Practical methods for standardized pathological characterization of residual disease for clinical trials of breast cancer include multidisciplinary communication; clinical marking of the tumor site; and mapping tissue sections. Pathologic complete response, the current AJCC/UICC system, and the Residual Cancer Burden system are recommended for quantification of residual disease.
Keywords: breast cancer, neoadjuvant systemic therapy, residual disease, pathologic complete response, pathologic assessment, response evaluation
Abstract
Neoadjuvant systemic therapy (NAST) provides the unique opportunity to assess response to treatment after months rather than years of follow-up. However, significant variability exists in methods of pathologic assessment of response to NAST, and thus its interpretation for subsequent clinical decisions. Our international multidisciplinary working group was convened by the Breast International Group-North American Breast Cancer Group (BIG-NABCG) collaboration and tasked to recommend practical methods for standardized evaluation of the post-NAST surgical breast cancer specimen for clinical trials that promote accurate and reliable designation of pathologic complete response (pCR) and meaningful characterization of residual disease. Recommendations include multidisciplinary communication; clinical marking of the tumor site (clips); and radiologic, photographic, or pictorial imaging of the sliced specimen, to map the tissue sections and reconcile macroscopic and microscopic findings. The information required to define pCR (ypT0/is ypN0 or ypT0 yp N0), residual ypT and ypN stage using the current AJCC/UICC system, and the Residual Cancer Burden system were recommended for quantification of residual disease in clinical trials.
introduction
Response to neoadjuvant systemic therapy (NAST) is an excellent indicator of outcome [1], especially when evaluated by breast cancer subset [2, 3]. The US Food and Drug Administration (FDA) has recommended pathologic complete response (pCR) as an end point for accelerated approval of new agents for neoadjuvant treatment of high-risk early-stage breast cancer, and recently approved pertuzumab based on the increase in pCR rate [4–6]. An FDA meta-analysis failed to show significant improvement in event-free or overall survival related to improved pCR rates in most included trials [1]. Therefore, accelerated approval can be based on an improved pCR rate; but improved event-free survival remains the end point for full approval. This new regulatory pathway emphasizes the importance of standardized, reproducible methods of pathology evaluation and reporting for neoadjuvant clinical trials. To use pCR to demonstrate treatment efficacy of novel therapies, we must have a standard definition and approach to pCR assessment. Moreover, with data emerging on regional recurrence risk based on response in the breast and lymph nodes, decisions about subsequent therapy might be based on pathologic assessment of response [7].
Post-NAST changes are complex. Several reviews of the different classification systems for post-NAST specimens are available [8–11]. Careful, systematic evaluation of the post-NAST specimen in the context of clinical and imaging findings is required for accurate diagnosis. Individual pathologists' experience with NAST specimens and standardization appears to affect results. For example in a 2004 study [12], using the Chevallier system [13], pCR rates dropped—from 16% and 10% for arms A and B, respectively, to 8% and 6%—from local to central pathology review. Similarly, in the I-SPY 1 trial, pCR rates fell by almost 10% after training in the Residual Cancer Burden (RCB) system, which requires a standardized approach for pathologic evaluation to map gross and microscopic findings (L. J. Esserman, personal communication). A standard approach to post-NAST pathologic assessment of breast cancer would improve comparisons between clinical trials, enable accumulation of more robust evidence in controversial areas of practice such as specimen handling, and better serve each patient.
methods
To build consensus on a more standard characterization of residual disease in breast cancer neoadjuvant trials, the BIG-NABCG collaboration convened an international working group of pathologists, radiologists, surgeons, gynecologists, and medical and radiation oncologists. Members were selected via BIG-NABCG leadership and working group co-chair nomination, as well as referred by sites involved in neoadjuvant trials. The working group reviewed standard operating procedures (SOPs) for pathologic assessment from 28 major NAST breast cancer trials and 25 trial sites, finding a variety of approaches (supplementary Material S1, available at Annals of Oncology online). Moreover, sites submitting SOPs noted a need for a standard. The working group developed practical recommendations for the post-NAST pathologic assessment of breast cancer in neoadjuvant clinical trials.
Detailed recommendations for pathologists, including more in-depth discussion of their evidence basis, are published in our pathology-focused paper [14]. This paper summarizes the recommendations for pathologic assessment for a multidisciplinary audience, addresses the prerequisites for accurate pathologic assessment, and explains how a standardized approach would benefit the entire medical team.
The majority of available evidence pertains to neoadjuvant chemotherapy. However, we did not identify existing data or conceptual reasons to limit this standardization effort to neoadjuvant chemotherapy only. When evidence was found lacking, the recommendations represent a consensus opinion for a pragmatic approach based on personal experience and our review of the SOP's of major NAST breast cancer trials.
recommendations
While the basic principles are similar in neoadjuvant and adjuvant situations, NAST specimens are generally more challenging. Therefore, multidisciplinary communication is essential before, during, and after NAST. These recommendations are intended for clinical trials; but they can be optionally incorporated into routine practice because, in our opinion, standardization is most effective when uniformly applied.
initial diagnosis
A percutaneous image-guided core-needle biopsy (CNB) is strongly recommended. The CNB must be adequate for an unequivocal diagnosis of invasion.
Pretreatment tumor size (T stage) is based on imaging and physical examination. Histologic type, tumor grade, estrogen receptor (ER), progesterone receptor (PR), HER2, and other parameters used to determine neoadjuvant treatment should be evaluated on the pretreatment CNB.
To ensure accurate diagnosis and ancillary tests, an adequate number of sufficiently thick, nonfragmented cores obtained with an appropriate-gauge needle are needed. Samples obtained from different parts of the tumor, for example by angling the needle, may be helpful.
Ideally, baseline CNBs for research are obtained at the time of diagnostic biopsy. A separate research biopsy is an alternative. Clinical trials often incorporate research biopsies at additional time points (e.g. after the first cycle or mid-course). We endorse the recommendations from a previous BIG-NABCG working group addressing this topic [15].
However, removing too much tumor for diagnosis by oversampling of tumor with wide-gauge needles interferes with response assessment.
