Abstract
Inflammation and oxidative stress are believed to contribute to the pathology of several chronic diseases including hypercholesterolemia (elevated levels of cholesterol in blood) and atherosclerosis. HMG-CoA reductase inhibitors of plant origin are needed as synthetic drugs, such as statins, which are known to cause adverse effects on the liver and muscles. Amaranthus viridis (A. viridis) has been used from ancient times for its supposedly medically beneficial properties. In the current study, different parts of A. viridis (leaf, stem, and seed) were evaluated for potential anti-HMG-CoA reductase, antioxidant, and anti-inflammatory activities. The putative HMG-CoA reductase inhibitory activity of A. viridis extracts at different concentrations was determined spectrophotometrically by NADPH oxidation, using HMG-CoA as substrate. A. viridis leaf extract revealed the highest HMG-CoA reductase inhibitory effect at about 71%, with noncompetitive inhibition in Lineweaver-Burk plot analysis. The leaf extract showed good inhibition of hydroperoxides, 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO), and ferric ion radicals in various concentrations. A. viridis leaf extract was proven to be an effective inhibitor of hyaluronidase, lipoxygenase, and xanthine oxidase enzymes. The experimental data suggest that A. viridis leaf extract is a source of potent antioxidant and anti-inflammatory agent and may modulate cholesterol metabolism by inhibition of HMG-CoA reductase.
1. Introduction
Various forms of free radicals, such as alkoxy (RO) and nitric oxide (NO) radicals, are believed to contribute to the pathogenesis of chronic diseases. Hypercholesterolemia, which is characterized by the presence of high levels of cholesterol in the blood, is also believed to arise from oxidative stress. The enzyme, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, is a rate-limiting enzyme that catalyzes the conversion of HMG-CoA to mevalonate in the cholesterol biosynthesis pathway [1]. Statins competitively inhibit HMG-CoA reductase and are efficient in reducing the serum levels of low density lipoprotein (LDL) cholesterol. However, long-term consumption of statins causes serious side effects such as liver and muscles damage [2].
Edible medicinal plants with antioxidant and anti-inflammatory abilities can play a crucial role in the management of hypercholesterolemia [3]. Essentially, practicing healthy diets, including the consumption of desirable quantities of edible antioxidants, enables the body to neutralize radicals and offset damage associated with oxidative stress [4]. Flavonoids and phenolic acids are classes of polyphenolic substances with known antioxidant properties, including inhibition of oxidative enzymes, scavenging of free radicals, and induction of anti-inflammatory actions [5]. The advantages of using plants for medicinal purposes relate to their safety, availability, and economical benefits [6].
Amaranthus viridis L. (A. viridis), locally known as “bayam pasir,” belongs to the family of Amaranthaceae. It is a branched glabrous herb which is distributed in most of the tropical countries [7]. A. viridis has been traditionally used to reduce labour pain and as an antipyretic in India and Nepal [8]. Other traditional usages are as analgesic, antiulcer, antirheumatic, antileprotic, and antiemetic agent [9]. It is also believed to treat eye diseases, psoriasis, eczema, asthma, and respiratory problems [10].
In the present study, the HMG-CoA reductase inhibitory activity of different parts of A. viridis (leaf, stem, and seed) was tested. Ferric thiocyanate (FTC), thiobarbituric acid (TBA), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and nitric oxide (NO) scavenging activity, and ferric-reducing antioxidant power (FRAP) assays were used to measure the antioxidant activity while hyaluronidase, xanthine oxidase, and lipoxygenase inhibition assays were performed to determine the anti-inflammatory potential of A. viridis extract. The objectives of this study are to investigate anti-HMG-CoA reductase, antioxidant, and anti-inflammatory effects of A. viridis, focusing on the therapeutic potential relating to hypercholesterolemia.
2. Materials and Methods
2.1. Chemicals
All the chemicals and reagents used in this study were of analytical reagent grade and were purchased from Sigma-Aldrich and Merck.
2.2. Extraction of A. viridis
A. viridis was collected from various regions of Selangor, Malaysia. The plant was botanically identified and the plant voucher specimen was deposited in the Institute of Bioscience, Universiti Putra Malaysia. The plant was washed and separated into leaf, stem, and seed. The parts of A. viridis were air-dried and ground using a blender (Panasonic MX 8967) and subjected to methanol 50% (v/v) distillation for 48 h. After filtration, the extracts were isolated using a separatory funnel. The crude methanol extracts were then concentrated using a rotary evaporator (Heidolph) under reduced pressure at 40°C and freeze-dried at −40°C [11].
2.3. Enzyme Assay
HMG-CoA reductase inhibitory activities of A. viridis (leaf, stem, and seed) were determined based on spectrophotometric measurements. Each crude extract (50 μg) was mixed with reaction mixture containing NADPH (400 μM), HMG-CoA substrate (400 μM), and potassium phosphate buffer (100 mM, pH 7.4) containing KCl (120 mM), EDTA (1 mM), and DTT (5 mM), followed by the addition of HMG-CoA reductase (2 μL). The reactants were incubated at 37°C and absorbance was measured at 340 nm after 10 min. Simvastatin (Sigma, Missouri, US) was used as positive control and distilled water as negative control. The HMG-CoA reductase inhibition (%) was calculated using the following formula [12]:
| (1) |
2.4. Kinetic Study
The inhibition mode of A. viridis leaf extract was determined using various concentrations of HMG-CoA reductase (0.3, 0.6, 0.9, and 1.2 mmol/L) and three different concentrations of A. viridis (0.05, 0.15, and 0.25 mg/mL). The assay was analyzed using Lineweaver-Burk plot analysis according to the Michaelis-Menten kinetics [12].
2.5. Total Phenolic Content (TPC)
The TPC of A. viridis extracts was determined according to Meda et al. [13] using Folin-Ciocalteu assay. Briefly, A. viridis extracts (100 mg) were dissolved in methanol (10 mL). The extracts (100 μL), sodium carbonate (7.5% (w/v), 2 mL), and Folin-Ciocalteu reagent (tenfold dilution, 2.5 mL) were mixed, vortexed, and incubated at 40°C for 30 min. The absorbance was measured at 760 nm using a UV-visible spectrophotometer (Pharmaspec UV-1650 PC, Shimadzu, Japan). TPC of A. viridis extracts were expressed as mg gallic acid equivalents/g dry weight.
2.6. Total Flavonoid Content (TFC)
The TFC of A. viridis leaf extracts was determined according to Ismail et al. [14] using aluminium calorimetric method. A. viridis extracts (100 mg) were dissolved in methanol (10 mL). The extracts (100 μL) were mixed with sodium nitrite (5% (w/v), 300 μL) and incubated at room temperature for 5 min. Aluminium chloride (10% (w/v), 300 μL) and sodium hydroxide (1 N, 2 mL) were then added followed by the addition of distilled water up to a total volume of 5 mL. The absorbance was measured using a UV-visible spectrophotometer (Pharmaspec UV-1650 PC, Shimadzu, Japan) at 510 nm. TFC of A. viridis extracts were expressed as mg rutin equivalents/g dry weight.
