Abstract
Hydrogen peroxide (Η2Ο2) is produced during a variety of cellular procedures. In this paper, the regulatory role of Η2Ο2, in Escherichia coli phagocytosis by the human polymorphonuclears, was investigated. White blood cells were incubated with dihydrorhodamine (DHR) in order to study H2O2 synthesis and E. coli-FITC to study phagocytosis. Flow cytometry revealed increased synthesis of H2O2 in polymorphonuclears which incorporated E. coli-FITC. The blocking of H2O2 synthesis by specific inhibitors, N-ethylmaleimide (ΝΕΜ) for NADPH oxidase and diethyldithiocarbamate (DDC) for superoxide dismutase (SOD), decreased E. coli phagocytosis, as well. Immunoblot analysis of white blood cell protein extracts revealed that the blocking of NADPH oxidase and SOD decreased ERK-1/2 phosphorylation, while it had no effect on JNK and p38. Confocal microscopy showed that phosphorylation of MAPKs and phagocytosis solely occur in the polymorphonuclear and not in mononuclear cells. The use of specific MAPKs inhibitors showed that all of them are necessary for phagocytosis, but only phospho-p38 affects H2O2 synthesis. The blocking of JNK phosphorylation, in the presence of E. coli, evoked a further decrease of cytoplasmic p47 thus increasing its translocation onto the plasma membrane for the assembly of NADPH oxidase. It appears that newly synthesised H2O2 invigorates the phosphorylation and action of ERK-1/2 in E. coli phagocytosis, while phospho-JNK and phospho-p38 appear to regulate H2O2 production.
Abbreviations: MAPKs, mitogen activated protein kinases; JNK, Jun N-terminus kinase; ERK, extracellular-signal-regulated protein kinase; p-JNK, phospho-JNK; p-p38, phospho-p38; p-ERK, phospho-ERK
Keywords: Polymorphonuclears, Phagocytosis, Hydrogen peroxide, Signalling
Graphical abstract
Highlights
-
•
Phagocytosis by polymorphonuclears is accompanied by a targeted production of H2O2.
-
•
H2O2 signals E. coli phagocytosis by invigorating ERK phosphorylation.
-
•
Phosphorylation of p38, appears to be involved in H2O2 synthesis.
-
•
Phospho-JNK appears to support a regulating mechanism for NADPH oxidase assembly.
1. Introduction
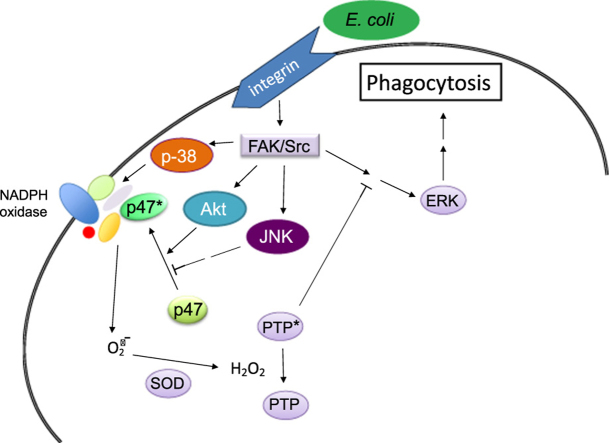
Neutrophils, the major group of the polymorphonuclear leucocytes, are responsible for the nonspecific immune response against bacterial invasion in the blood. After the chemotactic movement to the site of infection, neutrophils engulf and neutralise bacteria by phagocytosis [1]. Complications in this process may lead to frequent infections, often causing death. Bacteria is recognised directly by pattern recognition receptors (PRRs), which are expressed on the surface of the neutrophils. Engagement of these receptors and activation of adhesion molecules, such as integrins, trigger signal transduction pathways that initiate adhesion and phagocytosis [2], [3].
Neutrophil activation is linked with the production of superoxide and other secondarily derived reactive oxygen species (ROS), an oxygen-dependant process known as oxidative or respiratory burst [4], [5]. High levels of superoxide are generated upon the assembly of the multi-protein enzyme NADPH oxidase that catalysers the reduction of oxygen to superoxide, which is then converted to H2O2 by superoxide dismutase (SOD). The NADPH oxidase consists of two membrane bound protein subunits and a cytosolic complex of another four. Activation of NADPH oxidase is initiated by cytosolic complex phosphorylation, which induces a conformational change leading to the translocation of the complex towards the membrane subunits and formation of the active enzyme [4], [6]. The prevailing model in bacterial killing used to be that ROS attack and kill bacteria through their chemical reactivity. Many in vitro studies have proven that reactive oxygen species are toxic to a broad range of microbes, but the conditions used in these experiments did not always mimic the physiological situation in the neutrophil phagosome [7]. Recently, this model has been challenged and it is now accepted that ROS are signalling molecules, which regulate biochemical paths controlling basic cellular functions, such as proliferation and apoptosis [8], [9].
