Constitutive 4-HNE levels might contribute to adaptive response in protecting cancer cells against oxidative damage. Superoxide production is sensitive to the proton motive force and it can be significantly reduced by mild uncoupling of oxidative phosphorylation through action of mitochondrial uncoupling proteins (UCPs). UCP2, the widely expressed UCP, has been shown to be up-regulated in a number of aggressive human cancers and plays an important role in cancer initiation, progression, and metabolic reprogramming as an antioxidant defense [36]. It has been strongly supported by the fact that UCPs can be activated by ROS [37], [38] and several reports showed that ROS activated UCPs through a free radical chain reaction and 4-HNE formation; 4-HNE induced uncoupling was inhibited by inhibitors of UCPs [39], [40]. These studies suggest that 4-HNE may act as a feed-back mechanism through activating UCPs to mitigate excessive ROS production and oxidative damage in cancer.
ALDH2, located in the mitochondrial matrix, is better known for its critical role in ethanol metabolism. ALDH2 is also capable of metabolizing endogenous aldehydic products derived from lipid peroxidation, such as 4-HNE, thus protecting mitochondria from these toxic agents [41]. Chronic consumption of ethanol has been causally linked to the development of upper aero digestive track (UADT) cancers, liver cancer, colorectal cancer, and breast cancer. Studies from Asian countries confirmed a significant association between ALDH2 enzyme deficiency and UADT cancer risk [42], [43], [44]. Excessive ethanol consumption enhances lipid peroxidation by overwhelming antioxidant defense system in tissues and the aldehydic products of lipid peroxidation can covalently bind to proteins in tissues [45], [46]. Doorn et al. showed that 4-HNE was both a substrate and an inhibitor of ALDH2; inhibition of ALDH2 by 4-HNE is reversible at low concentration and become irreversible when the concentration of 4-HNE reaches 10 µM [47]. This inhibition appears to be a result of 4-HNE-cysteine adduct at the ALDH2 active site. Thus 4-HNE-induced ALDH2 inactivation may have an important role in the progression of some cancers.
4-HNE mitochondrial DNA adducts in cancer
Mitochondria are unique organelles because they contain their own DNA which is responsible for encoding 2 ribosomal RNAs, 22 transfer RNAs and 13 protein subunits of the ETC. Mitochondrial DNA (mtDNA) is organized into protein–DNA complexes called nucleoids within the mitochondrial matrix. This kind of structure can provide some protection to the genome while mtDNA remains in close proximity to the ECT which is considered the main source of ROS within the cell. Therefore, it is predictable that mtDNA is highly susceptible to oxidative damage by ROS. Indeed, more oxidative damage to mtDNA has been widely observed than those of nuclear DNA. However, whether these damages are directly from mitochondrial ROS remain to be clearly defined. Exposure to ROS can result in a number of oxidative modifications to DNA including the lesions 8-oxoguanine (8-oxo-dG, 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG), 4,6-diamino-5-formamidopyrimidine (FapyA), and thymine glycol. These mtDNA modifications can cause point mutations, deletions, and strand breaks; thus the unrepaired oxidative damage may lead to the production of dysfunctional proteins. 8-Oxo-dG represents the most commonly studied oxidative lesions and increased levels of 8-oxo-dG are found in mtDNA from patients with arsenical skin cancers [48], glioma [49], urinary bladder and renal cancer [50].
4-HNE, the product of lipid peroxidation, is mutagenic and genotoxic in viruses, bacteria and mammalian cells. It reacts with all four DNA bases but with different efficiency: G >C > A >T [44]. 4-HNE-dG represents the best biomarker of the genotoxic effects of 4-HNE and these adducts are primarily found in nuclear DNA. A classic example of etiological relevance of 4-HNE-dG in human cancers is 4-HNE-dG induced p53 mutation. P53 is a well-known tumor suppressor and a mutational hotspot in human cancers, especially in hepatocellular carcinoma. 4-HNE-dG adducts were preferentially formed at the third base of codon 249 in the p53 gene, causing gene mutation and affecting diverse biological processes including cell cycle arrest, apoptosis, DNA repair, and differentiation [51]. However, modification of mtDNA by 4-HNE and other lipid electrophile has yet to be discovered.