2.7. Antioxidant Activity of A. viridis Extracts
2.7.1. Ferric Thiocyanate (FTC)
FTC assay was performed according to the method described by Ismail et al. [14]. A. viridis extracts (4 mg) were dissolved in methanol (4 mL) and mixed with linoleic acid (2.5%, 4.1 mL), phosphate buffer (50 mM, pH 7, 8 mL), and distilled water (3.9 mL). The mixtures were kept in screw-cap vials which were placed in an oven maintained at 40°C in the dark. The mixtures (100 μL) were added to ethanol (75%, 9.7 mL) and ammonium thiocyanate (30%, 100 μL). Three minutes after the addition of ferrous chloride solution (2 × 102 M in 3.5% hydrochloric acid, 100 μL) to the reaction mixtures, the absorbance was measured at 500 nm using a UV-visible spectrophotometer (Pharmaspec UV-1650 PC, Shimadzu, Japan). The procedure was repeated (at 24 h interval) until the absorbance of the control sample reached its maximum value. Ascorbic acid and α-tocopherol were used as the standard antioxidants.
2.7.2. Thiobarbituric Acid (TBA)
TBA assay on A. viridis extracts was carried out according to the method described by Hendra et al. [15]. The test was performed instantly after the control sample (from FTC test) reached the maximum absorbance value. Aqueous trichloroacetic acid (20%, 1 mL) and aqueous thiobarbituric acid (0.67%, 2 mL) were added to the sample solution (2 mL) acquired from the FTC test. The mixtures were incubated in a boiling water bath for 10 min. After cooling, the mixtures were spun at 3000 rpm for 20 min. Absorbance of the supernatants was read at 532 nm using a UV-visible spectrophotometer (Pharmaspec UV-1650 PC, Shimadzu, Japan). For both FTC and TBA assays, the antioxidant activity was expressed as % inhibition as in the following formula:
| (2) |
where Abc is the absorbance of control and Abs is the absorbance of sample.
2.7.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
The free radical scavenging activity of A. viridis extracts was determined as described by Ismail et al. [14] using the DPPH assay. A. viridis extracts (100 μL) at various concentrations (50, 100, 150, 200, 250, and 300 μg/mL) were added to methanol (490 μL) and DPPH methanolic solution (4 mg/100 mL, 390 μL). The mixtures were then vortexed and allowed to stand at room temperature in the dark for 60 min. Absorbance of the mixtures was measured using a UV-visible spectrophotometer (Pharmaspec UV-1650 PC, Shimadzu, Japan) at 515 nm. Ascorbic acid and α-tocopherol were used as the standard antioxidants. Free radical scavenging activity of A. viridis extracts was expressed as % inhibition as in formula (2).
2.7.4. Nitric Oxide (NO) Scavenging Activity
The NO scavenging activity of A. viridis extracts was determined according to the method of Tsai et al. [16]. A. viridis extracts (60 μL) at various concentrations (50, 100, 150, 200, 250, and 300 μg/mL) were mixed with sodium nitroprusside (10 mM in phosphate buffered saline, 60 μL) in a flat-bottomed microtiter plate and incubated in the light for 150 min at room temperature. An equal volume of the Griess reagent was added to the wells to measure the nitrite content. A 546 was measured with a microtiter plate reader (Stat Fax 3200 Microplate Reader, Awareness Technology Inc., USA). Ascorbic acid and α-tocopherol were used as the standard antioxidants. The NO scavenging activity was expressed as % inhibition as in formula (2).
2.7.5. Ferric-Reducing Antioxidant Power (FRAP) Assay
The ferric-reducing effect of A. viridis extracts was determined according to the method suggested by Yen and Chen [17]. A. viridis extracts (1 mL) at various concentrations (50, 100, 150, 200, 250, and 300 μg/mL) were mixed with potassium phosphate buffer (0.2 M, pH 6.6, 2.5 mL) and potassium ferricyanide (1 g/100 mL, 2.5 mL). The mixtures were then incubated at 50°C for 20 min. After incubation, trichloroacetic acid (10%) was added to stop the reaction. An equal volume of distilled water was added to the mixtures followed by the addition of ferum chlorate (0.1 g/100 mL, 500 μL). The mixtures were then allowed to stand at room temperature for 30 min. A 700 was determined with a UV-visible spectrophotometer (Pharmaspec UV-1650PC, Shimadzu, Japan). The procedures were performed in triplicate and repeated with the standard antioxidants (ascorbic acid and α-tocopherol). The antioxidant activity (%) of the samples in FRAP assay was expressed as reducing power (%) according to the following formula:
| (3) |
where Abc is the absorbance of control and Abs is the absorbance of sample.
2.8. In Vitro Anti-Inflammatory Assay
2.8.1. Hyaluronidase Inhibition Assay
The assay was carried out according to the method described by Yahaya and Don [18] with slight modifications. All the solutions were freshly prepared before the assay. A. viridis samples (5 mg) were dissolved in dimethylsulphoxide (250 μL). The samples were prepared at various concentrations (100, 200, 300, 400, and 500 μg/mL) by dissolving in sodium phosphate buffer (200 mM, pH 7). Hyaluronidase (4 U/mL, 100 μL) was mixed with sample solution (25 μL) incubated at 37°C for 10 min. The reaction was initiated with the addition of substrate, hyaluronic acid solution (0.03% in 300 mM sodium phosphate, pH 5.4, 100 μL), and incubated at 37°C for 45 min. The undigested hyaluronic acid was then precipitated with acid albumin solution (bovine serum albumin (0.1%) in sodium acetate (24 mM), pH 3.8, 1 mL). After 10 min incubation at room temperature, A 600 was measured using a spectrophotometer (XMA 1200V, Seoul, Korea). The absorbance measurement in the absence of enzyme was used as a control value. Quercetin was used as the positive control to verify the performance of the assay. The assay was performed in triplicate. The percentage of hyaluronidase inhibition was determined using the following formula:
| (4) |
where Abc is the absorbance of control and Abs is the absorbance of sample.
2.8.2. Lipoxygenase Inhibition Assay
Lipoxygenase inhibitory activity was performed according to the method suggested by Pin et al. [19] with slight modification. A. viridis samples (10 mg) were dissolved in dimethylsulphoxide (500 μL). The samples were prepared at various concentrations (100, 200, 300, 400, and 500 μg/mL) by dissolving in sodium phosphate buffer (100 mM, pH 8). Sodium phosphate buffer (100 mM, pH 8, 2.46 mL), A. viridis samples (10 μL), and soybean lipoxygenase solution (167 U/mL, 20 μL) were mixed and incubated at 25°C for 10 min. The reaction was initiated with the addition of substrate, sodium linoleic acid solution (10 μL). The enzymatic conversion of sodium linoleic acid to (9Z,11E)-(13S)-13-hydroperoxyoctadeca-9,11-dienoate was measured by observing the change of absorbance at 234 nm for 6 min using a UV-vis spectrophotometer (Evolution 201, Madison, USA). A control was prepared by replacing samples (10 μL) with mixture of sodium phosphate buffer (2.74 mL) and dimethylsulphoxide (25 μL) into the quartz. Quercetin was used as the positive control to verify the performance of the assay. The assay was performed in triplicate. The percentage of lipoxygenase inhibition was determined according to formula (2).