The process of phagocytosis itself relies on the regulation of actin polymerisation early in uptake and during formation of the nascent phagosome [10]. This actin remodelling is under the control of several signalling pathways, which are maintained by kinases and phosphatases via modulation of the activities of the signalling molecules [11]. Mitogen-activated protein kinases (MAPKs) are upstream acting enzymes of actin remodelling [12]. There is abundant evidence that their activities are regulated by NADPH oxidase [13]. The produced H2O2, which is membrane-permeable and relatively stable, can diffuse away from the site of production and may also inactivates a group of enzymes called protein tyrosine phosphatases (PTPs) [7], [14].
In the present study, we investigated the role of H2O2 that is produced by Escherichia coli-challenged polymorphonuclear cells, as a signalling molecule contributing to the regulation of phagocytosis. This was based on the fact that H2O2 is involved in signal transduction via direct inactivation of PTPs and on the speculation that it might act as an indirect regulator of MAPKs activity.
2. Materials and methods
2.1. Chemicals
Affinity-purified rabbit polyclonal antibodies against human p47, p-JNK, p-p38, p-ERK1/2, and p38, were purchased from Cell Signalling Technology (Beverly, MA, USA). Goat anti-rabbit IgG-HRP, goat anti-mouse IgG-HRP and donkey anti-goat IgG-HRP (horseradish peroxidase conjugated) were obtained from Santa Cruz (Santa Cruz, CA, USA). All inhibitors, N-ethylmaleimide (ΝΕΜ), sodium diethyldithiocarbamate trihydrate (DDC), SB202190, SP600125, U0126 and DAPI stain were purchased from Sigma (St. Louis, MO, USA). Alexa Fluor® 647 donkey anti-rabbit IgG was purchased from Life Technologies (Carlsbad, CA, USA). Other materials were obtained as indicated.
2.2. FITC-labelling of E. coli
FITC-labelled E. coli (DH10B) was prepared after incubation of 108 heat-killed bacteria with 1 mg fluoroscein isothiocyanate (FITC), in 0.5 ml 0.5 M Na2CO3/0.5 M NaHCO3 at pH 9.5 for 30 min in the dark. FITC-conjugated E. coli was rinsed three times with phosphate-buffered saline, re-suspended in RPMI 1640 medium (GIBCO BRL, Grand Island, NY, USA) and stored in aliquots at −20 °C.
2.3. Isolation of white blood cells and preparation of lysates
Human peripheral white blood cells (WBCs) were isolated from freshly donated heparinated whole blood after hypo-osmotic lysis of red blood cells with an ammonium chloride-based lysing solution (BD Pharm Lyse, San Diego, CA, USA). One volume of blood was mixed with five volumes of lysis buffer. Samples were then centrifuged at 200 g for 6 min at 25 °C. Supernatant was aspirated and the same procedure was repeated once more. Sedimented WBCs were re-suspended in RPMI 1640 medium (GIBCO BRL, Grand Island, NY, USA). When it was necessary, isolated WBCs were lysed in PBS, by sonication at 4 °C. Insoluble material was removed by centrifugation (16,000 g, for 10 min at 4 °C) and supernatant was collected. Protein concentration was determined in lysates with a modified Bradford's solution containing 10% (w/v) Coomassie G250 (Merck, Darmstadt, Germany) in 5% (v/v) ethanol and 10% (v/v) H3PO4. O.D. was recorded at 595 nm.
2.4. Flow cytometry
Human peripheral blood leucocytes (3×106 cells/ml) were incubated in 200 μl RPMI 1640 medium containing 20% plasma, with either E. coli-FITC to measure phagocytosis or dihydrorhodamine 123 (DHR) to measure the production of H2O2, which oxidises DHR to fluorescent rhodamine 123. Cell suspensions were quenched with trypan blue 10%. Approximately 30,000 cells from each sample were analysed by flow cytometry, using a Coulter EPICS-XL-MCL cytometer (Coulter, Miami, FL, USA), selecting the polymorphonuclear cell population with the appropriate gating. Data was processed using the XL-2 software.
