Abstract
Coenzyme Q10 is a ubiquitous component of cellular membranes and belongs to the class of benzoquinones that mainly differ with regards to the length and composition of their hydrophobic tail. The characteristic quinone group can accept electrons from various biological sources and is converted by a one electron transfer to the unstable semiquinone or by a two electron transfer to the more stable hydroquinone. This feature makes CoQ10 the bona fide cellular electron transfer molecule within the mitochondrial respiratory chain and also makes it a potent cellular antioxidant. These activities serve as justification for its popular use as food supplement. Another quinone with similarities to the naturally occurring CoQ10 is idebenone, which shares its quinone moiety with CoQ10, but at the same time differs from CoQ10 by the presence of a much shorter, less lipophilic tail. However, despite its similarity to CoQ10, idebenone cannot be isolated from any natural sources but instead was synthesized and selected as a pharmacologically active compound in the 1980s by Takeda Pharmaceuticals purely based on its pharmacological properties. Several recent clinical trials demonstrated some therapeutic efficacy of idebenone in different indications and as a consequence, many practitioners question if the freely available CoQ10 could not be used instead. Here, we describe the molecular and pharmacological features of both molecules that arise from their structural differences to answer the question if idebenone is merely a CoQ10 analogue as frequently perpetuated in the literature or a pharmaceutical drug with entirely different features.
Keywords: Benzoquinone, Idebenone, Coenzyme Q10, Mitochondria, Antioxidant
Abbreviations: CoQ, coenzyme Q; DMD, Duchene Muscular Dystrophy; ETC, electron transport chain; NQO1, NAD(P)H-quinone oxidoreductase 1; ROS, reactive oxygen species
Graphical abstract
Highlights
-
●
The benzoquinones CoQ10 and idebenone have vastly different solubility.
-
●
Both molecules need to get activated by cellular reductases.
-
●
Due to their solubility both molecules are in different cellular compartments.
-
●
Therefore, both quinones are activated by different enzymes.
-
●
Thus, their solubility strongly determines their biological activities.
Introduction
Universally present in human cells, coenzyme Q10 (CoQ10) is a ubiquitous component of all cellular membranes. However, its best studied function lies within the mitochondrial energy producing system as an electron transport molecule. With only some rare exceptions CoQ10 is therefore essential to life. CoQ10 belongs to a class of compounds that are characterized by their quinone moiety but differ in length and composition of their hydrophobic tail (Fig. 1). The characteristic quinone group can accept electrons from various biological sources and is converted by a one electron transfer to the unstable semiquinone or by a two electron transfer to the more stable hydroquinone (Fig. 2). This feature makes CoQ10 the bona fide cellular electron transfer molecule within the mitochondrial respiratory chain. In addition, CoQ10 is also described as a potent cellular antioxidant. This activity, together with lower CoQ10 levels during ageing and some diseases serve as justification for its common use as food supplement. It is of interest that CoQ10 is only one among a large range of very similar molecules that are involved in a multitude of cellular functions. Many 1,4-benzoquinone-containing molecules similar to CoQ10 are selectively synthesized by cells from bacteria to eukaryotic cells. These molecules harbour different tail length ranging from 0 (CoQ0) to 10 (CoQ10) isoprenyl units. For example, the predominant form of coenzyme Q in rats is CoQ9 compared to CoQ10 in humans (Fig. 1). While the ubiquinone moiety present in CoQ10 is the major quinone in cells of animal origin, plants use an entirely different quinone moiety (plastoquinone) for photosynthesis, while still using CoQ10 within their mitochondria.
Fig. 1.
Chemical structure of the two quinones CoQ10 and idebenone. The ten ispopren unit-containing side chain of CoQ10 is responsible for major differences in solubility and molecular weight and as a consequence bioactivation. MW: molecular weight; LogD: partition coefficient at physiological pH.
Fig. 2.
Schematic representation of quinone bioactivation mainly by two-electron reduction (two red circles). While activation of CoQ10 preferentially occurs via the mitochondrial electron transport chain (mETC), idebenone is activated to the hydroquinone by the cytoplasmic NQO1 reductase. In contrast, one electron reduction (one red circle) to the unstable semiquinone is mostly done by the Cyp450 family in the absence of two-electron-transferring reductases and is not a favourable pathway as it generates oxidative radicals.
A synthetic quinone with similarities to the naturally occurring CoQ10 is idebenone (Fig. 1). Idebenone shares its quinone moiety with CoQ10, but at the same time differs from CoQ10 by the presence of a much shorter, less lipophilic tail. However, despite its similarity to CoQ10, idebenone is not synthesized by any organism and can therefore not be isolated from any natural sources. Thus, idebenone is a novel chemical entity, which was selected from a medicinal chemistry programme conducted in the 1980s by Takeda Pharmaceuticals as a pharmacologically active compound purely based on its pharmacological properties. Here, we describe the molecular and pharmacological features of idebenone that are shared with and at the same time separate it from CoQ10 to answer the question if idebenone is merely a CoQ10 analogue as frequently perpetuated in the literature or a drug with entirely different pharmacological properties.
