Abstract
AIM: To identify characteristic endoscopic findings and risk factors for cytomegalovirus (CMV)-associated colitis in patients with active ulcerative colitis (UC).
METHODS: A total of 149 UC patients admitted to the Department of Gastroenterology, Nagoya University Hospital, from January 2004 to December 2013 with exacerbation of UC symptoms were enrolled in this retrospective study. All medical records, including colonoscopy results, were reviewed. CMV infection was determined by the presence of CMV antigen, CMV inclusion bodies in biopsy specimens, or positive specific immunohistochemical staining for CMV. Multivariate analysis was used to identify independent risk factors for CMV colitis.
RESULTS: Multivariate analysis indicated independent associations with the extent of disease (pancolitis) and use of > 400 mg corticosteroids for the previous 4 wk. In contrast, no association was seen with sex, age at UC diagnosis, immunomodulator use, or infliximab use. Punched-out ulceration was also significantly associated with CMV infection in patients with active UC (odds ratio = 12.672, 95%CI: 4.210-38.143).
CONCLUSION: Identification of a total corticosteroid dose > 400 mg for 4 wk, extensive colitis and a specific endoscopic finding of punched-out ulcer might facilitate the more rapid diagnosis and timely initiation of antiviral therapy for CMV-associated colitis in patients with active UC.
Keywords: Colonoscopy, Risk factor, Ulcerative colitis, Antigenemia, Cytomegalovirus
Core tip: It has been reported that cytomegalovirus (CMV) infection can be associated with steroid resistance and be an exacerbating factor in ulcerative colitis (UC). This paper provides important information regarding characteristic endoscopic findings and risk factors for CMV-associated colitis in patients with active UC. A total corticosteroid dose > 400 mg for 4 wk and extensive colitis are associated with an increased risk of CMV-associated colitis. In addition, punched-out ulceration appears predictive of CMV-associated colitis in active UC.
INTRODUCTION
Cytomegalovirus (CMV), a member of the double-stranded DNA human herpes virus family, is reported to infect between 40% and 100% of the general population[1]. Primary CMV infection is asymptomatic or minimally symptomatic, and is followed by a latent state, similar to other herpes virus infections[2,3]. Most cases of symptomatic CMV infection are therefore caused by reactivation of latent virus[1-3].
Although active CMV infection can occur in immunocompetent individuals, it occurs most frequently in immunocompromised patients, such as those with acquired immunodeficiency syndrome, leukemia patients during chemotherapy, and patients on high-dose immunosuppressants (e.g., recipients of solid organ or bone marrow transplants)[1,4-7].
Powell et al[8] reported that CMV infection in patients with ulcerative colitis (UC) was associated with exacerbation of symptoms, while one early retrospective study reported the presence of CMV in surgical specimens of patients who underwent colectomy for the treatment of toxic megacolon or steroid-resistant UC[9]. However, the significance of CMV infection in inflammatory bowel disease (IBD) is still controversial, and the pathogenic role of CMV infection in IBD is debated: Some authors believe that CMV is only an “innocent bystander” and does not significantly impact outcome, whereas many other studies have reported a significant association between CMV infection and IBD[10-13].
Active CMV infection has been observed in UC patients receiving high-dose corticosteroid therapy[13-17]. From 27% to 100% of patients with steroid-refractory UC have been found to harbor CMV, and steroid resistance is one of the central characteristics of CMV infection in UC patients[9,16,18-21]. Moreover, multiple studies have concluded that CMV infection can be an exacerbating factor in UC patients and that UC prognosis is generally poor in patients with CMV if anti-viral therapy is not started at an early stage[2,3,13-15,21-23].
