This double-blind, phase 3 study assessed the efficacy and safety of ganitumab plus gemcitabine as first-line treatment of metastatic pancreatic cancer. The study was stopped after a preplanned futility analysis indicated a positive outcome was unlikely at primary analysis. Ganitumab plus gemcitabine had manageable toxicity but did not improve OS versus gemcitabine alone in unselected patients.
Keywords: ganitumab, gemcitabine, pancreatic cancer, IGF-1 receptor, biomarker
Abstract
Background
This double-blind, phase 3 study assessed the efficacy and safety of ganitumab combined with gemcitabine as first-line treatment of metastatic pancreatic cancer.
Patients and methods
Patients with previously untreated metastatic pancreatic adenocarcinoma were randomly assigned 2 : 2 : 1 to receive intravenous gemcitabine 1000 mg/m2 (days 1, 8, and 15 of each 28-day cycle) plus placebo, ganitumab 12 mg/kg, or ganitumab 20 mg/kg (days 1 and 15 of each cycle). The primary end point was overall survival (OS). Secondary end points included progression-free survival (PFS), safety, and efficacy by levels of circulating biomarkers.
Results
Overall, 322 patients were randomly assigned to placebo, 318 to ganitumab 12 mg/kg, and 160 to ganitumab 20 mg/kg. The study was stopped based on results from a preplanned futility analysis; the final results are reported. Median OS was 7.2 months [95% confidence interval (CI), 6.3−8.2] in the placebo arm, 7.0 months (95% CI, 6.2−8.5) in the ganitumab 12-mg/kg arm [hazard ratio (HR), 1.00; 95% CI, 0.82−1.21; P = 0.494], and 7.1 months (95% CI, 6.4−8.5) in the ganitumab 20-mg/kg arm (HR, 0.97; 95% CI, 0.76−1.23; P = 0.397). Median PFS was 3.7, 3.6 (HR, 1.00; 95% CI, 0.84−1.20; P = 0.520), and 3.7 months (HR, 0.97; 95% CI, 0.77–1.22; P = 0.403), respectively. No unexpected toxicity was observed with ganitumab plus gemcitabine. The circulating biomarkers assessed [insulin-like growth factor-1 (IGF-1), IGF-binding protein-2, and -3] were not associated with a treatment effect on OS or PFS by ganitumab.
Conclusion
Ganitumab combined with gemcitabine had manageable toxicity but did not improve OS, compared with gemcitabine alone in unselected patients with metastatic pancreatic cancer.
Clinical trial registration
ClinicalTrials.gov NCT01231347.
introduction
Pancreatic cancer is typically diagnosed at advanced stage and is associated with poor survival [1–3]. For over 15 years, gemcitabine-based treatment has been the standard-of-care in the first-line treatment of metastatic pancreatic cancer [4, 5]. Despite newer regimens (e.g. FOLFIRINOX and nab-paclitaxel plus gemcitabine [6, 7]), patient outcomes in first-line settings are unsatisfactory. Whereas targeted therapies are available for other tumor types, an unmet need remains for targeted treatments of metastatic pancreatic cancer.
The insulin-like growth factor (IGF)–1 receptor (IGF1R) pathway is a potential therapeutic target for pancreatic cancer, given the frequent overexpression in pancreatic tumors of IGF1R and its ligands IGF-1 and IGF-2 [8–10]. Ganitumab, an investigational, fully human, monoclonal antibody antagonist of IGF1R, prevents binding of IGF-1 and IGF-2 to IGF1R [11]. In a randomized phase 2 study, ganitumab combined with gemcitabine had manageable toxicity and showed a trend toward improved overall survival (OS; hazard ratio [HR], 0.67; P = 0.12) and progression-free survival (PFS; HR, 0.65; P = 0.072) compared with placebo in patients with metastatic pancreatic adenocarcinoma [12].