We strongly recommend clip placement at the time of diagnostic or research biopsy [16]. If, after the first or subsequent cycle(s) of chemotherapy, the decrease in tumor volume suggests a possible complete response and a clip was not placed previously, it is imperative to place a clip, even if mastectomy is planned. After completion of NAST, it may be difficult to identify the correct area in the breast or to ensure that the appropriate area was excised, if no clip was placed.
evaluation of the axilla pre-NAST
Systemic or local treatment decisions may be based on axillary status at presentation (pre-NAST). Pre-NAST sentinel lymph node biopsy (SLNB) is not recommended because assessment of nodal response in the axilla, a very important determinant of survival post-NAST, is unreliable after excision of a positive node. Furthermore, this invalidates the RCB score and the ypN stage, and potentially compromises comparisons of pCR results across different studies. This position should be balanced against the accuracy of SLNB post-NAST [17]. Post-NAST SLNB is strongly recommended.
So, to obtain maximum information about the axillary status pre-NAST for systemic or local treatment decisions, routine ultrasound of the regional nodal basins is strongly encouraged. Diagnosis of clinically or radiologically abnormal lymph nodes by fine-needle aspiration or CNB [18] is strongly recommended before NAST. Clip placement into the biopsied node may improve the accuracy of post-NAST SLNB. However, in clinically node-negative patients, it may be that pretherapeutic sentinel lymph node status may determine systemic or local treatment in some cases.
preoperative staging and surgery post-NAST
Preoperative imaging should be appropriate for the clinical stage at presentation, and is important to document the clinical extent of residual disease.
Surgical resection volume is based on preoperative imaging. All detectable residual disease should be removed by the surgery with clear margins [19–21]. In cases of complete radiologic response, the center of the tumor bed should be removed, including any radiologic clips. Pre- or intraoperative localization techniques and orientation of the specimen by the surgeon are imperative. In addition, marking the tumor bed with clips at the time of surgery is encouraged [22].
essential information accompanying the post-NAST surgical specimen
Table 1 lists the clinical information that should be available to the pathologist for optimal evaluation of the post-NAST specimen. The supplementary Material S2, available at Annals of Oncology online, includes a suggested template requisition form to send with the post-NAST specimen. At an absolute minimum, the specimen must be clearly marked as post-NAST and the pre-NAST location and size of the tumor must be indicated.
Table 1.
Essential information to be provided to the pathologist with the surgical specimen removed after neoadjuvant systemic therapy
| Essential and critical information to be provided or made available to the pathologist This information is very important to maintain a high quality of histopathological evaluation and to minimize turnaround time. A suggested requisition form is provided in the supplementary Material S2, available at Annals of Oncology online. | ||
|---|---|---|
| Comment | ||
| 1 | Clearly marked as post-NAST specimen. | In daily clinical routine, this information is often not passed along to the pathologist. The pathologist should be informed of any previous therapy (hormonal therapy, chemotherapy, radiation therapy, and/or other therapy) for the cancer. |
| 2 | Is this part of a clinical trial? Does the trial protocol recommend a grading system for response? |
Important to follow trial protocol. Pathologist needs to know this information in advance of the surgery in order to follow protocol for description, processing, and reporting of the specimen. If this is a drug registration trial, the pathologist should be blinded to the treatment arm, or arrange for independent blinded secondary review of the case by another colleague. |
| 3 | Results of previous core biopsies, especially if they were carried out in another hospital. | Core biopsy results: histologic type, grade, ER/PR, HER2 (and Ki67). Lab reference number. Ideally, slides should be available for review. |
| 4 | Pretreatment lymph node status and method of assessment. | This information is essential for a correct nodal status. If nodal status was assessed by sentinel lymph node biopsy or percutaneous biopsy (core-needle biopsy or fine-needle aspiration biopsy) before neoadjuvant treatment, what were the results (number of nodes sampled, number of positive nodes, size of largest metastasis)? Was a clip placed in the sampled lymph node? |
| 5 | Clinical tumor size(s) before and after chemotherapy. The information is best given as size in cm or mm, rather than clinical tumor stage. Different imaging modalities may provide different sizes. |
This information is important (i) to estimate the response to chemotherapy (=differences in tumor sizes) and (ii) to select the sampling area. If a large pretherapeutic tumor has been diagnosed, the pathologist will perform a more extensive sampling to rule out multifocal residual disease. If the clinical response is suggestive of a complete response, the pathologist will also perform a more extensive sampling. In contrast, if the clinical evaluation suggests no response, the histopathological turnaround time can be reduced, as the extensive sampling might not be necessary. For multifocal tumors, size of each tumor should be given. Imaging modality (mammography/US/MRI) and the chemotherapy cycle number at post-treatment imaging are informative, as are patterns of response (e.g. scattered versus concentric shrinking). |
| 6 | Location of the tumor/tumor bed/residual tumor after chemotherapy. The information is best given in a scheme/drawing. |
This information is important in particular for large resection specimens. Detailed description of the location with, for example for mastectomy specimens, ‘o'clock radius’ and distance from nipple is more helpful than just a quadrant. Procedure used for marking pretreatment tumor location should be noted. Location ideally marked/bracketed with clip (or ink) before treatment. Information on presence of calcifications should be provided because associated calcifications could be used to localize a lesion. The surgeon can mark the location with a stitch on the specimen. Specimen radiography can help localize lesions, clips, and calcifications. For multifocal tumors, location of each tumor should be given. |
| 7 | Information on close (e.g. <5 mm) margins based on intraoperative findings/specimen radiography. | This is particularly relevant for large specimens. Close (e.g. <5 mm) margins need a more extensive sampling. |
| 8 | Clinical and radiologic response to treatment in the axilla. | Clinical exam is sensitive to disease >1 cm in size. US is currently the imaging modality of choice for assessment of response in the axilla pre- and post-treatment and has the additional advantage of guiding percutaneous biopsy and clip placement to identify specific nodes for response evaluation at final histopathological evaluation. |
ER/PR, estrogen receptor, progesterone receptor; US, ultrasound; MRI, magnetic resonance imaging.
evaluation of the post-NAST surgical specimen
Pathologic evaluation of the post-NAST specimen must ensure that the surgery is adequate (identify tumor bed, assess margins), evaluate prognostic factors (document pCR or confirm size/extent of residual tumor, and allow microscopic estimate of residual tumor cellularity), and permit collection of research samples.
recommended data in the pathology report
Table 2 summarizes the recommended elements not always routinely included in the adjuvant setting but recommended in the pathology report of the post-NAST specimen. We strongly recommend that the manner of specimen processing and reporting allow for tumor staging and calculation of the RCB score [25, 26], as described below. In addition, we also encourage reporting using another system (e.g. Chevallier, Sataloff) [13, 27] when it is preferred locally. Particularly, whenever the Miller–Payne or Pinder systems [10, 28] are likely to be used, we recommend reporting the cellularity of the pre-NAST CNB. The US National Cancer Institute's Breast Oncology Local Disease (BOLD) Task Force has also recommended standardized data elements for collection in preoperative breast cancer clinical trials [29].