Thus, CMV infection may exacerbate UC and may even cause death if appropriate treatment is not given. Although the development of ganciclovir (GCV) antiviral therapy has improved outcomes of CMV-associated colitis[5,17,20], CMV infection must still be diagnosed early in corticosteroid-resistant UC patients so that antiviral therapy can be initiated as soon as possible. However, it is difficult to distinguish exacerbation of UC by CMV infection from exacerbation not associated with CMV on the basis of symptoms and signs alone. In such cases, UC symptoms, signs, and severity in patients at risk of CMV-associated colitis are routinely evaluated by endoscopy. While a few such studies have reported the absence of any characteristic endoscopic findings in patients with UC complicated by CMV infection[24], others have reported characteristic endoscopic features, including the absence of large single ulcers and the presence of longitudinal ulcers, microerosions, deep ulcers, pseudotumors, punched-out ulcers, mucosal defects, geographic ulcers, and irregular ulcers[1,25-30]. These studies have methodological differences, however, and no consensus on unique endoscopic features that can be used to facilitate early diagnosis of CMV-associated colitis in UC has yet been obtained.
Against this background, we conducted a retrospective review of all clinical and endoscopic findings in a large cohort of patients with moderate to severe UC with symptom exacerbation to identify risk factors and characteristic endoscopic findings of CMV-associated colitis.
MATERIALS AND METHODS
Patients
This study was a retrospective analysis of medical charts and endoscopic images obtained from patients diagnosed with moderate to severe (active) UC. From January 2004 to December 2013, a total of 171 UC patients were admitted to the Department of Gastroenterology, Nagoya University Hospital, with exacerbation of UC symptoms (Figure 1). The diagnosis of UC was based on clinical, endoscopic, radiological, and pathological criteria, and the severity of UC was assessed according to Stange et al[31], Truelove et al[32] and Dignass et al[33]. We routinely examine CMV antigenemia in such patients, and almost all undergo colonoscopy or sigmoidoscopy at admission[34-36]. Of the present 171 patients, we excluded 7 patients with a previous history of CMV-associated colitis or anti-CMV treatment, as well as 15 patients who had not undergone colonoscopy or examination using the antigenemia assay. Finally, 149 patients who received both a blood test for CMV antigenemia and endoscopic examination at admission were included in the analysis.
Figure 1.
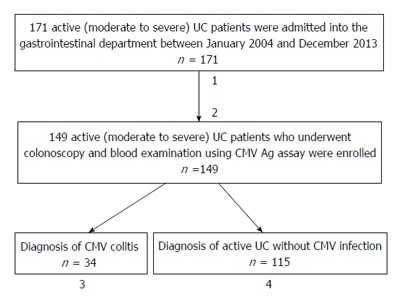
Clinical course of cytomegalovirus-associated colitis in patients with moderate to severe ulcerative colitis. Flow chart of the 171 patients admitted to our department with active UC. 1Seven patients with a history of CMV-associated colitis or anti-CMV treatment were excluded; 2Fifteen patients who had not undergone colonoscopy and examination using the CMV antigenemia assay were also excluded; 3Out of 34 UC patients with CMV-associated colitis, 26 received GCV antiviral therapy. After GCV therapy, 13 patients achieved remission, but 13 required colectomy. Eight patients did not receive GCV antiviral therapy, 4 of whom underwent colectomy; 4The remaining 115 UC patients not diagnosed with CMV-associated colitis received treatment for active UC, of which 81 achieved remission. Of the remaining patients, some improved but did not fulfill remission criteria, while others required a second treatment, hospitalization, or colectomy. CMV: Cytomegalovirus; UC: Ulcerative colitis; Ag: Antigenemia; GCV: Ganciclovir.
The following demographic and clinical data were obtained at the time of admission and classified according to the Montreal Classification[31,33]: Age at admission, age at diagnosis, sex, familial or spontaneous disease (familial disease was considered when at least one first- or second-degree relative was diagnosed with IBD), and disease localization (proctitis, left sided colitis, or pancolitis) as revealed by colonoscopy.
Endoscopic findings
Disease severity was assessed by colonoscopy. If ulcers were present, the shape and depth were described, and biopsies were obtained at the margin and base for histologic investigation. If no ulcers were detected, biopsies were obtained in the areas with the most severe inflammation. Colonic biopsy specimens were fixed, paraffinized, and stained with hematoxylin and eosin (HE) and specific immunohistochemical (IHC) staining with monoclonal antibody against CMV immediate early antigen[6,37]. Specimens were also evaluated for the presence of characteristic CMV inclusion bodies by experienced pathologists.