Pharmacokinetics
Despite the structural relatedness of CoQ10 and idebenone, both molecules differ significantly in their physicochemical properties (Fig. 1) (Table 1). The ten isoprenyl units of the tail (50 carbon atoms) of CoQ10 make this molecule practically insoluble in aqueous solutions, which is represented by a partition coefficient of nearly 20 [1]. Idebenone on the other hand has a much shorter tail (10 carbon atoms) and unlike CoQ10, also harbours a terminal hydroxyl group which provides the molecule with polarity. Both of those features are responsible for a partition coefficient of 3.9 for idebenone, which leads to a much higher solubility in aqueous solution. It is this difference in solubility that is largely responsible for all the functional differences between two molecules that are discussed in detail below.
Table 1.
Summary of structural and mechanistic differences between CoQ10 and idebenone.
| Parameter | CoQ10 | Idebenone |
|---|---|---|
| Chemical formula | C59H90O4 | C19H30O5 |
| Molecular weight (g/mol) | 863.49 | 338.44 |
| Solubility; log D (pH 7.4) | 19.12 | 3.91 |
| Ability to cross membranes | No | Yes |
| In vivo tmax | 6–8 h | 1–3 h |
| In vivo t1/2 | About 33 h | 10–15 h |
| Complex I inhibitor | No | Yes |
| Complex II substrate | Yes | Yes |
| Complex III substrate | Yes | Yes |
| Reduction by NQO1 | very low | Yes |
| Activation of G3PDH shuttle | Not reported | Yes |
| Rescue of ATP levels in the absence of functional complex I (the higher the better) | 0% | up to 80% |
| Reduction of lipid peroxide levels (the lower the better) | 93±5% | 45±7% |
| Effect on mitochondrial. membrane potential (ΔΨm) | 106% | 116% |
| Proposed mode(s) of action | Membrane-localized antioxidant, electron transport activity in mitochondrial respiratory chain | Antioxidant in multiple cellular compartments; redox function and energy rescue via alternative pathways |
It has to be stressed that CoQ10, unlike idebenone, is a physiological molecule that is synthesized by all cells of the body. The biosynthesis of CoQ10 is complex and shares some of the early steps with the cholesterol synthesis pathway [2]. Due to the very high lipophilicity of CoQ10 eleven specialized enzymes are presently known. These enzymes are crucial for the biosynthesis of the lipophilic CoQ10 and hand the synthetic intermediates of CoQ10 from one enzyme to the next. As a final step, these enzymes ensure that CoQ10 is effectively inserted into cellular membranes as no soluble form of CoQ10 exists [2]. Consequently, dietary CoQ10 faces a number of hurdles with regards to transport to reach its proposed site of action in cellular membranes. Despite its frequent use as food supplement, there are only few reports on the pharmacokinetics of CoQ10 in humans. Dietary CoQ10 is slowly absorbed from the intestinal tract, evidenced by a plasma Tmax of 6–8 h [3] and it is eliminated with a half-life of about 33 h [4]. A second plasma peak was described to occur about 24 h after oral administration, which likely reflects enterohepatic recycling. There is some suggestion that using chronic ingestion of high doses via the diet can increase CoQ10 concentrations at least in heart and brain tissue of rodent models [5], although it has to be noted that CoQ9 and not CoQ10 is the predominant form found in rodents. In rodents CoQ10 levels are kept at about 10% of those of CoQ9 under physiological conditions and it is long known that dietary CoQ10 is converted back to CoQ9 in rats [6]. Furthermore, there is some evidence for metabolism of dietary CoQ10, which highlights that the results described above cannot be easily translated to the human situation where CoQ10 is the predominant quinone. Consequently, due to the lack of data of CoQ10 metabolite production, reliable information on tissue levels for dietary CoQ10 is not available.
Based on the synthetic nature of idebenone, detailed investigations that took metabolic conversion into account by separately measuring the intact idebenone and metabolites provided reliable data for unmodified idebenone levels in plasma and tissues. Studies in animal models have demonstrated a wide biodistribution of intact, unmetabolized idebenone with the highest levels found in liver and kidney and the lowest in heart and brain [7]. In patients, idebenone is rapidly absorbed with a tmax of 1–3 h and also eliminated faster than CoQ10, with a half-life between 10 to 13 h [8]. Although there is uncertainty around the relevance and accuracy of CoQ10 measurements and metabolism, the reported pharmacokinetic differences between idebenone and CoQ10 are likely a direct consequence of the major difference in solubility of both molecules.
Different roles in electron transport
The most prominent role of CoQ10 is as an electron carrier in the mitochondrial electron transport chain (ETC). Under physiological conditions, CoQ10 accepts electrons mainly from complexes I and II and transports them to complex III. Upon donating the electrons to complex III, CoQ10 is able to be reduced by complexes I and II again. This cyclic activity is essential for the mechanism of mitochondrial energy production and hence lower CoQ10 levels negatively impact on cellular energy levels, which is evidenced by the severe phenotype of the reported CoQ10-deficiency disorders. Biochemical evidence suggests that throughout this cyclic electron transport process CoQ10, due to its highly lipophilic nature, is firmly anchored to and embedded within in the inner mitochondrial membrane.
