Abstract
Oxidative stress plays a major part in the pathogenesis of drug-induced liver injury. Yet, overcoming it with other xenobiotics impose additional risks. In this study, we consider the use of natural-occurring and purified Vitamin E analogs as hepatoprotective agents. Vitamin E is well-known for its intrinsic antioxidant property even though the differential effect of specific analogs of tocopherol (TP) and tocotrienol (T3) is still not ascertained. This study investigates the protective effect of T3 analogs (α-, δ-, γ−) in comparison with α-TP followed by assessing the underlying mechanisms of the cytoprotective T3 analog(s) in two xenobiotics-induced liver injury models using (1) acetaminophen (APAP)- and (2) hydrogen peroxide (H2O2). Both α-TP and α-T3 exerted cytoprotective effects while only lower concentration of γ-T3 was effective in inhibiting both toxicants induced injury. α-TP/α-T3 protected hepatocytes from APAP and H2O2-induced liver injury through arresting free radicals and inhibiting oxidative stress (inhibition of reactive oxygen species, lipid peroxidation and mitochondrial permeability transition). There was also demonstrable inhibition of the apoptotic pathway (inhibition of caspse-3 activity and overexpression of Bcl-XL), accompanied with an induction of liver regeneration (PCNA and NF-kB). The cellular uptake of α-T3 was higher than α-TP at the same treatment dosage after 24 h. Overall, α-T3 seems to be a more potent hepatoprotective analog among the tocotrienols and α-TP at the same in vitro treatment dosage. In summary, these results suggest that α-TP/α-T3 elicit hepatoprotective effects against toxicants-induced damage mainly through activation of antioxidant responses at an early stage to prevent the exacerbation of injury.
Keywords: Tocotrienol, Tocopherol, Antioxidant, Drug-induced liver injury
Abbreviations: α-TP, α-tocopherol; α-T3, α-tocotrienol; T3, tocotrienol; TP, tocopherol; DILI, drug-induced liver injury; APAP, acetaminophen; H2O2, hydrogen peroxide; PCNA, proliferating cell nuclear antigen; PMSF, phenylmethanesulfonyl fluoride; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DMEM/F12, Dulbecco’s modified Eagle's medium/Ham's F12; ITS, insulin, transferrin, selenium; DMSO, dimethylsulfoxide; PBS, phosphate buffered saline; BSA, bovine serum albumin; qRT-PCR, quantitative real time-polymerase chain reaction; TNF-α, tumor necrosis factor alpha; IL-6, interleukin 6 (IL-6); iNOS, inducible nitric oxide synthase; NAPQI, N-acetyl-p-benzoquinoneimine; HPLC, high performance liquid chromatography; TGF-α, transforming growth factor alpha; PMC, 2,2,5,7,8-pentamethyl-6-chromal; FLD, fluorescence detector; DAD, diode array detector; MCB, monochlorobimane, GSH, L-glutathione reduced; GST, glutathione-s-transferase; ROS, reactive oxygen species; LPO, lipid peroxidation; MPT, membrane potential transition; Nrf-2, nuclear factor erythroid 2-related factor; HO-1, Heme oxygenase-1; mrpw, multiple reads per well; SEM, standard error of means
Research highlights
-
•
Purified T3 analogs were compared for their hepatoprotective effects against two toxicants-induced liver injuries.
-
•
α-TP/α-T3 and lower concentration of γ-T3 exerted significant cytoprotective effects.
-
•
α-TP/α-T3 inhibits oxidative stress and apoptosis while induces liver regeneration.
-
•
α-T3 is the most potent hepatoprotective analog among T3 and α-TP at same dose.
-
•
α-TP/α-T3 prevented toxicants-induced injury mainly through antioxidant responses.
Introduction
Liver plays a central role in the metabolism of xenobiotics (drugs). As the primary site of Phase I and II enzyme activities, some drugs can be transformed into hepatotoxic drug metabolites due to the “first pass effect” through the liver. Therefore, liver is the organ most susceptible to drug-induced injury. Today, drug-induced liver injury (DILI) accounts for more than 50% of acute liver failure in the United States and has become a major clinical problem [1]. Acetaminophen (APAP, also known as paracetamol) is the drug most often implicated in DILI [2]. Most DILI involves oxidative stress as a part of the mechanism of cellular injury. Majority of these oxidative stress events can arise from the generation of reactive intermediates from drug metabolism [3], depletion of antioxidants [4], increased redox recycling of drugs [5] and interference of mitochondrial respiration by reactive metabolites [6]. This central role of oxidative stress in DILI presents the opportunity for natural antioxidants to quench and scavenge free radicals to prevent the deleterious effects of the toxicants. This approach can potentially trump the use of other xenobiotics, which may themselves, elicit untoward side health effects.
Vitamin E is well-known for its distinctive antioxidant properties. Being highly lipophilic, it is effective at alleviating oxidative damage particularly in lipid-rich environment like cellular membranes. Nature-derived Vitamin E is chemically diverse with distinct isoforms including α, β, γ and δ-tocopherols (TP) and tocotrienols (T3). T3 analogs are structurally similar to TP and differ only in having an unsaturated isoprenoid side chain rather than a saturated phytyl tail [7]. Recently, a growing number of studies reported that T3 possess numerous vital functions that are either not observed in TP or more potent than TP [8]. For instance, T3 has substantial cholesterol-lowering properties [9], [10], anticancer and tumor-suppressing activities, but not TP [11], [12]. On the other hand, α-T3 which demonstrated the most potent neuroprotection among Vitamin E analogs [13], was also shown to be cardioprotective [14], [15] and has the ability to protect against stroke [16]. Importantly, α-T3 was found to possess more potent antioxidant properties than other T3 analogs [17], [18] and α-TP [19], [20]. Based on this information, it is speculated that they may be particularly important for the protection against oxidative stress arising from drugs. However, the potential protective effect of individual T3 analogs and their ability to respond to different mechanisms of liver injury has never been investigated. Therefore, this work set out to first explore the potential cell death inhibitory effect of T3 analogs in comparison with α-TP followed by assessing the underlying mechanisms of the cytoprotective T3 analog(s) using liver cell culture models of well-defined xenobiotics-induced liver injury models.
Materials and methods
Materials and reagents
Dexamethasone, nicotinamide, gentamicin, HEPES, EDTA, glycerin, Triton-X, sodium chloride (NaCl), sodium fluoride, sodium orthovanadate, phenylmethanesulfonyl fluoride (PMSF), aprotinin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), acetaminophen (APAP), hydrogen peroxide (H2O2), monochlorobimane (MCB), l-glutathione reduced (GSH) and glutathione-s-transferase (GST) were obtained from Sigma Chemical (St. Louis, MO). Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/F12) and Superscript III First-strand Synthesis System were products of Invitrogen (Carlsbad, CA). Insulin, transferrin, selenium cocktail (ITS) was from BD (Franklin Lakes, NJ). Dimethylsulfoxide (DMSO) was obtained from Merck (Darmstadt, Germany). SYBR Green PCR master mix was obtained from Applied Biosystems (Warrington, UK). RNeasy® mini kit was product of Qiagen (Hilden, Germany). Phosphate buffered saline (PBS) and all primers were synthesized by 1st BASE Oligos (Singapore). Primary antibodies were purchased from the following companies: proliferating cell nuclear antigen (PCNA) and NF-kB p65, Cell Signaling Technology (Danvers, MA); Bcl-xL (H-5), Nrf-2 (C-20), p-Met (Tyr 1234), Santa Cruz Biotechnology (Dallas, TX); HO-1, Enzo Life Science (Farmingdale, NY); β-actin, Abcam (Cambridge, UK). CM-H2DCFDA and JC-1 dye were purchased from Molecular Probe (Carlsbad, CA); TBARS assay kit was obtained from Cayman Europe (Tallinn, Estonia) while Caspase-Glo® 3/7 Reagent assay kit was purchased from Promega (Madison, WI).
Preparation and quantification of vitamin E derived α-TP and T3
T3 and TP analogs were supplied by Davos Life Science Pte. Ltd., Singapore. The appearance of the pure compounds was oily liquids. They were dissolved in absolute ethanol (100 mM) and stored at −20 °C. Using the corresponding T3 analogs as the reference standard, the purity of T3 and α-TP analogs was verified to be ≥97% by HPLC.
Cell lines and culture conditions
Immortalized murine transforming growth factor alpha (TGF-α) transgenic hepatocyte (TAMH) cells [21], (obtained as a kind gift from Prof Nelson Fausto, University of Washington, USA), was used as a metabolically competent liver cell line that reproduced features of cytotoxicity to support this investigation. Although transgenic, this cell line still maintained normal hepatocyte morphology and remained non-tumorigenic even after prolonged passage and culturing [22]. TAMH cells were maintained in DMEM/F12 supplemented with 5 mg/ml insulin, 5 mg/ml transferrin, 5 ng/ml selenium, 100 nM dexamethasone, 10 mM nicotinamide and 0.01% (v/v) gentamicin. Cells were maintained at 37 °C in a humidified 95% air and 5% CO2 atmosphere, passaged and when reached 80–90% confluency.