2.8.3. Xanthine Oxidase Inhibition Assay
Xanthine oxidase inhibitory activity was determined according to the method of Yahaya and Don [18], with slight modifications. A. viridis samples (10 mg) were dissolved in dimethylsulphoxide (500 μL). The samples were prepared at various concentrations (100, 200, 300, 400, and 500 μg/mL) by dissolving in potassium phosphate buffer (50 mM, pH 7.5). Potassium phosphate buffer (50 mM, pH 7.5, 2.38 mL), A. viridis samples (10 μL), and xanthine oxidase solution (0.4 U/mL, 10 μL) were mixed and incubated at 25°C for 10 min. The reaction was initiated by the addition of the substrate, xanthine solution (100 μM, 100 μL). The enzymatic conversion of xanthine to uric acid and hydrogen peroxides was measured at A 295 using UV-vis spectrophotometer (Evolution 201, Madison, USA). A control was prepared by replacing samples (10 μL) with a mixture of potassium phosphate buffer (2.39 mL) and dimethylsulphoxide (25 μL) into the quartz. Quercetin was used as a positive control to validate the performance of the assay. The assay was performed in triplicate. The percentage of xanthine oxidase inhibition was determined according to formula (2).
2.9. IC50 Value Determination
The sample concentration providing 50% of inhibition (IC50) was determined through interpolation of linear regression analysis, with lower IC50 values suggesting higher antioxidant and anti-inflammatory activities, and vice versa.
2.10. Statistical Analysis
Data were expressed as mean ± SD from values determined in triplicate. One-way analysis of variance (ANOVA) and the accompanying Dunnett test (SPSS, version 19) were applied to compare values of A. viridis extracts with those of the positive controls, and IC50 was analyzed by using nonlinear regression.
3. Results
3.1. HMG-CoA Reductase Inhibitory Effect
A. viridis leaf, seed, and stem showed 72%, 35%, and 22% inhibitory effects, respectively, on HMG-CoA reductase activities (Figure 1). The leaf extract showed the highest inhibition and was further analyzed using Lineweaver-Burk plot analysis. Enzyme kinetics revealed noncompetitive inhibition on HMG-CoA reductase activity (Figure 2). Km value of HMG-CoA for HMG-CoA reductase was 1.0 mM. V max value of the control was 0.0164. V max values for A. viridis leaf extract at concentrations of 0.05, 0.15, and 0.25 mg/mL were 0.0074, 0.0049, and 0.0035, respectively.
Figure 1.
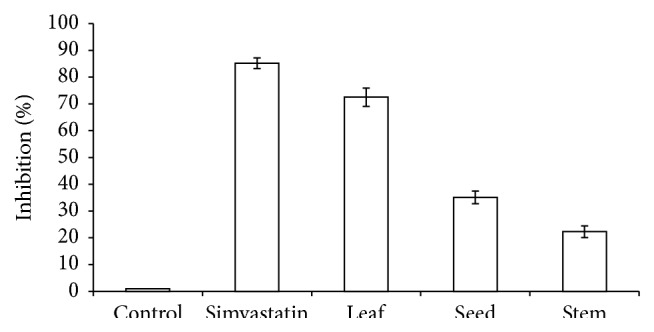
Inhibition of HMG-CoA reductase by A. viridis extracts. Distilled water was used as a negative control and simvastatin was used as a positive control. All data are presented as the mean ± SD of samples tested in triplicate.
Figure 2.
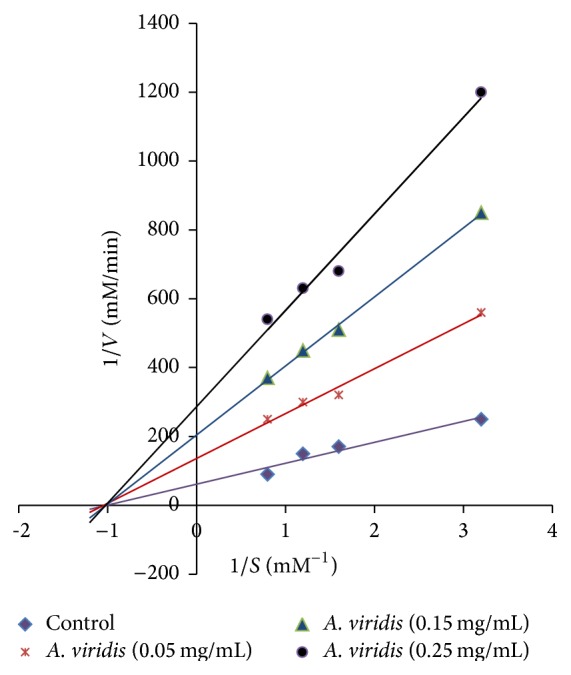
The Lineweaver-Burk plot analysis of HMG-CoA reductase in the presence of A. viridis leaf extract (0.05, 0.15, and 0.25 mg/mL) and HMG-CoA (0.3, 0.6, 0.9, and 1.2 mmol/L) at 340 nm.
3.2. Total Phenolic and Total Flavonoid Content
Total phenolic and total flavonoid content was determined using Folin-Ciocalteu and aluminium calorimetric assays, respectively. A. viridis leaf extract showed the highest phenolic and flavonoid content, which was approximately 85.83 mg GAE/g DW and 152.12 mg rutin equivalent/g DW, respectively. The results are summarized in Table 1.
Table 1.
Total phenolic and flavonoid content of different parts of A. viridis.
| Sample | Total phenolic contenta (mg/g DW) | Total flavonoid contentb
(mg/g DW) |
|---|---|---|
| Leaf | 85.83 ± 1.56 | 152.12 ± 1.41 |
| Stem | 26.38 ± 0.34 | 50.97 ± 0.75 |
| Seed | 38.12 ± 0.89 | 81.36 ± 0.36 |
a: gallic acid equivalent; b: rutin equivalent. The analyses were performed in three replications.
3.3. Antioxidant Activities
The inhibitory activity of A. viridis extracts with respect to hydroperoxides was measured by the ferric thiocyanate assay (Figure 3). The leaf extract was found to have the best tendency to inhibit hydroperoxides compared to stem and seed. The results from FTC and TBA were almost similar in the sense that leaf extract was superior to stem and seed in terms of the potential to inhibit hydroperoxides (Figure 4).
Figure 3.
