Abstract
The 30% of patients whose indolent follicular lymphoma transforms to aggressive diffuse large B-cell lymphoma (DLBCL) have poor survival. Reliable predictors of follicular B-cell lymphoma transformation to DLBCL are lacking, and diagnosis of those that will progress is challenging. microRNA (miRNA), which regulate gene expression, have critical functions in the growth and progression of many cancers and contribute to the pathogenesis of lymphoma. Using five paired samples from patients that presented with follicular lymphoma and progressed to DLBCL, we identified specific miRNA differentially expressed between the two. Specifically, miR-17-5p levels were low in follicular lymphoma and increased as the disease transformed. In contrast, miR-31 expression was high in follicular lymphoma and decreased as the lymphoma progressed. These results were confirmed in additional unpaired cases of low-grade follicular lymphoma (n=13) and high-grade follicular lymphoma grade 3 or DLBCL (n=17). Loss of miR-31 expression in DLBCL was not due to deletion of the locus. Changes in miR-17-5p and miR-31 were not correlated with immunophenotype, genetics, or status of the MYC oncogene. However, increased miR-17-5p expression did significantly correlate with increased expression of TP53 protein, which is indicative of mutant TP53. Two pro-proliferative genes, E2F2 and PI3KC2A, were identified as direct mRNA targets of miR-31, suggesting that these may contribute to follicular lymphoma transformation. Our results indicate that changes in miR-31 and miR-17-5p reflect the transformation of follicular lymphoma to an aggressive large B-cell lymphoma and may, along with their targets, be viable markers for this process.
Keywords: B-cell lymphoma, DLBCL, follicular lymphoma, microRNA, transformation
Introduction
Follicular lymphoma (FL), a low-grade non-Hodgkin B-cell lymphoma, accounts for 20% of all lymphomas [1]. Translocation of the anti-apoptotic gene BCL2 to the immunoglobulin heavy chain locus IGH, t(14:18) occurs in 85% of cases [2]. Additionally, chromosomal rearrangements of the transcriptional repressor BCL6 are present in 5–15% of FL [3]. A 28% rate of transformation at 10 years of low-grade FL (WHO grades FL1 and FL2) into a more aggressive large cell lymphoma, either WHO grade 3 FL (FL3) or diffuse large B-cell lymphoma (DLBCL), was reported with a cumulative rate of transformation of 3% per year [4]. Follicular lymphoma grade 3 is characterized by neoplastic follicles that contain greater than 15 large transformed cells per high power field (FL3A) or are composed solely of large transformed cells (FL3B). DLBCL consists of sheets of large transformed B cells with loss of the follicular growth pattern [1]. Both of these transformed lymphomas progress rapidly, require aggressive chemotherapy and have poor survival rates [4]. Multiple pathways of transformation to DLBCL have been proposed [5–9], yet what distinguishes one FL that transforms from one that does not progress still remains unclear. Elucidation of mechanisms of transformation may suggest novel therapeutic targets to prevent or arrest transformation. In addition, early markers predicting the likelihood of FL transformation would help to initiate aggressive therapy before the patient developed widespread involvement by FL3 or DLBCL.
miRNA are small non-coding RNA that regulate gene expression post-transcriptionally by binding to the 3′ untranslated region (UTR) of mRNA, inhibiting translation and/or facilitating mRNA degradation. They are critical modulators of many biological processes, such as cell growth, differentiation, and apoptosis, linked to the development and progression of many tumor types, including lymphomas [10]. The miR-17-92 cluster is induced by the oncogene MYC, which drives numerous malignancies including Burkitt lymphoma and many DLBCL [11, 12]. Overexpression of the miR17-92 polycistron specifically in B cells induces B-cell lymphoma development, leading to its oncomiR label [13, 14]. Other miRNA are thought to be tumor suppressor miRNA, since their expression inhibits tumor development or progression [10]. Still other miRNA, such as miR-31, have been linked to both oncogenic and tumor suppressive functions in different cancers [15].
There have been attempts to distinguish different lymphoma subtypes by the miRNA they express, which has led to miRNA signatures characterizing FL and DLBCL [5, 16]. Here, we compare miRNA expression from paired samples of low-grade FL and aggressive FL3 or DLBCL biopsied when the patient progressed. Our analyses of these lymphomas identify miR-17-5p and miR-31 as differentially regulated miRNA in FL compared to DLBCL. Our data show that as FL progresses to aggressive large B-cell lymphoma, miR-17-5p increases and miR-31 decreases, establishing these two miRNAs as markers of FL progression.