Given the structural similarity to CoQ10 at the level of the quinone moiety (Fig. 1), it was always assumed that idebenone also has similar properties with regards to cellular electron transport. Contrary to this notion however, there is evidence that in the presence of physiological levels of mitochondrial CoQ10, idebenone directly modulates mitochondrial respiration and energy production, which suggest that idebenone has characteristics that are distinct from those of CoQ10. It has to be noted that the majority of reports that observed effects of idebenone on respiratory activity used isolated mitochondria. Since we now know that idebenone also facilitates important redox-functions outside the mitochondria [9], these studies using isolated mitochondria not only misrepresent the activity of idebenone but are also responsible for many conflicting reports, Therefore only the most relevant studies will be mentioned briefly below.
Roles in the electron transport chain
Sugiyama et al. [10] were the first to observe in isolated rat brain mitochondria that idebenone decreased state 3 respiration in a concentration-dependent manner when using a complex I substrate. However, when using a complex II substrate, the authors described that the respiratory and phosphorylating activities of isolated mitochondria were left unchanged. Sugiyama et al. [10] also demonstrated that in line with CoQ10, reduced idebenone is rapidly converted back to the oxidized quinone form through oxidation by complex III of the respiratory chain. Although idebenone markedly inhibited complex I−III (NADH-cytochrome c reductase) activity in this system, the authors also reported a surprising stimulation of complex I activity by idebenone. However, given the low basal NADH-ubiquinone reductase activity observed, rather than measuring mitochondrial complex I activity, it is more likely that other quinone oxidoreductases such as NQO1 were detected, which co-purified with the mitochondrial preparation [9]. Overall, despite the use of different experimental systems by different investigators, there is consensus that idebenone is an efficient substrate for the complexes II and III and in contrast to CoQ10, a relatively slow substrate for complex I [10], [11], [12].
Idebenone as complex I inhibitor
In fact, more than just being an inefficient substrate, multiple studies consistently detected inhibition of complex I by idebenone, in contrast to the function of CoQ10 [10], [11], [12], [13], [14], [15], [16], [17], [19], [20]. Recent data confirm this inhibitory activity of idebenone using the sophisticated electrochemical detection of proton translocating activity of isolated mitochondrial membranes [16]. This inhibition of complex I by idebenone is thought to be based on the slow release of reduced idebenone from the CoQ10 binding site within complex I, which therefore interferes with the physiological reduction of CoQ10 [15]. One possible explanation for this inhibitory activity is based on the size of the quinone binding pocket of complex I. The long lipophilic tail of CoQ10 safely secures the molecule in the mitochondrial membrane, while still allowing the quinone moiety to enter into the quinone binding pocket of complex I. Idebenone on the other hand can be expected by its much shorter tail to completely enter the binding pocket, which likely results in a much longer time within the pocket [15]. This difference in tail size and the arising difference in its interaction with complex I make idebenone, quite contrary to CoQ10, a competitive inhibitor of complex I.
Activation of alternative pathways by idebenone
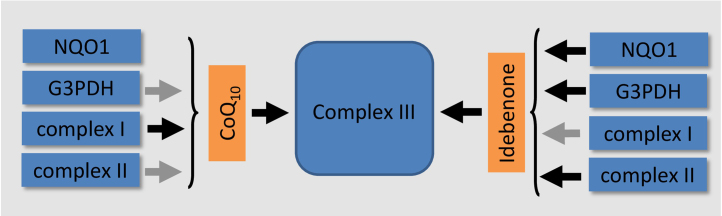
Given the importance of complex I for energy production, it appears counterintuitive that inhibition of complex I by idebenone could be associated with any beneficial therapeutic effects, unless idebenone could compensate this inhibition by utilizing other metabolic pathways to generate energy. In fact, there is evidence from several studies that idebenone can activate different complex I-independent metabolic pathways. One of those idebenone-preferred pathways facilitates complexes II–III based respiration; a mechanism that could support mitochondrial energy production in the presence of dysfunctional complex I [11]. Indeed, this activity was substantiated and later extended by several reports illustrating that idebenone utilizes and activates further complex I-independent metabolic pathways in the presence of CoQ10 [1], [9], [17], [18] (Fig. 3). One of those is the glycerophosphate (G3PDH) shuttle. This mechanism supplies extra energy from a non-mitochondrial source into the mitochondria and is predominantly active in tissues with high energy demand. First described by James et al. [12] and studied in more detail by Rauchova et al. [17], [19], [20], idebenone efficiently activates this metabolic pathway in vitro and in vivo in the presence of physiological levels of CoQ10 by a so far unknown mechanism.
Fig. 3.
Schematic representation of the different electron transport pathways favoured by the two quinones CoQ10 and idebenone. Black arrow: favoured pathway; grey arrow: minor pathway.