4-HNE mitochondrial lipid adducts in cancer
Mitochondria are dynamic organelles surrounded by two membranes with a well-defined lipid composition; most of these lipids are synthesized in the endoplasmic reticulum. However, de novo lipid synthesis and remodeling of mitochondrial lipids are important for maintaining the structural integrity and function of mitochondria. The most abundant phospholipids in mitochondrial membrane are phosphatidylcholine (PC) and phosphatidylethanolamine (PE), representing about 40–55% and 30–45% of total mitochondrial phospholipids, respectively. CL accounts for around 15% of mitochondrial phospholipids. Other lipids, such as phosphatidylserine (PS), sphingolipids and sterols, are present at modest or even trace amounts [52]. Phospholipids containing amino group, such as PE and PS, can undergo both a Michael addiction and a Schiff base formation with 4-HNE [53]. Since the low amount of PS in mitochondria, PE become the primary targets of 4-HNE adduction. PE Michael adducts were found in human urine and blood in oxidative stress related situations such as aging and diabetes [54], while the biological relevance is much less documented compared to protein and DNA adducts. Guichardant et al. [55] demonstrated that 4-HNE adduction made PE a poor substrate for secreted phospholipase A2 and was not cleaved by phospholipase D. Plasmalogen, an important subclass of PE, is covalently modified by 4-HNE and this modification may alter its antioxidant potential. However, all these lipid modification by 4-HNE have not been shown to be derived from mitochondrial lipids.
Therapeutic strategies for manipulation of ROS and lipid peroxidation in cancer: implication for mitochondrial 4-HNE
Evidence provided thus far clearly demonstrated that 4-HNE played an important role in cancer through mitochondria. 4-HNE at low concentrations can protect cancer cells against further damage (e.g., UCPs reduce superoxide production) whereas cells undergo apoptosis or even necrosis at high concentrations when adduct load on mitochondrial macromolecules overwhelms the protective mechanisms. Thus, strategies focusing on manipulating mitochondrial ROS generation, lipid peroxidation, and 4-HNE formation may provide therapeutic value to prevent or treat cancer.
While moderate ETC inhibition and subsequent increase in mitochondrial ROS production promote tumor cell migration and metastasis, specific scavenging of mitochondrial superoxide can be a therapeutic option to prevent spontaneous tumor metastasis. Studies by Paolo et al. showed that treatment with mitochondrial specific superoxide scavenger mitoTEMPO, an SOD2 mimetic, prevented the metastatic phenotype in human and mouse cancer cells [32]. MitoTEMPO impairs the formation of ROS including H2O2 and lipid peroxides [56]. Similar to mitoTEMPO, mitoQ, a mitochondria-targeted form of coenzyme Q10, is currently being tested in clinical trials [57]. These findings may offer a rationale to test mitochondria-targeted superoxide scavengers in metastasis prevention trials.
Robust ROS formation is a common mechanism for some anticancer drugs to kill cancer cells, such as DOX and arsenic trioxide (As2O3). However, only limited reports have taken into account of the roles played by the lipid peroxidation products induced by ROS. Reports showed that some PUFAs, such docosahexaenoic acid (DHA), can sensitize various tumor cells such as breast cancer, ovarian cancer, and cervical cancer, to ROS-inducing anticancer agents. The cytotoxic effect of combined treatments was due to induction of apoptosis, preceded by increased production of intracellular aldehydes of lipid peroxidation. These effects can be abolished by treatment of antioxidant vitamin E [58]. A recent study demonstrated that ketogenic diet (high in LAs, and low in carbohydrates and protein) enhanced radio-chemo-therapy responses in lung cancer xenografts by a mechanism that may involve increased oxidative stress [59]. Ketogenic diets may enrich mitochondrial CL with LA and at the same time force cells to rely on mitochondrial oxidative metabolism for energy production. According to our studies, LA-containing CL is an abundant source of 4-HNE under oxidative stress condition and predisposes cancer cells to undergo apoptosis. Indeed tumors from mice treated with ketogenic diet and radiation showed significantly increased levels of 4-HNE-modified proteins. Therefore, these results suggest that ketogenic diet (high in PUFAs, i.e., DHA, LA, and arachidonic acid) could serve as an effective adjuvant for improving responses to radio-chemo-therapies in the treatment of cancers by a mechanism linking mitochondrial 4-HNE formation, oxidative stress, and lipid peroxidation.
