Abstract
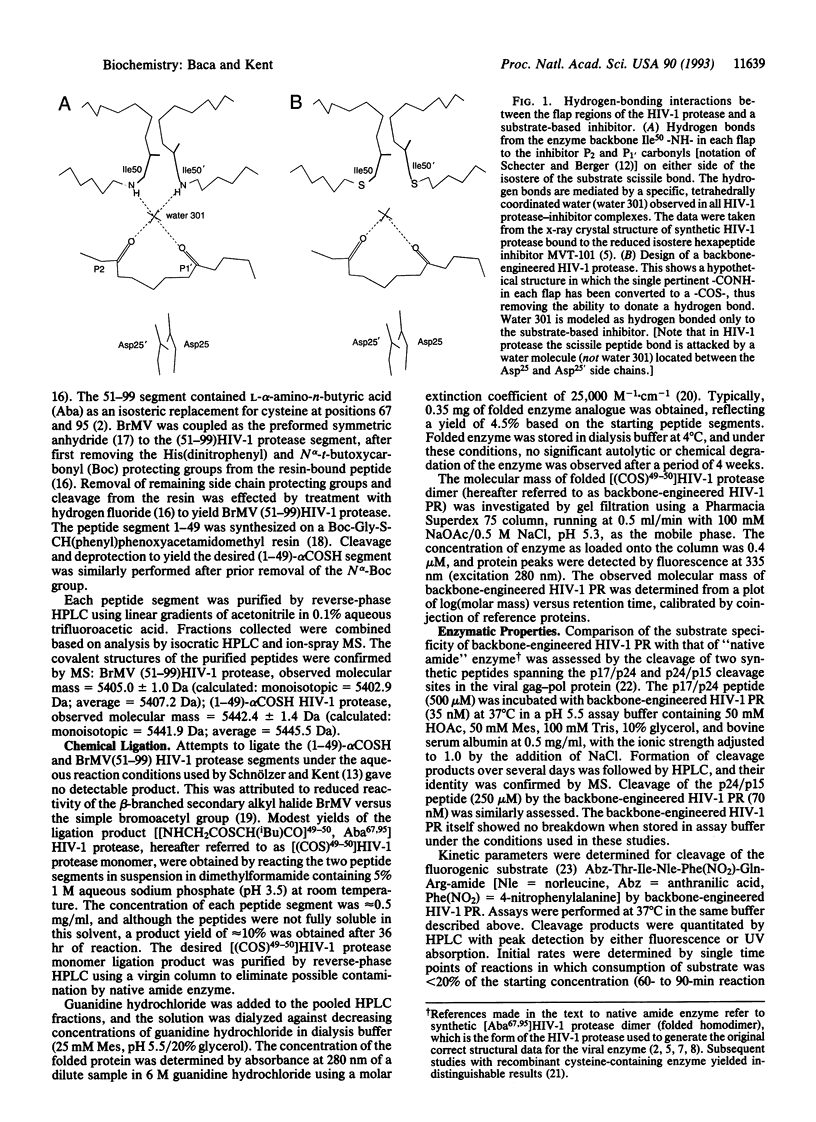
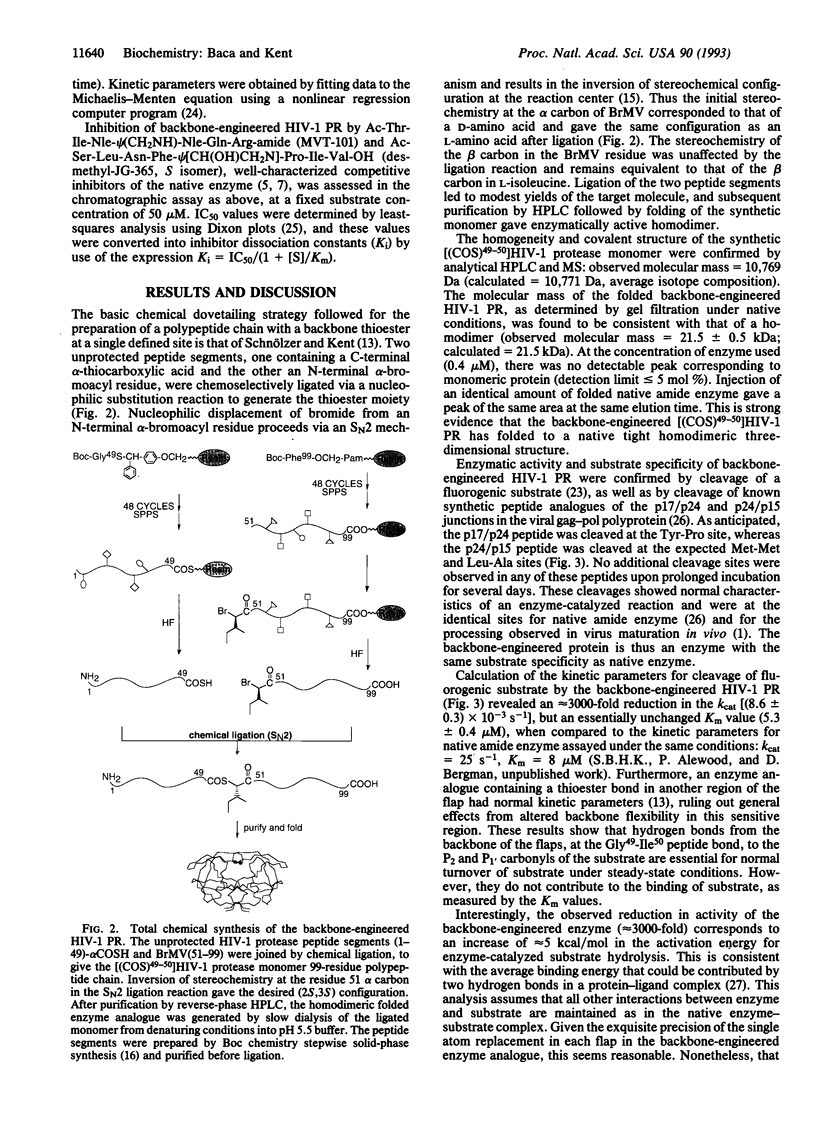
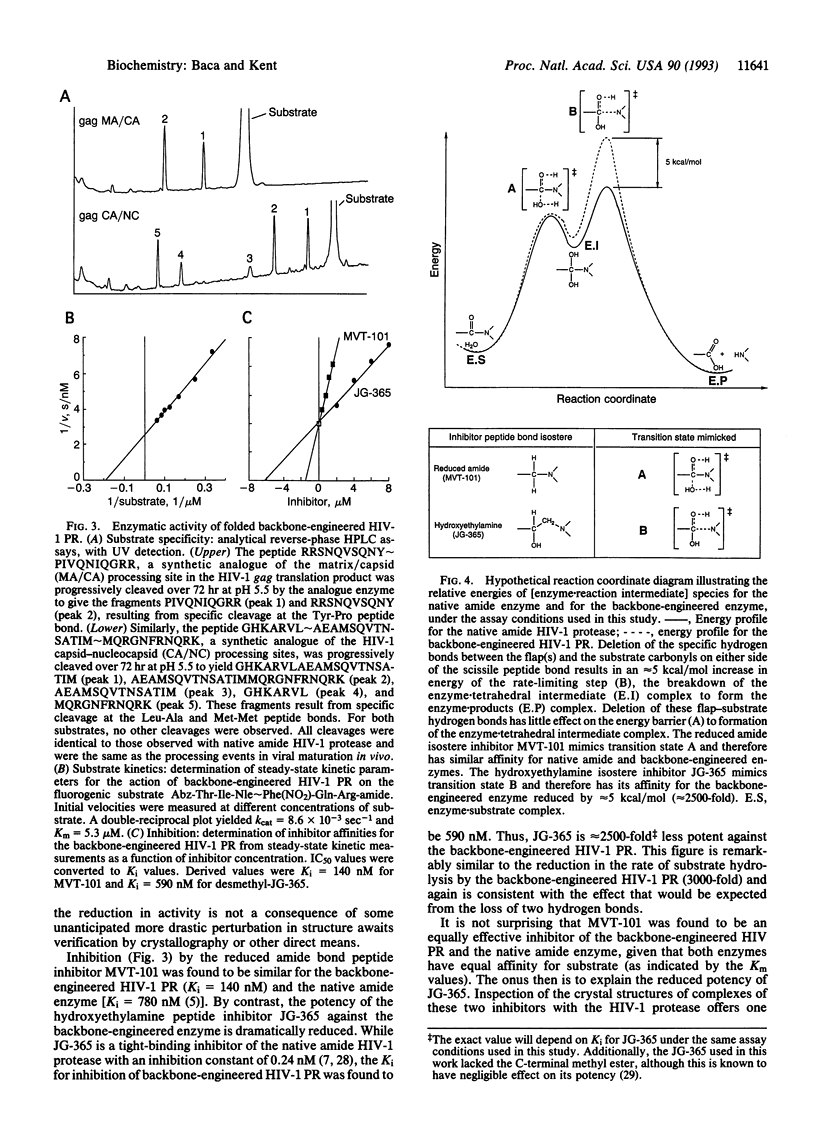
An analogue of "HIV-1 protease" was designed in which the ability to donate important water-mediated hydrogen bonds to substrate was precisely and directly deleted. Chemical ligation of unprotected peptide segments was used to synthesize this "backbone-engineered" enzyme. The functionally relevant amide -CONH- linkage between residues Gly49-Ile50 in each flap of the enzyme was replaced by an isosteric thioester -COS- bond. The backbone-engineered enzyme had normal substrate specificity and affinity (Km). However, the catalytic activity (kcat) was reduced approximately 3000-fold compared to the native amide bond-containing enzyme. Inhibition by the reduced peptide bond substrate analogue MVT-101 was unaffected compared with native enzyme. By contrast, the normally tight-binding hydroxyethylamine inhibitor JG-365 bound to the backbone-engineered enzyme with an approximately 2500-fold reduction in affinity. The reduced catalytic activity of the -Gly49-psi(COS)-Ile50-backbone-engineered enzyme analogue provides direct experimental evidence to support the suggestion that backbone hydrogen bonds from the enzyme flaps to the substrate are important for the catalytic function of the HIV-1 protease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baca M., Alewood P. F., Kent S. B. Structural engineering of the HIV-1 protease molecule with a beta-turn mimic of fixed geometry. Protein Sci. 1993 Jul;2(7):1085–1091. doi: 10.1002/pro.5560020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R. The structure and function of the aspartic proteinases. Annu Rev Biophys Biophys Chem. 1990;19:189–215. doi: 10.1146/annurev.bb.19.060190.001201. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G. Regression analysis of nonlinear Arrhenius plots: an empirical model and a computer program. Comput Biol Med. 1984;14(4):447–455. doi: 10.1016/0010-4825(84)90045-3. [DOI] [PubMed] [Google Scholar]
