Abstract
BACKGROUND
Clinical studies indicate that blood pressure (BP)-lowering effects of radiofrequency (RF) renal denervation (RD) are sustained for up to 2 years, although a recent clinical trial failed to find a major effect compared to sham treatment. In most previous studies, the efficacy of RD has not been assessed. The current study determined whether RD in different regions of the renal artery causes different degrees of RD as assessed with renal norepinephrine (NE) levels.
METHODS AND RESULTS
Unilateral RD was performed on 14 pigs divided into 3 groups: RD near the ostium, in the main renal artery near the bifurcation, and in extrarenal branches of the renal artery. After 2 weeks post-RD, the pigs were euthanized, renal cortex tissue was collected for NE measurement, and renal arteries were prepared for histological analysis. Renal NE decreased by 12% with RD at the ostium, 45% with RD near the bifurcation in the main renal artery, and 74% when RD was performed in extrarenal artery branches. The number of renal nerves was greatest in extrarenal branches and in the main artery compared to the ostium and the average distance from the lumen was greatest for nerves at the ostium and least at the branches.
CONCLUSIONS
RF RD lowers renal NE more significantly when performed in branches of the renal artery closer to the kidney. Increased efficacy of RF RD in extrarenal arterial branches may be due to the greater number of nerves in close proximity to the artery lumen in the branches.
Keywords: blood pressure, hypertension, norepinephrine, radiofrequency ablation, renal denervation, renal nerves.
Catheter-based radiofrequency (RF) renal denervation (RD) has been used in clinical trials to treat patients whose hypertension is not normalized with pharmaceutical treatment. Patients treated with EnligHTN RD system have been shown to have an average decrease in office blood pressure (BP) of −27mm Hg systolic and −11mm Hg diastolic and ambulatory BP reduction of −7/−4mm Hg after 1 year of follow-up.1 However, a recent large clinical trial, Symplicity HTN-3, failed to demonstrate a major fall in BP after RF RD in patients with resistant hypertension2; various explanations for the lack of efficacy of RD have been proposed to explain these surprising findings, including the efficacy of the RD procedure.
In most previous studies of RF RD, the efficacy of denervation has not been verified. Renal norepinephrine (NE) spillover has been used in a small number of human studies to determine efficacy of RF RD, while renal tissue NE reduction is sometimes used in animal studies. We recently showed that catheter-based RD in obese dogs decreased renal NE content by 42%.3 Krum et al. 4 have shown that catheter-based RD leads to a 47% decrease in renal NE spillover following RD. For RF RD to be effective, it is critical to have sufficient RF energy delivered to the appropriate sites along the renal artery. However, verification of the efficacy of RD has not been adequately tested in most previous studies.
The most common locations used to deliver RF energy to the renal nerves are across the main renal artery, prior to any branching. While this approach may cause a significant decrease in renal NE, it is not known if the main renal artery is the most efficient site for RF-based RD to lower renal tissue NE and cause effective denervation of the kidney. Thus, the current study was conducted to determine if performing catheter-based RD near the ostium, in the main renal artery near the bifurcation, or in the extrarenal arterial branches would have the greatest effect on renal NE levels, a reliable index of functional denervation of the kidneys.
METHODS
All procedures involving animals were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee. Pigs underwent unilateral catheter-based RD so that the contralateral kidney could serve as a control. The pigs were preanesthetized with acepromazine and atropine and then anesthetized with telazol and xylazine. After placement of an endotracheal tube, isoflurane anesthesia was administered for the duration of the surgical procedure. An 8F JR4 guide catheter was placed in the femoral artery and advanced to the level of the renal arteries using angiography. Once in the renal artery an image was taken, the guide catheter was withdrawn from that renal artery and placed in the contralateral renal artery. If there were no anatomic anomalies, the kidney to be denervated was chosen at random. In addition, the location of the denervation along the renal artery was chosen at random. RD was performed at the ostium (n = 4), in the main renal artery near the bifurcation (n = 5), or within the 2 main extrarenal branches of the renal artery (n = 5, Figure 1A).
Figure 1.
Areas of denervation along the renal artery. Locations used for RD (A) and the approximate areas defined for the histological analysis (B). Abbreviation: RD, renal denervation.