2.5. SDS-PAGE and immunoblot analysis
Samples of equal protein amount were analysed on a typical 12% acrylamide-0.12% bisacrylamide slab gel and electroblotted onto Immobilon P polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA, USA). Membranes were incubated in 5% non-fat dry milk for 1 h at 37 °C. Subsequently, membranes were incubated overnight at 4 °C with primary antibody diluted 1:1000 in PBS, 5% (w/v) BSA and 0.1% (v/v) Tween-20. Membranes were washed and incubated with HRP-linked secondary antibody (1:2000) for 1 h at 37 °C. Immunoreactive proteins were visualised on X-ray film by enhanced chemiluminescence methodology (Amersham, UK). Prestained protein markers, broad range, were used to indicate the size of the protein bands (Lonza, Rockland, MA, USA).
2.6. Confocal microscopy
Isolated WBCs treated with E. coli-FITC, were allowed to attach on Polysine® slides (Thermo Scientific, Braunschweig, Germany) for 20 min at 25 °C. The slides were washed with PBS to remove non-adherent cells. The monolayers were fixed with formaldehyde 3.7% for 10 min. WBCs were embedded in 0.01% Triton X-100 for 5 sec, washed and treated with a protein-blocking agent for 10 min at RT to reduce non-specific binding. Following saturation, slides were incubated with rabbit anti-human p-JNK or p-p38 or p-ERK1/2 (1:200 in PBS) for 1 h at 37 °C in a humid atmosphere. Following antibody treatment, the slides were washed with PBS and further incubated for 1 h at 37 °C, in a humid atmosphere in the dark, with a secondary Alexa Fluor® 647 donkey anti-rabbit IgG antibody (1:500 in PBS). WBCs monolayers were then washed with PBS, mounted with 20 μl MOWIOL (Calbiochem, Darmstadt, Germany) and 2 μl DAPI (1 µg/ml) and observed under a confocal microscope (Leica Microsystems) to examine E. coli-FITC and p-JNK or p-p38 or p-ERK1/2 distribution and localisation.
2.7. Statistical analysis
Quantisation of blot results was performed with the Scion Image processing and analysis programme free software. Comparison of the mean values among groups was performed by means of unpaired Student t-test. Each experiment included triplicate measurements for each condition tested. All results are expressed as mean±SE. Values of p less than 0.05 were accepted as significant.
3. Results
3.1. Hydrogen peroxide regulates phagocytosis via ERK phosphorylation
White Blood cells were isolated in RPMI (3×106 cells/ml) containing 20% plasma, to resemble whole Blood conditions. to study phagocytosis, E. coli-FITC was added in 200 μl WBC suspension, at a final concentration of 3×107 bacteria/ml. to study H2O2 synthesis, 200 μl WBC suspension was pre-incubated with 15 μM DHR followed by the addition of non fluorescent E. coli at a final concentration of 3×107 bacteria/ml. all samples were incubated for 15 min at 37 °C under mild agitation and were then processed to flow cytometric analysis. endogenous fluorescence was estimated in controls without the presence of bacteria or DHR. with the appropriate gating, WBC subpopulations were distinguished and phagocytosis was estimated by using the median X value of the respective fluorescence distribution. As expected, polymorphonuclear cells were the major phagocytic cells in the Blood compared to lymphocytes and monocytes (Table 1). it was also obvious that phagocytosis (E. coli-FITC fluorescence) had been accompanied by a de novo and analogous H2O2 synthesis (oxidised DHR fluorescence). In lymphocytes and monocytes, fluorescence was vaguely detectable, for both phagocytosis and H2O2 production (Table 1).
Table 1.
Phagocytosis and H2O2 synthesis in white blood cell types.
| Polymorphonuclears | Lymphocytes | Monocytes | |
|---|---|---|---|
| Number/30,000 cells | 18,300±1500 | 9600±1500 | 2100±780 |
| E. coli-FITC (AFU) | 82±8 | 3.6±1.1 | 0.9±0.2 |
| Mean AFU/cell | 4.5×10−3 | 0.3×10−3 | 0.4×10−3 |
| Oxidised DHR (AFU) | 66±7 | 4.3±1.1 | 0.8±0.2 |
| Mean AFU/cell |
3.6×10−3 |
0.4×10−3 |
0.3×10−3 |
AFU: Arbitrary Fluorescence Units≡median fluorescence distribution.