Table 2.
Elements not always routinely included in the adjuvant setting but recommended in the pathology report of the post-NAST specimena
| Report the elements as for any other type of specimen, plus the following: | |||
|---|---|---|---|
| Comment | |||
| 1. | Size | (A) Two dimensions of largest cross section of entire area involved by (possibly scattered) residual invasive tumor foci (=largest distance between invasive tumor cell foci) and (B) Extent of largest contiguous focus of invasive carcinoma as recommended by AJCC 7th edition [23] 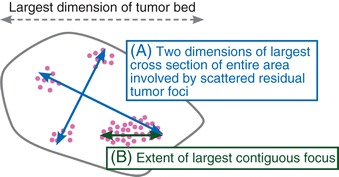
|
In the opinion of the working group, the largest dimension in (A) (longest blue arrow), together with tumor cellularity, is likely a better indicator of response than measurement (B) [19, 24]. The report should clearly state how the size was determined and which dimension was used for staging, especially in cases with scattered residual disease, where there is possible interobserver variability due to differences in guidelines regarding how size should be measured. (A) is needed to calculate the Residual Cancer Burden (RCB) score. |
| 2. | Cellularity |
|
Assessment of average cancer cellularity across the largest cross section of the residual tumor bed (that contains residual cancer) is needed to calculate the Residual Cancer Burden (RCB) score. |
| 3. | Tumor bed |
|
|
| 4. | Lymph node metastasis |
|
The largest distance between tumor cell foci including intervening areas of fibrosis. Size of largest metastasis is needed to calculate the Residual Cancer Burden (RCB) score. |
| 5. | Treatment effect |
|
|
aThis table discusses only those elements specific to NAST that may not be routinely included in pathology reports for non-NAST specimens. A complete list of elements recommended in the pathology report of the post-NAST specimen can be found in our pathology-focused paper [14]. Information about size, cellularity, and lymph node metastasis is required for quantification of residual disease.
extent of sampling
Accurate, reproducible documentation of pathologic response to NAST requires adequate sampling of the correct area of the breast. Overly exhaustive sampling and histologic evaluation of the entire tumor bed are not required and not as efficient or informative as informed mapping of the specimen. Clinical and imaging information, as well as marking of the tumor site, are critical in the selection of the areas to sample (Table 1). Furthermore, images of the sliced resection specimen (with a scale for measurement) are useful as maps on which to annotate the tissue sections that correspond to the different slides. This greatly helps the pathologist to reconstruct the extent and location of disease after reviewing the slides under the microscope. This technique is critical for more standardized and accurate staging of the residual tumor and calculation of RCB, and generally requires fewer tissue blocks to be processed, less time, and less expense.
Figure 1 summarizes possible patterns of tumor response in the breast and associated sampling problems affecting determination of extent and cellularity of residual disease. Because of these issues, we recommend systematic sampling with mapping of the specimen as described below and further detailed in our pathology-focused paper [14]. This pragmatic approach is a consensus based on personal experience and our review of the SOPs of major NAST breast cancer trials.
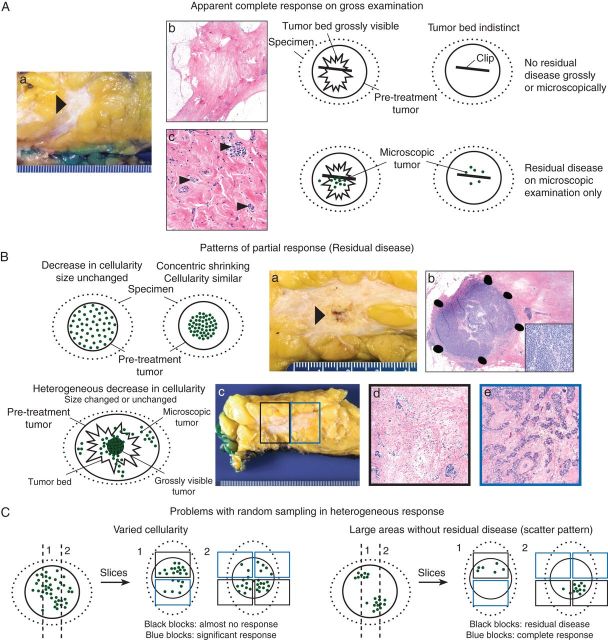
Figure 1.
Patterns of response in the breast and problems related to sampling for histologic evaluation: schematic overview with gross and microscopic illustrations. Photos courtesy of Veerle Bossuyt. (A) In some cases with complete response, a residual tumor bed is visible. In others, the tumor bed is indistinct and sampling of the correct area can only be confirmed by thorough clinical and imaging correlation and identification of a clip. Often, residual microscopic disease is identified when there is no residual tumor grossly. (a) Gross photograph of tumor bed (arrow). (b) Low-power hematoxylin and eosin (H&E) slide of this tumor bed. No residual tumor is identified. (c) High-power of H&E slide of the tumor bed from a different patient with rare residual invasive carcinoma cells (small arrows). (B) A partial response ranges from a decrease in cellularity with unchanged tumor size to concentric tumor shrinking with unchanged tumor cellularity. Often the decrease in cellularity is heterogeneous and residual disease extends beyond the grossly visible tumor bed. (a) Gross photograph of tumor bed with residual tumor (arrow). (b) H&E slides (low and high power) of different patient with residual invasive carcinoma concentrated in a nodule with high cellularity within the tumor bed (concentric shrinking). (c) Gross photograph of most common pattern of residual disease with scattered residual tumor across a fibrous tumor bed. (d,e) Medium power of H&E slides from two different blocks of the tumor bed (black and blue boxes) illustrating that cellularity often varies greatly from block to block. (C) When decrease in cellularity is heterogeneous, random sampling can lead to very different estimates of cellularity. When the decrease in cellularity is so heterogeneous that there are apparent areas with complete response (no residual disease) and apparent multiple foci of residual tumor (scatter pattern), there are interobserver variability and inconsistencies among guidelines in size measurements and when to consider multiple foci. For example, for AJCC staging, the largest contiguous focus of invasive carcinoma should be measured [23]. Intervening areas of fibrosis are specifically excluded, whereas other systems include these areas [24–26, 32, 33]. Moreover, there can be interobserver variability in how much fibrosis to allow within this largest contiguous focus.
