Abstract
Aim: To conduct a double-blind, placebo-controlled randomized clinical trial of baclofen in the treatment of alcohol dependence. Methods: Out of 69 participants consecutively screened, 42 alcohol-dependent patients were randomized to receive placebo, baclofen 30 mg/day or baclofen 60 mg/day for 12 weeks. All subjects were offered BRENDA, a structured psychosocial therapy for alcohol dependence that seeks to improve motivation for change, enhance strategies to prevent relapse and encourage compliance with treatment. Results: Intention-to-treat analyses revealed that alcohol consumption (heavy drinking days, drinks per drinking day) significantly reduced across all three groups during the treatment period. There were no statistically significant advantages to treatment on time to first heavy drinking day (relapse) (P = 0.08), nor time to first drink (lapse) (P = 0.18). A post hoc analysis stratifying according to whether there had been a comorbid anxiety disorder, revealed a beneficial effect of baclofen 30 mg/day versus placebo on time to lapse and relapse (P < 0.05). There was also a beneficial effect for baclofen 60 mg/day relative to placebo on time to relapse in this comorbid group (P < 0.05). Both doses of baclofen were well tolerated. There were no serious adverse events. Conclusions: In spite of the small sample for a 3-arm clinical trial, this study suggests a specific role of baclofen in alcohol-dependent individuals with comorbid anxiety. Replication in larger, fully-powered studies is required.
INTRODUCTION
In terms of years lost, alcohol is considered to be the most harmful substance in the world when associated death, disability, suffering and the full range of social harms are considered (Nutt et al., 2010). However, although alcohol use disorders are leading causes of preventable death, treatment options are still limited. At present there are only a few pharmacological treatments specifically indicated for alcohol dependence in Europe, the USA and Australia.
Baclofen is a selective GABAB receptor agonist currently indicated for the treatment of muscle spasticity. Preclinical studies have found that baclofen reduces self-administration of alcohol, acquisition, maintenance and reinstatement of alcohol-drinking behavior and modifies motivational cues for alcohol (Colombo et al., 2004; Maccioni and Colombo, 2009).
In humans, Addolorato and colleagues were the first group to conduct a double-blind, placebo-controlled, randomized clinical trial (n = 39) of baclofen in the treatment of alcohol dependence (Addolorato et al., 2002). This study indicated significantly higher rates of abstinence and reduced alcohol intake following 4 weeks of baclofen (30 mg/day) treatment. Following this, a preliminary open-label 12-week treatment study demonstrated that baclofen (30 mg/day) was well-tolerated in alcohol-dependent participants and resulted in significant reductions in drinks per drinking day, self-reported craving and anxiety (Flannery et al., 2004). Subsequently, Addolorato et al. (2007) similarly reported higher rates of abstinence and significantly reduced craving after 12 weeks of treatment with baclofen (30 mg/day) compared with placebo (n = 84) in alcoholic patients with liver cirrhosis. These findings were confirmed in a subset of alcoholic patients with cirrhosis and HCV infection (Leggio et al., 2012). Additionally, human laboratory studies have demonstrated the safety of baclofen when co-administered with alcohol (Evans and Bisaga, 2009; Leggio et al., 2013). Additionally, a preliminary study suggested that baclofen may amplify the biphasic effects of alcohol (sedation and stimulation) and reduce alcohol self-administration without reducing alcohol cue-induced craving (Leggio et al., 2013).
More recently, Garbutt et al. (2012) found no beneficial effect of baclofen in 80 alcohol-dependent patients randomized to 12 weeks of baclofen (30 mg/day) versus placebo for most alcohol outcome measures including time to lapse, time to relapse, days abstinent or the percentage of heavy drinking days. This study showed an overall treatment effect, that is, all patients significantly improved their drinking outcomes regardless of the medication condition (Garbutt et al., 2012), which was possibly due to the enrollment of a population with a lower severity of alcohol dependence, compared with the previous positive studies (Addolorato et al., 2002, 2007).
As such, different severity of alcohol dependence has been suggested as a possible key factor influencing baclofen response, including factors such as severity of drinking, withdrawal and anxiety (Leggio et al., 2010). Notably, Garbutt et al. (2012) observed a significant reduction in state anxiety in patients allocated to baclofen treatment. Previous studies have demonstrated the efficacy of GABAB subtype receptor agonists, including baclofen, in treating anxiety in patients with PTSD, panic disorder and in treating anxiety in alcohol-dependent patients (Breslow et al., 1989; Krupitsky et al., 1993; Drake et al., 2003). It is possible that baclofen may influence subjective expression of craving and consequently risk of relapse by suppressing anxiety and stress associated with alcohol dependence. Anxiety is one of the main symptoms of alcohol withdrawal syndrome, and a few studies have reported an effect of baclofen (30 mg/day) in significantly reducing alcohol withdrawal symptoms (Addolorato et al., 2006; Lyon et al., 2011).
The International Baclofen Intervention Study (IBIS) was originally planned as a multisite double-blind placebo-controlled randomized clinical trial to assess the effects of two doses of baclofen (30 mg/day, 60 mg day and placebo) in alcoholism. While six sites in Europe and Australia initially joined the consortium only three sites (Italy, Australia & Austria) were able to start the study and recruit patients; therefore the study was significantly underpowered. The data for the Italian study site have been reported previously (Addolorato and Leggio, 2010). While there was no baclofen effect on the primary outcomes of alcohol consumption possibly due to the underpowered sample, a post hoc analysis (Addolorato et al., 2011) observed a beneficial dose-response effect of baclofen (30 mg/day versus 60 mg/day) in reducing the number of drinks per drinking day relative to placebo over a 12-week treatment period (n = 42).