An additional idebenone-dependent metabolic pathway that transfers energy equivalents from the cytosol directly into the mitochondrial respiratory chain, was reported recently [1,9,18]. Here, upon entering the cell, idebenone is efficiently reduced by the cytoplasmic enzyme NADH-quinone oxidoreductase 1 (NQO1) as part of the cellular response to detoxify quinones and to prevent production of ROS. The resulting active form of idebenone subsequently enters the mitochondria to become re-oxidized by complex III of the mitochondrial electron transport chain. In line with this “catalytic” model of repeatedly donating electrons derived from the cytoplasma directly to complex III, idebenone is able to directly circumvent complex I−III‐dependent electron transport [9], [18] (Fig. 3). Indeed, under conditions of acute rotenone treatment, which efficiently inactivates complex I function and abolishes cellular energy levels, this NQO1-dependent activation of idebenone is able to increase mitochondrial membrane potential and restore cellular ATP levels in the presence of physiological levels of CoQ10 (Table 1) [1,9,18].
Overall, in the presence of CoQ10, idebenone treatment leads to a shift away from complex I-dependent respiration towards alternative pathways that either use complex II dependent substrates or utilize cytoplasmic electron equivalents, which are fed directly into complex III. The combined results of this idebenone-modified metabolism lead to a largely complex I-independent form of respiration. It is again important to state that the ability of idebenone to activate these alternative pathways is absolutely dependent on a balanced solubility that allows it to shuttle between cytosol and mitochondrial membranes [1]. Consequently, at present there is no evidence to suggest that dietary CoQ10 can activate the alternative modes of energy production described for idebenone above. Given that complex I dysfunction is the major cause of mitochondrial dysfunction in a multitude of disorders ranging from classic mitochondrial diseases to neuromuscular disorders such as Duchene Muscular Dystrophy (DMD) and neurological disorders such as glaucoma [21], this functional difference between CoQ10 and idebenone rationalizes the use of idebenone.
Antioxidative activities of idebenone and CoQ10
The naturally occurring CoQ10 is described by numerous reports as a potent physiological antioxidant (reviewed by Littarru and Tiano [22]). Within the cell, CoQ10 can detoxify radicals and is important to protect cellular membranes against lipid peroxidation. This information is based on the study of human CoQ10 deficiency disorders that are associated with low levels of CoQ10, high levels of ROS and most importantly, which can be treated with exogenous CoQ10 supplementation. Consequently, CoQ10 is widely used in indications that are thought to be associated with elevated levels of oxidative stress [22], although its effectiveness as oral supplement or therapeutic compound is still disputed [23]. Since idebenone shares the identical quinone group with CoQ10, it is not surprising that it is also reported to be a potent anti-oxidant. The effects of idebenone on oxidative stress and lipid peroxidation were investigated by numerous studies that consistently reported a high degree of protection against oxidative stress in vitro and in vivo [17], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. Concentrations needed to achieve protection against ROS-induced toxicity by idebenone varied extensively depending on the cellular model used. Idebenone concentrations as low as 10 nM efficiently inhibited mitochondrial ROS formation [17], while using an ex-vivo retina model 1 µM idebenone fully protected against acute oxidative stress and cell death [28].
Importantly, lipid peroxidation-induced changes to mitochondrial membrane integrity are thought to directly inhibit mitochondrial respiratory function. Several studies demonstrated an inverse relationship between the extent of experimentally-induced lipid peroxidation and mitochondrial respiration, which could be normalized by idebenone treatment [10], [36]. Although, lipid peroxidation impaired the activity of complexes II, III and V, in this study idebenone treatment specifically protected complex III function, which is likely based on the interaction of reduced idebenone with complex III [36]. The authors therefore suggested that lipid peroxidation is a major contributing factor leading to impairment of complex III function and therefore, lipophilic antioxidants like idebenone are more likely to prevent this particular type of macromolecular damage.
This protective activity of idebenone in preventing ROS-induced mitochondrial dysfunction was also tested in human tissue [37]. However, in contrast to the results by Cardoso et al. [36], idebenone also protected complex II activity against ROS-induced injury in this system [37]. The authors noted that this protective effect was dependent on the conversion of idebenone into the reduced quinol form by the respiratory chain.
It is important to point out that evidence for the antioxidant activity of both idebenone and CoQ10 are largely derived from in vitro and ex vivo studies. The few studies that looked at antioxidant function in vivo did so by demonstrating reduced biomarkers of oxidative stress in intact organisms in response to treatment with both molecules. This obviously harbours the possibility that both molecules could prevent oxidative stress by indirect mechanisms such as the upregulation of endogenous antioxidative defence mechanisms. However, at least one paper has reported a direct anti-oxidative activity of idebenone in vivo using electron spin resonance in the presence of CoQ10 [26], which suggests that at least idebenone can act as a bona fide antioxidant in vivo.