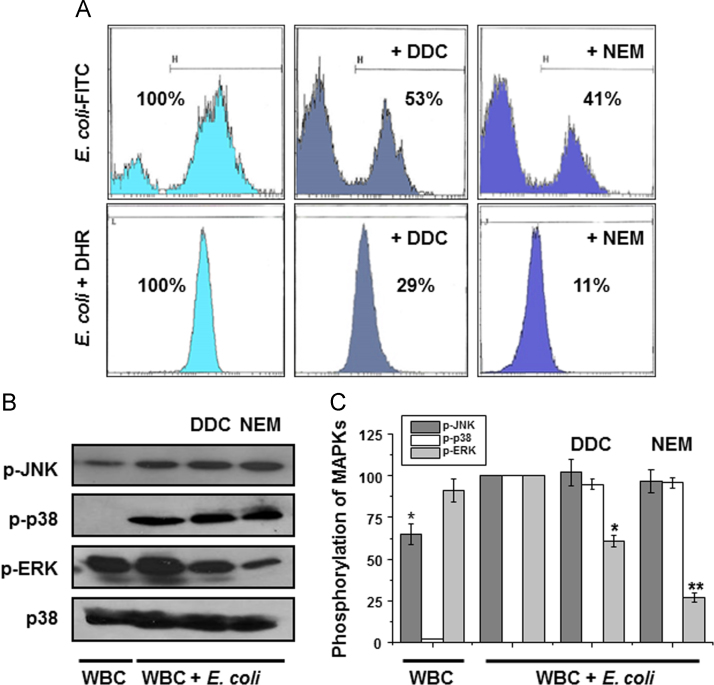
To investigate whether the produced H2O2, during E.coli phagocytosis, acted as an active molecule and was not just a side product, isolated WBCs (3×106 cells/ml) were pre-incubated in 200 μl RPMI medium containing 20% plasma, with either 150 µM NEM (NADPH oxidase inhibitor) or 100 µM DDC (SOD inhibitor) for 10 min at 37 °C. Then, E. coli-FITC (3×107 bacteria/ml) was added and incubated for 15 min more, under agitation. Control experiments were performed without the presence of inhibitors. E. coli-FITC phagocytosis was determined by flow cytometry. To ensure the effect of the inhibition on H2O2 synthesis, similar experiments with the same inhibitors were also performed in the presence of 15 μM DHR during pre-incubation, followed by the addition of E. coli. Combined results showed that the inhibitors of the enzymes, responsible for the synthesis of H2O2, decreased both E. coli-FITC phagocytosis and H2O2 production (Fig. 1A). This data was strong evidence that H2O2, produced by E. coli-challenged WBCs, had acted as a positive regulator of phagocytosis. Unfortunately any attempt to increase E. coli phagocytosis by adding H2O2 in the incubation medium, lead to the lost of the homogeneity of PMNs, thus making impossible the flow cytometry study.
Fig. 1.
Hydrogen peroxide affects E. coli phagocytosis and MAP kinases phosphorylation. Isolated WBCs were pre-incubated with specific inhibitors of H2O2 synthesis, namely DDC for SOD and NEM for NADPH oxidase. In two series of experiments, cells were incubated with either E. coli-FITC for phagocytosis (A, upper row) or DHR and E. coli for H2O2 synthesis (A, lower row). Control experiments were performed without inhibitors. Both inhibitors decreased H2O2 synthesis and phagocytosis of E. coli-FITC (translocation of plots to the left). Percentages are the average out of three independent experiments. WBCs protein crude extracts were immunoblotted with anti p-JNK, anti p-p38 anti p-ERK1/2 (B). p38 was used as an equal protein load indicator. Results are mean values±SE from three independent experiments (C). (*), p<0.01; (**), p<0.001.
Polymorphonuclear cells uptake bacteria via distinct pathways, in which MAP kinases activation is involved [15], [16]. Hence, a question was raised as to any potential role of the H2O2 in MAP kinases phosphorylation. Whole blood samples were pretreated with either NEM or DDC, as previously, and were then cultured in the presence of E. coli. White blood cells were isolated, lysed and protein crude extracts were immunoblotted for p-JNK, p-p38 and p-ERK1/2 (Fig. 1B). It appeared that phosphorylation had been increased in all three MAPKs in the presence of bacteria (Fig. 1B, lane 2 compared to lane 1). The inhibition of H2O2 synthesis affected only ERK1/2 by decreasing its phosphorylation while no change was observed in JNK and p38 phosphorylation (Fig. 1B, lanes 3 and 4 compared to lane 2).