Catheter-based RD was performed using the EnligHTN System (St. Jude Medical) that consists of an expandable basket with 4 electrodes to deliver the RF energy. The RF energy was applied for 90 seconds per electrode in a temperature control mode set to a maximum temperature of 75 °C. When the delivery of the energy was completed, the basket was closed, the denervation catheter was removed, and the guide catheter was advanced into the artery for angiography to assess any vessel reaction. The guide catheter was removed and the incision closed. The pigs were then allowed to recover and euthanized 14 days later.
At the time of euthanasia, kidneys with the attached renal arteries were removed. Six sections of renal cortex were flash frozen from each kidney and stored for renal NE measurements. The frozen pieces of renal cortex were homogenized in glutathione and EDTA buffer, centrifuged to remove cell parts, and the supernatant collected. All steps were performed on ice or in a refrigerated centrifuge. Samples were analyzed for NE by high-performance liquid chromatography.
Histological analyses
The renal arteries were cut into equal transverse sections from the aorta to the kidney, fixed, and embedded in paraffin. This resulted in 6–13 blocks depending on the length of the renal artery. Five-micron sections were taken from all blocks processed. Sections were assigned to 1 of 3 areas: the distal (close to the kidney), the middle, and the proximal (Figure 1B). All sections were stained with hematoxylin–eosin. Sections were examined for the total number of nerves, number of injured nerves, cross-sectional area of the nerve, and the distance measured from nerves to the renal artery lumen–intima interface. The observer was blinded to the source of tissue in the sections.
Statistical analyses
Data are presented as mean ± SEM. Kidney NE content differences were tested with 1-way analysis of variance with Holm–Sidek multiple comparison post hoc test. Histological data for RD animals were compared using 1-way analysis of variance if normally distributed and Kruskal–Wallis 1-way analysis of variance on ranks if the data were not normally distributed. Statistical significance was considered at a value of P < 0.05.
RESULTS
Catheter-based RD was conducted without complications in all animals. Figure 2 shows a typical picture, obtained by fluoroscopy, of the renal artery (Figure 2A), placement of the basket catheter (Figure 2B), vasospasm occurring immediately following RD (Figure 2C), and the resolution of the vasospasm after approximately 20 minutes (Figure 2D).
Figure 2.
Renal arteriograms. Renal arteriograms showing the renal artery with contrast (A), the RD catheter basket seated in the renal artery (B), some vasospasm immediately following the application of the radiofrequency signal (C), and the resolution of the vasospasm (D). Abbreviation: RD, renal denervation.
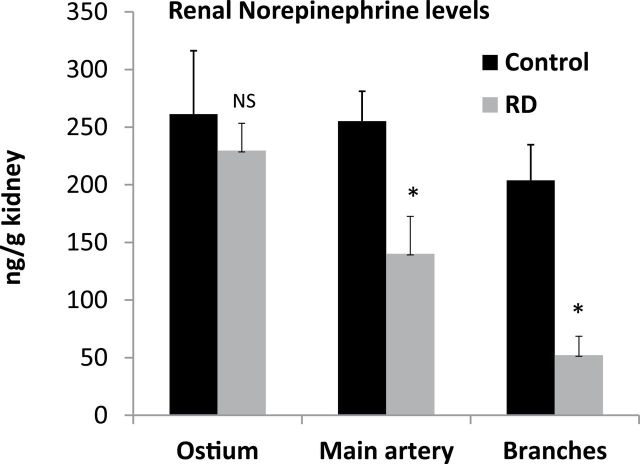
Catheter-based RD resulted in a significant decrease in renal NE content measured by high-performance liquid chromatography regardless of where in the artery the RD took place. However, as shown in Figure 3, RD in the extrarenal branches of the artery resulted in a 74% reduction (P < 0.05) in renal NE compared to 45% reduction (P < 0.05) when RD was performed near the bifurcation in the main renal artery and only 12% reduction (not significant) when performed at the ostium.
Figure 3.
Renal tissue norepinephrine. Renal tissue norepinephrine levels measured from non-denervated kidneys (control) and denervated kidneys (RD). Kidneys were denervated at the ostium, the main artery, or in the branches of the renal artery. *P < 0.05, NS = not significant. Abbreviation: RD, renal denervation.