Overall, most biochemical studies agree that both CoQ10 and idebenone can inhibit the generation of reactive oxygen species (ROS), however, given the differences in solubility and therefore cellular localization of both molecules, no information is available if their antioxidative activities are restricted to only certain cellular compartments. In this context, it is important to point out that nearly all described antioxidant effects of idebenone have been demonstrated in systems that display physiological levels of CoQ10. Positive effects of CoQ10 administration in cells with physiological levels of CoQ10 could suggest that the amount or localization of quinone, CoQ10 or idebenone, are the rate limiting factors for detoxification of ROS and that the quinone-dependent antioxidative activity is not saturated under physiological conditions. However, it could also suggest that idebenone is able to detoxify ROS in a manner distinct from that of CoQ10 or that the conditions for bioactivation of both molecules differ significantly based on their significant physicochemical differences described above.
Differences in bioactivation
It is important to point out that both idebenone and CoQ10 are only active as antioxidants or electron donors in the fully reduced hydroquinone form [32]. Therefore, both molecules can be regarded as pro-drugs that require bioactivation to become antioxidants. For their function as electron donors, the same requirement applies since only the activated hydroquinones can donate electrons into the electron transfer chain. Consequently, next to tissue distribution and cellular concentrations of both molecules, bioactivation appears to be a rate limiting step for their specific activities. In this context the extreme difference in the solubility of both molecules has to be remembered. As a consequence of its very high lipophilicity, CoQ10 is only present within cellular membranes [38], while idebenone with its much lower lipophilicity is found equally distributed in mitochondria and cytoplasm as shown for example in brain tissue [7], [39]. This difference in localization determines the access of both molecules to different reductases that localize to different cellular compartments. After entering the cell, idebenone, with its much higher solubility compared to CoQ10 is rapidly and exclusively activated by NQO1 [9]. On the other hand reduction of CoQ10 within mitochondrial membranes is dependent on the activity of the respiratory complexes I and II. Despite a report that CoQ10 can also be activated by NQO1, this activity, if at all specific, is at least 1000-fold lower compared to the reduction of idebenone by NQO1 [9], [40] and it is likely that extra-mitochondrial CoQ10 is reduced by another reductase altogether [41]. Therefore, in the context of mitochondrial disorders, it appears likely that efficient reduction of CoQ10 cannot be achieved since this bioactivation is largely dependent on intact mitochondrial activity. On the other hand, based on its mode of bioactivation by cytoplasmic NQO1, idebenone can still be efficiently reduced under conditions of mitochondrial dysfunction since NQO1 utilizes mitochondria-independent electron equivalents that are generated for example by glycolysis in the cytoplasm.
Can idebenone substitute for CoQ10?
As pointed out before, most studies on the activities of idebenone have been carried out in cells and tissues that contained physiological levels of CoQ10. Although, reduced CoQ10 levels have been described for the process of ageing, for most mitochondrial disorders and also in nearly all experimental systems where idebenone was tested, there is no evidence to suggest that CoQ10 levels are altered. Measurable results of idebenone-treatment therefore indicate that either idebenone has different protective activities compared to those of CoQ10 or that under physiological conditions only suboptimal levels of CoQ10 are available. The latter option would imply that idebenone simply acts by substituting for CoQ10. This possibility was tested and López et al. [42] clearly showed that in cells deficient in CoQ10 biosynthesis, idebenone was unable to substitute for CoQ10 in terms of normalizing electron flow or restoring ATP levels, which are the main functions of cellular CoQ10. Similar results were obtained in CoQ10 deficient mouse cells (genetic Coq7 mutants) where idebenone was also unable to rescue viability [43]. These pre-clinical results are strongly supported by a report of idebenone supplementation in a patient with CoQ10 deficiency [44]. After switching a young patient with a CoQ10 deficiency syndrome from CoQ10 supplementation to idebenone, his clinical and metabolic symptoms worsened markedly. Only after returning the patient to CoQ10 supplementation did his condition return to the state before idebenone treatment had commenced [44]. In another case, a patient with Leigh disease was treated with CoQ10, which coincided with a worsening of his condition that only normalized after commencing treatment with idebenone instead [45]. These results highlight that idebenone cannot be used as a CoQ10 replacement and that the protective activities of idebenone are unrelated to the activities shared with CoQ10. Based on these functional differences CoQ10 can therefore also not substitute for idebenone.
Conclusions
Based on their partial structural relatedness, CoQ10 and idebenone share the ability to act as potent antioxidants and to donate electrons to complex III of the ETC. Beyond this however, both molecules differ significantly with regards to pharmacokinetics, bioactivation and modulation of cellular energy production mainly due to their different tail structure resulting in different solubility in aqueous solutions. This feature leads to different subcellular localization of both molecules, which in turn affects their interactions with different proteins, enzymes and pathways. As a consequence, bioactivation of mitochondrial CoQ10 is strictly dependent on mitochondrial function, while idebenone is bioactivated predominantly in the cytoplasm and is not dependent on mitochondrial function. As another consequence of different molecular structure, CoQ10 largely drives complex I dependent respiration in contrast to idebenone, which favours alternative, complex I-independent pathways. Therefore, based on the current scientific information, including the studies and results described above, the synthetic idebenone harbours an entirely different repertoire of molecular activities compared to the natural CoQ10. As a consequence, idebenone and CoQ10 are unable to substitute for each other. Thus, the currently used habit of many authors of scientific publications and internet sites of referring to idebenone as a CoQ10 analogue lacks any scientific evidence.