Materials & Methods
Patient selection
Vanderbilt University Medical Center hematopathology/flow cytometry databases from 2000–2014 were searched for patients with a diagnosis of FL or DLBCL with IRB approval. Diagnostic slides from those patients with biopsies of both low-grade FL and FL3 or DLBCL were reviewed by two hematopathologists (MAT and SM-T). Confirmation of FL was based upon morphology and immunohistochemical or flow cytometric positivity for CD10, immunohistochemical positivity for BCL6, or cytogenetic demonstration of the t(14;18) IGH/BCL2 translocation. Only DLBCL with CD10 positivity were included, and DLBCL with cytogenetic documentation of MYC translocation were excluded. Of the 13 non-matched DLBCL, 5 had a history of FL prior to the DLBCL or residual regions of FL in the current biopsy (Table 1). Four additional cases had IGH/BCL2 translocation by FISH or PCR, suggesting a FL origin. Of the 13 non-matched FL1/FL2 patients, 10 had 1 to 3 year follow-up available, and of those 3 progressed to FL3/DLBCL (Table 1). Four of the FL1/FL2 had a previous FL3 or DLBCL. The paired samples from one patient that demonstrated the most uniform histology of FL1 and DLBLC were selected for the array analysis. The WHO criteria for grades 1–3 follicular lymphoma were used [1]. Pertinent patient information is listed in Table 1. The non-neoplastic normal lymph node samples were diagnosed as “reactive follicular hyperplasia.”
Table 1.
Patient and lymphoma characteristics
| IDa | Site | Gender | Age | DX | IHC | Genetics | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCL2 | BCL6 | MYC | P53 | IGH/BCL2 | Other | |||||
| 1A | LN | M | 71 | FL1 | + | ND | − | − | ||
| 1B | Spleen | M | 66 | DLBCL | + | ND | ND | ND | 6q–12p, 12q+, 18+ | |
| 2A | LN | F | 70 | FL2 | + | ND | ND | ND | ||
| 2B | LN | F | 71 | FL3A | + | ND | − | + | + by PCR | |
| 3A | LN | M | 70 | FL2 | + | ND | − | + | ||
| 3B | LN | M | 70 | FL3 | + | ND | − | + | ||
| 4A | LN | M | 54 | FL1 | + | ND | − | − | ||
| 4B | LN | M | 59 | DLBCL | + | + | ND | ND | + by FISH | |
| 5A | LN | M | 61 | FL2 | + | ND | − | − | ||
| 5B | Mesenteric | M | 68 | FL3A | + | + | − | − | + by PCR | |
| 6 | LN | M | 49 | FL2 | + | ND | − | − | ||
| 7b | LN | F | 63 | FL2 | + | ND | ND | ND | + by PCR | |
| 8b,c | LN | M | 84 | DLBCL | ND | + | + | + | ||
| 9c | LN | F | 61 | DLBCL | + | ND | − | + | + by PCR | |
| 10c | LN | F | 60 | DLBCL | + | ND | + | + | ||
| 11 | LN | F | 65 | FL2 | ND | ND | ND | ND | ||
| 12b | LN | F | 80 | DLBCL | + | ND | + | + | ||
| 13b | Tongue | F | 91 | FL2 | + | + | − | + | Hyper-diploid | |
| 14 | LN | M | 63 | FL1 | ND | ND | − | − | + by PCR | |
| 15 | Peri-orbital | M | 62 | FL1 | + | ND | − | − | ||
| 16d | LN | M | 42 | FL1 | + | ND | − | − | − by PCR | |
| 17b | LN | M | 51 | FL3B | + | ND | − | − | − by PCR | |
| 18 | LN | M | 51 | FL3B | − | + | ND | − | − by FISH | |
| 19 | LN | M | 64 | FL3 | + | ND | − | − | + by PCR | |
| 20c | LN | M | 73 | DLBCL | + | + | + | + | ||
| 21 | LN | F | 63 | FL2 | + | ND | − | − | ||
| 22d | LN | M | 65 | FL1 | + | ND | + | − | + by PCR | |
| 23 | LN | M | 65 | DLBCL | + | ND | ND | ND | + by FISH | 3 copies BCL6 |
| 24 | Ileal mass | M | 6 | DLBCL | + | ND | + | − | − by FISH | Variable copies of BCL6 and MYC |
| 25d | LN | M | 76 | FL3 | + | + | ND | ND | + by FISH | |
| 26 | Spleen | M | 88 | DLBCL | ND | + | ND | ND | ||
| 27 | LN | F | 43 | FL2 | ND | ND | ND | ND | ||
| 28 | LN | M | 63 | FL2 | + | + | ND | ND | ||
| 29 | LN | M | 56 | FL2 | + | ND | ND | ND | ||
| 30d | LN | M | 49 | FL2 | + | + | ND | ND | ||
| 31 | Soft tissue | M | 54 | DLBCL | + | ND | + | − | + by FISH | |
| 32 | Bone | F | 66 | DLBCL | + | + | − | − | + by FISH | Loss of 1 MYC, 1 BCL6 |
| 33c | Mesenteric | F | 49 | DLBCL | ND | ND | ND | ND | + by FISH | +8 |
| 34 | LN | F | 79 | DLBCL | ND | + | ND | ND | + by FISH | Extra MYC, IGH, BCL2 |
| 35 | Naso-pharynx | F | 87 | DLBCL | + | + | ND | ND | − by FISH | |
Sample IDs with an A and a B are from the same patient;
due to limited sample size only miR-31 or miR-17-5p expression was determined for these cases.