GAMMA (Gemcitabine and AMG 479 in Metastatic Adenocarcinoma of the Pancreas), a randomized, double-blind, placebo-controlled, phase 3 study, assessed the efficacy and safety of ganitumab combined with gemcitabine in first-line treatment of metastatic pancreatic adenocarcinoma. We report the final results of GAMMA, which was stopped early after a preplanned futility analysis demonstrated that a positive outcome was unlikely at primary analysis.
patients and methods
patients
GAMMA was conducted at 146 centers. Eligible patients (≥18 years) had previously untreated histologically or cytologically confirmed metastatic pancreatic adenocarcinoma; Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤1; and adequate hematologic, renal, hepatic, and cardiac function. Exclusion criteria were histology other than pancreatic adenocarcinoma; central nervous system metastases; external biliary drain; paracentesis or thoracentesis for malignant effusion within previous 14 days; prior or synchronous malignancy (except treated or inactive nonmelanoma skin cancer, lentigo maligna, cervical carcinoma, or prostatic intraepithelial neoplasia or malignancy cured ≥3 years); major or minor surgery within previous 30 or 7 days, respectively; and any previous systemic treatment of pancreatic cancer including adjuvant therapy. All patients provided written informed consent. The study protocol was approved by each site's ethics committee.
study design and treatment
Patients were randomly assigned 2 : 2 : 1 to receive intravenous gemcitabine 1000 mg/m2 plus either placebo, ganitumab 12 mg/kg, or ganitumab 20 mg/kg. Selected doses of ganitumab were based on a phase 2 exposure-response analysis [13]. Randomization was stratified by ECOG PS (0 versus 1), liver metastases (yes versus no), and region (Australia, Western Europe, USA, and Canada versus rest of world). Patients received gemcitabine on days 1, 8, and 15, and placebo/ganitumab on days 1 and 15 of each 28-day cycle.
dose adjustments
Gemcitabine could be withheld or reduced depending on timing and toxicity severity; ganitumab was withheld until gemcitabine was resumed. Ganitumab dose reductions up to 50% were allowed for toxicity; reductions were permanent. Ganitumab could be withheld or permanently discontinued for certain adverse events (AEs).
tumor assessment
Tumor response was based on investigator assessment (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1 [14]) of computed tomography or magnetic resonance imaging every 8 weeks.
safety assessment
All AEs occurring from enrollment until safety follow-up (30 days after the final treatment dose) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Clinical and laboratory assessments were performed at screening, day 1 of each cycle, and safety follow-up. Serum for assessment of anti-ganitumab antibodies [15] was collected on day 1 of cycles 1, 3, and 7 and at safety follow-up.
biomarker analysis
In a phase 2 study, ganitumab treatment was associated with enhanced OS in patients with higher baseline circulating total IGF-1, IGF-2, and IGF-binding protein-3 (IGFBP-3) and lower IGFBP-2 [16]. For biomarker assessment, serum was collected predose on days 1 and 8 of cycle 1; on day 1 of cycles 2, 3, 5, 7, and 9; and at safety follow-up. Serum baseline total IGF-1 as well as IGFBP-2 and -3 were measured using a competitive binding radioimmunoassay [15] and immunoassays [16], respectively.
statistical analysis
The primary end point was OS (time from randomization to death); patients living at the date of analysis were censored at the last date known alive. Key secondary end points included PFS per RECIST version 1.1 [14], OS at 12 months, objective response rate (ORR), duration of response (DOR), and incidence of AEs and laboratory abnormalities. Exploratory end points included pharmacodynamic response biomarkers (serum circulating IGF-1, IGFBP-2, and IGFBP-3).
The planned sample size was 825 patients. The primary analysis was scheduled after 642 deaths to provide 90% and 95% power to detect HRs of 0.73 and 0.65 for the ganitumab 12- and 20-mg/kg arms, respectively, with a one-sided significance level of 0.0125. The full analysis set and safety analysis set included all randomized patients, and all patients who received ≥1 dose of ganitumab or placebo, respectively. A planned interim efficacy futility analysis of OS was scheduled after ∼30% of events (188 deaths: 156 and 118 for ganitumab 12 and 20 mg/kg, respectively). An independent data monitoring committee reviewed the interim safety and efficacy analyses.
OS and PFS were compared using log-rank tests stratified by the randomization factors and Kaplan-Meier estimates. Cox proportional hazards models stratified by the randomization factors estimated HRs and two-sided 95% CIs for OS, PFS, and DOR. ORR differences and odds ratios (OR) between ganitumab and placebo arms were calculated. Median DOR was calculated for each treatment arm. Descriptive statistics are reported for safety data. Treatment effects in each biomarker group and interactions were estimated using a Cox proportional hazards model. Amgen Inc. and Axio Research (Seattle, WA, USA) conducted the statistical analyses in collaboration with the co-authors.