Here we aim to document the Australian site data from the IBIS study. Our primary hypothesis was that baclofen administered at 30 mg/day and 60 mg/day would increase abstinence and reduce relapse compared with placebo. Our secondary hypotheses were that baclofen would reduce measures of craving and anxiety over the 12-week treatment period. In addition, we performed secondary analyses investigating the efficacy of baclofen on alcohol outcomes in patients presenting with comorbid anxiety disorder. The potential importance of these analyses was suggested by (a) substantial levels of baseline anxiety and prevalence of comorbid anxiety disorders in the sample, (b) high rates of anxiety and comorbid anxiety disorder in the target population and (c) evidence suggesting a therapeutic role for GABAB subtype receptor agonists in treating anxiety.
METHODS
Design
This was a double-blind, placebo-controlled clinical, randomized trial in which alcohol-dependent participants received 10 or 20 mg t.i.d. baclofen or matching placebo for a 12-week period. The study was conducted over a 24-month period at Drug Health Services at Royal Prince Alfred Hospital, Sydney. The study was approved by the Human Ethics Review Committee of the Sydney Local Health District. The trial was registered in the Australian New Zealand Clinical Trials Registry (ACTRN12606000100594).
Participants
Potential male and female participants were identified by treating clinicians at the outpatient drug and alcohol unit. Inclusion criteria were the following: (a) men and women between the ages of 18 and 60 meeting DSM-IV criteria for current alcohol dependence, (b) ability to understand and provide written informed consent, (c) abstinence from alcohol for at least 3 days prior to randomization and resolution of any withdrawal symptoms, (d) desire to achieve abstinence or to reduce alcohol consumption, (e) evidence of a stable residence and (f) proof of an individual who could locate subject if needed. Exclusion criteria were: (a) clinically significant medical diseases that might interfere with the evaluation of the study medication or present a safety concern (e.g. kidney impairment, unstable hypertension, hypotension, diabetes mellitus, seizure disorder), (b) clinically significant psychiatric illness (e.g. psychotic disorder, bipolar disorder, severe depression), suicidal ideation or concurrent substance use disorder other than nicotine or cannabis, (c) concurrent use of any psychotropic medication (subjects who had been on a stable dose of selective serotonin reuptake inhibitors (SSRIs) for 2 months were still considered eligible), (d) concurrent use of anticonvulsants, insulin or oral hypoglycemic, (e) women who were pregnant or breastfeeding, (f) participation in any clinical trial within the last 60 days, (g) use of alcohol pharmacotherapy within the last 60 days and (h) court-mandated participation in alcohol treatment or pending incarceration.
Procedure
The consort diagram for the flow of participants is shown in Fig. 1. The treatment procedure and frequency of assessments were explained to all eligible individuals and a study information sheet was provided. Prior to full screening, individuals read and signed the informed consent. Screening procedures included a medical history and physical examination. Laboratory evaluations included bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, gamma-glutamyl transferase (GGT), sodium, potassium, chloride, blood urea nitrogen, creatinine, glucose, urinalysis, urine toxicology and human chorionic gonadotropin (β-HCG). Patients who had significant withdrawal symptoms with a Clinical Institute Withdrawal Assessment for alcohol (CIWA-Ar) (Sullivan et al., 1989) score of >10 were referred for detoxification treatment as appropriate. Participants then completed the baseline interview and self-report inventories. If eligible, participants were then scheduled for the initial treatment visit within 1 week.
Fig. 1.
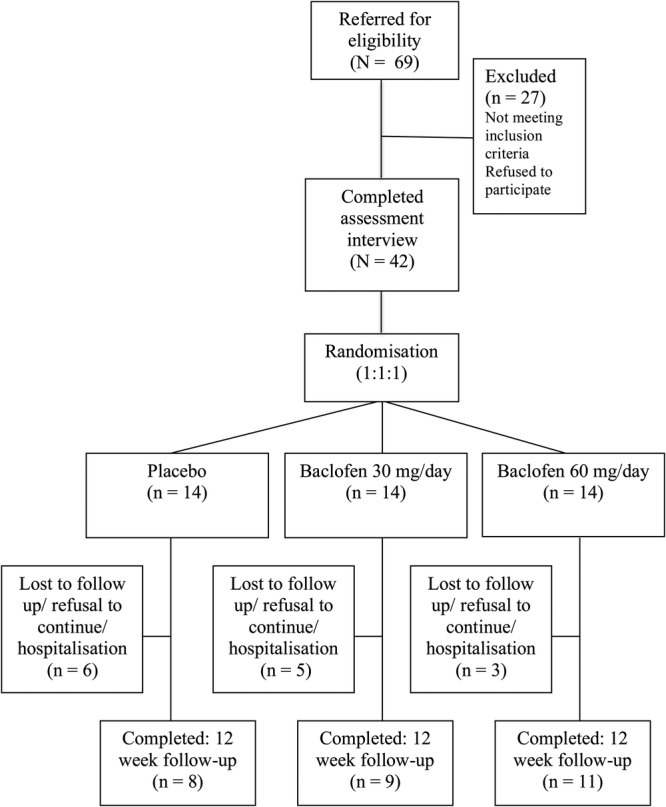
Flow of participants through the trial.