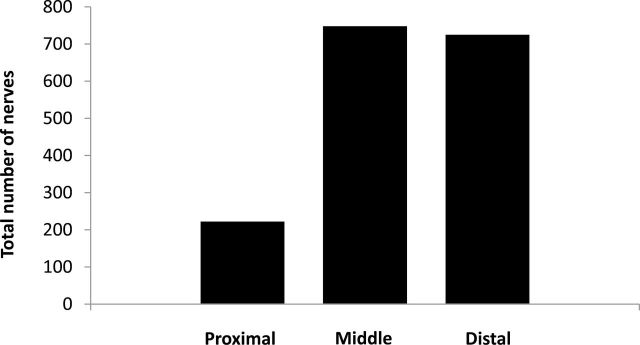
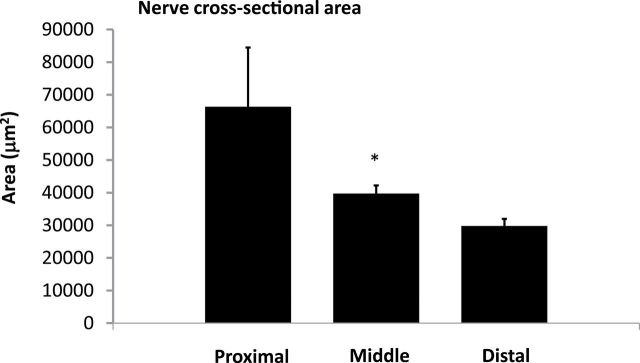
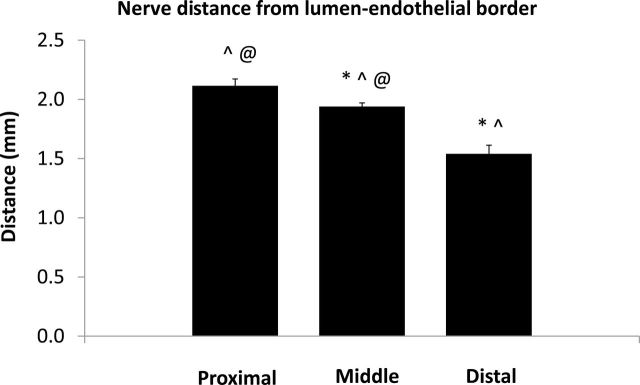
Figure 4 shows the total number of nerves examined from all sections from all animals in the proximal, middle, and distal areas. The most abundant supply of renal nerves was observed in the distal and middle areas of the renal artery and fewer nerves were found in the proximal area. While there were fewer nerves in the proximal area, the proximal nerves tended to be much larger than the nerves found in the middle and distal sections of the renal artery (Figure 5). In addition, the average distance measured from the renal artery endothelial/lumen junction to the renal nerves was greatest in the proximal area and smallest in the distal area of the renal artery (Figure 6).
Figure 4.
Total number of nerves in different sections of the renal artery. Total number of nerves observed in all sections from the proximal, middle, and distal sections of the renal artery.
Figure 5.
Cross-sectional area of nerves in different sections of the renal artery. Nerve cross-sectional area measured in sections coming from the proximal, middle, and distal sections of the renal artery. *P < 0.05 vs. distal.
Figure 6.
Nerve distance from lumen–endothelial border. Nerve distance from the renal artery endothelial–lumen border measured in sections coming from the proximal, middle, and distal areas of the renal artery. *P < 0.05 vs. proximal; ^P < 0.05 vs. middle; @P < 0.05 vs. distal.