results
patients
Between April 2011 and August 2012, 800 patients were randomized (placebo, n = 322; ganitumab 12 mg/kg, n = 318; ganitumab 20 mg/kg, n = 160). Patients' characteristics were balanced across treatment arms; 8%–9% of all patients had previously undergone surgery for pancreatic adenocarcinoma with curative intent (Table 1). Overall, 792 patients (99%) received ≥1 treatment dose and were included in the safety analysis (supplementary Figure S1, available at Annals of Oncology online). The study was stopped early (8 August 2012) following a preplanned interim analysis (2 August 2012) that, after crossing the futility boundaries, indicated a positive outcome was unlikely at the primary analysis. All patients then discontinued ganitumab and were subsequently managed with other therapies at their physicians' discretion. Post-trial therapy was not a trial end point and was not captured following safety follow-up.
Table 1.
Patients' demographics and baseline characteristicsa
| Placebo + gemcitabine (n = 322) | Ganitumab 12 mg/kg + gemcitabine (n = 318) | Ganitumab 20 mg/kg + gemcitabine (n = 160) | |
|---|---|---|---|
| Sex, n (%) | |||
| Men | 188 (58) | 159 (50) | 85 (53) |
| Women | 134 (42) | 159 (50) | 75 (47) |
| Median age, years (range) | 63 (36–83) | 62 (36–85) | 62 (31–81) |
| Race/ethnicity, n (%) | |||
| White | 253 (79) | 258 (81) | 129 (81) |
| Black | 3 (1) | 4 (1) | 0 (0) |
| Hispanic | 1 (<1) | 0 (0) | 0 (0) |
| Asian (excluding Japanese) | 34 (11) | 19 (6) | 14 (9) |
| Japanese | 30 (9) | 35 (11) | 16 (10) |
| Other | 1 (<1) | 2(1) | 1 (1) |
| Geographic region, n (%) | |||
| Western Europe | 126 (39) | 125 (39) | 66 (41) |
| Eastern Europe | 90 (28) | 94 (30) | 46 (29) |
| Japan | 30 (9) | 34 (11) | 16 (10) |
| United States | 26 (8) | 31 (10) | 13 (8) |
| Asia (excluding Japan) | 28 (9) | 16 (5) | 13 (8) |
| Australia | 13 (4) | 7 (2) | 4 (3) |
| South America | 5 (2) | 8 (3) | 1 (1) |
| Canada | 4 (1) | 3 (1) | 1 (1) |
| Median time since primary diagnosis, months (range) | 0.9 (0–29) | 0.8 (0–36) | 0.9 (0–25) |
| ECOG performance status, n (%) | |||
| 0 | 137 (43) | 140 (44) | 70 (44) |
| 1 | 184 (57) | 176 (55) | 90 (56) |
| 2 | 0 (0) | 2 (1) | 0 (0) |
| 3 | 1 (<1) | 0 (0) | 0 (0) |
| Prior surgery with curative intent for pancreatic adenocarcinoma, n (%) | 28 (9) | 26 (8) | 14 (9) |
| Tumor differentiation, n (%) | |||
| Well differentiated | 29 (9) | 24 (8) | 11 (7) |
| Moderately differentiated | 87 (27) | 68 (21) | 39 (24) |
| Poorly differentiated | 50 (16) | 50 (16) | 27 (17) |
| Unknown | 152 (47) | 171 (54) | 78 (49) |
| Other | 4 (1) | 5 (2) | 5 (3) |
| Lesions in the pancreas at screening, n (%) | |||
| Head only | 115 (36) | 124 (39) | 59 (37) |
| Head and body | 20 (6) | 21 (7) | 8 (5) |
| Head and tail | 4 (1) | 1 (<1) | 0 (0) |
| Head, body, and tail | 2 (1) | 5 (2) | 2 (1) |
| Body only | 71 (22) | 60 (19) | 30 (19) |
| Body and tail | 55 (17) | 45 (14) | 20 (13) |
| Tail only | 44 (14) | 50 (16) | 33 (21) |
| No tumor in pancreas | 11 (3) | 12 (4) | 8 (5) |
| Lesions outside the pancreas, n (%) | |||
| Liver | 249 (77) | 255 (80) | 125 (78) |
| Lung | 76 (24) | 78 (25) | 37 (23) |
| Lymph node | 97 (30) | 97 (31) | 37 (23) |
| Other | 104 (32) | 87 (27) | 51 (32) |
| Lesions within the peritoneal cavity, n (%) | 118 (37) | 116 (36) | 58 (36) |
ECOG, Eastern Cooperative Oncology Group.