Participants were allocated 1:1:1 as per a computer-generated randomization sequence conducted by the hospital clinical trials pharmacist. Randomized participants received upward and downward titrations of medication for the 84 days of treatment. Specifically, participants in the (a) baclofen 30 mg/day group took a dose of 5 mg, three times a day, for the first 3 days, a dose of 10 mg, three times a day, on Days 4–81, and finally a dose of 5 mg, three times a day, for the last 3 days; (b) baclofen 60 mg/day group took a dose of 5 mg, three times a day, for the first 3 days, a dose of 10 mg, three times a day on Days 4–7, a dose of 20 mg, three times a day, on Days 8–77, a dose of 10 mg three times a day on Days 78–81, and finally a dose of 5 mg, three times a day, for the last 3 days. The placebo pills, which were identical in appearance, were also titrated upward and downward to maintain the double blind. These upward and downward titrations of baclofen have been shown to reduce the likelihood of side effects (Addolorato et al., 2002). Subjects who experienced moderate side effects had their dose reduced according to physician judgment. Medication compliance, alcohol consumption and side effects were collected with diaries. Participants were medically monitored for adverse events and prescribed the study medication on a weekly (Weeks 0–4) and then biweekly (Weeks 4–12) basis.
Participants also received up to nine 30-min BRENDA therapy sessions. BRENDA is a structured psychosocial therapy for alcohol dependence that seeks to improve motivation for change, enhance strategies to prevent relapse and encourage compliance with treatment (Volpicelli et al., 2001). BRENDA sessions were delivered by a counselor who was trained and supervised by an experienced clinical psychologist. Individuals who wished to stop the medication were still encouraged to complete the study and receive BRENDA sessions.
Assessments
At the first visit the following assessments were administered: Recent (last 90 days) alcohol consumption was assessed by the alcohol time line follow-back method (TLFB) (Sobell et al., 1988); severity of alcohol dependence by the Alcohol Dependence Scale (Skinner and Allen, 1982); craving for alcohol measured by the Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999) and the Obsessive Compulsive Drinking Scale (OCDS) (Anton, 2000); and anxiety with the Spielberger Trait and State Anxiety Inventory (Spielberger, 1983). In addition, trained interviewers conducted a structured psychiatric diagnostic interview using the Mini International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998). The TLFB, PACS, STAI State and OCDS were performed at each visit (10 visits following the initial screening visit: weekly for Weeks 0–4 and then bi-weekly for Weeks 4–12 with an additional follow-up for drinking outcomes at Week 16).
Outcome measures
The primary outcome variables were: (a) time to relapse defined as ≥4 standard drinks on a single occasion for women, ≥5 standard drinks for men (standard drinks defined as 10 g of alcohol); (b) time to consumption of any alcohol (lapse); (c) self-reported amount of alcohol consumed expressed as the number of average drinks per drinking day, number of abstinent days, number of heavy drinking days (defined as ≥4 drinks for women, ≥5 drinks for men) (see Addolorato et al., 2011). Secondary outcomes included improvement in cravings for alcohol and clinically evident state anxiety detected at baseline. In addition, subjects were categorized into two groups depending on the absence or presence of an anxiety disorder according to the M.I.N.I. and subsequently analyzed for treatment effects on primary outcomes.
Statistical analyses
Baseline differences between the treatment groups were determined using analysis of variance (ANOVA) for continuous variables and the χ2 test for categorical and non-normally distributed variables. The main efficacy analyses for longitudinal alcohol use were conducted using the intention-to-treat (ITT) principle following the BMJ & Consort guidelines including all participants who took one dose of medication (Schulz et al., 2010). Although standard follow-up procedures were implemented, loss to follow-up and missing data occurred. A time survival model was employed to assess ITT differences with respect to dropouts (Ten Have et al., 1998). The χ2 test were employed to detect any significant differences on side-effect profile, treatment retention and medication compliance rates. Survival analyses (Kaplan–Meier estimates and log-rank test) were conducted to examine the effect of treatment on length of time to relapse and lapse whereby missing data were taken to have relapsed to baseline drinking levels. Mixed models were employed to determine differences between the three treatment groups on self-reported alcohol use (drinks per drinking day) and the psychological measures (STAI, OCDS; 10 time points: baseline-week 12). The treatment by time interaction examines whether treatment leads to a difference in the rate of change in the dependent variable and was the main effect of interest in the analyses. In the case of overall significant treatment × time differences, we examined the change scores from baseline using ANOVA with Bonferroni correction to determine where the treatment differences occur. Secondary survival analyses (Kaplan–Meier estimates and log-rank test) were also carried out on an intention-to-treat basis on time to lapse and relapse stratified by presence or absence of an anxiety disorder. When significant group effects were found, pairwise post hoc comparisons were made. Power was calculated for a large international trial based on an expected effect size (r = 0.94–0.95) as reported in studies at the time prior to study commencement (Addolorato et al., 2002, 2007) but was not calculated a priori for this local site sample size. All analyses were conducted with significance level at P < 0.05. Data were analyzed using SPSS 21 for Macintosh.