In vitro cell viability assay
Using 96-well plate, TAMH cells were seeded at a density of 6×103 cells/well in 200 µl DMEM/F12 medium. Vitamin E analogs with different concentrations of stock solutions were prepared and diluted at 1000-fold in the culturing media to the working concentration. For the cytotoxicity test, cells were treated with various concentrations (10–100 µM) of α-TP/T3 analogs with 0.1% ethanol vehicle for 24 h. Cell viability assays were performed after the incubation time to determine the cytotoxicity of each analog. In the concurrent treatment, TAMH cells were treated 2 mM APAP or 450 µM H2O2 concurrently with respective analog for 24 h before performing the cell viability test. Pre-treatment experiments involved 24 h incubation of the α-TP/T3 analogs. Thereafter, cells were subjected to a complete rinse with PBS followed by the APAP or H2O2 incubation for another 24 h. Each of the diluted α-TP/T3 analog mixture in the culturing medium was vortexed for 30 s before treated into each wells. Control cultures received ethanol vehicle (0.1%). Following incubation of toxicants with different concentrations of the α-TP/T3 analogs, the cell viability was evaluated by MTT assay. 20 ml of 5 mg/ml MTT was dissolved in PBS. After the incubation period, the media was aspirated and the formazan crystals in cells were dissolved in 200 µl of DMSO and 25 µl of Sorenson's buffer [23]. The absorbance was measured at 570 nm using Infinite® 200 PRO (Tecan, Switzerland) microtiter plate reader. Cell viability percentage was expressed as a ratio of cells exposed to different concentrations of toxicants with those of vehicle controls.
In vitro cellular uptake of vitamin E analogs (α-TP and T3 analogs)
TAMH cells (4×106) were seeded into T-75 flask overnight. Each flask of cells which contained 15 ml of DMEM/F12 medium was treated with 5, 10, 25, 50 and 100 µM of each analog for 24 h. Cells were harvested, washed with PBS twice before each sample cell pellet was lysed in 400 µl of lysis buffer (1% Triton X-100, pH 7.5, 20 mM HEPES buffer with 0.1 mM EDTA). 1 ml of 0.01% BHT (anti-oxidant) ethanol solution and 0.01 mg 2,2,5,7,8-pentamethyl-6-chromal (PMC) as internal control were added into the lysates. 3 ml of hexane were then added and the mixtures were vortexed for 5 min and centrifuged at 4000 rpm for 10 min. The extraction process was performed twice. The cellular Vitamin E in the upper hexane layer were extracted, dried, reconstituted in 600 µl hexane and analyzed with Agilent HPLC system using Lichrospher Si 60, 250×4 mm2, 5 µm cartridge normal phase column attached to fluorescence detector (FLD) and diode array detector (DAD) detector. Each α-TP or T3 was obtained by collection of each peak fraction during HPLC. The amount of α-, γ- and δ-T3 and α-TP were quantified against a standard curve established from purified standards.
Reverse transcription and quantitative real time-polymerase chain reaction (qRT-PCR)
In a 6 well plate, TAMH cells were seeded at a density of 5×105 cells/well and treated with 2 mM APAP or 350 µM H2O2 concurrently with 10 or 50 µM of α-TP or α-T3 for 24 h. All cells were harvested for total cell RNA extraction using RNeasy columns (Qiagen, Valencia, CA). The quality and quantity of total RNA was determined with NanoDrop (Thermo, Wilmington, DE), ensuring that RNAs with OD 260/280 >1.80 were used. First-strand cDNA was synthesized from 1 µg total RNA using Superscript First-Strand Synthesis System according to the protocols of the manufacturer. qRT-PCR was performed using BioRad CFX96 real time PCR system with SYBR Green master mix and primers as shown in Table 1. The thermal cycling condition comprised an initial denaturation at 95 °C (10 min), followed by 40 cycles at 95 °C (15 s) and 60 °C (60 s). Melting curves were generated at the end of 40 cycles to verify the purity of the PCR product. Data were obtained as average Ct values, and normalized against the geometric mean of GAPDH endogenous controls as ΔCt. Transcript differences between α-TP or α-T3 treated group and non-treated group were measured as fold changes using the comparative Ct method. Statistics were performed on Ct using REST software (Qiagen, Valencia, CA).
Table 1.
Sequences of primers used in real time PCR reaction [49].
| Gene | Forward primer (5′->3′) | Reverse primer (5′->3′) |
|---|---|---|
| In vitro hepatocytes (TAMH cells)/in vivo liver (mouse) | ||
| TNF-α | ATG AGC ACA GAA AGC ATG ATC | TAC AGG CTT GTA ACT CGA ATT |
| IL-6 | AGT TGC CTT CTT GGG ACT GA | TCC ACG ATT TCC CAG AGA AC |
| iNOS | CAC CTT GGA GTT CAC CCA GT | ACC ACT CGT ACT TGG GAT GC |
Western blots
TAMH cells were seeded at 2×106 in a 10 cm dish with DMEM/F12 medium overnight. Cells were then treated with respective concentration of APAP or H2O2 and α-TP/T3 analogs for 24 h. All cells were harvested for western blot analysis. Cell pellets were lysed with 100 µl of RIPA lysis buffer containing 50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 0.5 M sodium fluoride, 100 mM sodium orthovanadate, 100 mM PMSF and 20 mg/ml aprotinin. 10 µg/cell sample were resolved in 10% SDS-PAGE and immobilized on PVDF membrane. The membranes were blocked in 5% milk for 1 h and incubated in respective primary antibodies overnight (NF-κB 1:2000; Bcl-XL 1:1000; PCNA 1:10,000; pMet 1:400; HO-1 1:1000 and Nrf-2 1:2000), followed by respective secondary antibody (1:10,000) incubation for 1 h. All membranes were visualized using chemiluminescence substrate (Pierce Biotechnology, Rockford, IL). The bands intensities were normalized against actin and were quantified using ImageJ software [24].
Determination of GSH content
2×106 TAMH cells were seeded into 6-well plates overnight. Cells were treated 2 mM APAP or 450 µM H2O2 concurrently with 10 or 50 µM of α-TP or α-T3 for 24 h. Cells were then harvested and washed with PBS twice. 200 µl of lysis buffer (0.1% Triton-X/1 M Tris/HCl) were added. The cells were sonicated in a sonicating bath for 5 min and incubated in ice for 15 min before centrifugation. 10 µl of each supernatant or GSH standard were added into each well in a 96 well plate in duplicate. 90 µl of the MCB and GST mixture (final concentration 10 nmol of MCB and 0.1 unit of GST) were added into each well. The plate was incubated at 37 °C for 60 min before it was measured using fluorescent plate reader with excitation wavelength at 380 nm and emission wavelength at 470 nm.
Determination of intracellular reactive oxygen species (ROS)
2.5×104 TAMH cells were seeded into 96-well plates overnight. 2 mM APAP or 450 µM H2O2 were treated concurrently with 10 or 50 µM of α-TP or α-T3 for 24 h. The cells were then incubated with 25 µM CM-H2DCFDA for 45 min at 37 °C and washed three times with PBS. The plate was measured using Infinite® 200 PRO (Tecan, Switzerland) fluorescent plate reader with excitation wavelength at 485 nm and emission wavelength at 535 nm. Levels of intracellular ROS were then normalized against total cell number of their respective groups.
Determination of intracellular lipid peroxidation (LPO)
4×106 TAMH cells were seeded into T-75 flask overnight. 2 mM APAP or 450 µM H2O2 were treated concurrently with 50 µM α-TP or α-T3 in each flask for 24 h. The cellular MDA level was determined with a TBARS assay kit performed according to the manufacturer’s protocol. The MDA levels were measured using Infinite® 200 PRO (Tecan, Switzerland) fluorescent plate reader with excitation wavelength at 530 nm and emission wavelength at 540 nm.
Determination of membrane potential transition (MPT)
1.5×104 TAMH cells/well were seeded in a 96 well plate for overnight. 2 mM APAP or 450 µM H2O2 were treated concurrently with 10 or 50 µM of α-TP or α-T3 for 24 h. Medium were aspirated and the cells were incubated with 5 µM JC-1 at 37 °C for 60 min. Cells were then washed twice with PBS and 100 ml of PBS were added into each well. Red fluorescence (excitation 550 nm, emission 600 nm) and green fluorescence (excitation 485 nm, emission 535 nm) were read using fluorescence plate reader with multiple reads per well (mrpw) setting. Ratios of red fluorescence:green fluorescence were determined.
Caspase-3 activity assay
2.5×104 TAMH cells were seeded into 96 well plate overnight. 2 mM APAP or 450 µM H2O2 were treated concurrently with 10 or 50 µM of α-TP or α-T3 for 24 h. The caspase 3/7 level was determined with a Caspase-Glo® 3/7 Reagent assay kit performed according to the manufacturer’s protocol. The caspase 3/7 levels were normalized against total cell number of their respective groups.
Statistical analysis
Data were expressed as means±standard error of means (SEM) and analyzed using one way ANOVA. Statistical significance of difference was accepted at the p-values of less than 0.05.