aFull analysis set.
exposure
The median number of cycles for placebo or ganitumab treatment was 3; the median number of gemcitabine cycles was 4 and 3 in the placebo and ganitumab arms, respectively. The median relative dose intensity of ganitumab was ∼1.0; median relative dose intensity of gemcitabine was consistent among treatment arms (supplementary Table S1, available at Annals of Oncology online).
efficacy
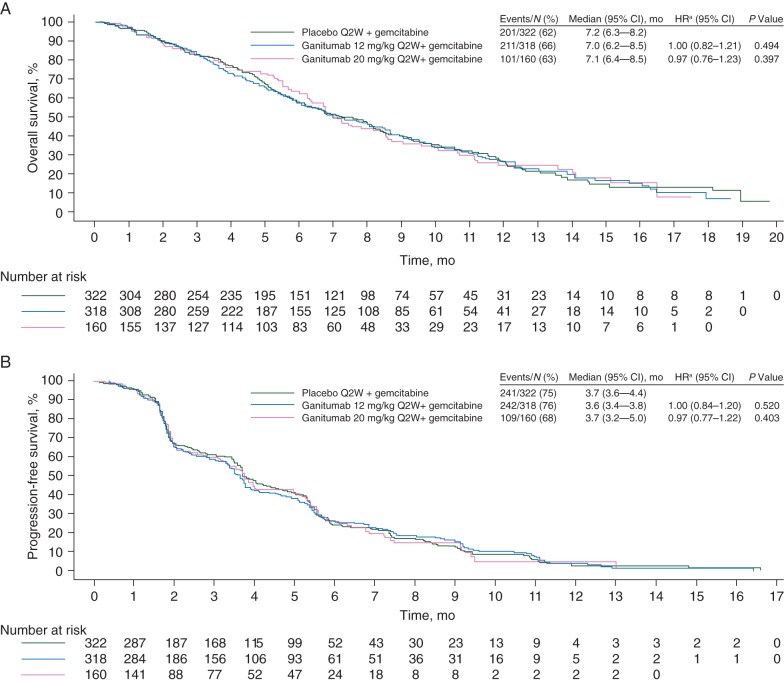
At the final analysis, 513 patients (64%) had died (placebo, n = 201; ganitumab 12 mg/kg, n = 211; ganitumab 20 mg/kg, n = 101). Median OS was 7.2 months (95% confidence interval (CI), 6.3−8.2) in the placebo arm, 7.0 months (95% CI, 6.2−8.5) in the ganitumab 12-mg/kg arm (HR, 1.00; 95% CI, 0.82−1.21; P = 0.494), and 7.1 months (95% CI, 6.4−8.5) in the ganitumab 20-mg/kg arm (HR, 0.97; 95% CI, 0.76−1.23; P = 0.397; Figure 1). Median PFS was 3.7 months (95% CI, 3.6−4.4) in the placebo arm, 3.6 months (95% CI, 3.4−3.8) in the ganitumab 12-mg/kg arm (HR, 1.00; 95% CI, 0.84−1.20; P = 0.520), and 3.7 months (95% CI, 3.2−5.0) in the ganitumab 20-mg/kg arm (HR, 0.97; 95% CI, 0.77−1.22; P = 0.403; Figure 1). Consistent OS and PFS results were observed using multivariate models adjusted for baseline prognostic factors (supplementary Table S2, available at Annals of Oncology online).
Figure 1.
Kaplan-Meier curves for overall survival (A) and progression-free survival (B) by treatment group in the full analysis set. Patients who had not died (A) or who had not progressed or died (B) at the date of their last assessment were censored. Q2W: every 2 weeks. aCox proportional hazards model adjusted for stratification covariates (Eastern Cooperative Oncology Group performance status, 0 or 1; presence of liver metastases, yes or no).