previous FL1/FL2 or adjacent FL;
subsequent FL3/DLBCL;
abbreviations: LN, lymph node; DX, diagnosis; IHC, immunohistochemistry; FL, follicular lymphoma, grade 1, 2, 3A, or 3B; DLBCL, diffuse large B-cell lymphoma; PCR, polymerase chain reaction; FISH, fluorescence in situ hybridization; ND, no data.
miRNA isolation and expression analysis
Total RNA was isolated from paraffin sections of archival lymphoma biopsies (RecoverAll, Ambion). For cell lines, miRNA was isolated with Trizol (Life Technologies) as we previously reported [17]. TaqMan microRNA low density arrays (TLDA; 365 human miRNA and two snoRNA; Applied Biosystems) were performed in duplicate by the Vanderbilt Technologies for Advanced Genomics core facility on a FL1 and a DLBCL biopsied at separate times from a single patient (1A and 1B in Table 1). Data were analyzed with RQ manager software, and the individual qRT-PCR reactions were reviewed to assess the quality of each reaction. qRT-PCR for specific miRNA were performed using the Applied Biosystems TaqMan microRNA assay as previously reported [17]. Small RNA controls, RNU24 for patient samples and RNU6 for cell lines, were used to normalize the data. Where indicated, miRNA data were normalized to results from normal lymph node. Unpaired and paired t-tests were performed to determine significance.
Fluorescence In Situ Hybridization (FISH)
FISH probes for the MIR31 locus (chromosome 9p21) and the CDKN2A locus (chromosome 9p22) were prepared by labeling BAC-PAC clones RP11-354P17 and RP11-149I2, respectively, (Children’s Hospital and Research Center). DNA was labeled by nick translation (Abbott Laboratories, Des Plaines, IL) using Spectrum Green dUDP for RP11-354P17 (MIR31) and Spectrum Orange dUDP for RP11-149I2 (CDKN2A) following manufacturer’s protocol. Paraffin tissue sections were processed according to standard protocols, including a protease step. Probe hybridization was performed in a HYBrite (Abbott) with denaturation at 73°C for 6 min and overnight hybridization at 37°C. The slides were washed, DAPI counterstained, and viewed by fluorescence microscopy with Applied Imaging CytoVision software.
Immunohistochemistry (IHC)
IHC was performed with heat-induced antigen retrieval on the Leica Bond Max IHC stainer using the Epitope Retrieval 2 solution. Antibodies included rabbit anti-human c-MYC monoclonal (Y69, ab32072, Abcam Laboratories) (20 min retrieval, 1 hr incubation with 1:600 dilution); mouse anti-human p53 monoclonal (DO-7, Leica Biosystems) (30 min retrieval, 30 min incubation with ready to use (RTU) antibody); mouse anti-human BCL2 monoclonal (124, bcl-2-100-D5, Leica) (30 min retrieval, 15 min incubation with RTU antibody); and mouse anti-human BCL6 monoclonal (LN22, PA0204, Leica) (10 min retrieval, 15 minute incubation with RTU antibody). The Leica Bond Polymer Refine Detection (DAB) system was used for visualization. Samples were considered positive if >25% of tumor cells were positive for the antibody, except for MYC for which >40% positivity is the standard cut-off [18].
Luciferase assays
Genomic DNA from normal human white blood cells was used to clone 3′-UTR sequences of E2F2 and PI3KCA2 into the pMIR-REPORT Luciferase miRNA Expression Reporter Vector (Life Technologies). Luciferase assays were conducted as previously described [19].
Results
Altered expression of miR-31 and miR-17-5p as FL transform
To determine whether miRNA would distinguish a low-grade FL from one that had transformed to a large B-cell lymphoma (FL3 or DLBCL), we measured in duplicate the expression of miRNA in a FL grade 1 and a DLBCL from the same patient by TLDA. Of the 365 miRNA on the TLDA, inspection of individual reactions demonstrated a successful reaction in both lymphoma samples for 207 miRNAs. Of these miRNA, 38 showed a 5-fold or greater decrease and 4 had a 5-fold or greater increase (Fig. 1) in DLBCL compared to FL1. These results suggest the miRNA expressed in low-grade FL changes as the lymphoma transforms to a more aggressive lymphoma, with a preponderance of the miRNAs demonstrating decreased expression with transformation.
Figure 1. More miRNA decrease than increase as FL transforms to large B-cell lymphoma.
miRNA expression in a low grade FL (FL1, 1A in Table 1) and a FL that had transformed to a diffuse large B-cell lymphoma (DLBCL, 1B in Table 1) from the same patient were assessed by TLDA in duplicate. miRNA levels were normalized to RNU24 and the fold difference in miRNA expression in DLBCL compared to FL is graphed on a log scale for those miRNA with 5 fold or greater change in expression. Grey bars indicate those that were subjected to additional evaluation (see Figure 2).