Results
Characterization of cytotoxicity and cellular uptake of tocotrienol (T3) analogs in TAMH cells
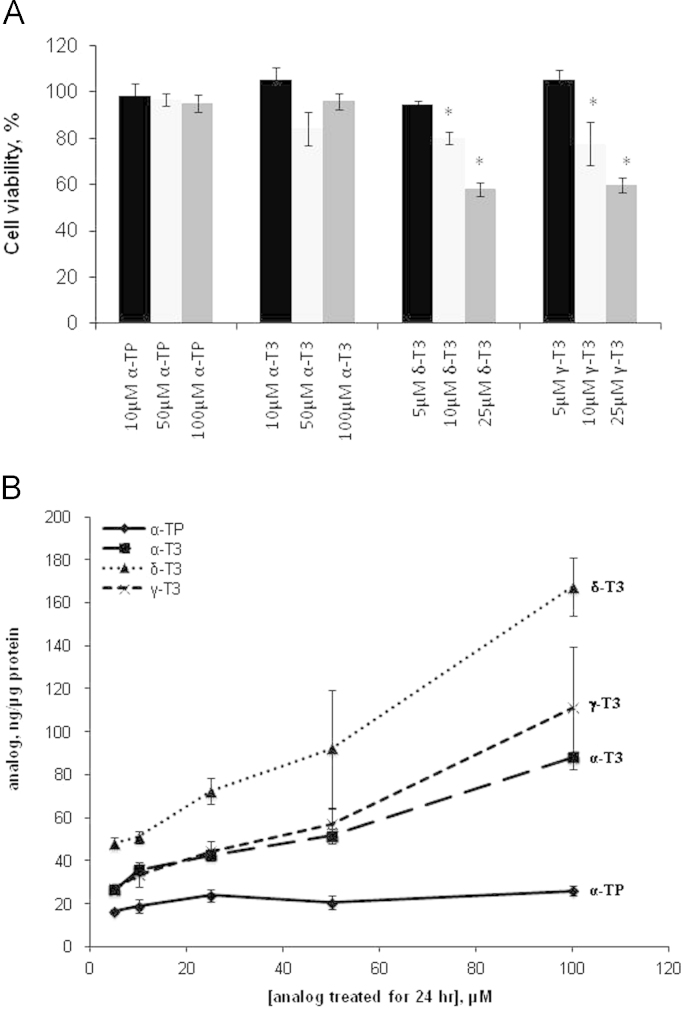
Preliminary characterization of T3 analogs and α-TP in terms of cytotoxicity and cellular uptake were performed in TAMH cells as a starting point. Cytotoxicity assay as shown in Fig. 1A revealed that α-TP and α-T3 were not toxic at up to a concentration of 100 µM with the cell viability maintained above 80%. On the contrary, significant reduction in cell viability was observed in δ-T3 and γ-T3 at concentration of 10 µM and above (p<0.05). Apart from the cytotoxicity characterization, we determined the cellular uptake of Vitamin E analogs to account for uptake differences as a potential confounding variable to the biological effectiveness of each analog in the cells. δ-T3 held the highest uptake among the analogs across all tested concentrations whereas the uptake of α-T3 and γ-T3 reach a plateau from 5 to 25 µM (Fig. 1B). The uptake of α-TP increased slightly from 5 to 25 µM and remained unchanged at higher concentration, while the other T3 analogs increased progressively over the range of treated concentrations. This dose dependent study of cellular uptake of analogs revealed increased level of uptake α-T3, γ-T3 and δ-T3 compared to that of α-TP after incubation for 24 h across different concentrations.
Fig. 1.
Characterization of cytotoxicity and cellular uptake of vitamin E analogs. (A) Experiments on the cytotoxicity of vitamin E analogs were performed in TAMH hepatocytes. 5, 10, 25 µM of δ- and γ-T3 and 10, 50, 100 µM of α-TP and α-T3 were added and MTT were performed 24 h later. Cell viability was normalized against vehicle control group and expressed in percentage. n=6 per group; *p<0.05 versus control group. (B) Cellular uptake of α-TP, α-T3, δ-T3 and γ-T3 in TAMH hepatocytes were performed. The cells were cultured in DMEM/F12 medium with each analog at the indicated concentrations for 24 h and the cellular vitamin E analog content was measured using an HPLC system. Mean values of cellular vitamin E analog content are shown with standard error. n=3 per group.
Effect of T3 analogs on APAP and H2O2-induced cell death in TAMH cells
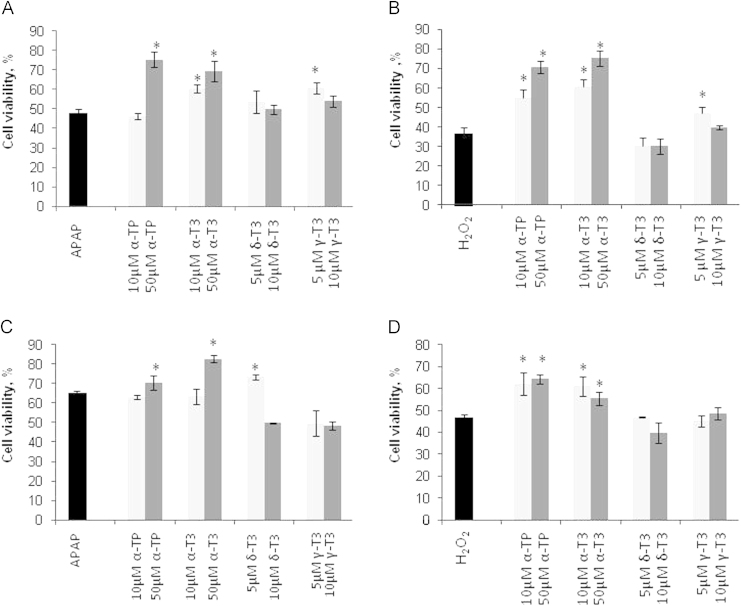
To evaluate T3 analogs responses towards different mechanisms of toxicity; two classical toxicants were used to induce toxicity: APAP represents liver injury model caused by both covalent modification of protein targets and oxidative stress mediated injury pathways [25] while H2O2 represents exclusive oxidative stress-induced liver injury pathway. Firstly, TAMH cultures were exposed concomitantly to APAP or H2O2 in the presence or absence of α-T3/α-TP (10 and 50 µM) or δ-T3/γ-T3 (5 and 10 µM) for 24 h. In both toxicity models, α-TP and α-T3 demonstrated dose dependent suppression of APAP- and H2O2-induced toxicity (Fig. 2A and B). However, 10 µM of α-TP only demonstrated significant recovery in cell viability after oxidative stress injury but not in APAP injury. Apart from that, lower concentration (5 µM) but not higher concentration (10 µM) of γ-T3 was effective in inhibiting both toxicants induced injury. δ-T3 did not exhibit any protection in the concurrent treatment study.
Fig. 2.
Cytoprotective effect of vitamin E analogs in concurrent and pre-treatment of APAP- and H2O2-induced injury in TAMH hepatocytes. 5, 10 And 50 µM of respective vitamin E analogs were added into respective concentration of APAP (A,C) and H2O2 (B,D) concurrently (A,B) and 24 h before treatment (C,D). MTT assays were performed 24 h later. Cell viability was normalized against vehicle control group and expressed in percentage. n=6 per group; *p<0.05 versus APAP or H2O2 at 0 µM treatment group.
Secondly, pre-treatment experiment was performed by first incubating the analogs for 24 h followed by 24 h of APAP or H2O2 incubation. The pre-treatment model was set up to rule out the possibility that α-TP or T3 analogs may react directly with H2O2 in the medium before they were taken into the cells, thereby confounding the assessment whether any observed protective effects arise from a cell-based mechanism. Based on the result shown in Fig. 2C, only 50 µM of α-TP and α-T3 and 5 µM of δ-T3 preserved significant higher cell viability after APAP injury. On the other hand, the effects of α-TP and α-T3 against H2O2 on cell viability were found to be more significant where the effective dose were observed at 10 μM and above (Fig. 2D). In contrast to the concurrent treatment study, γ-T3 did not exhibit any inhibitory effect against either toxicant in this pre-treatment study. Overall, the cytoprotection against APAP- and H2O2-induced liver injury in TAMH cells were consistent across α-TP and α-T3 while α-T3 showed the highest percentage recovery compared to the α-TP.