Conclusions
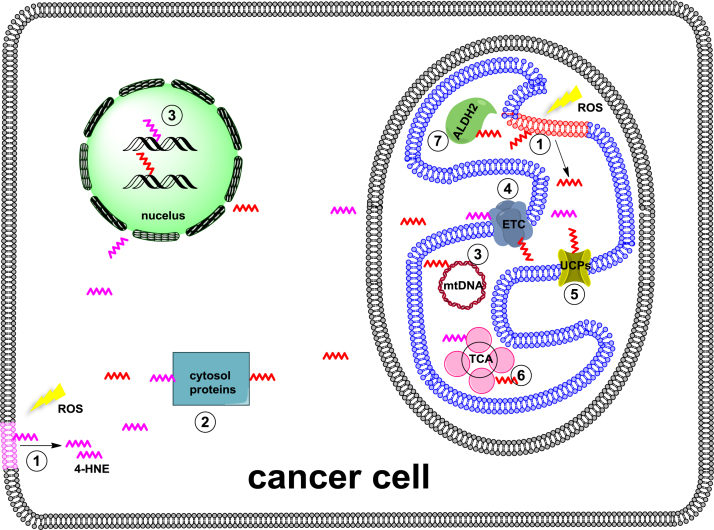
Lipid peroxidation-derived 4-HNE is a prototypical reactive lipid electrophile that readily forms covalent adducts with nucleophilic functional groups in macromolecule such as proteins, DNA, and lipids (Fig. 3). A body of work have shown that generation of 4-HNE macromolecule adducts plays important pathological roles in cancer through interactions with mitochondria. First of all, mitochondria are one of the most important cellular sites of 4-HNE production, presumably from oxidation of abundant PUFA-containing lipids, such as L4CL. Emerging evidence suggest that this process play a critical role in apoptosis. Secondly, in response to the toxicity of 4-HNE, mitochondria have developed a number of defense mechanisms to convert 4-HNE to less reactive chemical species and minimize its toxic effects. Thirdly, 4-HNE macromolecule adducts in mitochondria are involved in the cancer initiation and progression by modulating mitochondrial function and metabolic reprogramming. 4-HNE protein adducts have been widely studied but the mtDNA modification by lipid electrophiles has yet to emerge. The biological consequence of PE modification remains to be defined, especially in the context of cancer. Last but not the least, manipulation of mitochondrial ROS generation, lipid peroxidation, and production of lipid electrophiles may be a viable approach for cancer prevention and treatment.
Fig. 3.
A schematic view of 4-HNE macromolecule adducts in cancer cell. 4-HNE macromolecule adducts are involved in cancer initiation, progression, metabolic reprogramming, and cell death. 4-HNE (depicted as a zigzag line) is produced through ROS-induced lipid peroxidation of mitochondrial and plasma membranes. Biological consequences of 4-HNE adduction: 1, reducing membrane integrity; 2, affecting protein function in cytosol; 3, causing nuclear and mitochondrial DNA damage; 4, inhibiting ETC activity; 5, activating UCPs activity; 6, reducing TCA activity; 7, inhibiting ALDH2 activity.
Acknowledgements
This work is supported by grants from National Natural Science Foundation of China (31170809), National Key Basic Research Program of China (973 Program, # 2012CB524905), Ministry of Science and Technology of China (2012BAK01B00), and Hundred Talents Program from CAS (2012OHTP07). H.Y. is an Associate Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine at Nanjing Medical University. H.Y. also acknowledges the financial support from COST CM1001 to attend 4-HNE Club meeting in 2014 at Toulouse, France.