Conflict of interest
N. Gueven acts as scientific consultant to Santhera Pharmaceuticals (Switzerland) that seeks to obtain market authorization for the use of idebenone in several neuromuscular indications.
K. Woolley and J. Smith have no conflicts of interest to declare.
Acknowledgements
We would like to thank the Schools of Chemistry and Medicine (Pharmacy) for their financial support.
References
- 1.Erb M., Hoffmann-Enger B., Deppe H., Soeberdt M., Haefeli R.H., Rummey C., Feurer A., Gueven N. Features of idebenone and related short-chain Quinones that Rescue ATP Levels under conditions of impaired mitochondrial complex I. PLOS One. 2012;7(4):e36153. doi: 10.1371/journal.pone.0036153. 22558363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Hekimi S. Molecular genetics of ubiquinone biosynthesis in animals. Critical Reviews in Biochemistry and Molecular Biology. 2013;48(1):69–88. doi: 10.3109/10409238.2012.741564. 23190198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhagavan H.N., Chopra R.K. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radical Research. 2006;40(5):445–453. doi: 10.1080/10715760600617843. 16551570 [DOI] [PubMed] [Google Scholar]
- 4.Tomono Y., Hasegawa J., Seki T., Motegi K., Morishita N. Pharmacokinetic study of deuterium-labelled coenzyme Q10 in man. International Journal of Clinical Pharmacology, Therapy and Toxicology. 1986;24(10):536–541. 3781673 [PubMed] [Google Scholar]
- 5.Miles M.V. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7(Suppl.):S72–S77. doi: 10.1016/j.mito.2007.02.012. 17446143 [DOI] [PubMed] [Google Scholar]
- 6.Kishi H., Nanamori N., Nishii S. Metabolism of exogenous coenzyme Q10 in vivo and the bioavailability of coenzyme Q10 preparations in Japan. In: Folkers K., Jamamura Y., editors. Biomedical and Clinical Aspects of Coenzyme Q. Elsevier; Amsterdam: 1964. pp. 131–142. [Google Scholar]
- 7.Torii H., Yoshida K., Kobayashi T., Tsukamoto T., Tanayama S. Disposition of idebenone (CV-2619), a new cerebral metabolism improving agent, in rats and dogs. Journal of Pharmacobio-dynamics. 1985;8(6):457–467. doi: 10.1248/bpb1978.8.457. 4057041 [DOI] [PubMed] [Google Scholar]
- 8.Di Prospero N.A., Sumner C.J., Penzak S.R., Ravina B., Fischbeck K.H., Taylor J.P. Safety, tolerability, and pharmacokinetics of high-dose idebenone in patients with Friedreich ataxia. Archives of Neurology. 2007;64(6):803–808. doi: 10.1001/archneur.64.6.803. 17562928 [DOI] [PubMed] [Google Scholar]
- 9.Haefeli R.H., Erb M., Gemperli A.C., Robay D., Courdier Fruh I., Anklin C., Dallmann R., Gueven N. NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy Levels. PLOS One. 2011;6(3):e17963. doi: 10.1371/journal.pone.0017963. 21483849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiyama Y., Fujita T. Stimulation of the respiratory and phosphorylating activities in rat brain mitochondria by idebenone (CV-2619), a new agent improving cerebral metabolism. FEBS Letters. 1985;184(1):48–51. doi: 10.1016/0014-5793(85)80650-5. 3987906 [DOI] [PubMed] [Google Scholar]
- 11.Esposti M.D., Ngo A., Ghelli A., Benelli B., Carelli V., McLennan H., Linnane A.W. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Archives of Biochemistry and Biophysics. 1996;330(2):395–400. doi: 10.1006/abbi.1996.0267. 8660670 [DOI] [PubMed] [Google Scholar]
- 12.James A.M., Cochemé H.M., Smith R.A., Murphy M.P. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. Journal of Biological Chemistry. 2005;280(22):21295–21312. doi: 10.1074/jbc.M501527200. 15788391 [DOI] [PubMed] [Google Scholar]
- 13.Brière J.J., Schlemmer D., Chretien D., Rustin P. Quinone analogues regulate mitochondrial substrate competitive oxidation. Biochemical and Biophysical Research Communications. 2004;316(4):1138–1142. doi: 10.1016/j.bbrc.2004.03.002. 15044103 [DOI] [PubMed] [Google Scholar]
- 14.Fato R., Bergamini C., Leoni S., Lenaz G. Mitochondrial production of reactive oxygen species: role of complex I and quinone analogues. Biofactors. 2008;32(1–4):31–39. doi: 10.1002/biof.5520320105. 19096098 [DOI] [PubMed] [Google Scholar]