- Fruton J. S. The mechanism of the catalytic action of pepsin and related acid proteinases. Adv Enzymol Relat Areas Mol Biol. 1976;44:1–36. doi: 10.1002/9780470122891.ch1. [DOI] [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Griffiths J. T., Phylip L. H., Konvalinka J., Strop P., Gustchina A., Wlodawer A., Davenport R. J., Briggs R., Dunn B. M., Kay J. Different requirements for productive interaction between the active site of HIV-1 proteinase and substrates containing -hydrophobic*hydrophobic- or -aromatic*pro- cleavage sites. Biochemistry. 1992 Jun 9;31(22):5193–5200. doi: 10.1021/bi00137a015. [DOI] [PubMed] [Google Scholar]
- Hyland L. J., Tomaszek T. A., Jr, Meek T. D. Human immunodeficiency virus-1 protease. 2. Use of pH rate studies and solvent kinetic isotope effects to elucidate details of chemical mechanism. Biochemistry. 1991 Aug 27;30(34):8454–8463. doi: 10.1021/bi00098a024. [DOI] [PubMed] [Google Scholar]
- Hyland L. J., Tomaszek T. A., Jr, Roberts G. D., Carr S. A., Magaard V. W., Bryan H. L., Fakhoury S. A., Moore M. L., Minnich M. D., Culp J. S. Human immunodeficiency virus-1 protease. 1. Initial velocity studies and kinetic characterization of reaction intermediates by 18O isotope exchange. Biochemistry. 1991 Aug 27;30(34):8441–8453. doi: 10.1021/bi00098a023. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R., Hayakawa K., Gelb M. H. Crystallographic analysis of transition state mimics bound to penicillopepsin: difluorostatine- and difluorostatone-containing peptides. Biochemistry. 1992 Apr 21;31(15):3872–3886. doi: 10.1021/bi00130a019. [DOI] [PubMed] [Google Scholar]
- Jaskólski M., Tomasselli A. G., Sawyer T. K., Staples D. G., Heinrikson R. L., Schneider J., Kent S. B., Wlodawer A. Structure at 2.5-A resolution of chemically synthesized human immunodeficiency virus type 1 protease complexed with a hydroxyethylene-based inhibitor. Biochemistry. 1991 Feb 12;30(6):1600–1609. doi: 10.1021/bi00220a023. [DOI] [PubMed] [Google Scholar]