RESULTS
Sample characteristics
Of the 42 patients recruited, 14 were randomized to receive placebo, 14 to receive 30 mg/day baclofen and 14 to receive 60 mg/day baclofen (Fig. 1). One patient was excluded from the analysis post-randomization given that no baseline data were obtained. There were no significant differences between treatment groups at baseline (see Table 1). However, there was a significant trend for differences between treatment groups on baseline OCDS subscales despite randomization. We therefore conducted a sensitivity analysis placing this variable as a covariate in primary and secondary outcome analyses; however, this yielded no significant differences in the results. Overall, the mean age was 46.85 and 64% male.
Table 1.
Baseline characteristics
| Placebo (n = 14) | Baclofen 30 mg/day (n = 14) | Baclofen 60 mg/day (n = 14) | P-value | |
|---|---|---|---|---|
| Age, years | 46.00 (39.48–52.52) | 47.86 (42.18–53.54) | 46.64 (39.83–53.46) | 0.90 |
| Sex, % F | 36% | 50% | 79% | 0.17 |
| Drinks per drinking day | 14.30 (8.11–20.50) | 15.55 (8.21–16.88) | 15.15 (8.48–21.82) | 0.78 |
| Heavy drinking days per week | 4.31 (2.86–5.76) | 4.72 (3.33–6.11) | 3.98 (2.58–5.37) | 0.76 |
| Alcohol withdrawal symptoms (CIWA-Ar) | 0.73 (0.01–1.47) | 4.33 (0.16–8.83) | 2.25 (0.28–4.22) | 0.18 |
| Alcohol Dependence Scale (ADS) score | 17.92 (12.79–23.05) | 19.00 (11.98–26.02) | 17.14 (13.58–20.70) | 0.87 |
| PACS craving score | 15.23 (11.08–19.38) | 18.41 (15.41–21.43) | 15.29 (11.26–19.31) | 0.37 |
| Spielberger State Trait Anxiety Inventory (STAI) | ||||
| State Anxiety | 36.62 (30.68–42.55) | 42.23 (33.39–51.07) | 35.93 (30.33–41.53) | 0.32 |
| Trait Anxiety | 43.08 (35.71–50.44) | 46.92 (40.20–53.65) | 39.50 (33.79–45.21) | 0.23 |
| Obsessive Compulsive Drinking Scale (OCDS) | ||||
| Obsessive Subscale | 7.00 (4.10–9.90) | 9.13 (6.54–11.71) | 5.44 (3.90–6.99) | 0.05 |
| Compulsive Subscale | 12.50 (7.50–17.50) | 16.63 (13.87–19.83) | 12.00 (8.90–15.10) | 0.10 |
| Total OCDS | 19.50 (12.17–26.83) | 25.75 (20.98–30.52) | 17.44 (12.98–21.91) | 0.06 |
| Median serum GGT, U/l, (N < 60) | 61.0 | 45.5 | 64.0 | 0.85 |
| Serum ALT, U/l (N < 55) | 47.15 (20.06–74.24) | 31.42 (22.89–39.94) | 69.8 (9.81–129.79) | 0.25 |
Data represent mean and 95% confidence intervals.
CIWA-Ar, Clinical Institute Withdrawal Assessment for alcohol; PACS, Penn Alcohol Craving Scale; GGT, gamma-glutamyl transpeptidase; ALT, alanine aminotransferase.
Subject retention and treatment compliance
Twenty-eight (67%) patients out of the enrolled 42 completed the follow-up interviews (up to Week 12–16). Nineteen patients (45%) completed the entire treatment protocol (receipt of all scripts and BRENDA sessions). Eighty-five percent of subjects showed 80% compliance (67 out of 84 days) and the mean length of time of medication out of 84 days was 71.8 days for placebo, 73.8 days for 30 mg/day baclofen and 70.9 days for 60 mg/day baclofen. According to intention-to-treat, the mean length of time on medication was 43.67 days for subjects randomized to placebo (n = 14), 47.83 for those randomized to 30 mg/day baclofen (n = 14) and 55.83 for those randomized to 60 mg/day baclofen (n = 14). Subjects discontinued the treatment protocol either due to relapse, hospital admission or inability or refusal to attend follow-up. The median number of BRENDA sessions completed was 2.5 for placebo, 3 for baclofen 30 mg/day and 3.5 for baclofen 60 mg/day. There were no significant differences between groups on study retention (χ2 = 1.5, P = 0.47) or medication compliance rates (χ2 = 0.77, P = 0.68), the number of BRENDA sessions attended (F = 1.93, P = 0.826) or days on medication (F = 1.04, P = 0.36). Time-survival analysis revealed that the treatment retention rates did not significantly differ between randomized groups over the follow-up assessments (χ2 = 1.21, P = 0.55).
Safety and tolerability
The profile of side effects reported by subjects is shown in Table 2. No serious or severe side effects leading to drug cessation were observed and no patients discontinued treatment because of side effects. Regardless of medication, the most commonly reported side effect was drowsiness (19%). There were no significant differences between treatment groups with respect to the number of subjects experiencing each side effect listed (P's < 0.15). No new side effects were observed at drug discontinuation.
Table 2.