3.2. MAP kinase phosphorylation in WBCs during E. coli-FITC phagocytosis
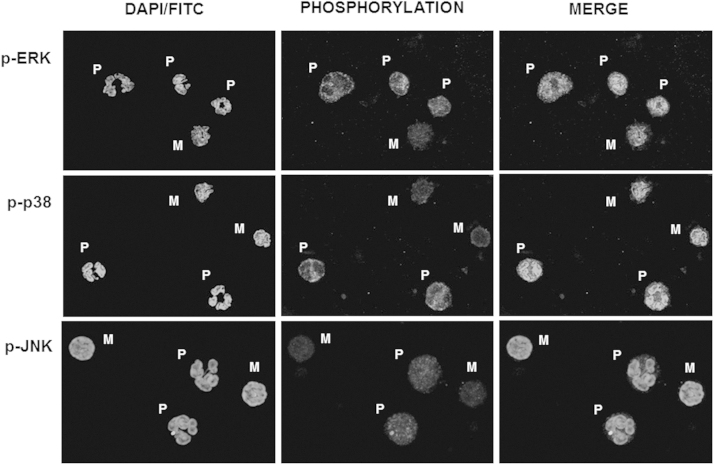
With the selective gating, flow cytometry clearly showed that phagocytosis and the accompanied H2O2 synthesis, is achieved by polymorphonuclear cells (Table 1). On the other hand, to investigate protein phosphorylation during phagocytosis, immunoblot analysis was performed in total WBC protein crude extracts. The next step was to ensure that these changes in phosphorylation, take place only in PMNs due to the presence of bacteria. Since their isolation was not feasible to confirm those results, we further studied JNK, p38 and ERK1/2 phosphorylation, during E. coli phagocytosis, by immunocytochemistry. Isolated WBCs were cultured with E. coli-FITC in RPMI containing 20% plasma, for 15 min at 37 °C. Cells were then allowed to attach on polysine slides and immunostained with either anti p-JNK, anti p-p38 or anti p-ERK1/2 and then with a secondary fluorescent antibody. Samples were covered with MOWIOL mounting medium, containing 2 μl DAPI (1 μg/ml). Confocal microscopy revealed phosphorylation of all MAPKs (Fig. 2, middle column – coloured cytoplasm) only in PMNs (Fig. 2, left column – coloured multilobed nuclei) regardless of the presence of phagocytosed E. coli-FITC (Fig. 2, right column – light spots). The absence of coloured cytoplasm in mononuclear cells (lymphocytes and monocytes) implies that p-JNK, p-p38 and p-ERK1/2 bands which appeared in immunoblot analysis, are related to changes taking place solely in polymorphonuclear cells.
Fig. 2.
MAP kinases phosphorylation in E. coli phagocytosis. Isolated WBCs were incubated with E. coli-FITC. Cells were then fixed on glass slides, in triplicate and indirectly immunostained for p-JNK or p-38 or p-ERK1/2 (middle column). Dapi dye was used to mark nuclei and discriminate blood cell types. E. coli-FITC (left column) phagocytosis was observed under confocal microscope. Phosphorylation of the three MAP kinases is evident only in polymorphonuclear cells (multi-lobbed nuclei) regardless of phagosytosis (right column). P: polymorphonuclear cells; M: mononuclear cells.
3.3. MAP kinase phosphorylation interferes with H2O2 synthesis in phagocytosis
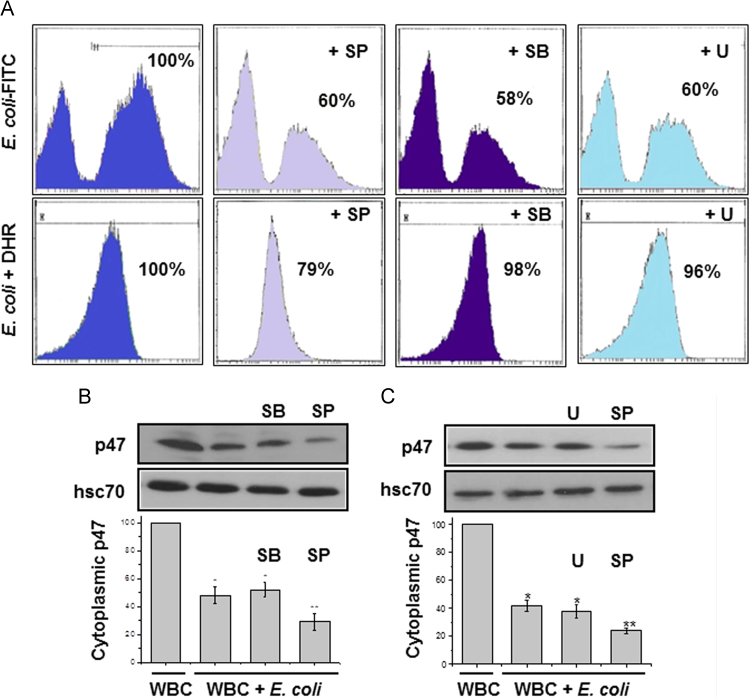
To investigate whether MAPKs phosphorylation affects E. coli phagocytosis and H2O2 synthesis, isolated white blood cells (3×106 cells/ml) were pre-incubated in 200 μl RPMI medium containing 20% plasma, with specific inhibitors for each one of MAPK phosphorylation, 20 μM SB202190 ( p38), 5 μM SP600125 (JNK) and 10 μM U0126 (ERK1/2) for 10 min at 37 °C. Then, E. coli-FITC (3×107 bacteria/ml) was added and incubated for 15 min more, under agitation. Controls were performed without inhibitors. E. coli-FITC phagocytosis was determined by flow cytometry. To investigate any influence of those inhibitors on H2O2 synthesis, similar experiments with the same inhibitors were also performed in the presence of 15 μM DHR during pre-incubation, followed by the addition of non fluorescent E. coli. Combined results showed that all inhibitors decreased E. coli-FITC phagocytosis but only the inhibition of p38 phosphorylation decreased basal H2O2 production (Fig. 3A). This data shows that phagocytosis is regulated by MAP kinases but only p38 appears to regulate phagocytosis via H2O2 synthesis.