- Lapatto R., Blundell T., Hemmings A., Overington J., Wilderspin A., Wood S., Merson J. R., Whittle P. J., Danley D. E., Geoghegan K. F. X-ray analysis of HIV-1 proteinase at 2.7 A resolution confirms structural homology among retroviral enzymes. Nature. 1989 Nov 16;342(6247):299–302. doi: 10.1038/342299a0. [DOI] [PubMed] [Google Scholar]
- Meek T. D. Inhibitors of HIV-1 protease. J Enzyme Inhib. 1992;6(1):65–98. doi: 10.3109/14756369209041357. [DOI] [PubMed] [Google Scholar]
- Miller M., Schneider J., Sathyanarayana B. K., Toth M. V., Marshall G. R., Clawson L., Selk L., Kent S. B., Wlodawer A. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science. 1989 Dec 1;246(4934):1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- Muir T. W., Kent S. B. The chemical synthesis of proteins. Curr Opin Biotechnol. 1993 Aug;4(4):420–427. doi: 10.1016/0958-1669(93)90007-j. [DOI] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Pearl L. H. The catalytic mechanism of aspartic proteinases. FEBS Lett. 1987 Apr 6;214(1):8–12. doi: 10.1016/0014-5793(87)80003-0. [DOI] [PubMed] [Google Scholar]
- Polhuijs M., Tergau A. C., Mulder G. J. Chiral inversion and stereoselective glutathione conjugation of the four 2-bromo-3-methylvaleric acid stereoisomers in the rat in vivo and in vitro. J Pharmacol Exp Ther. 1992 Mar;260(3):1349–1354. [PubMed] [Google Scholar]
- Rich D. H., Green J., Toth M. V., Marshall G. R., Kent S. B. Hydroxyethylamine analogues of the p17/p24 substrate cleavage site are tight-binding inhibitors of HIV protease. J Med Chem. 1990 May;33(5):1285–1288. doi: 10.1021/jm00167a003. [DOI] [PubMed] [Google Scholar]