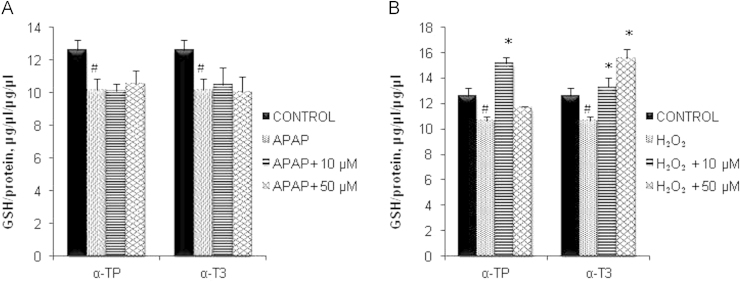
Effect of α-TP and α-T3 on GSH activity
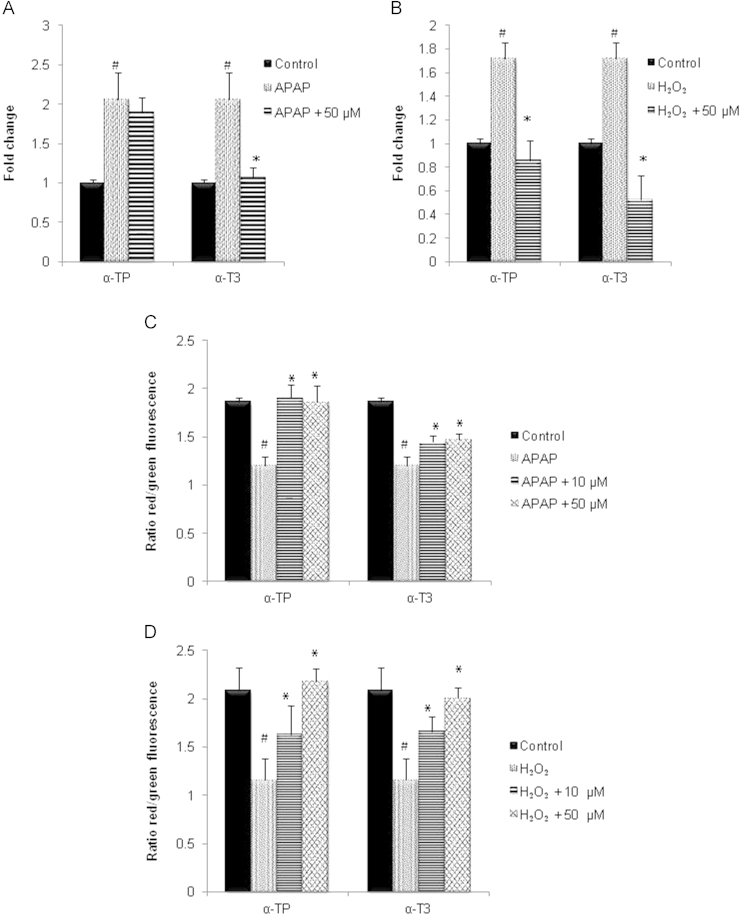
Given the greater protection manifested by concurrent treatment approach, subsequent experiments were performed using this mode of treatment to achieve more observable and biologically significant effects. To further investigate the underlying mechanisms of antioxidant α-T3 against APAP and H2O2 toxicity, cellular GSH levels were determined. Fig. 3A and B demonstrates significant decreased in intracellular GSH after 24 h incubation of APAP or H2O2. Results showed that the depletion of GSH by APAP was not inhibited despite exposure to cytoprotective concentration of α-TP or α-T3 (Fig. 3A). However, 10 µM of α-TP and both concentrations of α-T3 exhibited antioxidant activity against H2O2 injury, maintaining its intracellular GSH level and inducing higher generation of GSH (Fig. 3B). Interestingly, higher dosage of α-TP reversed the situation where no significant higher level of intracellular GSH was detected after the H2O2 injury.
Fig. 3.
Effects of α-TP and α-T3 in GSH after APAP- and H2O2-induced injury in TAMH hepatocytes. 0, 10 and 50 µM of each analog were added into TAMH cells concurrently with APAP (A) and H2O2 (B) and cellular GSH levels were performed 24 h later. n=3 per group; #p<0.05 versus control, *p<0.05 versus APAP or H2O2 without analog treatment.
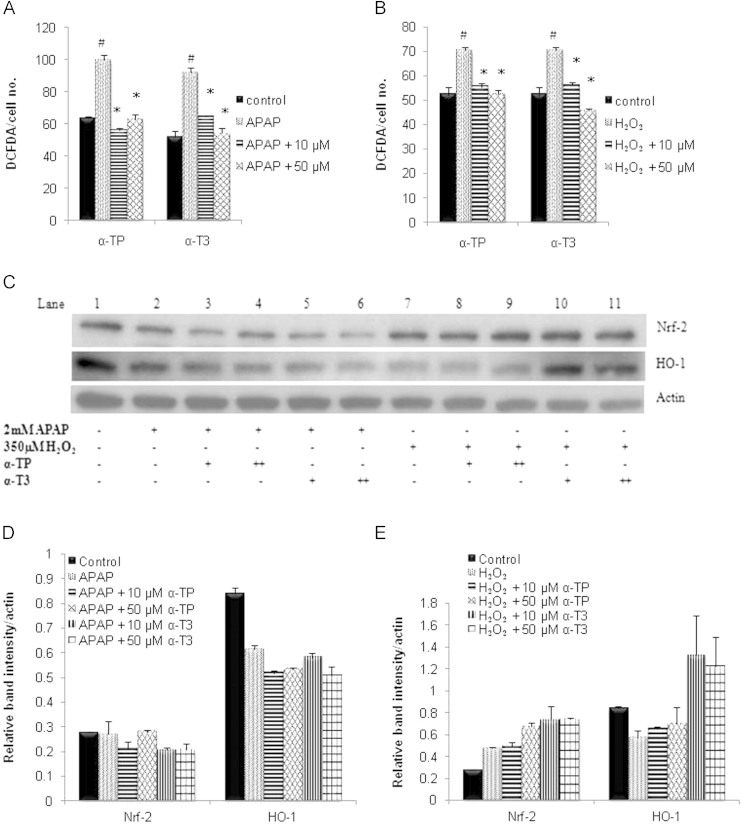
Anti-oxidative effects of α-TP and α-T3 on intracellular ROS and antioxidant genes activities
Subsequently, the intracellular ROS level and antioxidant genes activities were measured to examine the antioxidant effects of α-TP or α-T3. Firstly, the intracellular ROS level was determined by fluorescent probe CM-H2DCFDA and the results revealed that both APAP and H2O2 induced death was preceded by a significant increase in intracellular ROS (Fig. 4A and B). Conversely, the rise in ROS was suppressed by both α-TP and α-T3 in a dose-dependent manner as compared to its basal level.
Fig. 4.
Effect of α-TP and α-T3 in intracellular ROS and antioxidant gene activity after APAP- and H2O2-induced injury in TAMH hepatocytes. 0, 10 And 50 µM of each analog were added into TAMH cells concurrently with APAP (A) and H2O2 (B) and intracellular ROS levels were performed 24 h later and normalized against each group’s total number of viable cells. n=3 per group; #p<0.05 versus control, *p<0.05 versus APAP or H2O2 without analog treatment. (C) Expression of Nrf-2 and HO-1 were determined by immunoblotting after 24 h concurrent treatment of (+) 10 µM and (++) 50 µM of α-TP/α-T3 in APAP- or H2O2-injury models. Blot shown here was representative from a number of experiments, n=3. Densitometric analysis was performed by normalizing the intensity of the Nrf-2 and HO-1 bands to respective actin controls in the same samples and displayed as a bar graph for (D) APAP and (E) H2O2 injury model.
To further assess the influence of antioxidant response to α-TP/α-T3 against the oxidative stress, the protein expressions of antioxidant response genes HO-1 and Nrf-2 were carried out in an immunoblot assay. In the APAP model, both HO-1 and Nrf-2 proteins were downregulated and these expressions were not affected by the treatment of any protective concentrations of α-TP/α-T3 (Fig. 4C and D). On the other hand, H2O2 induced Nrf-2 expression while paradoxically reduced HO-1 expression. The 50 µM of α-TP triggered similar amount of Nrf-2 expression as compared to 10 or 50 µM of α-T3, and were significantly higher than H2O2 control (Fig. 4C and E). Even though 50 µM of α-TP induced Nrf-2 expression, HO-1 expression remained unchanged compared to the H2O2 control. On the contrary, HO-1 expression was highly induced following the increased in Nrf-2 expression after the treatment of α-T3.
Effects of α-TP and α-T3 on lipid peroxidation (LPO) and mitochondrial depolarization
Typically, overproduction of ROS mediates oxidative damage manifested by LPO, proteins oxidation and carbonylation, and DNA alterations, which in turn disrupt cellular function and integrity. To examine the involvement of α-TP and α-T3 in LPO, the levels of MDA formation was quantified. The release of MDA doubled after 24 h incubation with APAP and H2O2 as compared to the control (Fig. 5A and B). In the APAP injury model, only 50 µM of α-T3 managed to suppress the formation of LPO significantly (Fig. 5A). However, both α-TP and α-T3 prevented the H2O2 induced LPO where α-T3 appeared to be more effective (Fig. 5B).
Fig. 5.
Effect of α-TP and α-T3 in LPO formation and mitochondrial MPT after APAP- and H2O2-induced injury in TAMH hepatocytes. 0, 10 And 50 µM of each analog were added into TAMH cells concurrently with APAP (A,C) and H2O2 (B,D) and 24 h later the intracellular LPO levels were measured using TBARS assay (A and B) while the MPT levels were detected using JC-1 probe (C and D). The MPT levels were expressed in the red to green fluorescence ratio. n=3 per group; #p<0.05 versus control, *p<0.05 versus APAP or H2O2 without analog treatment.
To evaluate the mitochondria depolarization due to excessive ROS, JC-1 fluorescent probe was used where red fluorescent (emission at 590 nm) indicates healthy cells while green fluorescent (emission at 525 nm) indicates apoptotic cells. From Fig. 5C, treatment of APAP caused a decrease in red/green fluorescence intensity but the addition of α-TP inhibited the mitochondrial depolarization, resulting in a similar level to the control. Even though α-T3 also reversed the mitochondrial depolarization caused by APAP, the protective effect was not as great as α-TP. On the other hand, both α-TP and α-T3 demonstrated a similar extent of inhibition in a dose dependent manner against the H2O2 induced reduction in mitochondrial potential (Fig. 5D).