Fig. 3.
MAP kinases regulate E. coli phagocytosis and H2O2 synthesis.In two series of experiments, isolated WBCs were pre-incubated, with specific inhibitors of MAP kinases phosphorylation, SB202190 (p38), SP600125 (JNK) and U0126 (ERK1/2) and further incubated with either E. coli-FITC (A, upper row) or DHR and E. coli (A, lower row). Control experiments were performed without inhibitors. All inhibitors decreased phagocytosis of E. coli-FITC and at the same time only the inhibition of p38 phosphorylation decreased basal H2O2 synthesis (translocation of plot to the left). Percentages are the average out of three independent experiments. WBCs protein crude extracts were immunoblotted with anti-p47 cytoplasmic NADPH oxidase subunit. As an equal protein load indicator, hsc70 was used (B, C). Results are mean values±SE from three independent experiments (B). (*), p<0.01; (**), p<0.001.
The NADPH oxidase, the key enzyme for the synthesis of H2O2, consists of two membrane bound protein subunits and a cytosolic complex of another four. The activation of NADPH oxidase is initiated by the phosphorylation of the subunits of the cytosolic complex, leading to its translocation towards the membrane subunits and formation of the active enzyme [4], [6]. Since all three MAPKs appeared to be involved in E.coli phagocytosis but only p-p38 in H2O2 synthesis (Fig. 3A), the effect of ERK1/2, p38 and JNK phosphorylation in H2O2 enzymic production was investigated, by studying their role upon the p47 cytosolic subunit.
Three whole blood samples (200 μl) were pre-incubated with each one of MAPKs phosphorylation inhibitors, as before, for 5 min at 37 °C. Then, E. coli was added (3×107 bacteria/ml final concentration) and incubated for an additional 15 min, under agitation. Control experiments were performed without inhibitors or bacteria. WBCs were isolated and lysed by sonication in an ice bath. Protein crude extracts were immunoblotted with anti-p47 NADPH oxidase cytosolic subunit. It appeared that the presence of E. coli reduced the amount of cytosolic p47 (Fig. 3B and C, lane 2 compared to lane 1) as expected, due to the translocation of p47 phosphorylated molecules towards the plasma membrane for the assembly of NADPH oxidase. The inhibition of ERK1/2 and p38 phosphorylation did not influence that reduction (Fig. 3B and C, lane 3 compare to lane 2) while the inhibition of JNK phosphorylation lead to a further decrease of cytoplasmic p47 (Fig. 3B and C, lane 4 compared to lane 2). This data implies that p-JNK may be involved in the blockade of p47 phosphorylation and translocation and thus the assembly of NADPH oxidase. On the contrary, phosphorylation of ERK1/2 and p38 seems not to interfere with p47 subunit translocation. Since p-p38 appeared to affect H2O2 synthesis (Fig. 3A), it maybe be concluded that it probably acts on the phosphorylation of another subunit for the assembly of NADPH oxidase. It must be noted that any initial attempt to monitor the increase of p47 onto the membrane failed, since the protein extraction from the membrane fraction, for immunoblot analysis, was not obtainable.