Objective response was evaluable by the investigators in 769 patients (96%) with measurable disease at baseline (supplementary Table S3, available at Annals of Oncology online). ORR was 10% in the placebo arm, 16% in the ganitumab 12-mg/kg arm (OR, 1.69; 95% CI, 1.02−2.81), and 15% in the ganitumab 20-mg/kg arm (OR, 1.51; 95% CI, 0.80−2.81). The median DOR was 5.6 months (95% CI, 4.1−7.3) in the ganitumab 12-mg/kg arm and 5.5 months (95% CI, 2.1−9.3) in the ganitumab 20-mg/kg arm, compared with 3.0 months (95% CI, 1.9−5.5) in the placebo arm. In an ad hoc analysis using a stratified Cox proportional hazards model, the HR for DOR in the ganitumab 12-mg/kg arm versus the placebo arm was 0.49 (95% CI, 0.27−0.89; P = 0.019), and the HR for the ganitumab 20-mg/kg arm versus the placebo arm was 0.71 (95% CI, 0.30−1.56; P = 0.404).
safety
The incidence of treatment-emergent AEs is summarized in Table 2. AEs (any grade) occurring with a ≥5% greater incidence among all ganitumab-treated patients compared with placebo include hyperglycemia (22% versus 10%), thrombocytopenia (38% versus 30%), fatigue (36% versus 29%), vomiting (28% versus 23%), and diarrhea (23% versus 18%). The rates of treatment-emergent venous/arterial thromboembolic events were each ≤4% in each arm; the pulmonary embolism rate was balanced between arms and likewise ≤4% in each arm. Grade 3 and 4 treatment-emergent AEs occurred in 176 patients (56%) in the placebo arm, 214 (68%) in the ganitumab 12-mg/kg arm, and 94 (59%) in the ganitumab 20-mg/kg arm. AEs resulting in discontinuation of placebo, ganitumab, and/or gemcitabine occurred in 37 patients (12%) who received placebo, 48 (15%) who received ganitumab 12 mg/kg, and 17 (11%) who received ganitumab 20 mg/kg. Overall, on-treatment fatal AEs occurred in 33 patients (10%) who received placebo, 30 (10%) who received ganitumab 12 mg/kg, and 18 (11%) who received ganitumab 20 mg/kg. Two patients who received placebo had fatal AEs attributed by the investigators to gemcitabine (anemia and cardiac failure); two patients had fatal AEs attributed to ganitumab (cardiac failure and thrombotic thrombocytopenia purpura). Of 466 patients who received ganitumab with available serum samples, 18 (4%) developed anti-ganitumab–binding antibodies; no anti-ganitumab–neutralizing antibodies were detected.
Table 2.
Summary of treatment-emergent adverse events
| AEs,a n (%) | Placebo + gemcitabine (n = 317) |
Ganitumab 12 mg/kg + gemcitabine (n = 315) |
Ganitumab 20 mg/kg + gemcitabine (n = 160) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Anyb | 312 (98) | 123 (39) | 53 (17) | 308 (98) | 155 (49) | 59 (19) | 159 (99) | 78 (49) | 16 (10) |
| Nausea | 128 (40) | 7 (2) | 1 (<1) | 133 (42) | 5 (2) | 0 (0) | 71 (44) | 2 (1) | 0 (0) |
| Neutropenia | 109 (34) | 51 (16) | 14 (4) | 117 (37) | 54 (17) | 20 (6) | 52 (33) | 26 (16) | 5 (3) |
| Thrombocytopenia | 94 (30) | 16 (5) | 5 (2) | 126 (40) | 23 (7) | 4 (1) | 54 (34) | 8 (5) | 4 (3) |
| Fatigue | 93 (29) | 12 (4) | 0 (0) | 111 (35) | 15 (5) | 4 (1) | 62 (39) | 8 (5) | 0 (0) |
| Decreased appetite | 85 (27) | 5 (2) | 0 (0) | 94 (30) | 7 (2) | 1 (<1) | 37 (23) | 0 (0) | 0 (0) |
| Vomiting | 72 (23) | 11 (3) | 1 (<1) | 89 (28) | 12 (4) | 2 (1) | 46 (29) | 3 (2) | 0 (0) |
| Pyrexia | 69 (22) | 2 (1) | 0 (0) | 67 (21) | 1 (<1) | 2 (1) | 43 (27) | 1 (1) | 0 (0) |
| Anemia | 76 (24) | 19 (6) | 1 (<1) | 64 (20) | 10 (3) | 3 (1) | 36 (23) | 4 (3) | 1 (1) |
| Constipation | 62 (20) | 2 (1) | 0 (0) | 82 (26) | 3 (1) | 0 (0) | 29 (18) | 2 (1) | 0 (0) |
| Diarrhea | 58 (18) | 1 (<1) | 0 (0) | 84 (27) | 3 (1) | 0 (0) | 27 (17) | 2 (1) | 0 (0) |
| Abdominal pain | 59 (19) | 3 (1) | 1 (<1) | 69 (22) | 19 (6) | 0 (0) | 27 (17) | 3 (2) | 0 (0) |
| Hyperglycemia | 31 (10) | 7 (2) | 0 (0) | 63 (20) | 33 (10) | 4 (1) | 42 (26) | 23 (14) | 4 (3) |
| Peripheral edema | 58 (18) | 7 (2) | 0 (0) | 49 (16) | 1 (<1) | 0 (0) | 25 (16) | 1 (1) | 0 (0) |
| Alanine aminotransferase increased | 45 (14) | 1 (<1) | 0 (0) | 61 (19) | 0 (0) | 0 (0) | 24 (15) | 0 (0) | 0 (0) |