Anti-apoptotic effects of α-TP and α-T3 on Bcl-xL anti-apoptotic gene and caspase 3 activity
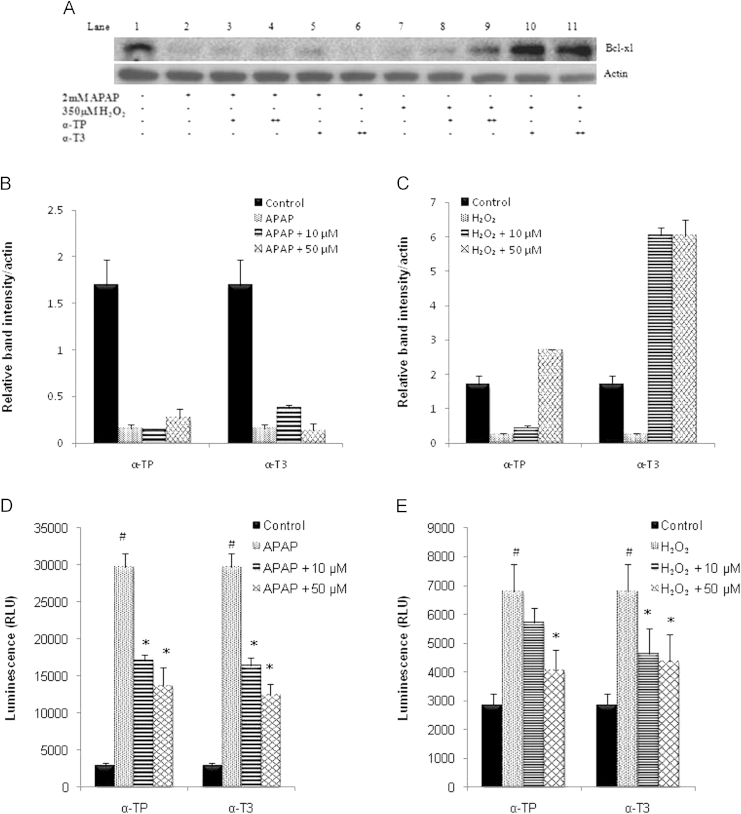
To explore the anti-apoptotic effects of these analogs, Bcl-xL expression, a mitochondrial anti-apoptotic protein predominant in hepatocytes, and caspase 3 activities were measured. From Fig. 6A, the expression of Bcl-xL was at a very low level after the treatment of APAP and H2O2 compared to the control. Additional treatment of α-TP or α-T3 did not result in higher expression of Bcl-xL in APAP model (Fig. 6B). On the other hand, 50 µM of α-TP demonstrated significantly higher expression of Bcl-xL in contrast to the H2O2 control. Likewise, both concentrations of α-T3 showed similar extent of Bcl-xL induction, with two-fold elevation as compared to 50 µM α-TP in the H2O2 model (Fig. 6C).
Fig. 6.
Effect of α-TP and α-T3 in Bcl-xL anti-apoptotic gene and on caspase 3 activity after APAP- and H2O2-induced injury in TAMH hepatocytes. (A) Expression of Bcl-xL was determined by immunoblotting after 24 h concurrent treatment of 10 and 50 µM of α-TP/α-T3 in APAP- or H2O2-injury model. Blot shown here was representative from a number of experiments, n=3. Densitometric analysis was performed by normalizing the intensity of Bcl-xL to respective actin controls in the same samples and displayed as a bar graph for (B) APAP and (C) H2O2 injury model. Caspase 3/7 was measured after 24 h treatment of 10 and 50 µM of α-TP/α-T3 in (D) APAP- or (E) H2O2-injury. n=3 per group; #p<0.05 versus control, *p<0.05 versus APAP or H2O2 without analog treatment.
On the other hand, caspase 3 activity was highly induced after the treatment of APAP and H2O2. Both α-TP and α-T3 analogs salvaged the injured cells by reducing the total caspase 3 activity in APAP model (Fig. 6D). Although 10 µM of α-TP did not significantly inhibit caspase 3 activity in the H2O2 model, 50 µM of α-TP as well as both α-T3 concentrations lowered the total caspase 3 activity to half of the activity detected in the H2O2 model (Fig. 6E).
Effects of α-TP and α-T3 on gene regulation in ROS induced inflammation
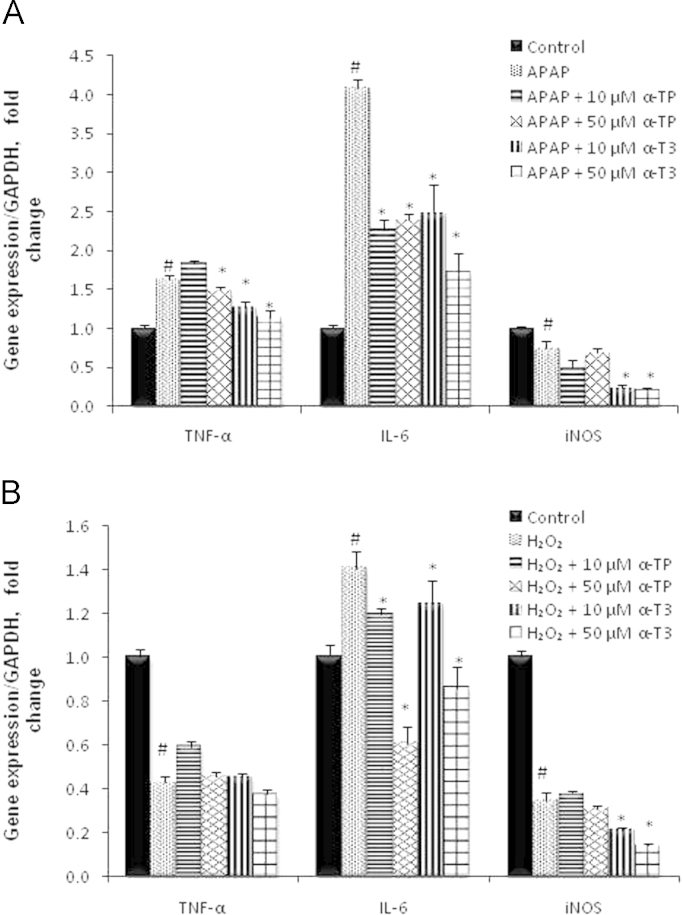
Tissue inflammation is frequently activated as a secondary response when cells undergo injury. Hence, by exploring the effect of α-TP/α-T3 on pro- and anti-inflammatory protein expressions, it is possible to determine the exact stage of the injury-recovery response paradigm where α-TP and α-T3 are involved in. In our injury model, hepatic regeneration markers such as pMet, PCNA, NF-kB and inflammatory cytokines like TNF-α, IL-6 and iNOS were induced upon liver injury. With the treatment of α-TP and α-T3, the expressions of TNF-α, IL-6 and iNOS were downregulated compared to the expression levels in APAP or H2O2 injury controls (Fig. 7A and B). 50 µM of α-T3 demonstrated lowest expressions among the tested analogs and concentrations across all the measured inflammatory responses. These results support that the protective effect of α-TP/α-T3 responded before the injury sets in, hence serving as preventive agents against APAP and H2O2 injury.
Fig. 7.
Effects of α-TP and α-T3 on qRT-PCR analysis of iNOS, TNF −α, and IL-6 inflammatory expression. Liver inflammatory responses in TAMH cells treated with or without 10 and 50 µM of α-TP/α-T3 concurrently in (A) APAP or (B) H2O2, for 24 h. All the expression were normalized against GAPDH expression of the same sample and presented as fold-increase over the controls. #p<0.05 versus control, *p<0.05 versus APAP or H2O2 without analog treatment.
Effects of α-TP and α-T3 on protein expressions in liver regeneration
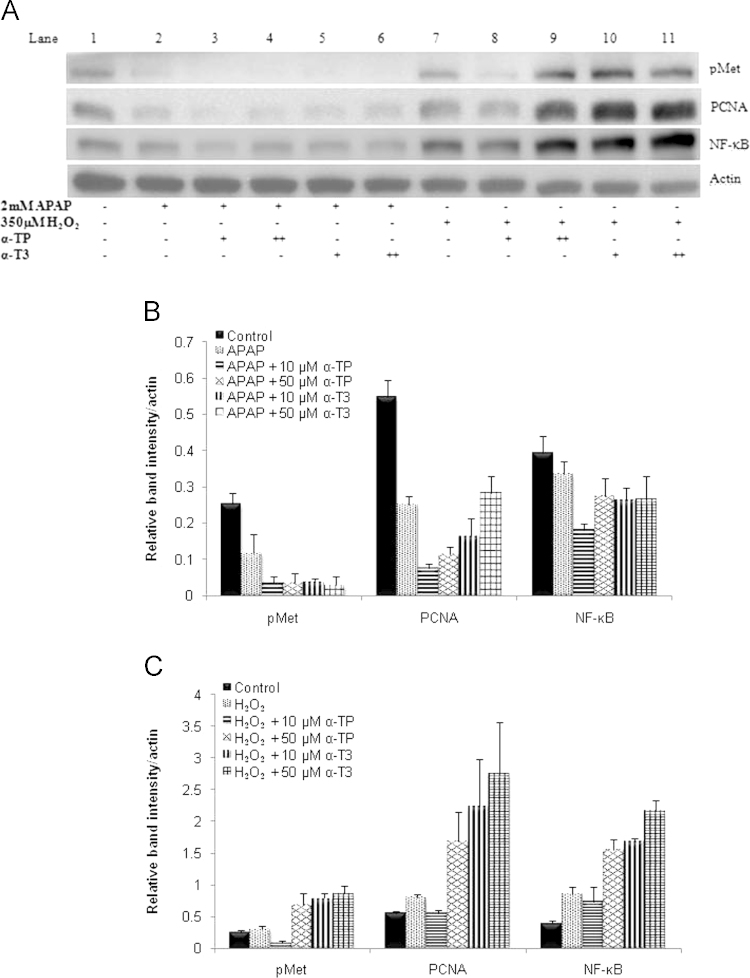
The role of liver regeneration as a complementary healing response to ROS prevention was considered in our model. To examine the regeneration effect, a subset of key liver regeneration markers, pMet, NF-kB and PCNA were monitored by immunoblot assay. As detailed in Fig. 8A and B, there were no significant difference across pMet, NF-kB or PCNA in the APAP injury model but the expressions were highly induced in a dose dependent manner for α-TP and α-T3 treatments in H2O2 injury model (Fig. 8A and C). The expressions of these proteins in α-T3 treatment were found to be higher than the α-TP treatment (Fig. 8A).