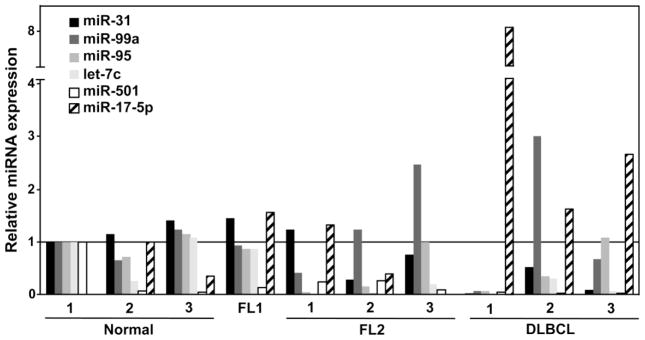
To validate the TLDA data, we measured the levels of the 6 miRNA (miR-31, miR-99a, miR-95, let-7c, miR-501, and miR-17-5p) that had the largest absolute difference in expression and lowest Ct values by performing Taqman qRT-PCR. Samples from patients with FL1, FL2, or DLBCL and three normal lymph nodes were evaluated. The expression of miR-17-5p, an oncogenic miRNA [10], showed a trend towards increased expression in the higher grade lymphomas (Fig. 2). In contrast, in the higher grade lymphomas, the levels of miR-31 decreased (Fig. 2). Let-7c levels showed decreased expression in FL2 and DLBCL compared to normal lymph nodes and FL1, while miR-99a and miR-95 did not show a consistent change in expression with the grade of lymphoma. miR-501 levels were variable in the normal samples and decreased in all the lymphomas regardless of grade.
Figure 2. miR-31 levels decrease and miR-17-5p levels increase as FL progresses.
miRNA levels in samples of the indicated lymphoma grade and normal lymph nodes were measured by qRT-PCR. Data were normalized first to an internal RNA control RNU24 and then to the results of RNA from normal lymph node #1, which was set at one.
Decreased miR-31 and increased miR-17-5p distinguish DLBCL from FL
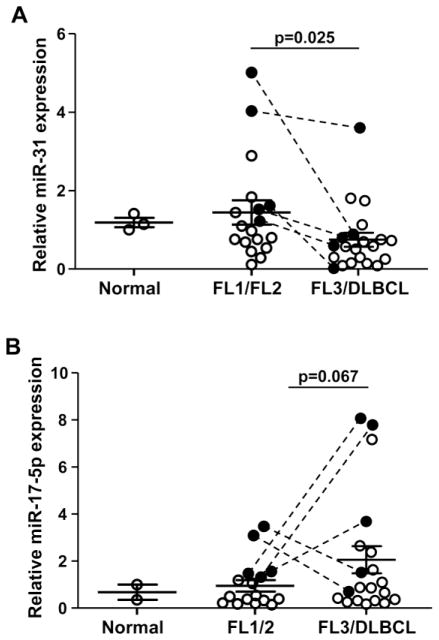
We focused on two of the miRNA that showed the greatest distinction between FL1/FL2 and DLBCL. MiR-31 was decreased and miR-17-5p was increased in the transformed lymphomas. Levels of miR-31 and miR-17-5p were then measured in 5 sets of matched samples from patients that were diagnosed with low-grade FL (FL1 or FL2) that progressed to FL3 or DLBCL. There were significantly reduced levels of miR-31 in FL3/DLBCL compared to FL1/FL2 (p<0.05, paired t-test; Fig. 3A). There was a trend toward significance for increased miR-17-5p in FL3/DLBCL (p=0.16, paired t-test; Fig. 3B). miR-31 and miR-17-5p levels were then measured in non-matched samples from additional patients with different grades of FL and DLBCL. Combining the matched and the unmatched samples, there was a significant decrease in miR-31 levels in FL3/DLBCL compared to FL1/FL2 (p=0.025 unpaired t-test; Fig 3A). There was an increase in miR-17-5p in FL3/DLBCL compared to FL1/FL2 that trended toward significance (p=0.067, unpaired t-test; Fig. 3B).
Figure 3. miR-17-5p increases and miR-31 decreases as the grade of FL increases.
miR-31 (A) and miR-17-5p (B) expression in matched (filled circle with line connecting) and unmatched samples of the indicated grade of FL were measured with qRT-PCR. Data were normalized to internal RNU24 levels and then to normal lymph node, which was set at 1. Each circle indicates a separate sample. Unpaired-t-tests were performed to determine significance.
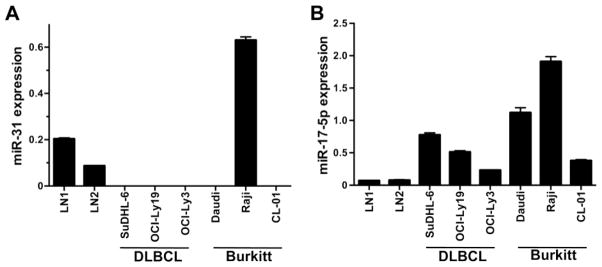
To further investigate changes in miR-31 and miR-17-5p levels in lymphomas, we evaluated miRNA expression in aggressive B-cell lymphoma cell lines derived from DLBCL and Burkitt lymphoma compared to normal human lymph nodes. qRT-PCR revealed a decrease in miR-31 in all lymphoma lines except one, Raji, compared to lymph node controls (Fig 4A). miR-17-5p showed increased expression in all DLBCL and Burkitt lymphoma lines (Fig. 4B). These data provide confirmation of the patient data for miR-31 and miR-17-5p.