References
- 1.Davies K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50(4–5):279–289. doi: 10.1080/713803728. 11327322 [DOI] [PubMed] [Google Scholar]
- 2.Yin H., Xu L., Porter N.A. Free radical lipid peroxidation: mechanisms and analysis. Chemical Reviews. 2011;111(10):5944–5972. doi: 10.1021/cr200084z. 21861450 [DOI] [PubMed] [Google Scholar]
- 3.Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochimica et Biophysica Acta. 1980;620(2):281–296. doi: 10.1016/0005-2760(80)90209-x. 6254573 [DOI] [PubMed] [Google Scholar]
- 4.Shoeb M., Ansari N.H., Srivastava S.K., Ramana K.V. 4-hydroxynonenal in the pathogenesis and progression of human diseases. Current Medicinal Chemistry. 2014;21(2):230–237. doi: 10.2174/09298673113209990181. 23848536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West J.D., Marnett L.J. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chemical Research in Toxicology. 2006;19(2):173–194. doi: 10.1021/tx050321u. 16485894 [DOI] [PubMed] [Google Scholar]
- 6.Barrera G., Pizzimenti S., Ciamporcero E.S., Daga M., Ullio C., Arcaro A., Cetrangolo G.P., Ferretti C., Dianzani C., Lepore A. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxidants & Redox Signaling. 2014 doi: 10.1089/ars.2014.6166. 25365742 [DOI] [PubMed] [Google Scholar]
- 7.Roede J.R., Jones D.P. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environmental and Molecular Mutagenesis. 2010;51(5):380–390. doi: 10.1002/em.20553. 20544880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guéraud F., Atalay M., Bresgen N., Cipak A., Eckl P.M., Huc L., Jouanin I., Siems W., Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radical Research. 2010;44(10):1098–1124. doi: 10.3109/10715762.2010.498477. 20836659 [DOI] [PubMed] [Google Scholar]
- 9.Chen Z.H., Niki E. 4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress. Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life. 2006;58(5–6):372–373. doi: 10.1080/15216540600686896. 16754333 [DOI] [PubMed] [Google Scholar]
- 10.Aldini G., Carini M., Yeum K.-J., Vistoli G. Novel molecular approaches for improving enzymatic and nonenzymatic detoxification of 4-hydroxynonenal: toward the discovery of a novel class of bioactive compounds. Free Radical Biology and Medicine. 2014;69(0):145–156. doi: 10.1016/j.freeradbiomed.2014.01.017. 24456906 [DOI] [PubMed] [Google Scholar]
- 11.Levonen A.-L., Hill B.G., Kansanen E., Zhang J., Darley-Usmar V.M. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radical Biology and Medicine. 2014;71(0):196–207. doi: 10.1016/j.freeradbiomed.2014.03.025. 24681256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W., Porter N.A., Schneider C., Brash A.R., Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: intramolecular peroxyl radical addition and decomposition. Free Radical Biology and Medicine. 2011;50(1):166–178. doi: 10.1016/j.freeradbiomed.2010.10.709. 21047551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong H., Lu J., Xia L., Zhu M., Yin H. Formation of electrophilic oxidation products from mitochondrial cardiolipin in vitro and in vivo in the context of apoptosis and atherosclerosis. Redox Biology. 2014;2(0):878–883. doi: 10.1016/j.redox.2014.04.003. 25061570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellot G.L., Liu D., Pervaiz S. ROS, autophagy, mitochondria and cancer: Ras, the hidden master? Mitochondrion. 2013;13(3):155–162. doi: 10.1016/j.mito.2012.06.007. 22750269 [DOI] [PubMed] [Google Scholar]
- 15.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. 21734707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y., Zhang L., Rong S., Qu H., Zhang Y., Chang D., Pan H., Wang W. Relation between gastric cancer and protein oxidation, DNA damage, and lipid peroxidation. Oxidative Medicine and Cellular Longevity. 2013;2013:543760. doi: 10.1155/2013/543760. 24454985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baradat M., Jouanin I., Dalleau S., Taché S., Gieules M., Debrauwer L., Canlet C., Huc L., Dupuy J., Pierre F.H. 4-hydroxy-2(E)-nonenal metabolism differs in Apc(+/+) cells and in Apc(Min/+) cells: it may explain colon cancer promotion by heme iron. Chemical Research in Toxicology. 2011;24(11):1984–1993. doi: 10.1021/tx2003036. 21967605 [DOI] [PubMed] [Google Scholar]