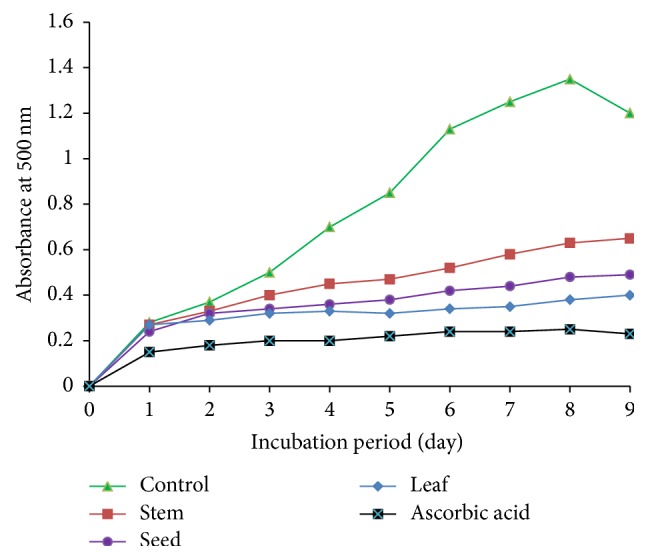
The inhibitory activity of A. viridis extracts on hydroperoxides in the ferric thiocyanate test.
Figure 4.
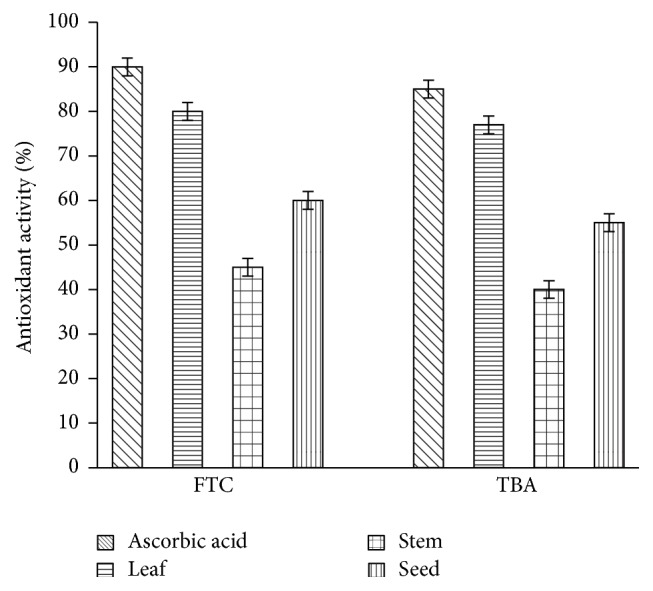
Total antioxidant activity assayed by ferric thiocyanate and thiobarbituric acid methods on day 9. The data represent the mean ± SD measurements of samples tested in triplicate.
A. viridis extracts exhibited DPPH radical scavenging activity at various concentrations (Figure 5). All samples exhibited DPPH scavenging activity in a dose-dependent manner. Leaf extract achieved its highest inhibition at about 70%, followed by seed (58%) and stem (39%) at a concentration of 300 μg/mL. IC50 values of leaf, seed, and stem were 115.74 μg/mL, 189.21 μg/mL, and >300 μg/mL, respectively.
Figure 5.
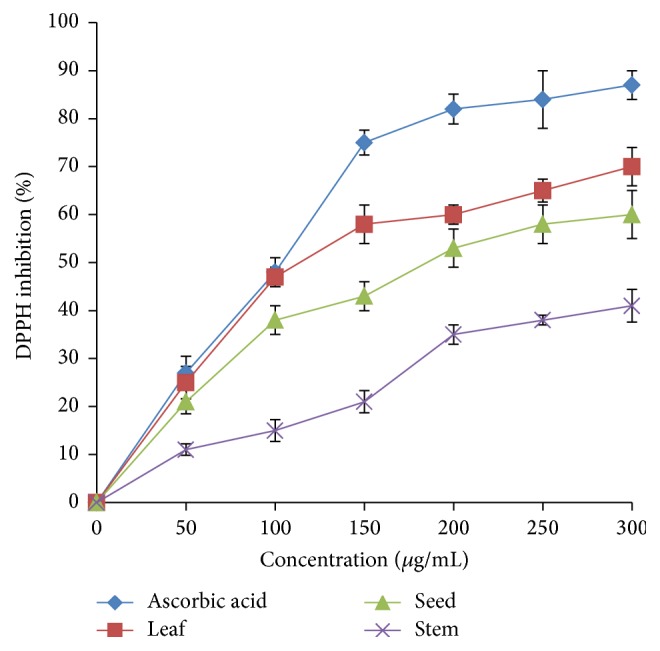
DPPH radical scavenging activity of A. viridis extracts at various concentrations. The data represent the mean ± SD measurements of sample tested in triplicate.
All samples exhibited NO scavenging activity in a dose-dependent manner (Figure 6). The leaf extract exhibited the highest inhibition, about 60%, followed by seed (48%) and stem (22%) at a concentration of 300 μg/mL. IC50 values of leaf, seed, and stem were 244.36 μg/mL, 299.40 μg/mL, and >300 μg/mL, respectively.
Figure 6.
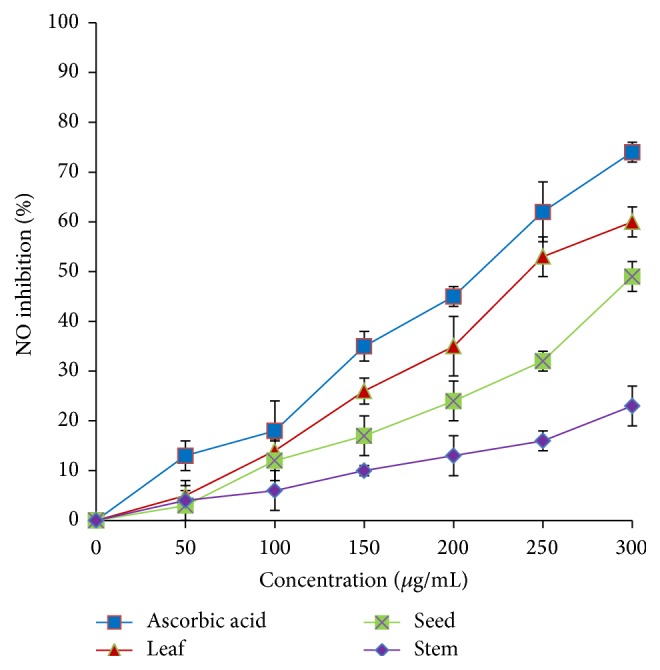
Nitric oxide scavenging activity of A. viridis extracts at various concentrations. The data represent the mean ± SD measurements of samples tested in triplicate.
A. viridis extracts also exhibited satisfactory potential to reduce ferric ions at different concentrations (Figure 7). A drastic increase in the reducing power was observed at a concentration of 100 μg/mL. The leaf extract exhibited the highest inhibition, about 85%, followed by seed (72%) and stem (48%) at a concentration of 300 μg/mL. IC50 values of leaf, seed, and stem were 77.32 μg/mL, 83.47 μg/mL, and >300 μg/mL, respectively. Table 2 summarizes IC50 values of A. viridis extracts and standards for DPPH and NO radical scavenging and ferric-reducing activities.