Figure 4. Aggressive B-cell lymphoma lines express reduced miR-31 and increased miR-17-5p levels.
Levels of miR-31 (A) and miR-17-5p (B) were measured by qRT-PCR in DLBCL and Burkitt’s lymphoma cell lines and normal lymph nodes. Data were normalized to RNU6b levels.
miR-31 is not deleted in DLBCL
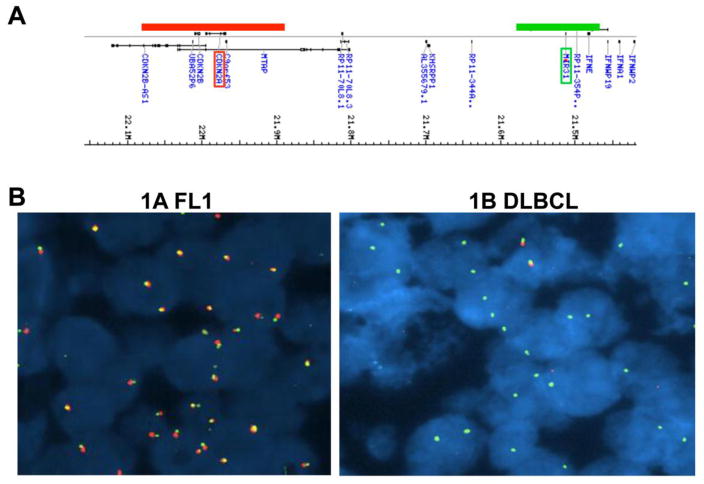
Deletions in the CDKN2A and CDKN2B loci encoding the cell cycle inhibitor p15INK4B and the tumor suppressors 16INK4A/p14ARF, respectively are detected in approximately 36% of DLBCL [20]. Since these loci are located near miR-31 on chromosome 9 (9p21.1 and 9p21.3, respectively; Fig. 5A), we evaluated whether the down regulation of miR-31 expression was a consequence of loss of the region encompassing both CDKN2A/B and MIR31. CDKN2A/B deletion was assayed by FISH for 15 DLBCL samples. Deletion of one copy of the CDKN2A/B locus was demonstrated in 3 DLBCL samples (Fig 5B; Table 1). However, all of the lymphoma samples had two copies of the MIR31 locus in the majority of cells (Fig 5B; Table 1). Therefore, the decrease in miR-31 expression in the transformed FLs was not due to deletion of the MIR31 locus.
Figure 5. miR-31 is not deleted with CDKN2A in DLBCL.
(A) Location on chromosome 9 of CDKN2A and MIR31 and the probes used for FISH. (B) FISH for CDKN2A (red) and MIR31 (green) was performed on paraffin sections of tissue from samples 1A (FL1) and 1B (DLBCL) from the same patient. A DAPI co-stain delineates the nuclei. A representative picture of each hybridization is shown.
Correlations of established markers of lymphoma to miR-31 or miR-17-5p expression
To determine other markers of disease that correlated to miRNA levels and progression of the lymphoma, we analyzed immunophenotype by flow cytometry and IHC, and levels of the oncogene MYC and the tumor suppressor TP53 protein by IHC. All cases demonstrated strong CD10 expression, a requirement to be included in this study. BCL2 expression was evaluated by IHC in 33 out of 40 samples, and was positive in all but one case (Table 1). The BCL2 negative case was also negative for the IGH/BCL2 rearrangement by FISH. This case was FL3 and had higher levels of miR-31 and miR-17-5p. Loss of BCL2 expression with transformation to DLBCL has been previously described [21]. BCL6 expression was evaluated by IHC in 13 of 40 samples and all were positive (Table 1); these cases expressed varying levels of miR-31 and miR-17-5p.