Diagnosis of CMV infection/CMV-associated colitis
CMV infection was defined by a positive CMV antigenemia assay, the presence of inclusion bodies in HE stained sections, or positive specific IHC staining for CMV. Diagnosis of CMV-associated colitis in patients with active UC was determined by active UC complicated by CMV infection.
Ethical considerations
The study protocol was approved by the institutional review board of Nagoya University Graduate School of Medicine.
Statistical analysis
Data are presented as mean ± SD or number (%) as appropriate. Categorical data were compared between groups using the χ2 or Fisher’s exact test. Continuous variables were compared using the Mann-Whitney U test. To identify candidate risk factors and characteristic endoscopic features for CMV-associated colitis, univariate analyses were conducted using Fisher’s exact test. All factors which were significant on univariate analysis were entered into multivariate logistic regression models constructed to identify significant independent risk factors and characteristic endoscopic features of CMV-associated colitis. For continuous variables, we found the best cut-off value with plotting the area under the receiver operating characteristic curve. The results are expressed as odds ratios (ORs) with 95%CIs. P-values less than 0.05 were considered statistically significant for all tests. All statistical analyses were performed using SPSS Statistics 21.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics
A total of 149 UC patients presenting with UC symptom exacerbation between January 2004 and December 2013 were included in the study. Of these, 34 (22.8%) tested positive on CMV antigenemia assay or had biopsy specimens with indicative of CMV infection. The clinical and demographical parameters of CMV-positive and CMV-negative patients are presented in Table 1. Univariate analysis revealed statistically significant group differences in age at UC diagnosis, age at admission, extent of disease (pancolitis), serum albumin level, systemic steroid dose on the day of admission, total systemic steroid dose for the week before admission, and total systemic steroid dose for 4 wk before admission. There were no significant group differences in sex ratio, disease duration, clinical course, total lifetime systemic steroid dose, immunomodulator use, infliximab use, or laboratory data at admission other than serum albumin level.
Table 1.
Clinical and demographic characteristics of patients with active ulcerative colitis (n = 149)
| CMV (+) n = 34 | CMV (-) n = 115 | P value | |
| Sex (male/female) | 19/15 | 64/51 | 0.981 |
| Age at UC diagnosis (yr) | 42.3 ± 14.4 | 29.0 ± 14.4 | < 0.001 |
| Age at admission (yr) | 46.9 ± 18.1 | 35.0 ± 15.6 | < 0.001 |
| Disease duration (yr) | 4.6 ± 4.9 | 6.0 ± 7.4 | 0.294 |
| Clinical course | |||
| Relapse | 23 (67.6%) | 79 (68.7%) | 0.908 |
| Chronic active | 4 (11.8%) | 11 (9.6%) | 0.708 |
| First attack | 7 (20.6%) | 25 (21.7%) | 0.886 |
| Disease extent | |||
| Extensive UC (pancolitis) | 28 (82%) | 52 (45%) | < 0.001 |
| Left-sided UC/proctitis | 6 (18%) | 63 (55%) | - |
| BMI at admission | 19.5 ± 3.2 | 18.9 ± 3.1 | 0.384 |