4. Discussion
It has now been accepted that H2O2 and other ROS are signalling molecules, which regulate biochemical paths in basic cellular functions and not by-products of normal cell catabolism that disturb the oxidative and anti-oxidative balance and lead to oxidative stress [8], [9]. The results of this work converge towards H2O2 being necessary for the regulation and the progression of phagocytosis by its interference in ERK phosphorylation. In the present data, the presence of E. coli results in MAPKs and p47 phosphorylation, among others. It has been reported that Akt phosphorylates p47 and mediates respiratory burst activity during phagocytosis [17]. Phospho-ERK1/2 deals with the progression of phagocytosis while p-p47 translocates onto the plasma membrane for the assembly of NADPH oxidase. The newly synthesised H2O2 inactivates protein tyrosine phosphatases (PTPs), working in favour of kinase activity in the cell, thus enhancing downstream ERK1/2 phosphorylation and phagocytosis. It can be concluded that the reduction of ERK1/2 phosphorylation, caused by the inhibition of H2O2 synthesis, is presumably due to the act of phosphatases, which were not deactivated by the normally produced H2O2. Phosphorylation of p38, occurring in the presence of bacteria, seems to be involved upstream in the NADPH oxidase assembly and the H2O2 synthesis but presumably in the phosphorylation of another subunit(s). A regulating mechanism, for p47 phosphorylation, seems also to be supported upstream by p-JNK. It must be noted that phosphorylated enzymes are observed in the presence of E. coli, even in polymorphonuclear cells without phagocytosed bacteria.
The results of this work were obtained with the use of enzyme inhibitors for the H2O2 synthesis. NEM was chosen due to its inhibitory effect on the activation of neutrophil NADPH oxidase [18]. A contradictory result presented a lack of oxidase inhibition by NEM [19]. However, it was not taken under consideration because we did not have access to EPR spectroscopy, in order to test NEM action in phagocytosis. Instead, flow cytometry was chosen to explore NADPH oxidase inhibition by NEM, since it had been referred to cause a reduction on the superoxide anion production, with the use of the specific fluorescent probe hydroethidine [20]. This has been confirmed in preliminary experiments in our lab (data not shown).
To investigate the role of H2O2 synthesis on MAPK phosphorylation, we proceeded with the combination of NEM action on NADPH activation and the specific inhibitor DDC for SOD that dismutes superoxide into H2O2. The decrease of H2O2 synthesis was evident in the presence of either NEM or DDC. The latter, as a chelator of Cu and Zn could be assumed to interfere with other ion inactivation as well, such as Fe. But in that case, H2O2 would increase because the Fenton reaction, in which H2O2 is decomposed, could not take place due to the chelation of Fe. Bivalent ions such as Cu and Zn are used by a variety of enzymes, as activators. It cannot be overlooked the possibility that other ions can also interfere with ROS production. In the future, supporting results could be obtained, from siRNA or gene silencing experiments.
The intracellular production of H2O2 and other ROS is affected by certain pathological conditions related to diseases which are connected to oxidative damage. It must not escape our notice the iron catalyzed decomposition of H2O2 that takes place via Fenton reaction, through a sequence of one-electron reduction of oxygen species leading to the production of very reactive free radicals. Iron-driven generation of oxygen-derived free radicals is known to induce oxidation of proteins, lipids and lipoproteins, nucleic acids, carbohydrates and other cellular components [21]. Cardiomyopathy, acute kidney disease, Friedreich ataxia, etc., are related to oxidative damage due to excessive intracellular iron. Antioxidants and chelators are effective and safe in the reversal of oxidative stress related to iron overload [22]. It would be of interest to investigate whether the use of certain antioxidants and chelators improve the phagocytic potential in these cases.
On the other hand, iron deficiency results in anaemia, impaired production of iron-containing proteins and inhibition of cell growth. Erythropoiesis-stimulating agents and iron have been available for decades to treat anaemia. The practice of intravenous iron supplementation has grown but this might lead to a small but significant fraction of redoxactive iron [23], [24]. This remark raises a question whether the H2O2 that is produced during phagocytosis, would be decomposed by intracellular redoxactive iron, thus, preventing its signalling role and action.
The prominent role of redox signalling, in phagocytosis, has been emerged in this paper. The signalling role of H2O2, rather than its toxic action against invading bacteria, has been clarified. We believe that the intravenous iron supplementation, and the use of antioxidants and iron chelators, which interfere in ROS production, should be given cautiously. Phagocytosis is the first line protection against pathogen invasion. Thus it is tempting to speculate that elucidation of the H2O2-mediated regulation of molecular mechanisms would provide a basis for the development or reconsideration of therapeutic strategies for the treatment of patients with frequent infections due to innate immunity dysfunctions.
Acknowledgements
This work was solely financed by the annual scheduled funding of the University of Patras through the Ministry of Education.