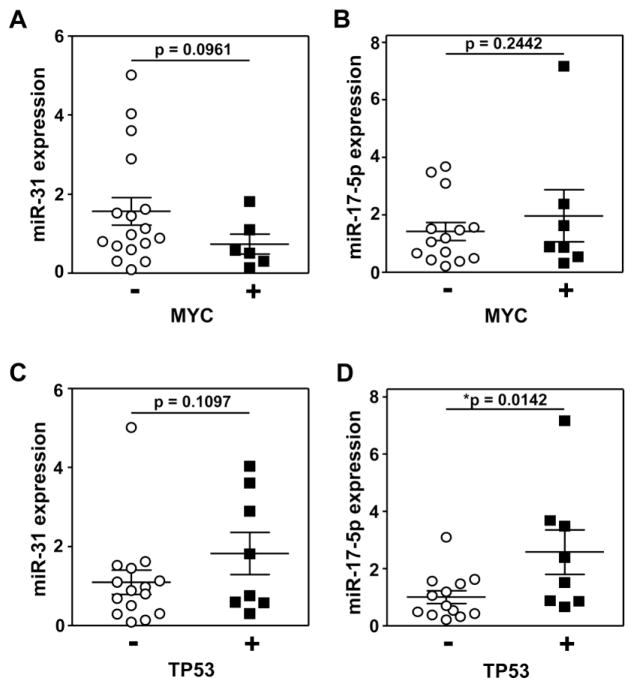
Alterations in MYC and TP53 are linked to lymphomagenesis [22, 23]. Additionally, it has been reported that the miR-17-92 cluster is a transcriptional target of MYC [24]. Therefore, we evaluated by IHC the expression of MYC and TP53 protein in the majority of the samples (24 for MYC, 25 for TP53). A similar percentage (61–64%) of FL1/2 and FL3/DLBCL samples were analyzed. Seven of the lymphomas evaluated were positive for MYC expression, defined as ≥40% positivity of the cells (Table 1). Only one FL1 case and no FL2 cases analyzed had increased MYC levels. Of the six MYC positive lymphomas for which there was sufficient tissue to evaluate miR-31, the data showed a trend towards significance of correlation between decreased miR-31 expression and increased MYC levels (Figure 6A). miR-17-5p was evaluated in all seven cases with increased MYC, and there was a slight trend towards increased miR-17-5p with increased MYC (Figure 6B). Elevated TP53 protein levels, indicative of mutant TP53 [25] and experimentally defined as TP53 expressed in ≥50% of cells, were detected by IHC in eight lymphomas (Table 1). None of the FL1 cases had increased TP53. There was a trend towards significance of correlation between elevated TP53 and increased miR-31 (Figure 6C). Notably, increased TP53 significantly positively correlated with increased miR-17-5p expression (p=0.014; Figure 6D). The data suggest there may be functional links between miR-31 and miR-17-5p and up-regulation of MYC and mutated TP53. The most significant correlation is between miR-17-5p and mutated TP53.
Figure 6. MYC and TP53 protein expression may correlate to miR-31 and/or miR-17-5p expression.
Samples of FL1, FL2, FL3, and DLBCL were subjected to IHC for MYC (A, B) and/or TP53 (C, D) as indicated in Table 1. miR-31 (A, C) and miR-17-5p (B, D) levels were measured by qRT-PCR and normalized to RNU24 levels. Each circle is a separate sample. Mean with SEM graphed; p values determined by t-tests.
miR-31 directly targets two mRNA linked to proliferation, E2F2 and PIK3C2A
miRNA functions are defined by their target mRNA. We utilized target prediction programs (e.g., TargetScan) to identify potential mRNA targets of miR-31 in lymphoma. Of potential targets identified, we focused on E2F2 and PIK3C2A due to their pro-growth functions [26, 27]. We assessed whether the mRNA encoded by these two genes were direct targets of miR-31 by luciferase assays in which the 3′-UTR of E2F2 or PIK3C2A was cloned 3′ to the luciferase coding sequence. Luciferase assays revealed that when miR-31, but not miR-17-5p, was present, luciferase activity was decreased in cells transfected with the E2F2 3′-UTR reporter vector (Fig 7A) or with the PIK3C2A 3′-UTR reporter vector (Fig 7B). The cell cycle inhibitor p21 (CDKN1A) has previously been shown to be a direct target of members of the miR-17-92 polycistron (e.g., miR-17-5p and 20a) [28], and our data support this result (Fig 7C). Therefore, E2F2 and PIK3C2A are direct targets of miR-31 and CDKN1A of miR-17-5p. These data suggest that the decrease in miR-31 and the increase in miR-17-5p should result in an increase in E2F2 and PI3KC2A and a decrease in p21, respectively, in FL as they transform.
Figure 7. E2F2 and PIK3CA are direct targets of miR-31.
Luciferase reporter assays were performed in triplicate in 292T cells following transfection with luciferase vectors encoding the 3′-UTR from E2F2 (A), PIK3C2A (B), and p21 (C) and miR-31 mimic, miR-17-5p mimic, miR-20a mimic, or RNA control (Cntrl) as indicated. Expression is relative to β-galactosidase activity. Error bars are SEM; A, *p=0.001; B, *p=0.018; C, *p=0.0005, **p=0.036, t-tests.
Discussion
We have investigated the role of miRNA in the progression of low grade FL (FL1 or FL2) to a more aggressive large B-cell lymphoma (FL3 or DLBCL). We identified miRNA whose levels change during this transformation and showed that many miRNA were down-regulated during this process, whereas fewer miRNA were up-regulated. These results suggest the miRNA may have a role in tumor suppression or progression, respectively. Specifically, we determined that the levels of miR-31 decreased and miR-17-5p increased with disease progression. These data and the identification of targets of these microRNA, provide new insight into the mechanism of FL progression that may have clinical utility as prognostic markers or therapeutic targets.
miR-31 can function as both an inhibitor and a facilitator of tumorigenesis, depending on the malignancy [15]. We determined that miR-31 levels decrease as FLs transform. Reduced miR-31 has also been reported in adult T cell leukemia, glioma, melanoma, mesothelioma, and malignancies of the bladder, esophagus, ovary, and prostate. However, miR-31 levels are increased in colorectal, lung, and pancreatic cancer and osteosarcoma (reviewed in [15]). Clearly, the targets of miR-31 must differ depending on the cell type and its effects on the particular cancer. We assessed potential targets by luciferase assay, and E2F2 and PIK3C2A were shown to be direct targets of miR-31. Therefore, miR-31 may be functioning as a tumor suppressor in this context, with reduction of miR-31 in the transforming lymphoma presumably allowing for increased expression of E2F2 and thus, the pro-proliferative genes that E2F2 regulates [26, 27]. In addition, lowering the level of the kinase PIK3C2A has been shown in other systems to inhibit proliferation and trigger apoptosis [29]. Therefore, relief of repression of PIK3C2A by reduction of miR-31 may contribute to transformation.