If the resection specimen is small (e.g. <30 g), it would be reasonable to process all of the excised tissue for microscopic evaluation. However, description or preferably an image of the sliced specimen should still be used to map the location of each tissue section. Using the techniques described below, it is often still possible to collect research samples.
Information on pre-NAST tumor size and location is critical. Systematic sampling should include macroscopically visible tumor/tumor bed and immediately adjacent tissue, to represent the area suspected of involvement by carcinoma before treatment (area of interest, AI). Extent of sampling is thus determined by the pretreatment size in addition to macroscopic pathologic evaluation, supplemented by any specimen radiography. Sometimes, additional sampling may be needed after reviewing the initial sections under the microscope.
Accurate description or diagrams (maps, ideally drawn on digital photographs or radiographs) must be used to reconstruct the specimen after microscopic evaluation for accurate measurements of extent of residual disease and estimates of cellularity, as well as to ensure adequacy of sampling (Figure 2). A cutoff of an entire cross section of the AI per 1 cm of pretreatment size or, for very large tumors, five representative blocks of a cross section of AI per 1–2 cm of pretreatment size, with a maximum of ∼25 blocks of AI, should be sufficient to confidently document pCR in most cases, provided a tumor bed or clip is identified.
Figure 2.
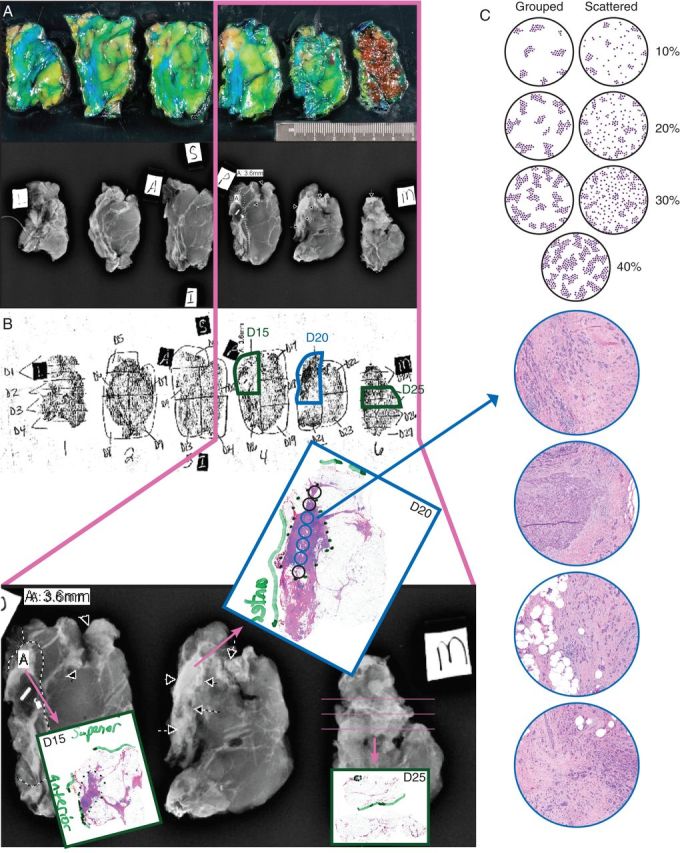
Example of mapping of a post-NAST lumpectomy specimen. The specimen is serially sliced and radiographed (A). A diagram allows the pathologist to correlate the microscopic findings with the gross findings and the specimen radiograph and to reconstruct the location of the microscopic residual disease in the specimen for size measurements (B) [30]. The cellularity is assessed across the largest cross section of residual microscopic disease and compared with a computer-generated standard to improve reproducibility (C) [25, 30]. (The average cellularity in this example is ∼30%.). Adapted by permission from the American Association for Cancer Research: Symmans WF. “Pathologic Evaluation After Neoadjuvant Chemotherapy: Standardizing Management of the Surgical Specimen and Assessing Response to Neoadjuvant Therapies: The Promises and Challenges of Pathologic Complete Response.” Regulatory Science and Policy Session, 8 April 2013. Washington, DC: American Association for Cancer Research (AACR) Annual Meeting 2013. http://webcast.aacr.org/console/player/20130?mediaType=audio&
Source: see ref. [30].
collection of tissue samples for research purposes
We generally recommend that dedicated research samples (to be frozen or otherwise prepared in a nonclinical manner) only be collected if there is grossly obvious residual invasive cancer. Such sites are the most likely to contain diagnostically expendable tumor tissue of sufficient cellularity for research use. One can thin a section and submit the trim for research. Another practical approach is to obtain small cylinders of tissue for research from the slices with a punch biopsy tool. Where the research tissue was collected can so be readily identified on the histology slides. A previous international working group has addressed the collection of biospecimens from NAST breast cancer clinical trials in detail [15].
tumor grade and type
Histologic tumor type can be more difficult to ascertain after NAST. NAST can cause nuclear hyperchromasia and pleomorphism and can alter the mitotic rate [10]; however, histologic grade should be compared with the pretreatment biopsy before assuming that findings are treatment-related.
tumor extent and cellularity
Tumor size/extent is often difficult to assess after NAST. Residual tumor is often softer and more difficult to see grossly. The residual carcinoma may be present as small foci scattered over a (ill-defined) tumor bed [31].
The most recent (7th edition) [23] American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) recommendation to measure the largest contiguous focus excluding areas with intervening fibrosis may result in a systematic artificial down-staging of tumors with a scattered response pattern if scattered tumor nests were part of a single tumor mass before treatment. There are currently no data linking this tumor measurement to survival outcome. Note that prior publications concerning prognosis of yp-TNM staging used the earlier (6th edition) [32] AJCC staging system, which considered the largest extent of residual cancer allowing for intervening fibrosis [24, 33]. To consider tumors multifocal, they should be separated by abundant normal breast or adipose tissue and should be measured independently and documented. In this situation, dimensions from the largest tumor deposit should be used for AJCC staging, with ‘m’ indicating the presence of multiple tumors.
Generally, tumor cellularity decreases with tumor size; but this is quite variable and so their combined results are more informative [19]. In some cases, tumor size may not decrease, but overall cellularity may decrease markedly (Figure 1). Comparison of pre- and post-treatment cellularity is the key element of several systems for classifying residual disease, including the Miller–Payne and Pinder systems [10, 28]. These systems, however, do not state how to deal with heterogeneity, and it can be tempting to only assess the most cellular areas of the tumor. Even pretreatment cellularity is often heterogeneous, with pretreatment CNB only partly representing the tumor. Similarly, changes in tumor cellularity induced by NAST are commonly heterogeneous (Figure 1). Systematic sampling as described above is therefore needed to accurately assess cellularity.