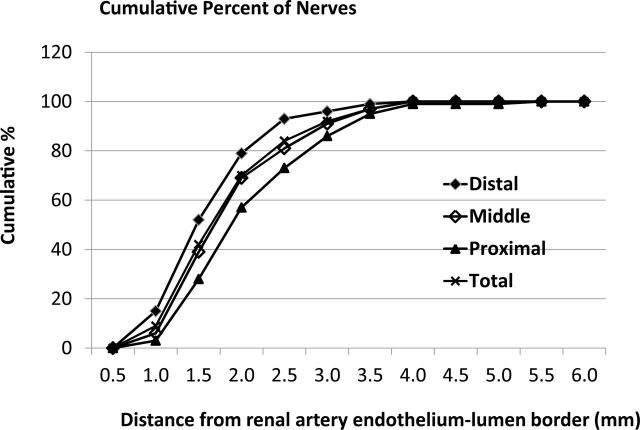
Figure 7 shows the cumulative percent of nerves as a function of distance from the lumen–intima interface. When examined from 0 to 3.0mm from the lumen–intima, there were 86%, 92%, and 96% of nerves included of the proximal, middle, and the distal segments, respectively, implying that a 3.0-mm lesion depth could result in more than 90% nerve injury provided circumferential lesions occurred. Similarly, a 2.5-mm lesion depth could lead to about 73% injury of the nerves in the proximal segment, while it could cause about 84% nerve injury if lesions occurred in the middle section circumferentially. However, up to 93% nerve injury would occur if lesions occurred in the distal segment circumferentially. Over 98% of the nerves examined were within 3.5mm measured from the lumen–intima interface regardless if the sample came from the near the ostium, the middle section, or the extrarenal branches of the renal artery.
Figure 7.
Cumulative percentage of nerves. Cumulative percentage of nerves from the renal artery endothelial–lumen interface for the different areas of sections analyzed.
DISCUSSION
The current study shows that in RF catheter-based RD, the location of energy delivery has an important effect on the efficacy of the denervation. Performing the RD with the catheter electrodes in the extrarenal branches produced the greatest decrease in renal NE, a reliable marker of renal sympathetic nerve denervation, when compared to RD originating in the main renal artery near bifurcation or near the ostium (Figure 3). This study also shows that the renal nerves are unevenly distributed along the length of the renal artery with nerves being larger and further away from the artery in the proximal portion of the renal artery, and smaller and closer to the artery in the distal areas toward the kidney.
Catheter-based RD has been shown to lower BP in patients with resistant hypertension1,5 and in obese dogs.3 The decrease in BP after RF RD is thought to be caused by damage to the renal sympathetic nerves, which, in turn, has multiple effects that tend to lower BP, including reductions in renal tubular reabsorption and decreased renin release. Although only a few studies have assessed the efficacy of RF RD procedures, Esler et al. found that renal NE turnover decreased by 47% in patients immediately following catheter-based RD. In addition, our laboratory has shown that catheter-based RF RD caused a 42% decrease in renal NE levels 8 weeks after RD in obese dogs.3 Both of these studies reported a significant decrease in BP after RD.
Although there have been no previous studies, to our knowledge, that have specifically examined the quantitative relationship between renal NE levels and BP reduction after RF RD, surgical RD appears to produce a greater decrease in BP in obese dogs than catheter-based RD. Studies by Kassab et al. 6 showed that surgical RD, which decreased renal NE by 92%, almost completely prevented the rise in BP in obese dogs. Lohmeier et al. 7 also found that surgical RD almost completely normalized BP in obese dogs with established hypertension. With RF-based RD, we observed a 42% fall in renal NE and a smaller decrease in BP than previously reported with surgical RD, which produced a much greater decrease in renal NE.3 Similar observations have been reported in clinical studies. Id et al. 8 recently reported that patients with accessory renal arteries had less BP reduction following RF-based RD than patients without accessory renal arteries. Thus, it appears that the amount of BP decrease following RF-based RD may be directly related to the efficiency of the denervation procedure.
Most studies in humans and animals using catheter-based RF RD to lower BP have performed the RD in the main renal artery. While RD in this location reduces renal tissue NE levels and renal NE spillover, the main renal artery may not be the optimal site for catheter-based RF RD. The current study suggests that performing catheter-based RD in the extrarenal branches provides a more efficacious treatment since RD in this location lowered renal NE by 74% (Figure 3).