- 15.King M.S., Sharpley M.S., Hirst J. Reduction of hydrophilic ubiquinones by the Flavin in mitochondrial NADH:ubiquinone oxidoreductase (complex I) and production of reactive oxygen species. Biochemistry. 2009;48(9):2053–2062. doi: 10.1021/bi802282h. 19220002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watzke N., Diekert K., Obrdlik P. Electrophysiology of respiratory chain complexes and the ADP–ATP exchanger in native mitochondrial membranes. Biochemistry. 2010;49(48):10308–10318. doi: 10.1021/bi1011755. 20958090 [DOI] [PubMed] [Google Scholar]
- 17.Rauchovà H., Vrbacký M., Bergamini C., Fato R., Lenaz G. Inhibition of glycerophosphate-dependent H2O2 generation in brown fat mitochondria by idebenone. Biochemical and Biophysical Research Communications. 2006;339(1):362–366. doi: 10.1016/j.bbrc.2005.11.035. 16300743 [DOI] [PubMed] [Google Scholar]
- 18.Giorgio V., Petronilli V., Ghelli A., Carelli V., Rugolo M., Lenaz G., Bernardi P. The effects of idebenone on mitochondrial bioenergetics. Biochimica et Biophysica Acta. 2012;1817(2):363–369. doi: 10.1016/j.bbabio.2011.10.012. 22086148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauchová H., Drahota Z., Bergamini C., Fato R., Lenaz G. Modification of respiratory-chain enzyme activities in brown adipose tissue mitochondria by idebenone (hydroxydecyl-ubiquinone) Journal of Bioenergetics and Biomembranes. 2008;40(2):85–93. doi: 10.1007/s10863-008-9134-1. 18368470 [DOI] [PubMed] [Google Scholar]
- 20.Rauchová H., Vokurková M., Drahota Z. Idebenone-induced recovery of glycerol-3-phosphate and succinate oxidation inhibited by digitonin. Physiological Research. 2012;61(3):259–265. doi: 10.33549/physiolres.932318. 22480420 [DOI] [PubMed] [Google Scholar]
- 21.Lee S., Sheck L., Crowston J.G., Van Bergen N.J., O’Neill E.C., O’Hare F., Kong Y.X., Chrysostomou V., Vincent A.L., Trounce I.A. Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Investigative Ophthalmology & Visual Science. 2012;53(4):2431–2437. doi: 10.1167/iovs.12-9596. 22427588 [DOI] [PubMed] [Google Scholar]
- 22.Littarru G.P., Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Molecular Biotechnology. 2007;37(1):31–37. doi: 10.1007/s12033-007-0052-y. 17914161 [DOI] [PubMed] [Google Scholar]
- 23.Madmani M.E., Yusuf Solaiman A., Tamr Agha K., Madmani Y. Coenzyme Q10 for heart failure. Cochrane Database System Reviews. 2014;6:CD008684. doi: 10.1002/14651858.CD008684.pub2. 24049047 [DOI] [PubMed] [Google Scholar]
- 24.Suno M., Nagaoka A. Inhibition of mitochondrial swelling and lipid peroxidation by a novel compound, idebenone (CV-2619) Japanese Pharmacology and Therapeutics. 1985;13:673–678. doi: 10.1254/jjp.35.196. [DOI] [PubMed] [Google Scholar]
- 25.Grieb P., Ryba M.S., Debicki G.S., Gordon-Krajcer W., Januszewski S., Chrapusta S.J. Changes in oxidative stress in the rat brain during post-cardiac arrest reperfusion, and the effect of treatment with the free radical scavenger idebenone. Resuscitation. 1998;39(1–2):107–113. doi: 10.1016/s0300-9572(98)00128-2. 9918457 [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto S., Mori N., Tsuchihashi N., Ogata T., Lin Y., Yokoyama H., Ishida S. Enhancement of nitroxide-reducing activity in rats after chronic administration of vitamin E, vitamin C, and idebenone examined by an in vivo electron spin resonance technique. Magnetic Resonance in Medicine. 1998;40(2):330–333. doi: 10.1002/mrm.1910400219. 9702715 [DOI] [PubMed] [Google Scholar]
- 27.Schulz J.B., Dehmer T., Schöls L., Mende H., Hardt C., Vorgerd M., Bürk K., Matson W., Dichgans J., Beal M.F., Bogdanov M.B. Oxidative stress in patients with Friedreich ataxia. Neurology. 2000;55(11):1719–1721. doi: 10.1212/wnl.55.11.1719. 11113228 [DOI] [PubMed] [Google Scholar]
- 28.Gil J., Almeida S., Oliveira C.R., Rego A.C. Cytosolic and mitochondrial ROS in staurosporine-induced retinal cell apoptosis. Free Radical Biology and Medicine. 2003;35(11):1500–1514. doi: 10.1016/j.freeradbiomed.2003.08.022. 14642398 [DOI] [PubMed] [Google Scholar]