Although it ignores pretreatment cellularity, the RCB system offers several advantages in addressing this heterogeneity. The RCB system standardizes sampling of specimens and interprets the average invasive cancer cellularity (by area) across the entire residual tumor bed. The residual tumor bed area is initially determined from the macroscopic evaluation combined with any specimen radiography, and revised after the corresponding tissue sections from that area have been studied under the microscope. Calibrating the observer's eyes to the online cellularity standard provided on the RCB website can be helpful [25]. The images in the publication for the Miller–Payne score are also helpful [28].
Assessment of RCB is quite reproducible [34]. This system helps to standardize gross and microscopic methods, and is more efficiently utilized in a prospective manner, rather than by retrospective review. As a first step, we advocate reporting the 2D size of the largest distance between residual tumor cell nests in a cross section of the entire area involved by residual tumor. The relevant sections should be recorded in the pathology report. Thus, the benefit of uniform sampling is achieved and RCB assessment, including average cellularity of the tumor bed area, can be carried out upon local or central review, as preferred.
margins
Assessment of margins may be less reliable post-NAST in cases with scattered response. Tumor bed extending to the margins should be documented.
evaluation of the axilla post-NAST
Post-NAST lymph node status is an important determinant of survival, regardless of response within the breast [7, 35–41]. The accuracy of SLNB after NAST is an important clinical research topic, especially for patients presenting with positive lymph nodes [17, 42].
Pathologic evaluation is the same as for non-NAST specimens, although lymph nodes may be more difficult to identify grossly. All surgically removed lymph nodes should be entirely submitted for histologic evaluation, sectioned at 2-mm intervals. Additional levels and immunohistochemistry (IHC) are not routinely required. The number of positive lymph nodes, size of the largest metastasis, and presence of micrometastasis and isolated tumor cells (ITCs) are predictors of worse survival and should be recorded [40, 43]. When ITCs (pN0i+) are present in the lymph nodes, this is not considered pCR. Molecular assays (e.g. OSNA) to evaluate sentinel lymph node (SLN) are not usually calibrated to detect ITCs [44] and are therefore not recommended post-NAST [45].
The presence of treatment effect in the lymph nodes may provide additional prognostic information and should be recorded [46]; however, this can be difficult to discern. Small fibrous scars suggestive of prior lymph node involvement or treatment effect can also be seen in patients without treatment [47]. NAST effect cannot always reliably be distinguished from previous biopsy site changes. Furthermore, granulomas can form around radiologic clips within lymph nodes and previously involved lymph nodes may look completely normal after NAST. The pathology report should state if a clip is identified and specify the histologic findings (involvement, possible treatment effect, or biopsy site changes) in the lymph node with the clip.
HR, HER2, and Ki67 post-NAST
Receptor status can differ between pre-NAST and post-NAST tumor samples. Two meta-analyses report discordant results of 13% and 18% for ER, 32% and 26% for PR, and 9% and 6% for HER2, respectively, before and after chemotherapy [48, 49]. Trastuzumab may increase the rate of negative conversion for HER2 [50, 51]. Reasons for this discordance include technical failure, intratumoral heterogeneity of marker expression, and changes induced by therapy. However, ER, PR, and HER2 assays are not 100% accurate and reproducible—i.e. repeating the assays will inevitably lead to some discordant results [52]. The reported rates of discordant results should be interpreted in the context of the expected discordance rate from technical variability in repeated measurements (∼10% for a 95% accurate test.)
In current practice, the choice of adjuvant therapy is dictated by the results at primary diagnosis. However, patients with residual disease that originally had negative receptor status can be re-tested to re-evaluate for eligibility for a targeted adjuvant treatment. Re-assessment of hormone receptor (HR) and HER2 in all cases with residual disease can be considered in clinical trials to gather high-quality data to clarify these issues. Otherwise, we recommend repeat testing only in circumstances where the clinical course or pathologic findings suggest repeat testing may yield a different result that would change treatment. If pre- and post-NAST results are discrepant, retesting of the pretreatment biopsy should also be considered.
Ki67 expression correlates with long-term outcome, whether natural prognosis or after endocrine [53] or chemo-endocrine therapy [54, 55]. Despite concerns about the analytical reproducibility of Ki67 measurements [56–58], the test is used at many institutions for basic risk assessment to tailor adjuvant therapy based on markedly low or high values that more reliably classify risk, and is a component of several multivariate prediction models in the post-NAST setting—e.g. the preoperative endocrine prognostic index (PEPI) and the residual proliferative cancer burden (RPCB) [53, 59].
pathologic complete response
To date, a variety of definitions of pCR have been used in neoadjuvant clinical trials in breast cancer, impeding cross-trial interpretation of data [5, 14]. In its guidance on the potential use of pCR to accelerate drug approval, the US FDA defines pCR as either ypT0/isypN0 or ypT0ypN0 [1, 5]. Indeed, there are excellent data and a strong consensus to include absence of disease in both the breast and the lymph nodes in a standard definition of pCR [1, 7, 35–41].
Whereas it is clear that patients with residual carcinoma in the lymph nodes only (ypT0/is ypN+) have a considerably inferior prognosis [1, 7, 35–41], the significance of residual in situ carcinoma [ductal carcinoma in situ (DCIS) alone] is not entirely clear. Both ypT0/is ypN0 and ypT0 ypN0 have comparable survival and are correlated with improved survival [1]. In a pooled analysis from the German Breast Group, absence of DCIS in addition to the absence of invasive carcinoma (ypT0 ypN0) identified a smaller group of patients with the best prognosis [3]. However, in patients treated at MD Anderson Cancer Center, there was no difference in survival between patients with ypT0 ypN0 and ypTis ypN0 [60]. Future studies should prospectively select either ypT0/is ypN0 or ypT0 ypN0 as a primary end point and state which definition is used; we would recommend also reporting the other as a secondary/exploratory end point. Pathologists should report DCIS, remembering to note if it is absent, so the data needed for further examination of outcomes associated with these two pCR definitions can be gathered.
Table 3 summarizes our recommendations for the assessment of pCR. IHC is not required but may be helpful to visualize tumor cells when hematoxylin and eosin (H&E) staining is inconclusive, or as part of an SOP for SLN evaluation. If residual tumor cells are present, they should be considered in the same manner whether identified on H&E or IHC. The companion pathology paper discusses occasionally controversial elements in detail [14].
Table 3.