Figure 7.
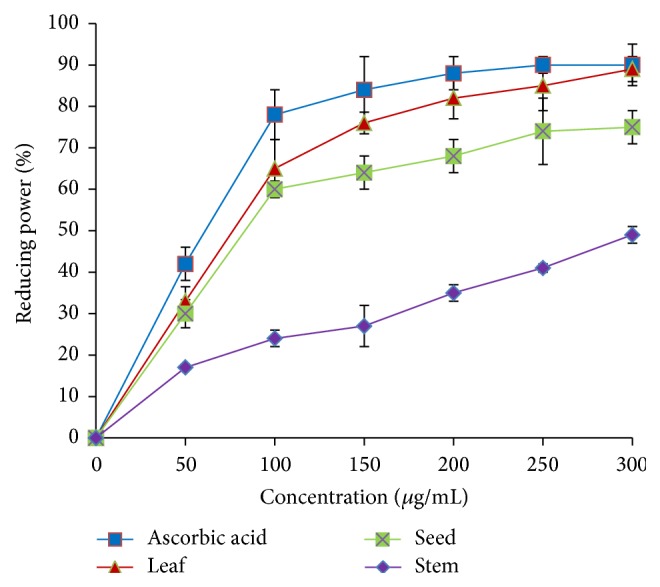
Ferric-reducing activity of A. viridis extracts at various concentrations. The data represent the mean ± SD measurements of samples tested in triplicate.
Table 2.
IC50 values (μg/mL) of A. viridis extracts and standard on radical scavenging and reducing power activities.
| Sample | DPPH | NO | FRAP |
|---|---|---|---|
| Ascorbic acid | 105.03 ± 1.31 | 217.64 ± 0.79 | 63.49 ± 1.75 |
| Leaf | 115.74 ± 1.64 | 244.36 ± 2.15 | 77.32 ± 0.96 |
| Stem | >300 | >300 | >300 |
| Seed | 189.21 ± 1.25 | 299.40 ± 1.26 | 83.47 ± 1.25 |
The analyses were performed in three replications.
3.4. Anti-Inflammatory Activities
The hyaluronidase inhibitory activity of the A. viridis extracts was investigated at different concentrations and was compared to quercetin (Figure 8). The inhibitory activity of the A. viridis extracts showed a gradual increase as the concentration increased. The leaf extract achieved about 70% inhibition at a concentration of 500 μg/mL, followed by seed (50%) and stem (35%). IC50 values of leaf extract and quercetin were 186.23 μg/mL and 101.64 μg/mL, respectively.
Figure 8.
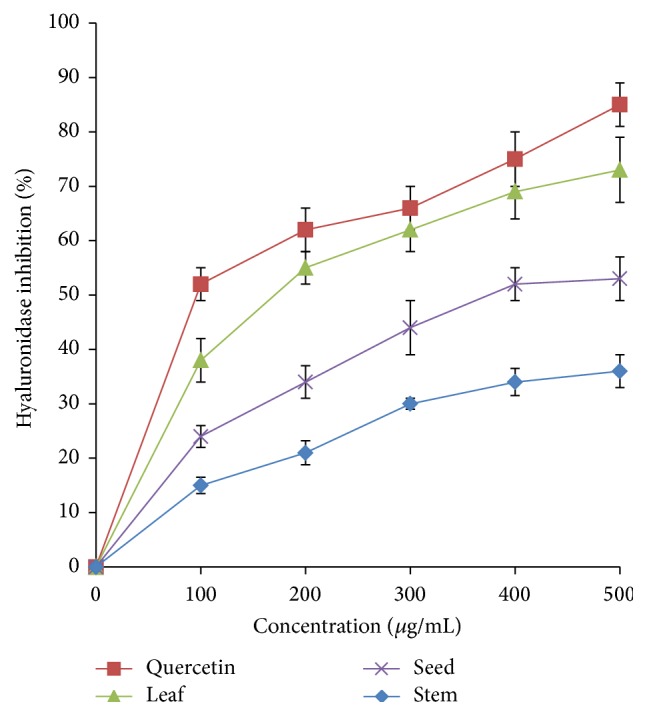
Hyaluronidase inhibitory activity of A. viridis extracts at various concentrations. The data represent the mean ± SD measurements of samples tested in triplicate.
Similarly, the investigation on lipoxygenase inhibitory activity revealed that A. viridis extracts inhibited the lipoxygenase enzyme in a dose-dependent manner. The highest inhibition, 82%, was exhibited by the leaf extract, followed by seed (60%) and stem (35%) at a concentration of 500 μg/mL (Figure 9). IC50 values of leaf extract and quercetin were 151.59 μg/mL and 98.36 μg/mL, respectively.
Figure 9.
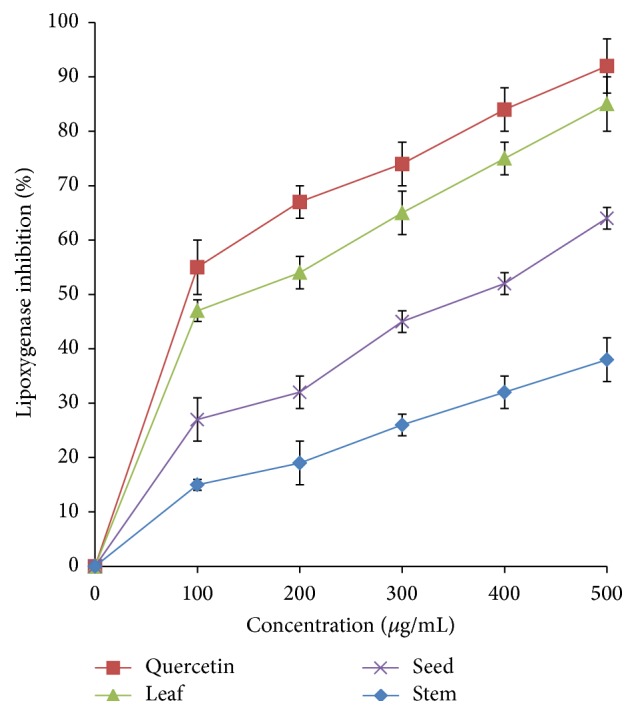
Lipoxygenase inhibitory activity of A. viridis extracts at various concentrations. The data represent the mean ± SD measurements of samples tested in triplicate.
A. viridis extracts exhibited anti-xanthine oxidase activity in a concentration-dependent manner (Figure 10). The highest inhibition, approximately 80%, was achieved by the leaf extract at a concentration of 500 μg/mL, followed by seed (58%) and stem (43%). IC50 values of leaf extract and quercetin were approximately 101.62 μg/mL and 68.67 μg/mL, respectively. Table 3 summarizes IC50 values of A. viridis extracts and standards for hyaluronidase, lipoxygenase, and xanthine oxidase inhibition assays.