Two of the most common causes for why a miRNA is expressed at lower levels in a cancer are deletion of its gene locus and altered methylation of its promoter. Our FISH data show that the reduction in miR-31 levels is not due to deletion of the MIR31 locus, even when the nearby CDKN2A/B locus was deleted. Therefore, altered epigenetic regulation of MIR31 is likley the cause of its reduced expression in DLBCL. In support of this concept, the promoter of MIR31 in breast and prostate cancer has been reported to be hypermethylated [30–32] or transcriptionally repressed due to methylation of H3K4 [33] or H3K27 [32], leading to reduced miR-31 expression.
The miR-17-92 polycistron is critical for B-cell development [34], is overexpressed or amplified in multiple cancers, and is an established oncogenic cluster for lymphoma [10, 11]. Overexpression of miR-17-92 in B cells initiates B-cell lymphoma development [13, 14], but its link to lymphoma progression has been unclear. Here we show that the levels of miR-17-5p, a member of this polycistron, increase as the grade of FL increases, indicating that it likely contributes to lymphoma progression. Moreover, multiple members of the miR-17-92 polycistron are reported to be expressed at higher levels in DLBCL compared to FL [5, 16], supporting its link to progression to a more aggressive lymphoma. Moreover, there was an association between increased miR-17-5p and elevated (mutant) TP53, which also indicates progressive disease. Genome wide copy number analysis showed amplifications at 13q31.3, including the miR-17-92 polycistron, in 7% of FL and 15% of transformed FL cases tested, demonstrating one mechanism for elevated expression [6]. Additionally, MYC overexpression has been shown to lead to increased expression of the miR-17-92 polycistron [24]. However, in our study the lymphomas that had the highest levels of miR-17-5p were not those that showed MYC overexpression by IHC. We also verified that p21 is a direct target of miR-17-5p; down-regulation of this cell cycle regulator is likely to contribute to the increased proliferation of the transformed lymphoma.
Thus our data showing that miR-31 decreases and miR-17-5p increases as FLs transform into a higher grade, more aggressive lymphoma provides insight into a complex transformative process. Additional investigation into the regulation of these miRNA and their targets should have significant clinical ramifications at the diagnostic, prognostic, and potentially therapeutic levels.
Acknowledgments
This work was supported by R01CA177786 (CME), American Cancer Society-Kirby Foundations Fund Postdoctoral Fellowship PF-13-088-01-CSM (MDE), the CTSA UL1TR000445 from the National Center for Advancing Translational Sciences, NCI Cancer Center Support Grant P30CA068485 utilizing the Vanderbilt Technologies for Advanced Genomics core facility, and the Vanderbilt Department of Pathology, Microbiology and Immunology.
Footnotes
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. [Google Scholar]
- 2.Horsman DE, Gascoyne RD, Coupland RW, Coldman AJ, Adomant SA. Comparison of cytogenetic analysis, southern analysis, and polymerase chain reaction for the detection of t(14;18) in follicular lymphoma. AJCP. 1995;103:472–8. doi: 10.1093/ajcp/103.4.472. [DOI] [PubMed] [Google Scholar]
- 3.Katzenberger T, Ott G, Klein T, Kalla J, Muller-Hermelink HK, Ott MM. Cytogenetic alterations affecting BCL6 are predominantly found in follicular lymphomas grade 3B with a diffuse large B-cell component. Am J Pathol. 2004;165:481–90. doi: 10.1016/S0002-9440(10)63313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montoto S, Davies AJ, Matthews J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25:2426–33. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 5.Lawrie CH, Chi J, Taylor S, et al. Expression of microRNAs in diffuse large B cell lymphoma is associated with immunophenotype, survival and transformation from follicular lymphoma. J Cell Mol Med. 2009;13:1248–60. doi: 10.1111/j.1582-4934.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouska A, McKeithan TW, Deffenbacher KE, et al. Genome-wide copy-number analyses reveal genomic abnormalities involved in transformation of follicular lymphoma. Blood. 2014;123:1681–90. doi: 10.1182/blood-2013-05-500595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okosun J, Bodor C, Wang J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet. 2014;46:176–81. doi: 10.1038/ng.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies AJ, Rosenwald A, Wright G, et al. Transformation of follicular lymphoma to diffuse large B-cell lymphoma proceeds by distinct oncogenic mechanisms. Br J Haematol. 2007;136:286–93. doi: 10.1111/j.1365-2141.2006.06439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Jenson SD, Lim MS, Elenitoba-Johnson KS. Application of SELDI-TOF mass spectrometry for the identification of differentially expressed proteins in transformed follicular lymphoma. Mod Pathol. 2004;17:670–8. doi: 10.1038/modpathol.3800100. [DOI] [PubMed] [Google Scholar]