- 18.Schneider C., Porter N.A., Brash A.R. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. Journal of Biological Chemistry. 2008;283(23):15539–15543. doi: 10.1074/jbc.R800001200. 18285327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider C., Tallman K.A., Porter N.A., Brash A.R. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. Journal of Biological Chemistry. 2001;276(24):20831–20838. doi: 10.1074/jbc.M101821200. 11259420 [DOI] [PubMed] [Google Scholar]
- 20.Yin H., Zhu M. Free radical oxidation of cardiolipin: chemical mechanisms, detection and implication in apoptosis, mitochondrial dysfunction and human diseases. Free Radical Research. 2012;46(8):959–974. doi: 10.3109/10715762.2012.676642. 22468920 [DOI] [PubMed] [Google Scholar]
- 21.Zhong H., Lu J., Xia L., Zhu M., Yin H. Formation of electrophilic oxidation products from mitochondrial cardiolipin in vitro and in vivo in the context of apoptosis and atherosclerosis. Redox Biology. 2014;2:878–883. doi: 10.1016/j.redox.2014.04.003. 25061570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W., Porter N.A., Schneider C., Brash A.R., Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: intramolecular peroxyl radical addition and decomposition. Free Radical Biology and Medicine. 2011;50(1):166–178. doi: 10.1016/j.freeradbiomed.2010.10.709. 21047551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalvez F., Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis: An International Journal on Programmed Cell Death. 2007;12(5):877–885. doi: 10.1007/s10495-007-0718-8. 17294083 [DOI] [PubMed] [Google Scholar]
- 24.Oberley T.D., Toyokuni S., Szweda L.I. Localization of hydroxynonenal protein adducts in normal human kidney and selected human kidney cancers. Free Radical Biology and Medicine. 1999;27(5–6):695–703. doi: 10.1016/s0891-5849(99)00117-3. 10490290 [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y., Miriyala S., Miao L., Mitov M., Schnell D., Dhar S.K., Cai J., Klein J.B., Sultana R., Butterfield D.A. Redox proteomic identification of HNE-bound mitochondrial proteins in cardiac tissues reveals a systemic effect on energy metabolism after doxorubicin treatment. Free Radical Biology and Medicine. 2014;72:55–65. doi: 10.1016/j.freeradbiomed.2014.03.001. 24632380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poli G., Schaur R.J., Siems W.G., Leonarduzzi G. 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Medicinal Research Reviews. 2008;28(4):569–631. doi: 10.1002/med.20117. 18058921 [DOI] [PubMed] [Google Scholar]
- 27.Siems W., Grune T. Intracellular metabolism of 4-hydroxynonenal. Molecular Aspects of Medicine. 2003;24(4–5):167–175. doi: 10.1016/s0098-2997(03)00011-6. 12892994 [DOI] [PubMed] [Google Scholar]
- 28.Chang B., Nishikawa M., Nishiguchi S., Inoue M. l-Carnitine inhibits hepatocarcinogenesis via protection of mitochondria. International Journal of Cancer. 2005;113(5):719–729. doi: 10.1002/ijc.20636. 15499623 [DOI] [PubMed] [Google Scholar]
- 29.Leake K., Singhal J., Nagaprashantha L.D., Awasthi S., Singhal S.S. RLIP76 regulates PI3K/Akt signaling and chemo-radiotherapy resistance in pancreatic cancer. PLOS One. 2012;7(4):e34582. doi: 10.1371/journal.pone.0034582. 22509328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.-L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biology. 2013;1(1):45–49. doi: 10.1016/j.redox.2012.10.001. 24024136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santidrian A.F., Matsuno-Yagi A., Ritland M., Seo B.B., LeBoeuf S.E., Gay L.J., Yagi T., Felding-Habermann B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. Journal of Clinical Investigation. 2013;123(3):1068–1081. doi: 10.1172/JCI64264. 23426180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porporato P.E., Payen V.L., Pérez-Escuredo J., De Saedeleer C.J., Danhier P., Copetti T., Dhup S., Tardy M., Vazeille T., Bouzin C. A mitochondrial switch promotes tumor metastasis. Cell Report. 2014;8(3):754–766. doi: 10.1016/j.celrep.2014.06.043. 25066121 [DOI] [PubMed] [Google Scholar]