Figure 10.
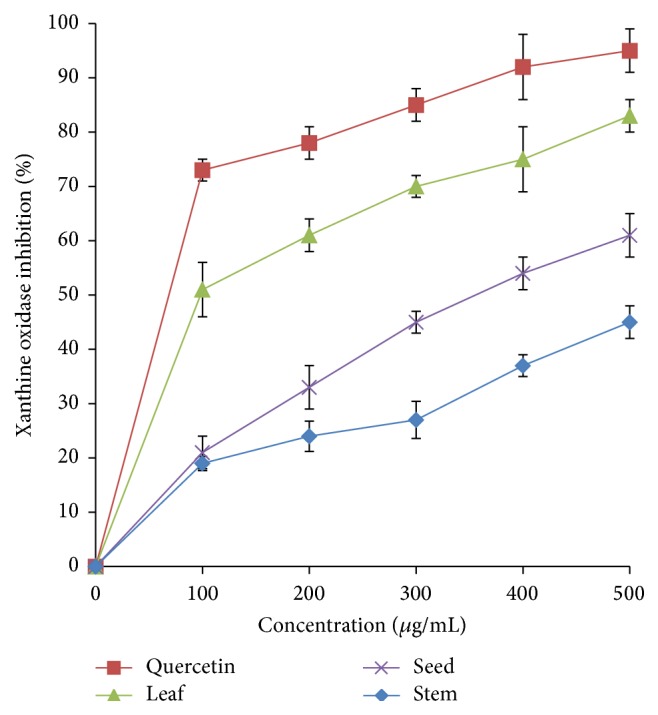
Xanthine oxidase inhibitory activity of A. viridis extracts at various concentrations. The data represent the mean ± SD measurements of samples tested in triplicate.
Table 3.
IC50 values (μg/mL) of A. viridis extract and standard on inhibitory activities of inflammatory enzymes.
| Sample | Hyaluronidase | Lipoxygenase | Xanthine oxidase |
|---|---|---|---|
| Quercetin | 101.64 ± 0.89 | 98.36 ± 1.83 | 68.67 ± 0.65 |
| Leaf | 186.23 ± 1.31 | 151.59 ± 0.82 | 101.62 ± 1.29 |
| Stem | >500 | >500 | >500 |
| Seed | 398.25 ± 2.33 | 394.52 ± 1.35 | 374.43 ± 2.15 |
The analyses were performed in three replications.
4. Discussion
Among the A. viridis extracts, the leaf extract showed the highest inhibition of 71% on HMG-CoA reductase activity. The inhibitory effect of A. viridis leaf extract is comparable to that of Basella alba leaf extract (74%) which was reported as a potent inhibitor of HMG-CoA reductase [11]. This observation is of great relevance since HMG-CoA reductase is the target enzyme for hypercholesterolemia. Plant extracts that suppress HMG-CoA reductase may possibly be used as hypocholesterolemic agent. Statins are synthetic drugs that competitively inhibit HMG-CoA reductase [20]. In contrast, A. viridis leaf extract was shown to inhibit HMG-CoA reductase in a noncompetitive manner. V max value was found to decrease as the leaf concentration increased, suggesting that the substrate was unable to bind to HMG-CoA reductase. The inhibitor interacts with the enzyme or enzyme-substrate complex in which the binding changes the shape of the active site and prevents the formation of cholesterol. The anti-HMG-CoA reductase activity of the A. viridis leaf extract reflects its potential in cholesterol reduction.
The presence of significant phenolic and flavonoid contents suggests the potential use of the A. viridis leaf extract as an alternative medicine. Phenolic compounds describe a class of secondary metabolites with a wide range of pharmacological activities [21]. Indeed, various biological roles of phenolic acids have been reported. They play important roles in secretion of bile and reduction of lipids and cholesterol levels [22]. Phenolics and flavonoids exhibit a wide range of biological activities, including anti-inflammatory [23], antioxidant [24], antidepressant [25], and antiulcer [26] activities. The results of the current study on A. viridis leaf are comparable to those of a study by Hendra et al. [15], who found that the plant Phaleria macrocarpa possessed a high phenolic and flavonoid content.
Antioxidants are compounds that impede oxidation of substrates, and this is characterized by the potential to trap free radicals [27]. The main role that antioxidants play within the body systems rests on their ability to react with free radicals. Free radicals are compounds with unpaired electrons. The reaction eliminates the reactive properties of the radical [28]. With slight modification, the tests used in the current study are acknowledged to be reliable in investigating the antioxidant and anti-inflammatory potential of various substances [18, 29]. In many instances, the methods that have been utilized in determining the antioxidant activities are oriented towards the tendency of the tested compounds to scavenge free radicals or complex metal ions [30, 31]. We believe that the fact that these methods have been used in the current study highlights the reliability of the study.
The radical scavenging and reducing activities of A. viridis extracts appear to have been related mostly to the flavonoid content. The standard, ascorbic acid, gave higher inhibition rate compared to A. viridis extracts. This suggests that the rate of inhibition of A. viridis extracts may be improved by increasing the concentration in all cases. The measurement of inhibitory activities of A. viridis extracts with respect to hydroperoxides, using the FTC assay, suggests that A. viridis leaf extract effectively inhibits hydroperoxides. The A. viridis leaf and ascorbic acid standard showed low absorbance values compared to the negative control, suggesting high levels of antioxidant activity. The differences in the potential to inhibit hydroperoxides were further assayed by FTC and TBA methods on day nine when the negative control's absorbance decreases. The similarity in the results between FTC and TBA suggests the reliability of the results. In the context of toxicity, despite the fact that hydrogen peroxide is unreactive in the body systems, it may be toxic to cells, inhibiting cell functioning abilities by yielding hydroxyl radicals [32]. In this regard, elimination of hydrogen peroxide is considered a crucial antidetoxification process [33].
A. viridis leaf exhibited the DPPH radical scavenging ability at various concentrations, and the inhibition rate of A. viridis extract can be further improved by increasing the concentration. Similar to the potential in inhibiting hydroperoxides, leaf extract exhibited good inhibition of DPPH, considering that it compared favorably with ascorbic acid in terms of its ability to scavenge DPPH radical. The results revealed that A. viridis leaf has certain components possessing desirable hydrogen donation capacity that are well placed to scavenge the DPPH radicals which can be linked to the significant concentrations of flavonoid and phenolic acid, as well as the presence of hydroxyl groups [34]. Thaipong et al. [35] further explained that the antiradical activity of phenolic acids depends on the presence of phenolic hydroxyl hydrogen on the molecular structure, as well as on the tendency of phenoxyl radicals to stabilize resulting from the donation of hydrogen.