| Aspartate aminotransferase increased | 40 (13) | 6 (2) | 2 (1) | 56 (18) | 23 (7) | 1 (<1) | 21 (13) | 7 (4) | 0 (0) |
| Asthenia | 45 (14) | 10 (3) | 0 (0) | 47 (15) | 10 (3) | 2 (1) | 18 (11) | 4 (3) | 0 (0) |
| Rash | 34 (11) | 0 (0) | 0 (0) | 44 (14) | 1 (<1) | 0 (0) | 24 (15) | 1 (1) | 0 (0) |
| Leukopenia | 32 (10) | 9 (3) | 0 (0) | 47 (15) | 14 (4) | 1 (<1) | 15 (9) | 4 (3) | 0 (0) |
| Weight decreased | 29 (9) | 1 (<1) | 0 (0) | 27 (9) | 2 (1) | 0 (0) | 23 (14) | 1 (1) | 0 (0) |
| Fatal AEs | 33 (10) | 30 (10) | 18 (11) | ||||||
AE, adverse event; FOLFIRI, leucovorin, 5-fluorouracil, and irinotecan.
aSafety analysis set. Includes adverse events occurring during treatment and up to 30 days from the last dose of investigational product. Adverse events were coded using MedDRA (v 15.0) and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
bOccurring in ≥10% of all patients.
biomarker assessment
Circulating pharmacodynamic biomarkers were assessed in 582 patients (73%; placebo, n = 243; ganitumab 12 mg/kg, n = 226; ganitumab 20 mg/kg, n = 113). Baseline circulating levels of total IGF-1, IGFBP-2, and IGFBP-3 were similar across treatment arms (supplementary Figure S2, available at Annals of Oncology online). Dividing patients into high and low subgroups based on whether their baseline levels of IGF-1, IGFBP-2, and IGFBP-3 were above or below median, respectively, did not result in a treatment effect on OS or PFS by ganitumab (supplementary Figure S3, available at Annals of Oncology online).
discussion
GAMMA was stopped early after a prespecified interim analysis indicated that the primary objective was unlikely to be met. The final results did not demonstrate improved efficacy by the addition of ganitumab to gemcitabine. Numerous other phase 3 studies have also failed to demonstrate clinical benefit from targeted therapies in metastatic pancreatic cancer [17]. These results are disappointing given the encouraging phase 2 study in which ganitumab 12 mg/kg combined with gemcitabine was associated with marginally improved 6-month survival (57% versus 50%) and improved OS (8.7 versus 5.9 months; HR, 0.64; P = 0.12) [12].
In the placebo, ganitumab 12-mg/kg, and ganitumab 20-mg/kg arms, median OS (7.2, 7.0, and 7.1 months) and PFS (3.7, 3.6, and 3.7 months) were similar to those in the gemcitabine-based control arms of other metastatic pancreatic cancer trials [6, 18]. In the Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT), compared with placebo, nab-paclitaxel combined with gemcitabine improved median OS (8.5 versus 6.7 months), PFS (5.5 versus 3.7 months), and ORR (23% versus 7%) [6]. Median OS and PFS (11.1 and 6.4 months, respectively) were also longer in a phase 3 study of first-line FOLFIRINOX in patients with metastatic pancreatic cancer than in GAMMA [18].
Early clinical data identified circulating IGF-1 as a predictive biomarker candidate for the anti-IGF1R monoclonal antibody figitumumab [19]. An analysis of phase 2 data suggested that high median baseline circulating total IGF-1, IGF-2, and IGFBP-3 (and low baseline circulating IGFBP-2) predicted the treatment effect of ganitumab combined with gemcitabine in patients with metastatic pancreatic cancer [16]. In GAMMA, dichotomization of patients based on median biomarker values did not reveal a treatment effect on OS or PFS by ganitumab combined with gemcitabine. Possibly, the median serum baseline level was not ideal to separate the high and low subgroups [16]. In the phase 3 Avastin and Tarceva in Advanced Pancreatic Cancer (AViTA) study, which did not meet its primary objective of increasing OS [20], improved OS and PFS was associated with the single-nucleotide polymorphism rs9582036 [21]. These results and other large studies of unselected patients with initially disappointing results underscore the need for validated predictive biomarkers for patient selection in trials of IGF1R-targeted therapies [22].