- 33.Dröse S., Zwicker K., Brandt U. Full recovery of the NADH:ubiquinone activity of complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica by the addition of phospholipids. Biochimica et Biophysica Acta. 2002;1556(1):65–72. doi: 10.1016/s0005-2728(02)00307-9. 12351219 [DOI] [PubMed] [Google Scholar]
- 34.Fry M., Green D.E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. Journal of Biological Chemistry. 1981;256(4):1874–1880. 6257690 [PubMed] [Google Scholar]
- 35.Eble K.S., Coleman W.B., Hantgan R.R., Cunningham C.C. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. Journal of Biological Chemistry. 1990;265(32):19434–19440. 2147180 [PubMed] [Google Scholar]
- 36.Valle A., Oliver J., Roca P. Role of uncoupling proteins in cancer. Cancers (Basel) 2010;2(2):567–591. doi: 10.3390/cancers2020567. 24281083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Echtay K.S., Murphy M.P., Smith R.A., Talbot D.A., Brand M.D. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. Journal of Biological Chemistry. 2002;277(49):47129–47135. doi: 10.1074/jbc.M208262200. 12372827 [DOI] [PubMed] [Google Scholar]
- 38.Krauss S., Zhang C.-Y., Scorrano L., Dalgaard L.T., St-Pierre J., Grey S.T., Lowell B.B. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. Journal of Clinical Investigation. 2003;112(12):1831–1842. doi: 10.1172/JCI19774. 14679178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteves T.C., Brand M.D. The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochimica et Biophysica Acta. 2005;1709(1):35–44. doi: 10.1016/j.bbabio.2005.06.002. 16005426 [DOI] [PubMed] [Google Scholar]
- 40.Echtay K.S., Esteves T.C., Pakay J.L., Jekabsons M.B., Lambert A.J., Portero-Otín M., Pamplona R., Vidal-Puig A.J., Wang S., Roebuck S.J., Brand M.D. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO Journal. 2003;22(16):4103–4110. doi: 10.1093/emboj/cdg412. 12912909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoval-Sánchez B., Rodríguez-Zavala J.S. Differences in susceptibility to inactivation of human aldehyde dehydrogenases by lipid peroxidation byproducts. Chemical Research in Toxicology. 2012;25(3):722–729. doi: 10.1021/tx2005184. 22339434 [DOI] [PubMed] [Google Scholar]
- 42.Asakage T., Yokoyama A., Haneda T., Yamazaki M., Muto M., Yokoyama T., Kato H., Igaki H., Tsujinaka T., Kumagai Y. Genetic polymorphisms of alcohol and aldehyde dehydrogenases, and drinking, smoking and diet in Japanese men with oral and pharyngeal squamous cell carcinoma. Carcinogenesis. 2007;28(4):865–874. doi: 10.1093/carcin/bgl206. 17071628 [DOI] [PubMed] [Google Scholar]
- 43.Ding J.-H., Li S.P., Cao H.X., Wu J.Z., Gao C.M., Su P., Liu Y.T., Zhou J.N., Chang J., Yao G.H. Polymorphisms of alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 and esophageal cancer risk in Southeast Chinese males. World Journal of Gastroenterology. 2009;15(19):2395–2400. doi: 10.3748/wjg.15.2395. 19452585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muto M., Takahashi M., Ohtsu A., Ebihara S., Yoshida S., Esumi H. Risk of multiple squamous cell carcinomas both in the esophagus and the head and neck region. Carcinogenesis. 2005;26(5):1008–1012. doi: 10.1093/carcin/bgi035. 15718256 [DOI] [PubMed] [Google Scholar]
- 45.Pawlosky R.J., Salem N., Jr. Perspectives on alcohol consumption: liver polyunsaturated fatty acids and essential fatty acid metabolism. Alcohol. 2004;34(1):27–33. doi: 10.1016/j.alcohol.2004.07.009. 15670662 [DOI] [PubMed] [Google Scholar]