Adverse event profile of the study participants
| Adverse eventa | Placebo, n (%) | Baclofen 30 mg/day, n (%) | Baclofen 60 mg/day, n (%) |
|---|---|---|---|
| Nil reported | 8 (57) | 10 (71) | 10 (71) |
| Somnolence/drowsiness | 3 (21) | 2 (14) | 3 (21) |
| Headaches | 1 (14) | 3 (21) | – |
| Fatigue | 1 (7) | 1 (7) | 2 (14) |
| Muscle aches | 1 (7) | 1 (7) | – |
| Constipation | – | 1 (7) | 1 (7) |
| Insomnia | 1 (7) | – | – |
| Dizziness | – | 1 (7) | – |
| Numbness in extremities | – | – | 1 (7) |
| Dry mouth | – | 1 (7) | 1 (7) |
| Nausea | – | 1 (7) | – |
| Anxiety | 1 (7) | – | – |
| Skin rash | – | – | 1 (7) |
aOne patient randomized to 10 mg t.i.d. experienced constipation, sedation, drowsiness and headaches while another patient randomized to 20 mg t.i.d. experienced drowsiness, fatigue and numbness in the extremities. However, following halving of the dose, both of these patients reported that these symptoms disappeared.
Main efficacy outcomes
Primary efficacy outcomes are listed in Table 3. Survival analysis revealed no significant difference between treatment groups in the number of days to relapse, although there was a trend toward significance in favor of baclofen (χ2 = 5.05, P = 0.08). Survival analysis revealed no significant difference between treatment groups in the number of days to first lapse (χ2 = 3.48, P = 0.18). Mixed models over the treatment period (10 time intervals) revealed an effect of time (F9,46.51 = 6.15, P < 0.0001) but no effect for treatment (F2,45.30 = 0.10, P = 0.91) nor treatment × time (F18,46.90 = 0.63, P = 0.86) for number of heavy drinking days during the previous week. For drinks per drinking day there was an effect of time (F9,49.97 = 4.05, P < 0.001) but no effect for treatment (F2,37.20 = 0.39, P = 0.68) nor treatment × time (F18,49.53 = 1.02, P = 0.45). For days abstinent during the previous week, there was an effect of time (F9,50.12 = 5.22, P < 0.0001) but no effect for treatment (F2,48.50 = 1.19, P = 0.31) nor treatment × time (F18,49.52 = 1.60, P = 0.10). Thus, alcohol consumption significantly decreased over the duration of the treatment period (time) but not at a significantly different rate between the three groups (treatment × time). At the 16 week follow-up (4 weeks post-treatment completion), ANOVA revealed that there were no significant differences between treatment groups for the number of heavy drinking days (F2,38 = 1.43, P = 0.26) nor for the drinks per drinking day (F2,38 = 1.72, P = 0.20) during the previous week.
Table 3.
Intention to treat outcomes
| Placebo (n = 14) | Baclofen 30 mg/day (n = 14) | Baclofen 60 mg/day (n = 14) | |
|---|---|---|---|
| Primary outcomes | |||
| Days to lapse+ | 3.14 (1.90–4.39) | 13.14 (2.79–23.49) | 17.64 (3.45–31.84) |
| Days to relapse+ | 7.07 (2.37–11.77) | 23.79 (9.62–37.95) | 19.17 (4.91–34.52) |
| Drinks per drinking dayx | 2.82 (0.01–5.65) | 5.86 (2.80–8.92) | 5.64 (3.20–8.08) |
| Heavy drinking days per weekx | 1.36 (0.32–3.04) | 2.07 (0.26–3.88) | 1.89 (0.43–3.34) |
| Secondary outcomes: | |||
| STAI State Anxietyx | 32.44 (22.59–42.29) | 33.18 (24.13–42.22) | 36.61 (28.24–44.98) |
| OCDS Obsessivex,* | 4.66 (2.20–7.12) | 4.08 (1.63–6.52)c | 4.47 (2.53–6.42) |
| OCDS Compulsivex | 6.98 (2.70–11.26) | 6.93 (2.67–11.19) | 8.22 (4.87–11.56) |
| Stratified for comorbid anxietyxx | |||
| Days to lapse+,** | |||
| Absence of comorbid anxiety | 3.57 (1.31–5.83) | 5.29 (0.00–13.36) | 15.27 (0.00–30.78) |
| Presence of comorbid anxiety | 2.71 (1.53–3.90) | 21.00 (3.12–38.88)a | 26.33 (0.00–65.70) |
| Days to relapse+,** | |||
| Absence of comorbid anxiety | 9.14 (0.00–18.36) | 17.14 (0.00–37.63) | 15.09 (0.56–29.62) |
| Presence of comorbid anxiety | 5.00 (2.70–7.30) | 30.43 (10.68–50.18)a | 36.67 (0.00–33.10)b |
Data represent mean and 95% confidence intervals.
STAI, Spielberger State Trait Anxiety Inventory; OCDS, Obsessive Compulsive Drinking Scale.
+Data derived from survival analyses whereby lost to follow-up and dropout were taken as relapsed to baseline drinking levels at the last appointment attended.
xWeek 12 means from mixed linear models over 10 time points during treatment.
xxPatients were categorized into two groups according to the absence (n = 25) or presence (n = 17) of an anxiety disorder as per the Mini International Neuropsychiatric Interview.
*P < 0.05, Mixed Model treatment × time interaction.
** = P < 0.01, Kaplan–Meier survival analysis.
aP < 0.01 30 mg/day baclofen versus placebo.
b = P < 0.05 60 mg/day baclofen versus placebo.
cP < 0.05 30 mg/day baclofen versus 60 mg/day baclofen.