- 10.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelicci PG, Knowles DM, 2nd, Magrath I, Dalla-Favera R. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1986;83:2984–8. doi: 10.1073/pnas.83.9.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin HY, Oda H, Lai M, et al. MicroRNA-17~92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathways. EMBO J. 2013;32:2377–91. doi: 10.1038/emboj.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu SK, Fassan M, Volinia S, Lovat F, Balatti V, Pekarsky Y, Croce CM. B-cell malignancies in microRNA Emu-miR-17~92 transgenic mice. Proc Natl Acad Sci U S A. 2013;110:18208–13. doi: 10.1073/pnas.1315365110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurila EM, Kallioniemi A. The diverse role of miR-31 in regulating cancer associated phenotypes. Genes Chromosomes Cancer. 2013;52:1103–13. doi: 10.1002/gcc.22107. [DOI] [PubMed] [Google Scholar]
- 16.Roehle A, Hoefig KP, Repsilber D, et al. MicroRNA signatures characterize diffuse large B-cell lymphomas and follicular lymphomas. Br J Haematol. 2008;142:732–44. doi: 10.1111/j.1365-2141.2008.07237.x. [DOI] [PubMed] [Google Scholar]
- 17.Arrate MP, Vincent T, Odvody J, Kar R, Jones SN, Eischen CM. MicroRNA biogenesis is required for Myc-induced B-cell lymphoma development and survival. Cancer Res. 2010;70:6083–92. doi: 10.1158/0008-5472.CAN-09-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–7. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 19.McGirt LY, Adams CM, Baerenwald DA, Zwerner JP, Zic JA, Eischen CM. miR-223 regulates cell growth and targets proto-oncogenes in mycosis fungoides/cutaneous T-cell lymphoma. J Invest Dermatol. 2014;134:1101–7. doi: 10.1038/jid.2013.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guney S, Bertrand P, Jardin F, Ruminy P, Kerckaert JP, Tilly H, Bastard C. Molecular characterization of 9p21 deletions shows a minimal common deleted region removing CDKN2A exon 1 and CDKN2B exon 2 in diffuse large B-cell lymphomas. Genes Chromosomes Cancer. 2011;50:715–25. doi: 10.1002/gcc.20893. [DOI] [PubMed] [Google Scholar]
- 21.Llanos M, Alvarez-Arguelles H, Aleman R, Oramas J, Diaz-Flores L, Batista N. Prognostic significance of Ki-67 nuclear proliferative antigen, bcl-2 protein, and p53 expression in follicular and diffuse large B-cell lymphoma. Med Oncol. 2001;18:15–22. doi: 10.1385/MO:18:1:15. [DOI] [PubMed] [Google Scholar]
- 22.Sewastianik T, Prochorec-Sobieszek M, Chapuy B, Juszczynski P. MYC deregulation in lymphoid tumors: molecular mechanisms, clinical consequences and therapeutic implications. Biochim Biophys Acta. 2014;1846:457–67. doi: 10.1016/j.bbcan.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Xu-Monette ZY, Medeiros LJ, Li Y, et al. Dysfunction of the TP53 tumor suppressor gene in lymphoid malignancies. Blood. 2012;119:3668–83. doi: 10.1182/blood-2011-11-366062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–43. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 25.Davidoff AM, Humphrey PA, Iglehart JD, Marks JR. Genetic basis for p53 overexpression in human breast cancer. Proc Natl Acad Sci U S A. 1991;88:5006–10. doi: 10.1073/pnas.88.11.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeGregori J. The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochim Biophys Acta. 2002;1602:131–50. doi: 10.1016/s0304-419x(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 27.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Lu L, Xu J, et al. Bortezomib induces apoptosis of endometrial cancer cells through microRNA-17-5p by targeting p21. Cell Biol Int. 2013;37:1114–21. doi: 10.1002/cbin.10139. [DOI] [PubMed] [Google Scholar]
- 29.Elis W, Triantafellow E, Wolters NM, et al. Down-regulation of class II phosphoinositide 3-kinase alpha expression below a critical threshold induces apoptotic cell death. Mol Cancer Res. 2008;6:614–23. doi: 10.1158/1541-7786.MCR-07-0262. [DOI] [PubMed] [Google Scholar]
- 30.Augoff K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer. 2012;11:5. doi: 10.1186/1476-4598-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrba L, Munoz-Rodriguez JL, Stampfer MR, Futscher BW. miRNA gene promoters are frequent targets of aberrant DNA methylation in human breast cancer. PLoS One. 2013;8:e54398. doi: 10.1371/journal.pone.0054398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin PC, Chiu YL, Banerjee S, et al. Epigenetic repression of miR-31 disrupts androgen receptor homeostasis and contributes to prostate cancer progression. Cancer Res. 2013;73:1232–44. doi: 10.1158/0008-5472.CAN-12-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vire E, Curtis C, Davalos V, et al. The breast cancer oncogene EMSY represses transcription of antimetastatic microRNA miR-31. Mol Cell. 2014;53:806–18. doi: 10.1016/j.molcel.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ventura A, Young AG, Winslow MM, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–86. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]