| Severity | |||
| Severe | 11 (32%) | 27 (23%) | 0.297 |
| Moderate | 23 (68%) | 88 (77%) | - |
| Laboratory data at admission | |||
| CRP (mg/dL) | 3.4 ± 4.1 | 3.8 ± 5.4 | 0.685 |
| WBC (× 103/μL) | 8.7 ± 3.7 | 9.9 ± 4.2 | 0.132 |
| Hemoglobin (g/dL) | 11.4 ± 1.8 | 11.7 ± 1.2 | 0.387 |
| Platelet (× 103/μL) | 321.0 ± 118.9 | 349.9 ± 120.2 | 0.219 |
| Total cholesterol (mg/dL) | 155.3 ± 39.7 | 155.1 ± 44.3 | 0.979 |
| Albumin (g/dL) | 3.0 ± 0.54 | 3.4 ± 0.68 | 0.002 |
| Medication | |||
| Total lifetime systemic steroid dose before admission (g) | 4.69 ± 5.80 | 4.86 ± 8.45 | 0.892 |
| Total systemic steroid dose for 4 wk before admission (mg) | 1083.4 ± 1113.5 | 245.5 ± 328.4 | < 0.001 |
| Total systemic steroid dose for 1 wk before admission (mg) | 260.7 ± 103.9 | 92.3 ± 117.0 | < 0.001 |
| Systemic steroid dose on the day at admission (mg) | 37.5 ± 15.0 | 13.9 ± 17.6 | < 0.001 |
| 5-ASA | 29 (85.3%) | 82 (71.3%) | 0.100 |
| SASP | 1 (2.9%) | 10 (8.7%) | 0.260 |
| Cytapheresis | 5 (15%) | 11 (9.6%) | 0.395 |
| Immunomodulator use | 8 (24%) | 20 (17%) | 0.421 |
| AZA | 4 (12%) | 16 (14%) | 0.747 |
| 6-MP | 2 (5.9%) | 2 (1.7%) | 0.177 |
| Tacrolimus | 2 (5.9%) | 2 (1.7%) | 0.177 |
| Infliximab use | 5 (15%) | 7 (6.1%) | 0.105 |
| Family history of IBD | 1 (2.9%) | 1 (0.87%) | 0.356 |
| PSC | 0 | 2 (1.7%) | - |
| Outcome | |||
| Ganciclovir use | 26 (76%) | 0 | - |
| Colectomy | 17 (50%) | 37 (32%) | 0.058 |
| Colectomy for cancer or dysplasia | 0 | 4 (3.5%) | - |
Values presented as mean ± SD or number (%) as appropriate. CMV: Cytomegalovirus; CRP: C-reactive protein; WBC: White blood count; BMI: Body mass index; 5-ASA: 5-aminosalicylate acid; SASP: Salicylazosulfapyridine; AZA: Azathioprine; 6-MP: 6-mercaptopurine; IBD: Inflammatory bowel disease; UC: Ulcerative colitis; PSC: Primary sclerosing cholangitis.
For multivariate analysis, we selected a total systemic steroid dose for 4 wk before admission as the most important factor among factors regarding steroid dose. This multivariate analysis using a logistic regression model identified pancolitis and a total systemic steroid dose > 400 mg for 4 wk before admission as significant independent risk factors for CMV infection (Table 2). Patients treated with more than 400 mg corticosteroid for UC exacerbation over the 4 wk prior to admission had a 27-fold greater risk of CMV-associated colitis and patients with extensive UC (pancolitis) had about a 3-fold greater risk. The other factors tested (age at UC diagnosis, age at admission, and serum albumin) were not significant risk factors by multivariate analysis.
Table 2.
Risk factors for cytomegalovirus-associated colitis among the 149 patients with active ulcerative colitis (multivariate analysis)
| Odds ratio | 95%CI | P value | |
| Age at UC diagnosis > 30 yr | 2.764 | 0.581-13.152 | 0.202 |
| Age at admission > 35 yr | 1.433 | 0.295-6.951 | 0.655 |
| Pancolitis | 3.419 | 1.077-10.856 | 0.037 |
| Albumin < 3.0 g/dL | 1.402 | 0.480-4.098 | 0.537 |
| Total systemic steroid dose for 4 wk before admission > 400 mg | 26.697 | 5.848-121.868 | < 0.001 |
UC: Ulcerative colitis; CMV: Cytomegalovirus.