Fig. 8.
Effects of α-TP and α-T3 on liver regeneration markers after APAP- or H2O2-induced injury in TAMH hepatocytes. (A) Expressions of pMet, NF-κB and PCNA were determined by immunoblotting after 24 h concurrent treatment of 10 and 50 µM of α-TP/α-T3 in APAP- or H2O2-injury models. Blot shown here was representative from a number of experiments, n=3. Densitometric analysis was performed by normalizing the intensity of pMet, NF-κB and PCNA to respective actin controls in the same samples and displayed as a bar graph for (B) APAP and (C) H2O2 injury model.
Discussion
This work set out to first evaluate analog-specific effects of Vitamin E in attenuating xenobiotic-induced liver injury, followed by assessing the underlying mechanisms of the cytoprotection. As such, we explored each analog’s hepatoprotective potential in well-defined models of toxicity −APAP and H2O2 administered to metabolically competent TAMH cells. Cytotoxicity of different T3 analogs were first explored in the cell viability followed by the cellular uptake assay and the effects were compared in parallel with α-TP. Accordingly, our experiments have demonstrated that α-T3 is the analog that consistently preserved the highest cell viability after the injury of APAP and H2O2 while γ-T3 and δ-T3 produced marginal and inconsistent protective effect. Notably, α-T3 exerted both preventive and protective effect against DILI. It acts as an antioxidant by reacting with ROS, protecting the cells from injury while inducing the remnant hepatocytes regeneration. The powerful antioxidant properties of α-T3 can be proven by its effectiveness against the H2O2-injury model compared to the APAP model. We qualified the antioxidant potential of α-T3 by firstly investigating the relative hepatoprotection capacities of each analog and the underlying mechanisms by which analog exerts its cytoprotection action.
The cytoprotective effect of α-T3 was found to be more potent compared to α-TP in both injury models. As claimed by Packer et al., the effectiveness of different Vitamin E analogs may involve two main features, i.e., the substituent on the chromanol nucleus “head” and the properties of the side chain “tail” [26]”. Based on the differences in the tail structure, it has been suggested that the unsaturated side chain of α-T3 contributes to the stronger disordering of membrane lipids which makes interaction of chromanols with lipid radicals more efficient and therefore, compared to α-TP, α-T3 distributed more uniformly within the membrane bilayer and thus possessed higher antioxidant efficacy against oxidative damage directed at lipid membranes [19]. Studies also indicated that α-T3 which is located nearer to the membrane surface may contribute to greater recycling efficiency of the chromanols from chromanoxyl radicals correlating with the inhibition of LPO [27]. However, these studies were performed in ex vivo model and thus the antioxidant efficacy might differ in contrast with in vitro or in vivo studies.
Barring any difference in free radical scavenging potential, the better protection shown by α-T3 could be attributable to higher cellular uptake rate or accumulation of α-T3 in the cells, a possibility reported in previous studies [28], [29]. Although γ-T3 has been shown to be more potent in other cases, the issue of uptake as a confounding effect remains elusive. In this study, similar cell viability was observed after the treatment of 50 µM α-TP in APAP and H2O2 for concurrent and pre-treatment study. One of the possible explanations to this could be due to the saturable uptake of α-TP at these higher concentrations, leading to similar amount of α-TP achieved in the cells for protective effect. Nevertheless, the cellular uptake shown in this study were in accordance with the reported findings where α-T3 has higher cellular uptake than α-TP in Jurkat cells [28] and primary cortical neurons [29]. The higher uptake of α-T3 could be due to the unsaturated side chain which allows them to be incorporated into the cell membrane more easily than α-TP [30]. By comparing at similar intracellular concentration attained, some reported identical cytoprotection and resistance effect against oxidative stress of α-T3 and α-TP [29], [31], [32]. Although the intracellular concentration remained a confounding factor in this study, it may be noteworthy that apart from the different cytoprotective effects of α-T3 and α-TP observed in this study could also be attributed to the difference in antioxidant potency or non-antioxidant function of each analog.
In the liver, APAP is metabolized by P450 into a reactive metabolite, NAPQI. CYP3E1 and CYP3A4 are important enzymes of the CYPP450 system responsible for this metabolism reaction. It has been reported that all the T3 but not TP analogs induced 3–5 fold of CYP3A4 expression in the primary hepatocytes [33]. α-T3 has also been reported to stimulate the upregulation of endogenous CYP3A4 and CYP3A5 more significantly than α-TP [34]. As a result, these enzymes will cause higher generation of NAPQI from APAP, signifying more injuries in the response to α-T3 exposure. Nevertheless, similar protective effects were observed after the treatment of α-T3 and α-TP. Therefore, these results may indicate higher anti-oxidant potency of α-T3 in preventing against the oxidative stress compared to α-TP.
To address the underlying antioxidant mechanisms of α-T3, the effect against GSH level followed by the ROS generation and its adverse effects were examined. It has been claimed that the regeneration of bioactive Vitamin E from its oxidized state is a GSH-dependent process [35]. Other clinical studies reported that the pharmacological doses of Vitamin E enhance red blood cell levels of reduced glutathione [36] and plasma GSH/GSSG ratio in humans [37], [38]. Parallel with these findings, the result in this study showed that the GSH level in H2O2 remained significantly higher after the treatment of both α-TP and α-T3 compared to the control, but not in the case of APAP. A plausible explanation for this effect could be that the GSH oxidation under the influence of H2O2 could have been restored by α-TP/α-T3 thus maintaining its high GSH level, whereas GSH committed to conjugation with electrophilic NAPQI in APAP model may not be readily restored by the same treatment.
Given the dichotomous role of GSH in oxidative stress and protein binding, a targeted investigation into ROS was performed. Despite the different outcome in GSH level, both α-TP and α-T3 were able to inhibit the elevation of intracellular ROS in both APAP and H2O2 injury models. It has been reported in a glutamate-induced neurotoxicity study that even though α-TP/α-T3 did not inhibit the decrease in GSH level, ROS level was significantly suppressed at the respective neuroprotection concentrations [29]. Similarly, α-T3 was reported to have no effect in sparing the glutamate-induced depletion of intracellular GSH but it completely prevented the accumulation of intracellular peroxides even if the α-T3 was treated 5 h after the glutamate treatment [39]. Collectively, the findings of these studies where prevention of ROS release and mitochondrial stress were manifested despite an initial GSH depletion might indicate that α-TP and α-T3 were acting on different levels of the cytoprotection cascade. In another words, apart from preventive measure of blocking the chemical insult caused by ROS, α-TP and α-T3 were also able to exert their function during injury and post-injury.
Based on the study of Khanna et al., α-T3 was able to prevent the loss of mitochondrial membrane potential arising from homocysteic acid and linoleic acid [40]. Moreover, α-T3 has been reported to exert more pronounced inhibitory effects on LPO in rat liver and murine microsomes [19], [41] and have higher potency in scavenging the very reactive HO × and lipid peroxyl radical (ROO●) in liposomes than α-TP [27]. In line with these studies, our findings demonstrated suppression of LPO and MPT formation by both α-T3 and α-TP, given that the α-T3 exhibits stronger antioxidant effect than α-TP. We are cognizant that TBARS assay for LPO quantification can be confounded by reaction of the thiobarbituric substrate with numerous complex substances especially in vivo system. Nevertheless, in this study, TBARS assay was solely performed on in vitro liver cell line where any interfering substances were minimal. Effect if any, was exerted uniformly across the various experimental arms. Therefore, the results indirectly reflected the lipid peroxidation activity and were reliable in comparison with the negative control.
Other than the antioxidant function, α-T3 was found to possess anti-apoptotic activity through inducing the Bcl-xL anti-apoptotic protein while blocking the caspase 3 activity in H2O2 model. However, this was not apparent in the APAP injury model. This difference could be due to the differential injury pathway elicited by each toxicant. Aside from inducing oxidative stress pathway, APAP also induces the mitochondria independent pathway, potentially through the induction of TNF-α and the activation of the extrinsic apoptotic cascade. This explains the findings where α-TP/α-T3 having minimal effect in Bcl-xL induction while demonstrating significant suppression of caspase 3 (the convergent point of the extrinsic and intrinsic apoptotic pathway) activity in APAP model. It is worth noting that from our findings, both α-TP and α-T3 are involved in the inhibition of mitochondria dependent and independent apoptosis process.
Overproduction of ROS is often associated with inflammation, which also plays an important and multiplier role in toxicant-induced acute liver injury. Following the exposure to hepatotoxic chemicals, the generated oxidative stress can trigger inflammatory cytokine responses in injured hepatocytes and Kupffer cells [42]. The release of cytokines activates a slew of inflammatory responses that orchestrate the removal of dead or dying cells, where it is essential for regeneration of lost tissue. Here, TNF-α and IL-6 were found to be highly induced after the exposure to APAP or CCl4 as toxicants [43], [44]. Similarly, the elevated TNF-α, IL-6 and iNOS genes in this study were downregulated after the treatment of α-TP/α-T3. Even though a monoculture system employed here prevented us to see the complete effect of inflammation, the perturbation of many markers that pointed towards anti-inflammation indicates that α-TP/α-T3 attenuated toxicant-induced liver injury. It is therefore considered that the protective effect of α-TP/α-T3 against the APAP and H2O2-induced injury took place at the ROS preventive or early injury stage.