Secondary outcomes
Secondary outcomes are depicted in Table 3. Mixed models revealed that, for STAI state anxiety, there was no effect of time, treatment nor treatment × time (P's > 0.45 = 0.82). For OCDS obsessive craving, there was an effect of time (F9,89.16 = 5.07, P < 0.0001) and treatment × time (F18,86.71 = 1.93, P < 0.05), but not for treatment (P = 0.60). For OCDS compulsive craving and total OCDS scores, there was an effect of time (F9,18.55 = 7.17, P < 0.0001; F9,27.83 = 4.73, P < 0.0001 respectively) but no effect for treatment or treatment × time (P's > 0.30). Thus, while obsessive and compulsive craving significantly decreased over the duration of the study period, there were significant treatment differences in the rate of change in obsessive but not compulsive craving during this time. ANOVA of change scores revealed a significant effect for OCDS obsessive craving baseline to follow-up change scores (F2,38 = 4.91, P < 0.05), with the 30 mg/day baclofen group demonstrating significantly greater change relative to the 60 mg/day baclofen group (P < 0.05) but not placebo (P = 0.15).
Stratification by presence of comorbid anxiety
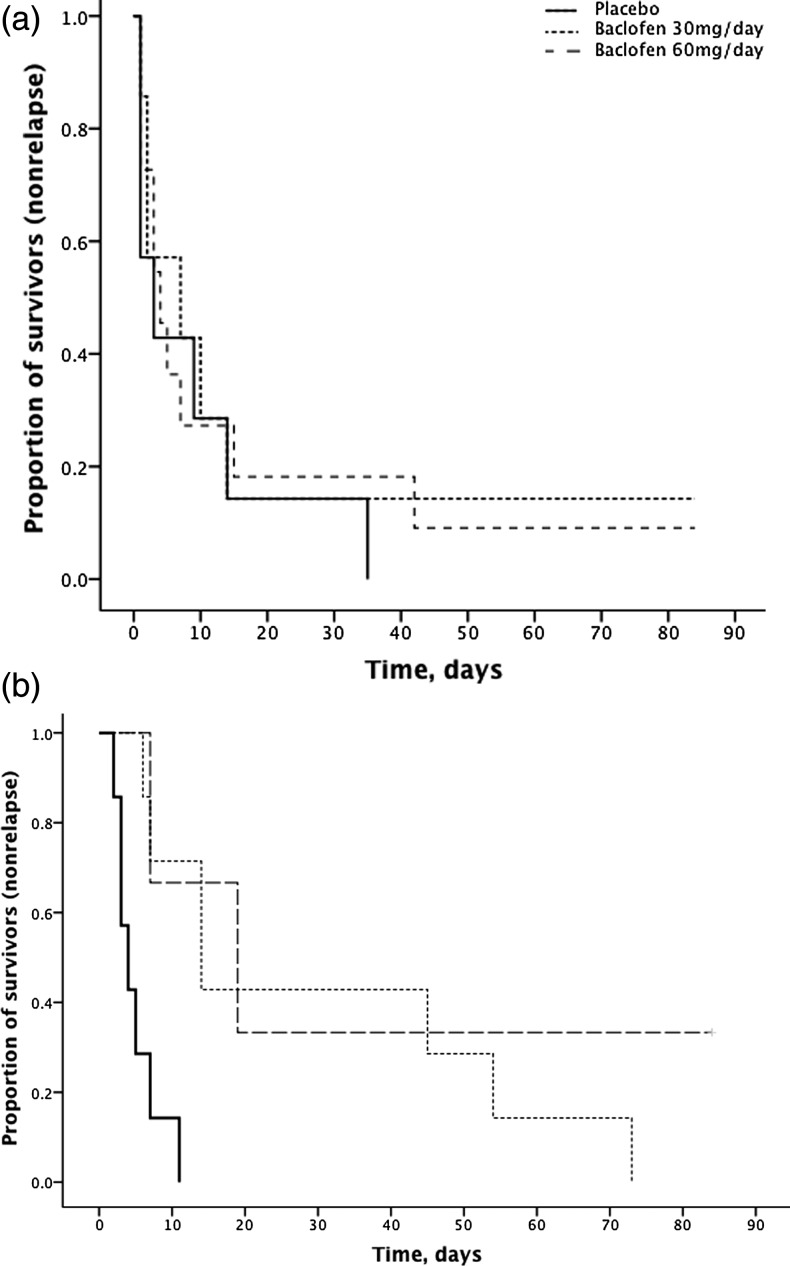
The M.I.N.I. diagnostic interview revealed that 41% (n = 17: placebo = 7, baclofen 10 mg = 7, baclofen 30 mg = 3) of the sample had a lifetime or current anxiety disorder (generalized anxiety disorder, social phobia, obsessive disorder, agoraphobia, panic disorder and/or PTSD). Data are depicted in Table 3. In a post hoc analysis, we found that subjects with anxiety comorbidity experienced a significant treatment effect in the number of days to lapse (χ2 = 6.48, P < 0.05) and relapse (χ2 = 12.43, P < 0.01). For lapse, significant differences emerged between baclofen 30 mg/day and placebo (χ2 = 7.04, P < 0.01). For relapse, significant differences emerged between baclofen 30 mg/day and placebo (χ2 = 9.10, P < 0.01) and baclofen 60 mg/day and placebo (χ2 = 4.55, P < 0.05). For alcohol patients with no anxiety comorbidity, there were no significant treatment effects in the number of days to lapse or relapse (P's > 0.43) (Fig. 2).