Endoscopic findings
To identify endoscopic findings characteristic of CMV-associated colitis in patients with active UC, we analyzed ulcerative features (e.g., deep ulcer, punched-out ulcer, geographical ulcer, longitudinal ulcer, and mucosal defect) and mucosal features (e.g., mucopurulent exudate, spontaneous bleeding, cobblestone-like appearance, and post inflammatory polyp). Characteristic colonoscopic features of CMV-associated colitis included deep ulcer, punched-out ulcer, geographical ulcer, longitudinal ulcer, and mucosal defect (Figure 2). We defined endoscopic findings according to published reports[28,38]. Deep ulcer was defined as deep excavated ulceration near or beyond muscularis propria with or without slightly raised edges. Punched-out ulcer was defined as ulceration with an almost round shape and clear demarcation. Geographical ulcer was defined as ulceration with an irregular pattern and a branched shape. Longitudinal ulcer was defined as ulceration with a longitudinal spread along the lumen of the colon. Mucosal defect was defined as a wide area of defect with a longitudinal and/or transverse spread, indicating that more than one-fourth of the mucosa in the endoscopic field was defective. The accuracy, sensitivity, specificity, positive predictive value, and negative predictive value for each of these features were determined. Univariate analysis revealed that deep ulcer, punched-out ulcer, geographical ulcer, and spontaneous bleeding were more frequent in CMV-positive patients than in CMV-negative patients (Table 3).
Figure 2.
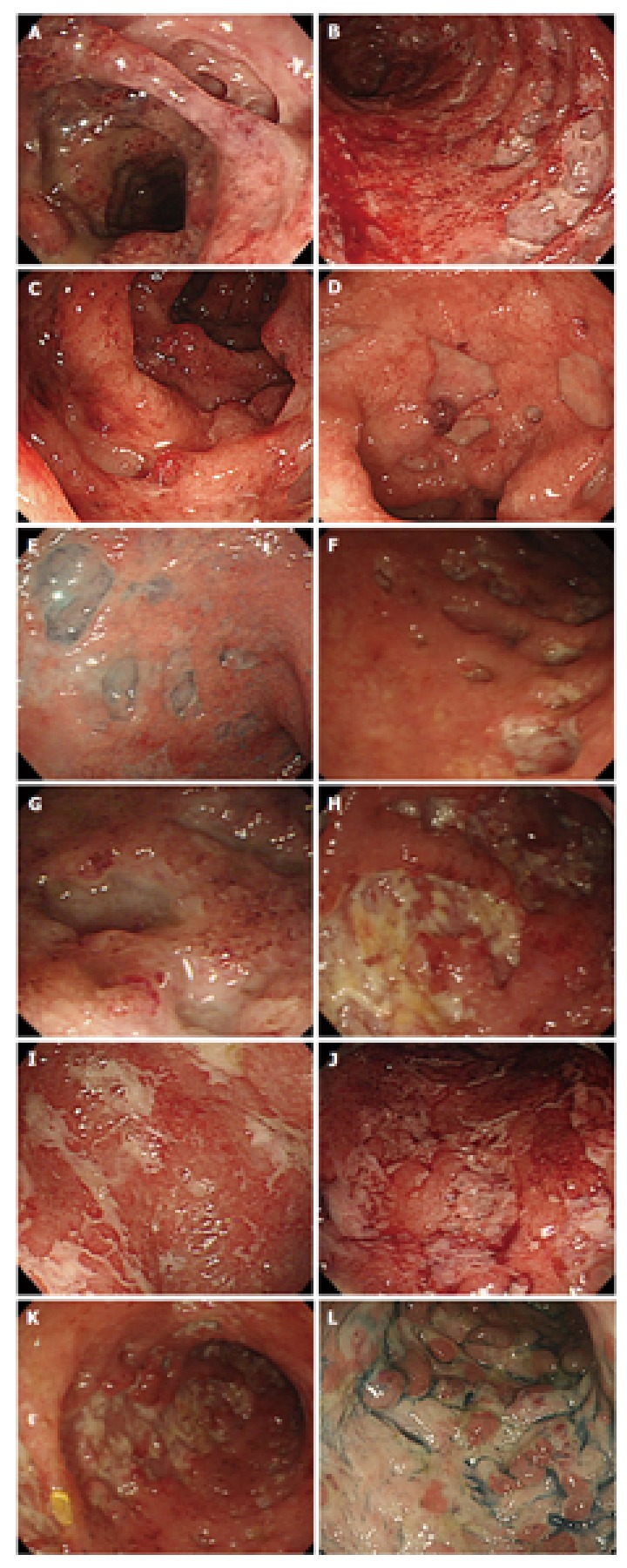
Endoscopic images of cytomegalovirus-associated colitis in patients with active ulcerative colitis. A-C: Deep ulcer; D-G: Punched-out ulcer; H-J: Geographical ulcer; K: Longitudinal ulcer; L: Mucosal defect.