- 46.Niemelä O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radical Biology and Medicine. 2001;31(12):1533–1538. doi: 10.1016/s0891-5849(01)00744-4. 11744326 [DOI] [PubMed] [Google Scholar]
- 47.Doorn J.A., Hurley T.D., Petersen D.R. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-Hydroxynon-2-enal and 4-Oxonon-2-enal. Chem. Res. Toxicol. 2006;19(1):102–110. doi: 10.1021/tx0501839. 16411662 [DOI] [PubMed] [Google Scholar]
- 48.Lee C.H., Wu S.B., Hong C.H., Chen G.S., Wei Y.H., Yu H.S. Involvement of mtDNA damage elicited by oxidative stress in the arsenical skin cancers. J. Invest. Dermatol. 2013;133(7):1890–1900. doi: 10.1038/jid.2013.55. 23370535 [DOI] [PubMed] [Google Scholar]
- 49.Ma W.W., Hou C.C., Zhou X., Yu H.L., Xi Y.D., Ding J., Zhao X., Xiao R. Genistein alleviates the mitochondria-targeted DNA damage induced by beta-amyloid peptides 25–35 in C6 glioma cells. Neurochem. Res. 2013;38(7):1315–1323. doi: 10.1007/s11064-013-1019-y. 23519932 [DOI] [PubMed] [Google Scholar]
- 50.Wada T., Tanji N., Ozawa A., Wang J., Shimamoto K., Sakayama K., Yokoyama M. Mitochondrial DNA mutations and 8-hydroxy-2′-deoxyguanosine content in Japanese patients with urinary bladder and renal cancers. Anticancer Research. 2006;26(5A):3403–3408. 17094459 [PubMed] [Google Scholar]
- 51.Hu W., Feng Z., Eveleigh J., Iyer G., Pan J., Amin S., Chung F.L., Tang M.S. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23(11):1781–1789. doi: 10.1093/carcin/23.11.1781. 12419825 [DOI] [PubMed] [Google Scholar]
- 52.Daum G., Vance J.E. Import of lipids into mitochondria. Progress in Lipid Research. 1997;36(2–3):103–130. doi: 10.1016/s0163-7827(97)00006-4. 9624424 [DOI] [PubMed] [Google Scholar]
- 53.Guichardant M., Bacot S., Molière P., Lagarde M. Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75(3):179–182. doi: 10.1016/j.plefa.2006.05.006. 16828271 [DOI] [PubMed] [Google Scholar]
- 54.Bacot S., Bernoud-Hubac N., Chantegrel B., Deshayes C., Doutheau A., Ponsin G., Lagarde M., Guichardant M. Evidence for in situ ethanolamine phospholipid adducts with hydroxy-alkenals. Journal of Lipid Research. 2007;48(4):816–825. doi: 10.1194/jlr.M600340-JLR200. 17220481 [DOI] [PubMed] [Google Scholar]
- 55.Guichardant M., Bernoud-Hubac N., Chantegrel B., Deshayes C., Lagarde M. Aldehydes from n-6 fatty acid peroxidation. Effects on aminophospholipids. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2002;67(2–3):147–149. doi: 10.1054/plef.2002.0412. 12324234 [DOI] [PubMed] [Google Scholar]
- 56.Rogers C., Davis B., Neufer P.D., Murphy M.P., Anderson E.J., Robidoux J. A transient increase in lipid peroxidation primes preadipocytes for delayed mitochondrial inner membrane permeabilization and ATP depletion during prolonged exposure to fatty acids. Free Radical Biology and Medicine. 2014;67:330–341. doi: 10.1016/j.freeradbiomed.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin H., Kanthasamy A., Ghosh A., Anantharam V., Kalyanaraman B., Kanthasamy A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochimica et Biophysica Acta. 2014;1842(8):1282–1294. doi: 10.1016/j.bbadis.2013.09.007. 24060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumgartner M., Sturlan S., Roth E., Wessner B., Bachleitner-Hofmann T. Enhancement of arsenic trioxide-mediated apoptosis using docosahexaenoic acid in arsenic trioxide-resistant solid tumor cells. International Journal of Cancer. 2004;112(4):707–712. doi: 10.1002/ijc.20462. 15382055 [DOI] [PubMed] [Google Scholar]
- 59.Allen B.G., Bhatia S.K., Buatti J.M., Brandt K.E., Lindholm K.E., Button A.M., Szweda L.I., Smith B.J., Spitz D.R., Fath M.A. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clinical Cancer Research. 2013;19(14):3905–3913. doi: 10.1158/1078-0432.CCR-12-0287. 23743570 [DOI] [PMC free article] [PubMed] [Google Scholar]