- Robey F. A., Fields R. L. Automated synthesis of N-bromoacetyl-modified peptides for the preparation of synthetic peptide polymers, peptide-protein conjugates, and cyclic peptides. Anal Biochem. 1989 Mar;177(2):373–377. doi: 10.1016/0003-2697(89)90068-7. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kent S. B. Enzymatic activity of a synthetic 99 residue protein corresponding to the putative HIV-1 protease. Cell. 1988 Jul 29;54(3):363–368. doi: 10.1016/0092-8674(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Alewood P., Jones A., Alewood D., Kent S. B. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int J Pept Protein Res. 1992 Sep-Oct;40(3-4):180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Kent S. B. Constructing proteins by dovetailing unprotected synthetic peptides: backbone-engineered HIV protease. Science. 1992 Apr 10;256(5054):221–225. doi: 10.1126/science.1566069. [DOI] [PubMed] [Google Scholar]
- Suguna K., Padlan E. A., Smith C. W., Carlson W. D., Davies D. R. Binding of a reduced peptide inhibitor to the aspartic proteinase from Rhizopus chinensis: implications for a mechanism of action. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7009–7013. doi: 10.1073/pnas.84.20.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A. L., Miller M. M., Green J., Rich D. H., Schneider J., Kent S. B., Wlodawer A. X-ray crystallographic structure of a complex between a synthetic protease of human immunodeficiency virus 1 and a substrate-based hydroxyethylamine inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8805–8809. doi: 10.1073/pnas.87.22.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M. V., Marshall G. R. A simple, continuous fluorometric assay for HIV protease. Int J Pept Protein Res. 1990 Dec;36(6):544–550. doi: 10.1111/j.1399-3011.1990.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- Yamashiro D., Li C. H. New segment synthesis of alpha-inhibin-92 by the acyl disulfide method. Int J Pept Protein Res. 1988 Mar;31(3):322–334. doi: 10.1111/j.1399-3011.1988.tb00040.x. [DOI] [PubMed] [Google Scholar]