Table 3.
Endoscopic findings in patients with active ulcerative colitis (n = 149)
| CMV (+) n = 34 | CMV (-) n = 115 | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | P value | |
| Deep ulcer | 17 (50.0%) | 14 (12.2%) | 79.2 | 50.0 | 87.8 | 54.8 | 85.6 | < 0.001 |
| Punched-out ulcer | 20 (58.8%) | 8 (7.0%) | 85.2 | 58.8 | 93.0 | 71.4 | 88.4 | < 0.001 |
| Geographical ulcer | 14 (41.2%) | 25 (21.7%) | 76.5 | 41.2 | 78.2 | 35.9 | 81.8 | 0.024 |
| Longitudinal ulcer | 11 (32.4%) | 24 (20.9%) | 68.5 | 32.4 | 79.1 | 31.4 | 79.8 | 0.165 |
| Mucosal defect | 6 (17.6%) | 10 (8.7%) | 74.5 | 17.6 | 91.3 | 37.5 | 78.9 | 0.139 |
| Mucopurulent exudate | 24 (70.6%) | 66 (57.4%) | 49.0 | 70.6 | 42.6 | 26.7 | 83.1 | 0.167 |
| Spontaneous bleeding | 14 (41.2%) | 19 (16.5%) | 73.8 | 41.2 | 83.5 | 42.4 | 82.8 | 0.002 |
| Cobblestone-like appearance | 5 (14.7%) | 7 (6.1%) | 75.8 | 14.7 | 93.9 | 41.7 | 78.8 | 0.105 |
| Post inflammatory polyp | 9 (26.5%) | 21 (18.3%) | 75.8 | 26.5 | 81.7 | 30.0 | 79.0 | 0.294 |
PPV: Positive predictive value; NPV: Negative predictive value; CMV: Cytomegalovirus.
Multivariate analysis showed that only punched-out ulcer was a significant independent predictor of CMV colitis (OR = 12.672, 95%CI: 4.210-38.143) (Table 4).
Table 4.
Characteristic endoscopic findings for cytomegalovirus-associated colitis in patients with active ulcerative colitis (multivariate analysis)
| Odds ratio | 95%CI | P value | |
| Deep ulcer | 2.128 | 0.678-6.680 | 0.196 |
| Punched-out ulcer | 12.672 | 4.210-38.143 | < 0.001 |
| Geographical ulcer | 1.919 | 0.664-5.542 | 0.229 |
| Spontaneous bleeding | 2.106 | 0.735-6.036 | 0.166 |
Patient outcomes
In the CMV-positive (CMV-associated colitis) group, 26 of the 34 patients (76.5%) received antiviral therapy with GCV. After GCV therapy, 13 of these patients achieved remission, while 13 required colectomy because of severe and refractory UC. Of the remaining 8 patients who did not receive GCV antiviral therapy, 4 underwent colectomy because of severe UC.
Among the CMV-negative group, 81 patients (70.4%) achieved remission with anti-inflammatory therapy (including relapse cases), while 37 (32.2%) eventually underwent colectomy during the course of follow-up. Among these 37 patients, 4 underwent colectomy for cancer or dysplasia.