Requirements for accurate and reliable histologic assessment of pathologic complete response (pCR)
| Assessment of pathologic complete response (pCR) | ||||
| pCR = No residual invasive carcinoma in the breast and in all sampled lymph nodes | ||||
| (ypT0/is ypN0 or ypT0 ypN0) [1, 5] | ||||
Requires adequate sampling of the correct area of the breast:
| ||||
| Immunohistochemistry is not routinely required but may be helpful. | ||||
| All surgically removed lymph nodes must be entirely submitted for histologic evaluation, sectioned at 2-mm intervals. (Additional levels and immunohistochemistry are not routinely required.) | ||||
| Occasionally controversial elements: | ||||
| pCR | NOT pCR | Insufficient evidence | Comment | |
| Ductal carcinoma in situ (DCIS) | x | x | pCR definitions vary [1, 3, 60]; adding pT0 or pTis clarifies the pCR definition | |
| Lobular carcinoma in situ | x | |||
| Lymphovascular invasion (LVI) | x | x | Very rarely a problem for designation as pCR or not because significant LVI-only residual disease without residual disease in the lymph nodes is extremely rare. | |
| Micro- and macrometastasis in lymph node(s) (pN1mic and above) | x | Residual disease in the lymph nodes confers a worse prognosis irrespective of the presence of disease in the breast [7, 35–41]. The significance of micrometastases and isolated tumor cells is different in the neoadjuvant setting than in the adjuvant setting [43]. |
||
| Isolated tumor cells in lymph node(s) (pN0i+) | x | |||
aThe FDA has recommended a minimum of one block per cm of pretreatment tumor size or at least 10 blocks in total, whichever is greater [5].
residual disease
Using pCR as the only indicator of response to NAST underestimates the clinical benefit a patient receives in terms of event-free survival. Simulations show that measures of residual disease can improve the power of neoadjuvant clinical trials and will improve estimates of survival benefits [61]. There is great interest to gain further prognostic information from the extent of residual disease through evaluation of, e.g. yp-stage, RCB, and PEPI, and also from the biology of residual disease, e.g. using Ki67 and multigene assays in residual disease [26, 53, 59, 62, 63].
Different classification systems can yield different estimates of future risk [41]. Systems that combine clinical, pathologic, and biomarker information pre- and post-NAST, thus incorporating information about pretreatment tumor burden, residual disease, and biology, will likely be the most useful [62]. For example, the clinical–pathologic stage-estrogen/grade (CPS-EG) combines pathologic stage post-NAST with clinical stage pre-NAST, nuclear grade, and ER status, and has been independently validated to identify patients with residual disease post-NAST who have a high risk of relapse [33]. Particularly in HR + HER2− breast cancer, pretreatment variables are prognostic beyond residual disease measures [62].
At present, we recommend both the RCB system and the current AJCC/UICC staging system to quantify residual disease in neoadjuvant trials. The RCB score incorporates pCR as a score of zero and combines findings in the primary tumor bed (size and average cellularity of largest cross section of residual tumor bed) and the regional lymph nodes (number of and size of largest metastases) to quantify increasing amounts of residual disease as an increasing continuous RCB score that is subdivided into four classes (0, I, II, and III) [26]. The quantitative RCB score can be incorporated into a multivariate model. RCB has been validated in several independent cohorts, and is prognostic at 5 years and beyond 10 years overall and in phenotypic subgroups [26, 62]. A prescriptive protocol for pathologists is available at http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3 [25].
conclusions
We propose a standardized evaluation of the post-NAST surgical specimen in breast cancer neoadjuvant clinical trials that can be optionally incorporated into routine practice and promotes accuracy and reproducibility of response assessment across institutions. Rather than exhaustive sampling, thorough sampling in the areas of the specimen identified by informed mapping, taking into account clinical and imaging information, is needed. The standard proposed also allows collection of research tissue and better serves the study of response to NAST. pCR and RCB have robust long-term prognostic data for breast cancer overall and within phenotypic subsets. The AJCC/UICC yp-staging system is internationally endorsed.
Clearly identifying resection specimens as post-NAST is essential. We recommend that the post-NAST pathology evaluation and pathology report include:
The information needed to determine pCR versus residual disease, using either of the definitions proposed by the FDA meta-analysis [1] (ypT0/is ypN0 or ypT0 ypN0).
AJCC/UICC ypT and ypN stage.
More detailed quantification of residual disease. Ideally, the RCB system and/or any other classification system that is locally preferred or required for a clinical trial protocol relevant to the patient is included in the report. When RCB is not reported, we advocate reporting the 2D size of the largest distance between residual tumor cell nests in a cross section of the entire area involved by residual tumor and identifying the relevant sections in the pathology report with at least a qualitative assessment of cellularity across this entire area.
We hope that direct, prospective comparisons of different classification systems will provide greater clarity for pathologic reporting of residual disease.
Finally, the pathologic assessment of residual disease forms an important component of multivariate approaches that combine pre- and post-treatment burden of disease and biological characteristics to better define prognosis after NAST.
disclosure
WFS filed Residual Cancer Burden (RCB) as intellectual property (Nuvera Biosciences), patenting the RCB equation. (The RCB calculator is freely available on the worldwide web.) WFS reports current stock in Nuvera Biosciences and past stock in Amgen.
GMG reports grants and personal fees from Roche, and personal fees from Sanofi Aventis, outside the submitted work. All remaining authors have declared no conflict of interest.
Supplementary Material
acknowledgements
We thank the BIG-NABCG leadership: Nancy E. Davidson, from University of Pittsburgh Cancer Institute and UPMC Cancer Center, Pittsburgh, Pennsylvania. Martine Piccart, of Institut Jules Bordet, Université Libre de Bruxelles, Brussels, Belgium. Larry Norton, Memorial Sloan-Kettering Cancer Center, New York, New York.