Finally, the induction of oxidative stress can also lead to impairment in liver regeneration. Recently, Nrf-2 has been shown to play a critical role in liver regeneration where Nrf-2-deficient mice demonstrated significant delay after partial hepatectomy [45]. Nrf-2 is known to regulate the cellular antioxidant defense system, thus reduction in Nrf-2 and its target antioxidant gene expression will result in enhanced oxidative stress. Apart from that, deficiency in Nrf-2 which demonstrated the increased susceptibility of the mice to APAP also suggests the important role of Nrf-2 in the regulation of GSH synthesis and cellular detoxification processes [46], [47]. HO-1 is an Nrf-2-targeted gene and the induction of HO-1 in acute and chronic hepatic inflammation rodent models resulted in improvement of liver damage and downregulation of proinflammatory cytokines [48]. As shown in this study, α-T3 possessed a stronger effect than α-TP and was more effective in inducing the suppressed endogenous antioxidative defense mechanisms of Nrf-2 and HO-1 against the H2O2 oxidative stress injury. At the same time, a significant trend of elevation in the regeneration markers of pMet, NF-kB and PCNA were observed. Nonetheless, the negative effect of α-TP/α-T3 in the Nrf-2 and HO-1 expression in APAP injury resulted in no difference in the regeneration markers. These findings are in agreement with the previous studies shown where Nrf-2 is crucial in inducing liver regeneration through regulating the antioxidative enzymes during injury.
Conclusion
In conclusion, α-TP/α-T3 demonstrated dose-dependent protective effects against APAP and H2O2-induced liver injury by arresting free radicals, blocking mitochondria stress, inhibiting oxidative stress and triggering endogenous anti-oxidative stress. These biochemical effects also triggered signals suggestive of anti-inflammatory responses and hepatocyte regeneration upon injury (Fig. 9). Overall, these molecular events staged at different time points of the injury process complements each other to achieve a more effective protective response. Finally, α-T3 seems to be a more potent hepatoprotective analog among the tocotrienols and α-TP at the same dosage in vitro, likely owing to its higher incorporation into the cells. Nevertheless, the potency at similar intracellular contents deserved a future investigation. Taken as a whole, both α-TP and α-T3 analogs demonstrated distinct and differential protective effect against oxidative stress in H2O2 compared to APAP model. Isoform-specific therapeutic advantages among Vitamin E could be more carefully investigated and exploited for future use.
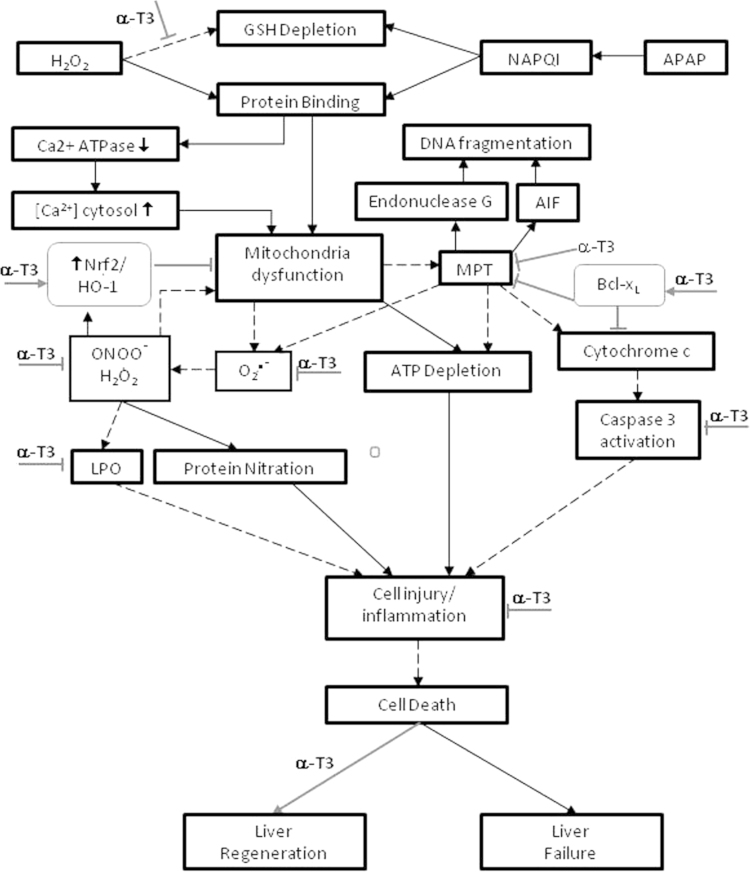
Fig. 9.
Proposed α-T3 protection pathways in APAP- and H2O2-induced liver injury in TAMH cells.
Conflicts of interest
The authors confirm that there are no conflicts of interest.
Acknowledgements
This work was supported by NMRC-NIG Grant R148-000-125-275 and Ministry of Education academic research Grant R148-000-187-112.
Contributor Information
Cheau Yih Tan, Email: A0068305@nus.edu.sg.
Tzuen Yih Saw, Email: judy.saw@davoslife.com.
Chee Wai Fong, Email: cw.fong@davoslife.com.
Han Kiat Ho, Email: phahohk@nus.edu.sg.
References
- 1.Daniels D., Grytdal S., Wasley A. Centers for Disease Control and Prevention (CDC) surveillance for acute viral hepatitis – United States, 2007. MMWR Surveill Summ. 2009;58(3):1–27. 19478727 [PubMed] [Google Scholar]
- 2.Ostapowicz G., Fontana R.J., Schiødt F.V., Larson A., Davern T.J., Han S.H., McCashland T.M., Shakil A.O., Hay J.E., Hynan L., Crippin J.S., Blei A.T., Samuel G., Reisch J., Lee W.M., U.S. Acute Liver Failure Study Group Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Annals of Internal Medicine. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. 12484709 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell J.R., Jollow D.J., Potter W.Z., Gillette J.R., Brodie B.B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. Journal of Pharmacology and Experimental Therapeutics. 1973;187(1):211–217. 4746329 [PubMed] [Google Scholar]
- 4.Grattagliano I., Bonfrate L., Diogo C.V., Wang H.H., Wang D.Q., Portincasa P. Biochemical mechanisms in drug-induced liver injury: certainties and doubts. World Journal of Gastroenterology. 2009;15(39):4865–4876. doi: 10.3748/wjg.15.4865. 19842215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fau D., Berson A., Eugene D., Fromenty B., Fisch C., Pessayre D. Mechanism for the hepatotoxicity of the antiandrogen, nilutamide. Evidence suggesting that redox cycling of this nitroaromatic drug leads to oxidative stress in isolated hepatocytes. Journal of Pharmacology and Experimental Therapeutics. 1992;263(1):69–77. 1403804 [PubMed] [Google Scholar]
- 6.Meyers L.L., Beierschmitt W.P., Khairallah E.A., Cohen S.D. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicology and Applied Pharmacology. 1988;93(3):378–387. doi: 10.1016/0041-008x(88)90040-3. 3368917 [DOI] [PubMed] [Google Scholar]
- 7.Sen C.K., Khanna S., Rink C., Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitamins and Hormones. 2007;76:203–261. doi: 10.1016/S0083-6729(07)76008-9. 17628176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen C.K., Khanna S., Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sciences. 2006;78(18):2088–2098. doi: 10.1016/j.lfs.2005.12.001. 16458936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi A.A., Burger W.C., Peterson D.M., Elson C.E. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. Journal of Biological Chemistry. 1986;261(23):10544–10550. 3733719 [PubMed] [Google Scholar]
- 10.Qureshi A.A., Sami S.A., Salser W.A., Khan F.A. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161(1):199–207. doi: 10.1016/s0021-9150(01)00619-0. 11882333 [DOI] [PubMed] [Google Scholar]
- 11.Nesaretnam K., Guthrie N., Chambers A.F., Carroll K.K. Effect of tocotrienols on the growth of a human breast cancer cell line in culture. Lipids. 1995;30(12):1139–1143. doi: 10.1007/BF02536615. 8614304 [DOI] [PubMed] [Google Scholar]
- 12.Wada S., Satomi Y., Murakoshi M., Noguchi N., Yoshikawa T., Nishino H. Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Letters. 2005;229(2):181–191. doi: 10.1016/j.canlet.2005.06.036. 16098658 [DOI] [PubMed] [Google Scholar]
- 13.Osakada F., Hashino A., Kume T., Katsuki H., Kaneko S., Akaike A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology. 2004;47(6):904–915. doi: 10.1016/j.neuropharm.2004.06.029. 15527824 [DOI] [PubMed] [Google Scholar]
- 14.Das S., Lekli I., Das M., Szabo G., Varadi J., Juhasz B., Bak I., Nesaretam K., Tosaki A., Powell S.R., Das D.K. Cardioprotection with palm oil tocotrienols: comparison of different isomers. American Journal of Physiology – Heart and Circulatory Physiology. 2008;294(2):H970–H978. doi: 10.1152/ajpheart.01200.2007. 18083895 [DOI] [PubMed] [Google Scholar]
- 15.Das M., Das S., Wang P., Powell S.R., Das D.K. Caveolin and proteasome in tocotrienol mediated myocardial protection. Cellular Physiology and Biochemistry. 2008;22(1–4):287–294. doi: 10.1159/000149807. 18769056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S., Roy S., Slivka A., Craft T.K., Chaki S., Rink C., Notestine M.A., DeVries A.C., Parinandi N.L., Sen C.K. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36(10):2258–2264. doi: 10.1161/01.STR.0000181082.70763.22. 16166580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida Y., Niki E., Noguchi N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chemistry and Physics of Lipids. 2003;123(1):63–75. doi: 10.1016/s0009-3084(02)00164-0. 12637165 [DOI] [PubMed] [Google Scholar]
- 18.Kamal-Eldin A., Appelqvist L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31(7):671–701. doi: 10.1007/BF02522884. 8827691 [DOI] [PubMed] [Google Scholar]