The underlying cause of the lack of improved efficacy by ganitumab combined with gemcitabine is unclear. Although this randomized phase 3 trial failed to meet its primary end point, the original decision to proceed with the trial was based on extensive preclinical data linking the IGF pathway to pancreatic cancer pathogenesis and progression, as well as on the results of a completed randomized phase 2 trial suggesting a potential OS benefit (median OS, 8.7 versus 5.9 months for gemcitabine with ganitumab versus gemcitabine alone, respectively; stratified HR, 0.67; P = 0.12) [12]. Given the magnitude of the unmet need and the novelty of the mechanism of action, this phase 3 trial sought to bring forward a possible breakthrough therapy. Patients' characteristics were balanced across treatment arms and were generally consistent with the MPACT and FOLFIRINOX studies [6, 18]. Pancreatic adenocarcinoma is a heterogeneous disease characterized by several activating gene mutations [23]. Among these, KRAS mutations, present in ∼95% of pancreatic adenocarcinomas, appear to be an initiating event [23, 24]. The analysis of interactions between the IGF1R pathway and pathways downstream of KRAS (e.g. MEK [25]) during malignant transformation may help identify patients responsive to IGF1R-targeted therapy. Furthermore, combined targeting of IGF1R and other signaling pathways may be necessary to overcome potential resistance mechanisms [26], which may have played a role in GAMMA.
The safety profile of ganitumab plus gemcitabine was consistent with earlier observations [12, 15]; no unexpected toxicities were observed. The most frequent AEs in GAMMA (e.g. nausea, neutropenia, thrombocytopenia, and fatigue) and other AEs of potential concern (e.g. hyperglycemia) are easily managed and have been observed in previous ganitumab studies and with other IGF1R pathway–targeted agents [12, 27, 28].
In conclusion, ganitumab combined with gemcitabine was not associated with improved OS compared with gemcitabine alone in this unselected patient population with metastatic pancreatic cancer. Dichotomization of patients based on median values of potential circulating biomarkers did not demonstrate an enriched treatment effect on OS or PFS. Without a validated biomarker to guide patient selection, IGF1R-targeted therapy is not recommended for metastatic pancreatic cancer.
funding
This study was supported by Amgen Inc. in collaboration with Takeda Global Research & Development Center, Inc. There were no grants or applicable grant numbers for this study.
disclosure
CSF has served as an advisor/consultant for Amgen Inc., Takeda, Eli Lilly, Acceleron, Sanofi, Bayer, and Celgene; and has received research funding from the National Institutes of Health (grant numbers R01CA124908 and P50CA127003). TO has received research funding from Amgen Inc. and Takeda; and has received compensation for travel from Amgen Inc. and Takeda. HR has served as an advisor/consultant for Amgen Inc., Bayer, Celgene, Merck, Roche, and Sanofi. CS has served as an advisor/consultant for GlaxoSmithKline, Pfizer, Bayer, and Astellas; and has received compensation for travel from Pfizer, Bayer, and Astellas. MP has received honoraria from Amgen Inc., Sanofi, Bayer, and Merck; has served as an advisor/consultant for Amgen Inc., Bayer, and Sanofi; has served on speakers' bureau for Amgen Inc., Bayer, Sanofi, and Merck; and has received research funding from Amgen Inc. and Roche. GB has served as an advisor/consultant for Amgen Inc., Eli Lilly, and Nordic; and has received compensation for travel from Pfizer and Novartis. BM has received honoraria from Amgen Inc., Roche, Novartis, Astellas, GlaxoSmithKline, and Bayer; has served as an advisor/consultant for Roche, Novartis, Astellas, Bayer, and GlaxoSmithKline; and has received travel, accommodations, or expenses from Novartis, Roche, and Janssen. RN has received research funding from Amgen Inc. SAT has served on speakers' bureau for Sanofi Aventis, Novartis, Merck, and AstraZeneca; and has received research funding from Sanofi, Novartis, Merck, and AstraZeneca. EVC has received research funding from Amgen Inc. RL is employed by and owns stock in Amgen Inc. VH is employed by and owns stock in Amgen Ltd. JLG is employed by and owns stock in Amgen Inc. BB is employed by and owns stock in Amgen Inc. and has received compensation for travel from Amgen Inc. AC has participated in advisory boards for Amgen Inc. Bayer, Celgene, Roche, Eli Lilly, and Merck; and has received research funding from Amgen Inc. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors acknowledge the patients (and their families) who participated in GAMMA, the global network of GAMMA investigators, and Benjamin Scott, PhD, and James Balwit, MS, whose work was funded by Amgen Inc., for assistance in writing this manuscript.