We also thank the following for providing their input: Helena Earl of the University of Cambridge Department of Oncology, UK, Chief Investigator of Neo-tAnGo and ARTemis trials; Emilio Alba, Ana Lluch, and Joan Albanell of GEICAM (Spanish Breast Cancer Research Group), Spain; Keith Amos of the University of North Carolina, Chapel Hill; Véronique Becette of Institut Curie-Hôpital René Huguenin, France; Wojciech Biernat of the Medical University of Gdansk, Poland; Hervé Bonnefoi of Institut Bergonié, France; Aman Buzdar of MD Anderson Cancer Center, Texas; Paul Cane of Guy's and St. Thomas' Hospitals, London; Sarah Pinder of Guy's and St. Thomas' Hospitals, London; Lesley Carson of Aberdeen Royal Infirmary, Foresterhill, NHS Grampian, Aberdeen, UK; Diana Dickson-Witmer of Christiana Care, Delaware; Gyungyub Gong of Asan Medical Center, University of Ulsan College of Medicine, Korea; Jimmy Green of Pathology Sciences Medical Group, Norfolk, Virginia; Chih-Yi Hsu of Taipei Veterans General Hospital, Taipei, Taiwan; Ling-Ming Tseng of Taipei Veterans General Hospital, Taipei, Taiwan; Judith Kroep of Leiden University Medical Center, Netherlands; A. Marilyn Leitch of UT Southwestern Medical Center, Texas; Venetia Sarode of UT Southwestern Medical Center, Texas; Eleftherios Mamounas of the National Surgical Adjuvant Breast and Bowel Project; Paul Kelly Marcom of Duke University, North Carolina; Paolo Nuciforo of Vall d'Hebron Institute of Oncology, Barcelona, Spain; Soonmyung Paik of the Yonsei University College of Medicine, Seoul, Korea, and the National Surgical Adjuvant Breast and Bowel Project; Vicente Peg of Vall d'Hebron University Hospital, Barcelona, Spain; David Peston of Charing Cross Hospital, London; Jean-Yves Pierga of Institut Curie, France; Miguel Quintela-Fandino of Centro Nacional de Investigaciones Oncológicas, Spain; Roberto Salgado of Institut Jules Bordet, Belgium; William Sikov of Women and Infants Hospital, Breast Health Center, Rhode Island; Jeremy Thomas of Western General Hospital, NHS Lothian, Edinburgh, UK; Gary Unzeitig of Laredo Breast Care, Texas; Jelle Wesseling of Netherlands Cancer Institute; and Marc Wilt of Centre Paul Strauss, Strasbourg, France.
We also thank Rebecca Enos of the EMMES Corporation for information gathering and for coordination and administrative support of the BIG-NABCG Residual Disease Characterization Working Group. We also thank the Breast Cancer Research Foundation (BCRF) for its support of the BIG-NABCG collaboration, including the BIG-NABCG meeting where this working group was proposed.
references
- 1.Cortazar P, Zhang L, Untch M et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 2.Esserman LJ, Berry DA, DeMichele A et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012; 30: 3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Minckwitz G, Untch M, Blohmer JU et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 4.Esserman LJ, Woodcock J. Accelerating identification and regulatory approval of investigational cancer drugs. JAMA 2011; 306: 2608–2609. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Guidance for industry: pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM305501.pdf (30 October 2014, date last accessed).
- 6.U.S. Food and Drug Administration News Release. FDA approves Perjeta for neoadjuvant breast cancer treatment: first drug approved for use in preoperative breast cancer. 2013. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm370393.htm (30 October 2014, date last accessed).
- 7.Mamounas EP, Anderson SJ, Dignam JJ et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012; 30: 3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan F. Evaluation and reporting of breast cancer after neoadjuvant chemotherapy. Open Pathol J 2009; 3: 58–63. [Google Scholar]
- 9.Marchio C, Sapino A. The pathologic complete response open question in primary therapy. J Natl Cancer Inst Monogr 2011; 2011: 86–90. [DOI] [PubMed] [Google Scholar]
- 10.Pinder SE, Provenzano E, Earl H, Ellis IO. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology 2007; 50: 409–417. [DOI] [PubMed] [Google Scholar]
- 11.Sahoo S, Lester SC. Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med 2009; 133: 633–642. [DOI] [PubMed] [Google Scholar]
- 12.Dieras V, Fumoleau P, Romieu G et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol 2004; 22: 4958–4965. [DOI] [PubMed] [Google Scholar]
- 13.Chevallier B, Roche H, Olivier JP et al. Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol 1993; 16: 223–228. [PubMed] [Google Scholar]
- 14.Provenzano E, Bossuyt V, Viale G et al. Standardization of pathologic evaluation and reporting of post-neoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol 2015; in press. [DOI] [PubMed] [Google Scholar]
- 15.Loi S, Symmans WF, Bartlett JM et al. Proposals for uniform collection of biospecimens from neoadjuvant breast cancer clinical trials: timing and specimen types. Lancet Oncol 2011; 12: 1162–1168. [DOI] [PubMed] [Google Scholar]
- 16.Braeuning MP, Burke ET, Pisano ED. Embolization coils as tumor markers for mammography in patients undergoing neoadjuvant chemotherapy for carcinoma of the breast. AJR Am J Roentgenol 2000; 174: 251–252. [DOI] [PubMed] [Google Scholar]
- 17.Kuehn T, Bauerfeind I, Fehm T et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14: 609–618. [DOI] [PubMed] [Google Scholar]
- 18.Houssami N, Ciatto S, Turner RM et al. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg 2011; 254: 243–251. [DOI] [PubMed] [Google Scholar]
- 19.Rajan R, Poniecka A, Smith TL et al. Change in tumor cellularity of breast carcinoma after neoadjuvant chemotherapy as a variable in the pathologic assessment of response. Cancer 2004; 100: 1365–1373. [DOI] [PubMed] [Google Scholar]
- 20.Peintinger F, Kuerer HM, McGuire SE et al. Residual specimen cellularity after neoadjuvant chemotherapy for breast cancer. Br J Surg 2008; 95: 433–437. [DOI] [PubMed] [Google Scholar]
- 21.Chagpar AB, Middleton LP, Sahin AA et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg 2006; 243: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coles CE, Wilson CB, Cumming J et al. Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: audit on behalf of the IMPORT Trial Management Group. Eur J Surg Oncol 2009; 35: 578–582. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC et al. (eds). American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition New York, NY: Springer; 2009. [Google Scholar]
- 24.Carey LA, Metzger R, Dees EC et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst 2005; 97: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 25.Residual Cancer Burden calculator and associated documents [Guide for Measuring Cancer Cellularity, Examples of Gross & Microscopic Evaluation, Pathology Protocol for Macroscopic and Microscopic Assessment of RCB]. Houston, Texas: MD Anderson Cancer Center; http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3 (30 October 2014, date last accessed). [Google Scholar]
- 26.Symmans WF, Peintinger F, Hatzis C et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25: 4414–4422. [DOI] [PubMed] [Google Scholar]
- 27.Sataloff DM, Mason BA, Prestipino AJ et al. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg 1995; 180: 297–306. [PubMed] [Google Scholar]
- 28.Ogston KN, Miller ID, Payne S et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 2003; 12: 320–327. [DOI] [PubMed] [Google Scholar]
- 29.National Cancer Institute. Breast Oncology Local Disease (BOLD) Task Force of the Breast Cancer Steering Committee. NCI BOLD Task Force Common Data Elements (CDEs) http://www.cancer.gov/aboutnci/organization/ccct/steering-committees/breast-cancer/ (30 October 2014, date last accessed).