Fig. 2.
Survival curve for time to relapse for participants randomized to either placebo, baclofen 30 mg/day or baclofen 60 mg/day for 12 weeks stratified for (a) ‘no comorbid anxiety’ (n = 25) and (b) ‘comorbid anxiety’ (n = 17). Intention-to-treat analysis. In participants with ‘comorbid anxiety’, significant treatment effects (χ2 = 12.43, P < 0.01) emerged between baclofen 30 mg/day and placebo (χ2 = 9.10, P < 0.01) and baclofen 60 mg/day and placebo (χ2 = 4.55, P < 0.05). Patients were categorized into two groups according to the absence or presence of an anxiety disorder (generalized anxiety disorder, social phobia, obsessive disorder, agoraphobia, panic disorder and/or PTSD) as per the Mini International Neuropsychiatric Interview conducted at baseline.
DISCUSSION
This study explored the effects of two doses of baclofen in the treatment of alcoholic patients. Overall, alcohol consumption significantly decreased across the 3-month study protocol regardless of treatment group. There were no significant effects of treatment on the primary outcome of time to lapse while a trend was observed for low-dose baclofen to reduce the time to relapse. There were no treatment effects on other drinking outcomes such as standard drinks per drinking day and heavy drinking days. The minimal effect in the current results of baclofen in improving drinking outcomes relative to placebo is consistent with Garbutt et al. (2012) but somewhat inconsistent with the Italian studies which have found higher rates of abstinence and reduced alcohol intake in baclofen-treated patients (Addolorato et al., 2002, 2007).
Interestingly, the current results suggest that the baclofen treatment response on alcohol outcomes was most effective in patients with comorbid anxiety: there was a significant effect for baclofen to increase time to lapse and time to relapse in these patients.
It has been proposed that variations in severities of dependence may explain previous conflicting results with baclofen treatment (Addolorato et al., 2002, 2011; Garbutt et al., 2012) and that baclofen may be more effective in those alcohol-dependent patients with increased levels of anxiety (Leggio et al., 2010). This suggests that the presence of anxiety is an indicator of severity of dependence. However, it is important to note that the level of dependence severity and alcohol consumption was high in the current study similar to previous positive studies (Addolorato et al., 2002, 2011) yet no significant effect of baclofen was observed in the overall sample. Accordingly, it is possible that the mechanism by which baclofen was effective in improving alcohol outcomes specifically for patients with comorbid anxiety may encompass clinical constructs of addiction that are supplementary or distinct to dependence severity.
It has recently been suggested that potential effects of baclofen to reduce measures of alcohol consumption in patients affected by comorbid anxiety may be due, at least in part, to a relief of anxiety symptoms (Agabio and Colombo, 2014). Previous studies have shown the efficacy of GABAB subtype receptor agonists, including baclofen, in alleviating anxiety in patients with PTSD (Drake et al., 2003), panic disorder (Breslow et al., 1989; Manteghi et al., 2014) and alcohol-dependent patients (Breslow et al., 1989; Krupitsky et al., 1993; Addolorato et al., 2002; Flannery et al., 2004; Garbutt et al., 2012). Anxiety symptoms often disappear rapidly after detoxification for some patients (Allan et al., 2002) while persisting severe anxiety symptoms may lead to an increased risk of relapse (Driessen et al., 2001). Willinger et al. (2002) demonstrated high trait anxiety to be of significant predictive value for relapse to drinking. Similarly, previous reports have found that, during treatment for alcohol dependence, people with comorbid anxiety disorder experience shorter time to relapse and greater long-term alcohol consumption (Cornelius et al., 1997; Greenfield et al., 1998; Haver, 2003).
Although the neurobiological mechanisms that underlie anxiety are not fully understood, studies have shown that dysfunction in limbic areas such as the amygdala and prefrontal interconnections such as the orbitofrontal and medial prefrontal cortex might be responsible (Cannistraro and Rauch, 2003; Milad and Rauch, 2007). Baclofen's action on these structures (Franklin et al., 2012; Young et al., 2014) could therefore serve to restore this function in alcohol patients with high levels of persisting anxiety, areas also hyper-responsive to drug cues (Franklin et al., 2012), which may dampen subjective expression of craving during stress and consequently risk of relapse. Preclinical studies have found that the actions of baclofen are modulated by anxiety status (high anxiety versus low anxiety rats) whereby high anxiety rats are particularly responsive to baclofen-induced locomotor behavioral changes (Falco et al., 2014). A larger sample size is required to further elucidate the interactive relationship between comorbid anxiety disorders, baclofen treatment and alcohol consumption.
Limitations of the current study are the small sample size, limited power and low treatment retention rate. Given that we found a trend for a significant effect of baclofen in increasing time to relapse in line with our significant effect of baclofen in reducing craving, it is possible that a larger sample size and higher treatment retention rate would yield an effect on primary drinking outcomes. However, power was calculated based on an expected large effect size (r = 0.94–0.95) as reported in the published studies at the time prior to commencement of the study (Addolorato et al., 2002, 2007) and indeed we were able to detect large effect sizes for the beneficial treatment effect of baclofen relative to placebo in reducing craving in the sample overall and also in reducing lapse and relapse in those patients with comorbid anxiety.