- 29.Ranganathan S., Harmison G.G., Meyertholen K., Pennuto M., Burnett B.G. Mitochondrial abnormalities in spinal and bulbar muscular atrophy. Human Molecular Genetics. 2009;18(1):27–42. doi: 10.1093/hmg/ddn310. 18824496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kernt M., Arend N., Buerger A., Mann T., Haritoglou C., Ulbig M.W., Kampik A., Hirneiss C. Idebenone prevents human optic nerve head astrocytes from oxidative stress, apoptosis, and senescence by stabilizing BAX/Bcl-2 ratio. Journal of Glaucoma. 2013;22(5):404–412. doi: 10.1097/IJG.0b013e31824caf90. 23661043 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed M.A. Neuroprotective effects of idebenone against pilocarpine-induced seizures: modulation of antioxidant status, DNA damage and Na(+), K (+)-ATPase activity in rat hippocampus. Neurochemical Research. 2014;39(2):394–402. doi: 10.1007/s11064-014-1236-z. 24414170 [DOI] [PubMed] [Google Scholar]
- 32.Mordente A., Martorana G.E., Minotti G., Giardina B. Antioxidant properties of 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone (idebenone) Chemical Research in Toxicology. 1998;11(1):54–63. doi: 10.1021/tx970136j. 9477226 [DOI] [PubMed] [Google Scholar]
- 33.Suno M., Nagaoka A. Inhibition of lipid peroxidation by a novel compound, idebenone (CV-2619) Japanese Journal of Pharmacology. 1984;35(2):196–198. doi: 10.1254/jjp.35.196. 6748380 [DOI] [PubMed] [Google Scholar]
- 34.Suno M., Nagaoka A. Inhibition of lipid peroxidation by a novel compound (CV-2619) in brain mitochondria and mode of action of the inhibition. Biochemical and Biophysical Research Communications. 1984;125(3):1046–1052. doi: 10.1016/0006-291x(84)91389-5. 6517932 [DOI] [PubMed] [Google Scholar]
- 35.Heitz F.D., Erb M., Anklin C., Robay D., Pernet V., Gueven N. Idebenone protects against retinal damage and loss of vision in a mouse model of Leber’s hereditary optic neuropathy. PLOS One. 2012;7(9):e45182. doi: 10.1371/journal.pone.0045182. 23028832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardoso S.M., Pereira C., Oliveira R. Mitochondrial function is differentially affected upon oxidative stress. Free Radical Biology and Medicine. 1999;26(1–2):3–13. doi: 10.1016/s0891-5849(98)00205-6. 9890635 [DOI] [PubMed] [Google Scholar]
- 37.Rustin P., Kleist-Retzow J.C., Chantrel-Groussard K., Sidi D., Munnich A., Rötig A. Effect of idebenone on cardiomyopathy in Friedreich's ataxia: a preliminary study. Lancet. 1999;354(9177):477–479. doi: 10.1016/S0140-6736(99)01341-0. 10465173 [DOI] [PubMed] [Google Scholar]
- 38.Lenaz G. A critical appraisal of the mitochondrial coenzyme Q pool. FEBS Letters. 2001;509(2):151–155. doi: 10.1016/s0014-5793(01)03172-6. 11741580 [DOI] [PubMed] [Google Scholar]
- 39.Becker C., Bray-French K., Drewe J. Pharmacokinetic evaluation of idebenone. Expert Opinion on Drug Metabolism & Toxicology. 2010;6(11):1437–1444. doi: 10.1517/17425255.2010.530656. 20955109 [DOI] [PubMed] [Google Scholar]
- 40.Siegel D., Bolton E.M., Burr J.A., Liebler D.C., Ross D. The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Molecular Pharmacology. 1997;52(2):300–305. doi: 10.1124/mol.52.2.300. 9271353 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T., Okuno M., Okamoto T., Kishi T. NADPH-dependent coenzyme Q reductase is the main enzyme responsible for the reduction of non-mitochondrial CoQ in cells. Biofactors. 2008;32(1–4):59–70. doi: 10.1002/biof.5520320108. 19096101 [DOI] [PubMed] [Google Scholar]
- 42.López L.C., Quinzii C.M., Area E., Naini A., Rahman S., Schuelke M., Salviati L., Dimauro S., Hirano M. Treatment of CoQ(10) deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PLOS One. 2010;5(7):e11897. doi: 10.1371/journal.pone.0011897. 20689595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Hekimi S. Mitochondrial respiration without ubiquinone biosynthesis. Human Molecular Genetics. 2013;22(23):4768–4783. doi: 10.1093/hmg/ddt330. 23847050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auré K., Benoist J.F., Ogier de Baulny H., Romero N.B., Rigal O., Lombès A. Progression despite replacement of a myopathic form of coenzyme Q10 defect. Neurology. 2004;63(4):727–729. doi: 10.1212/01.wnl.0000134607.76780.b2. 15326254 [DOI] [PubMed] [Google Scholar]
- 45.Haginoya K., Miyabayashi S., Kikuchi M., Kojima A., Yamamoto K., Omura K., Uematsu M., Hino-Fukuyo N., Tanaka S., Tsuchiya S. Efficacy of idebenone for respiratory failure in a patient with Leigh syndrome: a long-term follow-up study. Journal of the Neurological Sciences. 2009;278(1–2):112–114. doi: 10.1016/j.jns.2008.11.008. 19101701 [DOI] [PubMed] [Google Scholar]