The data suggest that the leaf extract had good scavenging activity on the NO radical, in which the effectiveness increased in a dose-dependent manner. The leaf extract effectively inhibited ferric ions and was as effective as ascorbic acid in scavenging ferric ion radicals at a concentration of 300 μg/mL. The performance of A. viridis leaf on NO and ferric ions inhibition has interestingly found concurring results; this finding is consistent with those reported earlier [21, 33]. Napoli and Lerman [36] have suggested that the inhibition of NO and ferric ion radicals is crucial in the management of hypercholesterolemia. The formation of these radicals can also result in pronounced vasomotor dysfunction which can further lead to atherosclerotic lesions [37, 38].
IC50 values (μg/mL) of A. viridis extract revealed its good radical scavenging and reducing power activities. These results are comparable with those obtained using Phaleria macrocarpa, an antioxidant rich medicinal plant [15]. The A. viridis leaf extract exhibited a desirable ability to inhibit hydroperoxides, DPPH, NO, and ferric ions; this shows that it has a strong antioxidant capacity and could be helpful in treating the diseases caused by radicals, such as hypercholesterolemia and atherosclerosis.
The degradation of extracellular matrix plays an essential role in the development of various diseases possessing an inflammatory background [39]. Hyaluronidase is one of the most vital enzymes in this process and plays a major role in controlling the size and concentration of depolymerised hyaluronan chains, which are capable of altering the activities of many pathological processes. Hyaluronidase can cause increased endothelial permeability which can lead to atherogenesis [40]. The results from the current study suggested that the A. viridis leaf extract possessed high antihyaluronidase activity.
Lipoxygenases (LOXs) composed of nonheme iron-containing dioxygenases are important enzymes in leukotrienes biosynthesis, which have been believed to play a key role in the pathophysiology of inflammatory diseases [41]. Cipollone et al. [42] found that the LOXs expressions increased in the carotid atherosclerotic plaques of patients. It is suggested that LOXs are promising targets for therapeutic approaches focused on inflammation reduction in atherosclerotic plaques [43]. The A. viridis leaf extract was proven to be an effective inhibitor of lipoxygenase enzyme.
Xanthine oxidase, an oxygen free radical, has been commonly associated with being an important route in the oxidative injury to tissues, particularly ischemia-reperfusion [44]. Xanthine oxidase is involved in the metabolism of xanthine to uric acid. Uric acid plays a pathogenetic role in cardiovascular diseases; hyperuricemia is generally associated with deleterious effects on vascular function [45]. The inhibition of xanthine oxidase may exert therapeutic effects on impaired vascular function. The data from the present study suggest that the A. viridis leaf extract exhibited satisfactory potential as an inhibitor of the xanthine oxidase enzyme.
An examination of IC50 values (μg/mL) of A. viridis extract on inhibition of the enzymes activities reveals its potential as an anti-inflammatory agent. Since leaf extract compares favorably with quercetin in inhibiting hyaluronidase, lipoxygenases, and xanthine oxidase, it may possess beneficial effects in protecting the vascular endothelial cells from oxidative stress, inflammation, and atherosclerosis.
5. Conclusion
A. viridis leaf extract appears to be a potent inhibitor of HMG-CoA reductase in a noncompetitive manner. The presence of phenolic and flavonoid content suggests that A. viridis can be used as an antioxidant in alternative medicine. Indeed, A. viridis leaf extract also exhibited good potential to reduce hydroperoxides, DPPH, NO, ferric ions, hyaluronidase, lipoxygenase, and xanthine oxidase at various concentrations. An examination of IC50 values (μg/mL) of A. viridis leaf extract and standards on radical scavenging, reducing power, and enzymes inhibitory activities suggests its potential as an antioxidant and anti-inflammatory agent. Thus, in the management of hypercholesterolemia, A. viridis leaf extract appears to possess appreciable anti-HMG-CoA reductase activity and beneficial antioxidant and anti-inflammatory effects. Investigation in an in vivo model could further confirm the potential of A. viridis leaf extract in treating hypercholesterolemia.
Acknowledgments
The research is supported by Universiti Putra Malaysia, Putra Grants: 9438200. Shamala Salvamani is supported by the Ministry of Higher Education of Malaysia.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Friesen J. A., Rodwell V. W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biology. 2004;5(11):248.1–248.7. doi: 10.1186/gb-2004-5-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskaran G., Salvamani S., Azlan A., Ahmad S. A., Yeap S. K., Shukor M. Y. Hypocholesterolemic and anti-atherosclerotic potential of Basella alba leaf extract in hypercholesterolemia-induced rabbits. Evidence-Based Complementary and Alternative Medicine. 2015;2015:7. doi: 10.1155/2015/751714.751714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvamani S., Gunasekaran B., Shaharuddin N. A., Ahmad S. A., Shukor M. Y. Antiartherosclerotic effects of plant flavonoids. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/480258.480258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S.-M., Lin B.-H., Hsieh W.-M., et al. Structural identification and bioactivities of red-violet pigments present in Basella alba fruits. Journal of Agricultural and Food Chemistry. 2010;58(19):10364–10372. doi: 10.1021/jf1017719. [DOI] [PubMed] [Google Scholar]
- 5.Moundipa P. F., Beboy N. S. E., Zelefack F., et al. Effects of Basella alba and Hibiscus macranthus extracts on testosterone production of adult rat and bull Leydig cells. Asian Journal of Andrology. 2005;7(4):411–417. doi: 10.1111/j.1745-7262.2005.00056.x. [DOI] [PubMed] [Google Scholar]
- 6.Moundipa F. P., Kamtchouing P., Koueta N., Tantchou J., Foyang N. P. R., Mbiapo F. T. Effects of aqueous extracts of Hibiscus macranthus and Basella alba in mature rat testis function. Journal of Ethnopharmacology. 1999;65(2):133–139. doi: 10.1016/s0378-8741(98)00207-4. [DOI] [PubMed] [Google Scholar]
- 7.Girija K., Lakshman K. Anti-hyperlipidemic activity of methanol extracts of three plants of Amaranthus in triton-WR 1339 induced hyperlipidemic rats. Asian Pacific Journal of Tropical Biomedicine. 2011;1(1):S62–S65. doi: 10.1016/s2221-1691(11)60125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turin M. Ethnobotonical notes on Thangmi plant names and their medicinal and ritual uses. Contributions to Nepalase Studies. 2003;30(1):19–52. [Google Scholar]
- 9.Kumar B. S. A., Lakshman K., Jayaveea K. N., et al. Antidiabetic, antihyperlipidemic and antioxidant activities of methanolic extract of Amaranthus viridis Linn in alloxan induced diabetic rats. Experimental and Toxicologic Pathology. 2012;64(1-2):75–79. doi: 10.1016/j.etp.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Ashok Kumar B. S., Lakshman K., Jayaveera K. N., et al. Pain management in mice using methanol extracts of three plants belongs to family Amaranthaceae . Asian Pacific Journal of Tropical Medicine. 2010;3(7):527–530. doi: 10.1016/s1995-7645(10)60127-7. [DOI] [Google Scholar]