references
- 1.SEER Stat Fact Sheets: Pancreas Cancer. National Cancer Institute; http://seer.cancer.gov/statfacts/html/pancreas.html (3 March 2014, date last accessed). [Google Scholar]
- 2.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol 2007; 14: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 3.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 2003; 21: 3402–3408. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma v.1.2014. Fort Washington, PA: National Comprehensive Cancer Network, 2014. [Google Scholar]
- 5.Seufferlein T, Bachet JB, Van Cutsem E, Rouqier P, ESMO Guidelines Working Group. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23(Suppl 7): vii33–vii40. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abraxane® (nab-paclitaxel). Full Prescribing Information. Summit, NJ: Celgene Corporation, 2013. [Google Scholar]
- 8.Arnaldez FI, Helman LJ. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am 2012; 26: 527–542, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res 1995; 55: 2007–2011. [PubMed] [Google Scholar]
- 10.Hakam A, Fang Q, Karl R, Coppola D. Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci 2003; 48: 1972–1978. [DOI] [PubMed] [Google Scholar]
- 11.Beltran PJ, Mitchell P, Chung YA, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther 2009; 8: 1095–1105. [DOI] [PubMed] [Google Scholar]
- 12.Kindler HL, Richards DA, Garbo LE, et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol 2012; 23: 2834–2842. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Deng H, Tang R, et al. Exposure-response (E-R) analysis to facilitate phase III (P3) dose selection for ganitumab (GAN, AMG 479) in combination with gemcitabine (G) to treat metastatic pancreatic cancer (mPC). J Clin Oncol 2011; 29: abstr 4049. [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 15.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol 2009; 27: 5800–5807. [DOI] [PubMed] [Google Scholar]
- 16.McCaffery I, Tudor Y, Deng H, et al. Putative predictive biomarkers of survival in patients with metastatic pancreatic adenocarcinoma treated with gemcitabine and ganitumab, an IGF1R inhibitor. Clin Cancer Res 2013; 19: 4282–4289. [DOI] [PubMed] [Google Scholar]
- 17.Furuse J. Pancreatic cancer: is combination treatment better? Clin Pract 2013; 10: 695–700. [Google Scholar]
- 18.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 19.Hixon ML, Gualberto A, Demers L, et al. Correlation of plasma levels of free insulin-like growth factor 1 and clinical benefit of the IGF-IR inhibitor figitumumab (CP-751, 871). J Clin Oncol 2009; 27: abstr 3539. [Google Scholar]
- 20.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009; 27: 2231–2237. [DOI] [PubMed] [Google Scholar]
- 21.Lambrechts D, Claes B, Delmar P, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol 2012; 13: 724–733. [DOI] [PubMed] [Google Scholar]
- 22.Pollak M. The insulin receptor/insulin-like growth factor receptor family as a therapeutic target in oncology. Clin Cancer Res 2012; 18: 40–50. [DOI] [PubMed] [Google Scholar]
- 23.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006; 20: 1218–1249. [DOI] [PubMed] [Google Scholar]
- 24.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 1988; 53: 549–554. [DOI] [PubMed] [Google Scholar]
- 25.Appleman VA, Ahronian LG, Cai J, Klimstra DS, Lewis BC. KRAS(G12D)- and BRAF(V600E)-induced transformation of murine pancreatic epithelial cells requires MEK/ERK-stimulated IGF1R signaling. Mol Cancer Res 2012; 10: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst 2012; 104: 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atzori F, Tabernero J, Cervantes A, et al. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2011; 17: 6304–6312. [DOI] [PubMed] [Google Scholar]
- 28.Cohn AL, Tabernero J, Maurel J, et al. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol 2013; 24: 1777–1785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.