In summary, the current results found a minimal effect of baclofen on drinking outcomes in the overall sample of alcohol-dependent patients with or without anxiety. The study does, however, support the efficacy of baclofen in the relapse prevention of alcoholism among patients with anxiety comorbidity, a treatment effect that is clinically important and worthy of a fully powered study.
Funding
This trial was funded by a New South Wales Health Drug and Alcohol grant (PH, AB, KM).
Conflict of interest statement
None declared.
REFERENCES
- Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des. 2010;16:2113–7. doi: 10.2174/138161210791516440. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomised controlled study. Alcohol Alcohol. 2002;37:504–08. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Abenavoli L, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006;119 doi: 10.1016/j.amjmed.2005.08.042. 276.e13–8. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–22. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46:312–7. doi: 10.1093/alcalc/agr017. [DOI] [PubMed] [Google Scholar]
- Agabio R, Colombo G. GABAB receptor ligands for the treatment of alcohol use disorder: preclinical and clinical evidence. Front Neuro Sci. 2014;8:140. doi: 10.3389/fnins.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan C, Smith I, Mellin M. Changes in psychological symptoms during ambulant detoxification. Alcohol Alcohol. 2002;37:241–44. doi: 10.1093/alcalc/37.3.241. [DOI] [PubMed] [Google Scholar]
- Anton RF. Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95:211–7. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- Breslow MF, Fankhauser MP, Potter RL, et al. Role of gamma-aminobutyric acid in antipanic drug efficacy. Am J Psychiatry. 1989;146:353–6. doi: 10.1176/ajp.146.3.353. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37:8–25. [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, et al. Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–14. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics. A double-blind, placebo-controlled trial (see comments) Arch Gen Psychiatry. 1997;54:700–5. doi: 10.1001/archpsyc.1997.01830200024004. [DOI] [PubMed] [Google Scholar]
- Drake RG, Davis LL, Cates ME, et al. Baclofen treatment for chronic posttraumatic stress disorder. Ann Pharmacother. 2003;37:1177–81. doi: 10.1345/aph.1C465. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, et al. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–55. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Evans SM, Bisaga A. Acute interaction of baclofen in combination with alcohol in heavy social drinkers. Alcohol Clin Exp Res. 2009;33:19–30. doi: 10.1111/j.1530-0277.2008.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco AM, McDonald CG, Smith RF. Anxiety status affects nicotine- and baclofen-induced locomotor activity, anxiety, and single-trial conditioned place preference in male adolescent rats. Dev Psychobiol. 2014;56:1352–64. doi: 10.1002/dev.21217. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati H. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;28:1289–95. [PubMed] [Google Scholar]
- Flannery BA, Garbutt JC, Cody MW, et al. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–23. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Shin J, Jagannathan K, et al. Acute baclofen diminishes resting baseline blood flow to limbic structures: a perfusion fMRI study. Drug Alcohol Depend. 2012;125:60–6. doi: 10.1016/j.drugalcdep.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2012;34:1849–57. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, et al. The effect of depression on return to drinking. Arch Gen Psychiatry. 1998;55:259–65. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Haver B. Comorbid psychiatric disorders predict and influence treatment outcome in female alcoholics. Eur Addict Res. 2003;9:39–44. doi: 10.1159/000067735. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Ivanov VB, et al. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug Alcohol Depend. 1993;33:157–63. doi: 10.1016/0376-8716(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets. 2010;9:33–44. doi: 10.2174/187152710790966614. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37:561–4. doi: 10.1016/j.addbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, McGeary JE, et al. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav. 2013;103:784–91. doi: 10.1016/j.pbb.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon JE, Khan RA, Gessert CE, et al. Treating alcohol withdrawal with oral baclofen: a randomized, double-blind, placebo-controlled trial. J Hosp Med. 2011;6:469–74. doi: 10.1002/jhm.928. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Colombo G. Role of the GABA(B) receptor in alcohol-seeking and drinking behavior. Alcohol. 2009;43:555–8. doi: 10.1016/j.alcohol.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Manteghi AA, Hebrani P, Mortezania M, et al. Baclofen add-on to citalopram in treatment of posttraumatic stress disorder. J Clin Psychopharmacol. 2014;34:240–3. doi: 10.1097/JCP.0000000000000089. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–61. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–65. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Br Med J. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GL, et al. Reliability of a timeline followback method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–57. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Ten Have TR, Kunselman AR, Pulkstenis EP, et al. Mixed effects logistic regression models for longitudinal binary response data with informative drop-out. Biometrics. 1998;54:367–83. [PubMed] [Google Scholar]
- Volpicelli JR, Pettinati HM, McLellan AT, et al. Combining Medication and Psychosocial Treatments for Addictions: the BRENDA Approach. New York: Guilford; 2001. [Google Scholar]
- Willinger U, Lenzinger E, Hornik K, et al. Anxiety as a predictor of relapse in detoxifed alcohol-dependent patients. Alcohol Alcohol. 2002;37:609–12. doi: 10.1093/alcalc/37.6.609. [DOI] [PubMed] [Google Scholar]
- Young KA, Franklin TR, Roberts DC, et al. Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J Neurosci. 2014;34:5038–43. doi: 10.1523/JNEUROSCI.4977-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]