Initially, it was not clear why performing RD in the extrarenal branches of the renal artery would result in a further decrease in renal NE compared to RD performed in the main renal artery near the bifurcation or near the ostium. We hypothesized that the renal nerves in the extrarenal artery branches may be closer to the renal artery lumen and thus closer to the source of RF energy generated by catheter-based RD. In addition, there are only a few studies that have examined the distribution of renal sympathetic nerves along the longitudinal axis of the renal artery.9–12 Thus, we examined histological cross-sections, cut from the entire length of the renal artery, for nerve numbers, size, and distribution. We found that the nerves located in the proximal renal artery histological sections were further away from the endothelium–lumen interface of the artery. Atherton et al. 9 reported that the renal nerves were farthest from the renal artery luminal surface in the proximal regions and got closer to the renal artery in the more distal regions of the artery in human cadavers. In our study in pigs the renal nerves near the extrarenal branches were also closer to the artery lumen than those in the middle section of the main artery (Figure 6). Atherton et al. 9 also reported that the number of renal nerves increased along the length of the renal artery going from the proximal to the distal sections in humans. We also found that there were fewer nerves along the proximal renal artery compared to the middle and distal histological samples of the renal artery. Renal nerves in the proximal sections of the renal artery were also much larger than more distal nerves. There was no significant difference found in the cross-sectional area or the total number of nerves observed in the middle or distal areas (Figures 4 and 5).
The distance of the nerves from the renal artery lumen and the number and size of the nerves in proximal area of the renal artery could explain the minimal decrease in renal NE after performing catheter-based RF RD near the ostium. Atherton et al. 9 reported that approximately 90% of the renal nerves are located within 2.0mm of the endothelial–lumen border of the renal artery. In that study, it appears that the nerves were only examined out to 2.5mm so there may have been nerves that were missed beyond the first 2.5mm. Virmani10 examined human cadaver renal arteries and found that nerves were located up to 8–10mm from the lumen–endothelial surface. The distribution of renal nerves around the renal artery in pigs is similar to what we reported for obese dogs with over 98% of the renal nerves located within 3.5mm of the renal artery lumen–intima interface.
RF energy delivered from a catheter in the renal artery creates heat that penetrates the artery wall and surrounding tissue, causing injury to the nerves. The heat is greatest at the electrode/artery interface and decreases as the energy travels through the tissue. The depth of this penetration is determined by various parameters of the generator, including the wattage and duration of RF energy delivery, and the contact between the electrode and the artery lumen. Thus, with all other factors being equal, the more nerves that are close to the electrode–artery interface, the more nerve injury that will occur from the RF energy that is delivered. While this is true for RF energy, this relationship may not be as predictable for other catheter-based methods of inducing renal nerve renal injury.
When the distance of renal nerves from the renal artery lumen was analyzed, we found that the nerves in middle sections were closer to the renal artery endothelial–lumen interface than those in the proximal segment, while nerves in the distal section were the closest to the endothelial–lumen interface (Figure 7). In fact, in the distal sections, 79% of the nerves were located within 2.0mm from the endothelial–lumen interface compared to about 69% in the middle and 57% proximal sections. This implies that RD could be more effective in the distal section (in the extrarenal branches); this was confirmed by much greater reductions in renal tissue NE when the RF RD procedure was performed in the extrarenal branches in the distal section of the renal artery.
One possible explanation for the increased effectiveness of RD performed in the renal artery branches is the possibility that more of the neurons located at the level of the branches actually go into the kidney and the nerves are the closest to the endothelium–lumen interface. Reddy et al. 13 have shown that the tunica media of the main renal artery has significant sympathetic innervation and that these nerve terminals are derived from the renal nerves. Therefore, it is likely that the closer the renal artery gets to the kidney, the greater the percentage of nerves that actually enter the kidney instead of branching off to innervate the vasculature or other structures.
Tzafriri et al. 15 also recently reported in pigs that the innervation pattern of nerves and ganglia should be taken into consideration when catheter-based RD is performed near the ostium. They showed that nerves and ganglia are farthest from the renal artery lumen and not homogeneously distributed around the renal artery near the aorta. In addition, when they performed catheter-based RD at the ostium only 1 of 8 animals had a significant decrease in renal NE, which correlated directly with the amount of renal nerve injury. These findings are consistent with our observations that endovascular methods of RD may be more effective when performed in the distal sections of the renal artery, near the bifurcation, compared to the more proximal portions.