- 19.Serbinova E., Kagan V., Han D., Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radical Biology and Medicine. 1991;10(5):263–275. doi: 10.1016/0891-5849(91)90033-y. 1649783 [DOI] [PubMed] [Google Scholar]
- 20.Serbinova E.A., Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods in Enzymology. 1994;234:354–366. doi: 10.1016/0076-6879(94)34105-2. 7808307 [DOI] [PubMed] [Google Scholar]
- 21.Coe K.J., Jia Y., Ho H.K., Rademacher P., Bammler T.K., Beyer R.P., Farin F.M., Woodke L., Plymate S.R., Fausto N., Nelson S.D. Comparison of the cytotoxicity of the nitroaromatic drug flutamide to its cyano analogue in the hepatocyte cell line TAMH: evidence for complex I inhibition and mitochondrial dysfunction using toxicogenomic screening. Chemical Research in Toxicology. 2007;20(9):1277–1290. doi: 10.1021/tx7001349. 17702527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J.C., Merlino G., Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proceedings of the National Academy of Sciences of the United States America. 1994;91(2):674–678. doi: 10.1073/pnas.91.2.674. 7904757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plumb J.A., Milroy R., Kaye S.B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Research. 1989;49(16):4435–4440. 2743332 [PubMed] [Google Scholar]
- 24.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. 22930834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid A.B., Kurten R.C., McCullough S.S., Brock R.W., Hinson J.A. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. Journal of Pharmacology and Experimental Therapeutics. 2005;312(2):509–516. doi: 10.1124/jpet.104.075945. 15466245 [DOI] [PubMed] [Google Scholar]
- 26.Packer L., Weber S.U., Rimbach G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. Journal of Nutrition. 2001;131(2):369S–373S. doi: 10.1093/jn/131.2.369S. 11160563 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y.J., Tsuchiya M., Wassall S.R., Choo Y.M., Govil G., Kagan V.E., Packer L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993;32(40):10692–10699. doi: 10.1021/bi00091a020. 8399214 [DOI] [PubMed] [Google Scholar]
- 28.Saito Y., Yoshida Y., Akazawa T., Takahashi K., Niki E. Cell death caused by selenium deficiency and protective effect of antioxidants. Journal of Biological Chemistry. 2003;278(41):39428–39434. doi: 10.1074/jbc.M305542200. 12888577 [DOI] [PubMed] [Google Scholar]
- 29.Saito Y., Nishio K., Akazawa Y.O., Yamanaka K., Miyama A., Yoshida Y., Noguchi N., Niki E. Cytoprotective effects of vitamin E homologues against glutamate-induced cell death in immature primary cortical neuron cultures: tocopherols and tocotrienols exert similar effects by antioxidant function. Free Radical Biology and Medicine. 2010;49(10):1542–1549. doi: 10.1016/j.freeradbiomed.2010.08.016. 20736061 [DOI] [PubMed] [Google Scholar]
- 30.Yoshida Y., Niki E., Noguchi N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chemistry and Physics of Lipids. 2003;123(1):63–75. doi: 10.1016/s0009-3084(02)00164-0. 12637165 [DOI] [PubMed] [Google Scholar]
- 31.Noguchi N., Hanyu R., Nonaka A., Okimoto Y., Kodama T. Inhibition of THP-1 cell adhesion to endothelial cells by alpha-tocopherol and alpha-tocotrienol is dependent on intracellular concentration of the antioxidants. Free Radical Biology and Medicine. 2003;34(12):1614–1620. doi: 10.1016/s0891-5849(03)00216-8. 12788481 [DOI] [PubMed] [Google Scholar]
- 32.Saito Y., Yoshida Y., Nishio K., Hayakawa M., Niki E. Characterization of cellular uptake and distribution of vitamin E. Annals of the New York Academy of Sciences. 2004;1031:368–375. doi: 10.1196/annals.1331.047. 15753172 [DOI] [PubMed] [Google Scholar]
- 33.Zhou C., Tabb M.M., Sadatrafiei A., Grün F., Blumberg B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metabolism and Disposition. 2004;32(10):1075–1082. doi: 10.1124/dmd.104.000299. 15269186 [DOI] [PubMed] [Google Scholar]
- 34.Landes N., Pfluger P., Kluth D., Birringer M., Rühl R., Böl G.F., Glatt H., Brigelius-Flohé R. Vitamin E activates gene expression via the pregnane X receptor. Biochemical Pharmacology. 2003;65(2):269–273. doi: 10.1016/s0006-2952(02)01520-4. 12504802 [DOI] [PubMed] [Google Scholar]
- 35.Scholz R.W., Graham K.S., Gumpricht E., Reddy C.C. Mechanism of interaction of vitamin E and glutathione in the protection against membrane lipid peroxidation. Annals of the New York Academy of Sciences. 1989;570:514–517. [Google Scholar]
- 36.Costagliola C., Libondi T., Menzione M., Rinaldi E., Auricchio G. Vitamin E and red blood cell glutathione. Metabolism. 1985;34(8):712–714. doi: 10.1016/0026-0495(85)90019-8. 4021803 [DOI] [PubMed] [Google Scholar]
- 37.Costagliola C., Menzione M. Effect of vitamin E on the oxidative state of glutathione in plasma. Clinical Physiology and Biochemistry. 1990;8(3):140–143. 2225721 [PubMed] [Google Scholar]
- 38.Barbagallo M., Dominguez L.J., Tagliamonte M.R., Resnick L.M., Paolisso G. Effects of vitamin E and glutathione on glucose metabolism: role of magnesium. Hypertension. 1999;34(4 2):1002–1006. doi: 10.1161/01.hyp.34.4.1002. 10523398 [DOI] [PubMed] [Google Scholar]
- 39.Sen C.K., Khanna S., Roy S., Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. Journal of Biological Chemistry. 2000;275(17):13049–13055. doi: 10.1074/jbc.275.17.13049. 10777609 [DOI] [PubMed] [Google Scholar]
- 40.Khanna S., Roy S., Parinandi N.L., Maurer M., Sen C.K. Characterization of the potent neuroprotective properties of the natural vitamin E alpha-tocotrienol. Journal of Neurochemistry. 2006;98(5):1474–1486. doi: 10.1111/j.1471-4159.2006.04000.x. 16923160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komiyama K., Iizuka K., Yamaoka M., Watanabe H., Tsuchiya N., Umezawa I. Studies on the biological activity of tocotrienols. Chemical and Pharmaceutical Bulletin. 1989;37(5):1369–1371. doi: 10.1248/cpb.37.1369. 2630104 [DOI] [PubMed] [Google Scholar]
- 42.Domitrović R., Jakovac H., Blagojević G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl(4)-intoxicated mice. Toxicology. 2011;280(1–2):33–43. doi: 10.1016/j.tox.2010.11.005. 21095217 [DOI] [PubMed] [Google Scholar]
- 43.Blazka M.E., Wilmer J.L., Holladay S.D., Wilson R.E., Luster M.I. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicology and Applied Pharmacology. 1995;133(1):43–52. doi: 10.1006/taap.1995.1125. 7597709 [DOI] [PubMed] [Google Scholar]
- 44.Bruccoleri A., Gallucci R., Germolec D.R., Blackshear P., Simeonova P., Thurman R.G., Luster M.I. Induction of early-immediate genes by tumor necrosis factor alpha contribute to liver repair following chemical-induced hepatotoxicity. Hepatology. 1997;25(1):133–141. doi: 10.1002/hep.510250125. 8985279 [DOI] [PubMed] [Google Scholar]
- 45.Beyer T.A., Xu W., Teupser D., auf dem Keller U., Bugnon P., Hildt E., Thiery J., Kan Y.W., Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO Journal. 2008;27(1):212–223. doi: 10.1038/sj.emboj.7601950. 18059474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enomoto A., Itoh K., Nagayoshi E., Haruta J., Kimura T., O’Connor T., Harada T., Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicological Sciences. 2001;59(1):169–177. doi: 10.1093/toxsci/59.1.169. 11134556 [DOI] [PubMed] [Google Scholar]
- 47.Chan K., Han X.D., Kan Y.W. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proceedings of the National Academy of Sciences of the United States America. 2001;98(8):4611–4616. doi: 10.1073/pnas.081082098. 11287661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sass G., Barikbin R., Tiegs G. The multiple functions of heme oxygenase-1 in the liver. Zeitschrift für Gastroenterologie. 2012;50(1):34–40. doi: 10.1055/s-0031-1282046. 22222796 [DOI] [PubMed] [Google Scholar]
- 49.Tan C.Y., Lai R.C., Wong W., Dan Y.Y., Lim S.K., Ho H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Research and Therapy. 2014;5(3):76. doi: 10.1186/scrt465. 24915963 [DOI] [PMC free article] [PubMed] [Google Scholar]