- 30.Symmans WF. Pathologic Evaluation After Neoadjuvant Chemotherapy: Standardizing Management of the Surgical Specimen and Assessing Response to Neoadjuvant Therapies: The Promises and Challenges of Pathologic Complete Response. Regulatory Science and Policy Session, 8 April 2013. American Association for Cancer Research (AACR) Annual Meeting: Washington, DC, USA; 2013. http://webcast.aacr.org/console/player/20130?mediaType=audio& (30 October 2014, date last accessed). [Google Scholar]
- 31.Mukhtar RA, Yau C, Rosen M et al. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol 2013; 20: 3823–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene FL, Page DL, Fleming ID et al. (eds). American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th edition New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 33.Mittendorf EA, Jeruss JS, Tucker SL et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol 2011; 29: 1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peintinger F, Sinn B, Hatzis C et al. Reproducibility of Residual Cancer Burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol 2015; May 1 [epub ahead of print], doi:10.1038/modpathol.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouzier R, Extra JM, Klijanienko J et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol 2002; 20: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 36.McCready DR, Hortobagyi GN, Kau SW et al. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg 1989; 124: 21–25. [DOI] [PubMed] [Google Scholar]
- 37.Rastogi P, Anderson SJ, Bear HD et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008; 26: 778–785. [DOI] [PubMed] [Google Scholar]
- 38.Buchholz TA, Tucker SL, Masullo L et al. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol 2002; 20: 17–23. [DOI] [PubMed] [Google Scholar]
- 39.Hennessy BT, Hortobagyi GN, Rouzier R et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 2005; 23: 9304–9311. [DOI] [PubMed] [Google Scholar]
- 40.Klauber-DeMore N, Ollila DW, Moore DT et al. Size of residual lymph node metastasis after neoadjuvant chemotherapy in locally advanced breast cancer patients is prognostic. Ann Surg Oncol 2006; 13: 685–691. [DOI] [PubMed] [Google Scholar]
- 41.Corben AD, Abi-Raad R, Popa I et al. Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy: a comparison between classifications and their practical application. Arch Pathol Lab Med 2013; 137: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 42.Boughey JC, Suman VJ, Mittendorf EA et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher ER, Wang J, Bryant J et al. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 2002; 95: 681–695. [DOI] [PubMed] [Google Scholar]
- 44.Feldman S, Krishnamurthy S, Gillanders W et al. A novel automated assay for the rapid identification of metastatic breast carcinoma in sentinel lymph nodes. Cancer 2011; 117: 2599–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Visser M, Jiwa M, Horstman A et al. Intra-operative rapid diagnostic method based on CK19 mRNA expression for the detection of lymph node metastases in breast cancer. Int J Cancer 2008; 122: 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman LA, Pernick NL, Adsay V et al. Histopathologic evidence of tumor regression in the axillary lymph nodes of patients treated with preoperative chemotherapy correlates with breast cancer outcome. Ann Surg Oncol 2003; 10: 734–739. [DOI] [PubMed] [Google Scholar]
- 47.Donnelly J, Parham DM, Hickish T et al. Axillary lymph node scarring and the association with tumour response following neoadjuvant chemoendocrine therapy for breast cancer. Breast 2001; 10: 61–66. [DOI] [PubMed] [Google Scholar]
- 48.Jabbour MN, Massad CY, Boulos FI. Variability in hormone and growth factor receptor expression in primary versus recurrent, metastatic, and post-neoadjuvant breast carcinoma. Breast Cancer Res Treat 2012; 135: 29–37. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N, Moran MS, Huo Q et al. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest 2011; 29: 594–598. [DOI] [PubMed] [Google Scholar]
- 50.Mittendorf EA, Wu Y, Scaltriti M et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 2009; 15: 7381–7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Minckwitz G, Darb-Esfahani S, Loibl S et al. Responsiveness of adjacent ductal carcinoma in situ and changes in HER2 status after neoadjuvant chemotherapy/trastuzumab treatment in early breast cancer—results from the GeparQuattro study (GBG 40). Breast Cancer Res Treat 2012; 132: 863–870. [DOI] [PubMed] [Google Scholar]
- 52.Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist 2010; 15: 1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellis MJ, Tao Y, Luo J et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008; 100: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones RL, Salter J, A'Hern R et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2009; 116: 53–68. [DOI] [PubMed] [Google Scholar]
- 55.von Minckwitz G, Schmitt W, Loibl S et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res 2013; 19: 4521–4531. [DOI] [PubMed] [Google Scholar]
- 56.Dowsett M, Nielsen TO, A'Hern R et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011; 103: 1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris L, Fritsche H, Mennel R et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007; 25: 5287–5312. [DOI] [PubMed] [Google Scholar]
- 58.Polley MC, Leung S, McShane LM et al. An international Ki67 reproducibility study. J Natl Cancer Inst 2013; 105: 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheri A, Smith IE, Johnston SR et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol 2015; 26: 75–80. [DOI] [PubMed] [Google Scholar]
- 60.Mazouni C, Peintinger F, Wan-Kau S et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol 2007; 25: 2650–2655. [DOI] [PubMed] [Google Scholar]
- 61.Hatzis C, Gould RE, Zhang Y et al. Predicting Improvements in Survival Based on Improvements in Pathologic Response Rate to Neoadjuvant Chemotherapy in Different Breast Cancer Subtypes [Abstract P6-06-37]. San Antonio, TX: San Antonio Breast Cancer Symposium; 2013. [Google Scholar]
- 62.Symmans WF, Wei C, Gould R et al. Long-term Prognostic Value of Residual Cancer Burden (RCB) Classification Following Neoadjuvant Chemotherapy [Abstract S6-02]. San Antonio, TX: San Antonio Breast Cancer Symposium; 2013. [Google Scholar]
- 63.Earl HM, Chin S, Dunning M et al. Neo-tAnGo science: a translational study of PAM 50 sub-typing in sequential fresh tissue samples during neoadjuvant chemotherapy [Abstract 1015]. J Clin Oncol 2013; 31: (suppl; abstr 1015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.