Another factor that may affect the efficiency of RF-based RD in different parts of the renal artery is the thickness of the artery. In the main renal artery, there is a substantial tunica media compared to the smaller branches of the renal artery closer to the kidney. Our data showed that 50% of the nerves located in the distal sections of the artery were injured when the RD occurred in the renal artery branches, while only 33% of the nerves in the middle sections were injured following RD in the main renal artery. More experiments are needed to determine the reasons that RD in the renal artery branches is more effective in lowering renal NE.
One limitation to the current study that might be perceived is that BP was not measured. However, the main goal of this study was to determine if performing RD in different locations in the renal artery would result in more effective lowering of renal NE. We chose to denervate 1 kidney while allowing the contralateral kidney to serve as a control for optimal statistical comparisons. In addition, our studies were conducted in normotensive pigs and we did not anticipate that denervation of only 1 kidney would produce significant changes in BP; our previous studies showed that denervation in normotensive animals had no significant effect on BP.14 The current study was not designed to test the safety of RD from the branches of the renal artery. While there were no safety issues 2 weeks after RD in the animals used in this study, further studies are needed to determine the long-term safety of RD performed in the renal artery branches prior to large clinical trials.
In conclusion, the current study shows that catheter-based RF RD lowers renal NE levels. However, catheter-based RD performed in the branches of the renal artery, closer to the kidney, produced the greatest decrease in renal NE. This may be due to RF-based RD causing a greater percentage of nerves to be injured at this more distal location because of the smaller size and close proximity of the nerves to the artery endothelium–lumen interface in the branches. These results suggest that future clinical trials using catheter-based RD should consider performing RD in the renal artery branches once safety has been confirmed.
DISCLOSURE
Drs Henegar and Hall are the Principal Investigators on a research grant from St. Jude Medical, Inc.
ACKNOWLEDGMENTS
The authors thank Mr Calvin Torrey for technical assistance with these studies.
REFERENCES
- 1. Papademetriou V, Tsioufis CP, Sinhal A, Chew DP, T. Meredith IT, Malaiapan Y, Worthley MI, Worthley SG. Catheter-based renal denervation for resistant hypertension 12-month results of the EnligHTN I First-in-Human Study using a multielectrode ablation system. Hypertension 2014; 64:565–572. [DOI] [PubMed] [Google Scholar]
- 2. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 3. Henegar JR, Zhang Y, De Rama R, Hata C, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens 2014; 27:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 5. Esler M, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomized controlled trial. Lancet 2010; 376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 6. Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 1995; 25:893–897. [DOI] [PubMed] [Google Scholar]
- 7. Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 2012; 59:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Id D, Kaltenbach B, Bertog SC, Hornung M, Hofmann I, Vaskelyte L, Sievert H. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091. [DOI] [PubMed] [Google Scholar]
- 9. Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat 2012; 25:628–633. [DOI] [PubMed] [Google Scholar]
- 10. Virmani R. Perirenal nerve distribution, density and quantification: implications for the evaluation of device safety and efficacy. Presented at TCT, Miami, FL, 23 October 2012. [Google Scholar]
- 11.Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, Virmani R, Joner. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64: 635–643. [DOI] [PubMed] [Google Scholar]
- 12. Tunstall R, Winsor-Hines D, Willard M, Huibregtse B. A preclinical comparative histological evaluation of the renal artery and nerves in the human cadaver and swine model. J Am Coll Cardiol 2012; 60(17_S). doi:10.1016/j.jacc.2012.08.238. [Google Scholar]
- 13. Reddy S, Kumar P, Prasad K. Histomorphometric and sympathetic innervation of the human renal artery: a cadaveric study. Urol Ann 2011; 3:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mizelle HL, Hall JE, Woods LL, Montani JP, Dzielak DJ, Pan YJ. Role of renal nerves in compensatory adaptation to chronic reductions in sodium intake. Am J Physiol 1987; 252:F291–F298. [DOI] [PubMed] [Google Scholar]
- 15. Tzafriri AR, Mahfoud F, Keating JH, Markham PM, Spognardi A, Wong G, Fuimaono K, Böhm M, Edelman ER. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol 2014; 64:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]