References
- 1.Kobayashi S.D., DeLeo F.R. Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip. Rev.: Syst. Biol. Med. 2009;1:309–333. doi: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi S.D., Voyich J.M., Burlak C., DeLeo F.R. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. (Warsz) 2005;53:505–517. [PubMed] [Google Scholar]
- 3.Schymeinsky J., Mócsai A., Walzog B. Neutrophil activation via β2 integrins (CD11/CD18): molecular mechanisms and clinical implications. J. Thromb. Haemost. 2007;98:262–273. [PubMed] [Google Scholar]
- 4.Quinn M.T., Ammons M.C., Deleo F.R. The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction. Clin. Sci. 2006;111:1–20. doi: 10.1042/CS20060059. [DOI] [PubMed] [Google Scholar]
- 5.Rigby K.M., DeLeo F.R. Neutrophils in innate host defence against Staphylococcus aureus infections. Semin. Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauseef W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 7.Rada B., Leto T.L. Oxidative innate immune defences by Nox/Duox family NADPH Oxidases. Contrib. Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbouti A., Amorgianiotis C., Kolettas E., Kanavaros P., Galaris D. Hydrogen peroxide inhibits caspase-dependent apoptosis by inactivating procaspase-9. Free Radic. Biol. Med. 2007;43:1377–1387. doi: 10.1016/j.freeradbiomed.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Galaris D., Skiada V., Barbouti A. Redox signalling and cancer: the role of “labile” iron. Cancer Lett. 2008;266:21–29. doi: 10.1016/j.canlet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Lee W.L., Harrison R.E., Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–1306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Park H., Ishihara D., Cox D. Regulation of tyrosine phosphorylation in macrophage phagocytosis and chemotaxis. Arch. Biochem. Biophys. 2011;510:101–111. doi: 10.1016/j.abb.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kustermans G., Piette J., Legrand-Poels S. Actin-targeting natural compounds as tools to study the role of actin cytoskeleton in signal transduction. Biochem. Pharmacol. 2008;76:1310–1322. doi: 10.1016/j.bcp.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 14.Denu J.M., Tanner K.G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield P.J., Shayman J.A., Boxer L.A. Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood. 2000;95:2407–2412. [PubMed] [Google Scholar]
- 16.Zhong B., Jiang K., Gilvary D.L., Epling-Burnette P.K., Ritchey C., Liu J., Jackson R.J., Hong-Geller E., Wei S. Human neutrophils utilize a Rac/Cdc42-dependent MAPK pathway to direct intracellular granule mobilization toward ingested microbial pathogens. Blood. 2003;101:3240–3248. doi: 10.1182/blood-2001-12-0180. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q., Powell D.W., Rane M.J., Singh S., Butt W., Klein J.B., McLeish K.R. Akt phosphorylates p47phox and mediates neutrophils respiratory burst activity in human. J. Immunol. 2003;170:5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- 18.Park J.W., Park H.L., Lee S.M. Possible target components for the inhibitory effect of N-ethylmaleimide on the activation of neutrophil NADPH oxidase. Biochem. Mol. Biol. Int. 1998;45:699–707. doi: 10.1080/15216549800203102. [DOI] [PubMed] [Google Scholar]
- 19.Janiszewski M., Pedro M.A., Scheffer R.C., Van Asseldonk J.H., Souza L.C., Da Luz P.L., Augusto O., Laurindo F.R. Inhibition of vascular NADH/NADPH oxidase activity by thiol reagents: lack of correlation with cellular glutathione redox status. Free Radic. Biol. Med. 2000;29:889–899. doi: 10.1016/s0891-5849(00)00393-2. [DOI] [PubMed] [Google Scholar]
- 20.Walrand S., Valeix S., Rodriguez C., Ligot P., Chassagne J., Vasson M.P. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin. Chim. Acta. 2003;331:103–110. doi: 10.1016/s0009-8981(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 21.Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat. Res. 2003;531:81–92. doi: 10.1016/j.mrfmmm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Kontoghiorghe C.N., Kolnagou A., Kontoghiorghes G.J. Antioxidant targeting by deferiprone in diseases related to oxidative damage. Front. Biosci. (Landmark Ed) 2014;19:862–885. doi: 10.2741/4253. [DOI] [PubMed] [Google Scholar]
- 23.Del Vecchio L., Locatelli F. Anemia in chronic kidney disease patients: treatment recommendations and emerging therapies. Expert Rev. Hematol. 2014;7:495–506. doi: 10.1586/17474086.2014.941349. [DOI] [PubMed] [Google Scholar]
- 24.Slotki I., Cabantchik Z.I. The labile side of Iron supplementation in CKD. J. Am. Soc. Nephrol. 2015 doi: 10.1681/ASN.2015010052. (May 21. pii: ASN.2015010052. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]