- 11.Baskaran G., Salvamani S., Ahmad S. A., Shaharuddin N. A., Pattiram P. D., Shukor M. Y. HMG-CoA reductase inhibitory activity and phytocomponent investigation of Basella alba leaf extract as a treatment for hypercholesterolemia. Drug Design, Development and Therapy. 2015;9:509–517. doi: 10.2147/dddt.s75056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gholamhoseinian A., Shahouzehi B., Sharifi-Far F. Inhibitory activity of some plant methanol extracts on 3-hydroxy-3-methylglutaryl coenzyme a reductase. International Journal of Pharmacology. 2010;6(5):705–711. doi: 10.3923/ijp.2010.705.711. [DOI] [Google Scholar]
- 13.Meda A., Lamien C. E., Romito M., Millogo J., Nacoulma O. G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry. 2005;91(3):571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 14.Ismail H. I., Chan K. W., Mariod A. A., Ismail M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chemistry. 2010;119(2):643–647. doi: 10.1016/j.foodchem.2009.07.023. [DOI] [Google Scholar]
- 15.Hendra R., Ahmad S., Oskoueian E., Sukari A., Shukor M. Y. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) scheff fruit. BMC Complementary and Alternative Medicine. 2011;11(1):110–120. doi: 10.1186/1472-6882-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai P.-J., Tsai T.-H., Yu C.-H., Ho S.-C. Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea. Food Chemistry. 2007;103(1):181–187. doi: 10.1016/j.foodchem.2006.08.013. [DOI] [Google Scholar]
- 17.Yen G.-C., Chen H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry. 1995;43(1):27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- 18.Yahaya Y. A., Don M. M. Evaluation of Trametes lactinea extracts on the inhibition of hyaluronidase, lipoxygenase and xanthine oxidase activities in vitro . Journal of Physical Science. 2012;23(2):1–15. [Google Scholar]
- 19.Pin K. Y., Chuah A. L., Rashih A. A., et al. Antioxidant and anti-inflammatory activities of extracts of betel leaves (Piper betle) from solvents with different polarities. Journal of Tropical Forest Science. 2010;22(4):448–455. [Google Scholar]
- 20.Carbonell T., Freire E. Binding thermodynamics of statins to HMG-CoA reductase. Biochemistry. 2005;44(35):11741–11748. doi: 10.1021/bi050905v. [DOI] [PubMed] [Google Scholar]
- 21.Soobrattee M. A., Neergheen V. S., Luximon-Ramma A., Aruoma O. I., Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2005;579(1-2):200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Kamal-Eldin A., Frank J., Razdan A., Tengblad S., Basu S., Vessby B. Effects of dietary phenolic compounds on tocopherol, cholesterol, and fatty acids in rats. Lipids. 2000;35(4):427–435. doi: 10.1007/s11745-000-541-y. [DOI] [PubMed] [Google Scholar]
- 23.Miles E. A., Zoubouli P., Calder P. C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition. 2005;21(3):389–394. doi: 10.1016/j.nut.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daudt R., Von Poser G. L., Neves G., Rates S. M. K. Screening for the antidepressant activity of some species of Hypericum from South Brazil. Phytotherapy Research. 2000;14(5):344–346. doi: 10.1002/1099-1573(200008)14:5<344::aid-ptr586>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Benzie I. F. F. Evolution of dietary antioxidants. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2003;136(1):113–126. doi: 10.1016/s1095-6433(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 27.Amic D., Davidovic-Amic D., Beslo D., Trinajstic N. Structure-radical scavenging activity relationships of flavonoids. Croatica Chemica Acta. 2010;76(1):55–61. [Google Scholar]
- 28.Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry and Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Dudonné S., Vitrac X., Coutiére P., Woillez M., Mérillon J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Journal of Agricultural and Food Chemistry. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 30.Heim K. E., Tagliaferro A. R., Bobilya D. J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 31.Pietta P.-G. Flavonoids as antioxidants. Journal of Natural Products. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B. Encyclopedia of Molecular Cell Biology and Molecular Medicine. 2006. Free radicals in biochemistry and medicine. [Google Scholar]
- 33.Halliwell B., Clement M. V., Long L. H. Hydrogen peroxide in the human body. FEBS Letters. 2000;486(1):10–13. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- 34.Larson R. A. The antioxidants of higher plants. Phytochemistry. 1988;27(4):969–978. doi: 10.1016/0031-9422(88)80254-1. [DOI] [Google Scholar]
- 35.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins B. D. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 2006;19(6-7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 36.Napoli C., Lerman L. O. Involvement of oxidation-sensitive mechanisms in the cardiovascular effects of hypercholesterolemia. Mayo Clinic Proceedings. 2001;76(6):619–631. doi: 10.1016/S0025-6196(11)62413-0. [DOI] [PubMed] [Google Scholar]
- 37.Ling W. H., Cheng Q. X., Ma J., Wang T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. Journal of Nutrition. 2001;131(5):1421–1426. doi: 10.1093/jn/131.5.1421. [DOI] [PubMed] [Google Scholar]
- 38.Wassmann S., Laufs U., Bäumer A. T., et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37(6):1450–1457. doi: 10.1161/01.HYP.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 39.Adair-Kirk T. L., Senior R. M. Fragments of extracellular matrix as mediators of inflammation. The International Journal of Biochemistry and Cell Biology. 2008;40(6-7):1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunnergren K. P., Fairman R. P., DeBlois G. G., Glauser F. L. Effects of protamine, heparinase, and hyaluronidase on endothelial permeability and surface charge. Journal of Applied Physiology. 1987;63(5):1987–1992. doi: 10.1152/jappl.1987.63.5.1987. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y., Gaynor R. B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. Journal of Clinical Investigation. 2001;107(2):135–142. doi: 10.1172/jci11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cipollone F., Mezzetti A., Fazia M. L., et al. Association between 5-lipoxygenase expression and plaque instability in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(8):1665–1670. doi: 10.1161/01.ATV.0000172632.96987.2d. [DOI] [PubMed] [Google Scholar]
- 43.Lai X.-F., Qin H.-D., Guo L.-L., Luo Z.-G., Chang J., Qin C.-C. Hypercholesterolemia increases the production of leukotriene B4 in neutrophils by enhancing the nuclear localization of 5-lipoxygenase. Cellular Physiology and Biochemistry. 2014;34(5):1723–1732. doi: 10.1159/000366373. [DOI] [PubMed] [Google Scholar]
- 44.Sanhueza J., Valdes J., Campos R., Garrido A., Valenzuela A. Changes in the xanthine dehydrogenase/xanthine oxidase ratio in the rat kidney subjected to ischemia-reperfusion stress: preventive effect of some flavonoids. Research Communications in Chemical Pathology and Pharmacology. 1992;78(2):211–218. [PubMed] [Google Scholar]
- 45.Khosla U. M., Zharikov S., Finch J. L., et al. Hyperuricemia induces endothelial dysfunction. Kidney International. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]