DISCUSSION
In this retrospective study of 149 UC patients presenting with exacerbation of symptoms, we identified extensive UC (pancolitis) and 4 wk of high-dose steroid treatment as independent risk factors for CMV-associated colitis in active UC. The only endoscopic finding indicative of CMV-associated colitis by multivariate analysis was punched-out ulcer. To our knowledge, this is the first study to identify both risk factors and characteristic endoscopic findings for CMV-associated colitis in patients with moderate to severe UC. These factors may help facilitate both the timely diagnosis and treatment of UC complicated by CMV infection.
We evaluated total systemic steroid dose over the patient’s lifetime, as well as dose over the 4 wk before admission, over the previous week before admission, and on the day of admission. Between CMV-positive and CMV-negative patients, total systemic steroid dose over the 4 wk prior to admission (total dose > 400 mg) was an independent risk factor for CMV-associated colitis in active UC patients. Furthermore, neither immunomodulator nor infliximab use was associated with CMV-associated colitis. However, this study included only a few cases treated by immunomodulators or infliximab, and additional studies are required to confirm these results. Nonetheless, the finding that immunomodulator and infliximab use did not alter the risk of CMV-associated colitis is important, because it suggests an alternative treatment regimen for patients with moderate to severe UC rather than using high-dose corticosteroids for corticosteroid-refractory cases or corticosteroid-resistant cases. Given that tumor necrosis factor (TNF)-α from monocytes and dendritic cells plays an important role in the reactivation of CMV and that infliximab is a potent blocker of TNF-α, we consider that this combination therapy may be particularly effective[7,39]. However, the efficacy of infliximab for UC patients with concomitant CMV infection remains controversial, as there have been few case reports and no controlled clinical trials.
Pancolitis was significantly associated with CMV infection in active UC, consistent with the theory that CMV is prone to proliferate in granulation tissue[9]. Some studies reported that CMV was readily found in granulation tissue and tissue from deep ulcers, suggesting that CMV can penetrate inflamed mucosa via mononuclear cells and then proliferate in the mucosa[2,9,40,41]. It is thus possible that a more extensive UC lesion may lead to wider CMV infection.
In general, there is no clear consensus on the diagnostic criteria for CMV infection in active UC. There are several methods of detecting CMV infection, including histology with IHC, serology, CMV culture, polymerase chain reaction (PCR) detection of the CMV genome, and CMV antigenemia[6,34-37,42]. Each method offers advantages and disadvantages in the precise diagnosis of CMV infection. For example, histological examination is a relatively easy method, but its sensitivity is lower (10%-87%) than PCR. In contrast, PCR for CMV genes is highly sensitive, but the method is time-consuming and its selectivity is low given the ubiquity of CMV infection. CMV culture is too slow. In contrast, CMV antigenemia is relatively sensitive (60%-100%) and easy to measure within a short period, and has also been used to monitor CMV infection in heart transplant recipients and for the early diagnosis of CMV infection in renal transplant recipients[43]. Moreover, results of CMV antigenemia are good indication for antiviral therapy[44,45].
Accordingly, we adopted CMV antigenemia and histology, including IHC for CMV, to detect CMV infection in our analysis. Results showed that 33 of the 34 CMV-associated colitis patients (97.1%) were positive for CMV antigenemia. Histology including IHC is considered the objective standard for the diagnosis of CMV infection. In our study, however, among the 34 patients with CMV-associated colitis whose biopsy specimens were stained with HE and a CMV antibody, only 8 patients were positive by histology. Only 7 were positive by both CMV antigenemia and hitology. We therefore suggest that our combination of CMV antigenemia and histology including IHC for CMV is an appropriate strategy for diagnosis of CMV infection/CMV-associated colitis in active UC patients.
Colonoscopy is usually performed in patients with exacerbation of UC symptoms because direct observation of the colonic mucosa provides detailed information on disease status and is useful for judging disease severity and treatment efficacy. The rapid and accurate diagnosis of CMV-associated colitis in UC patients is critical, because its treatment strategy differs markedly from that for UC exacerbation not associated with CMV infection. A few reports have documented the endoscopic findings of CMV-associated colitis, but several failed to find features able to rapidly distinguish CMV-associated colitis from unrelated active UC. Endoscopic findings of UC concomitant with CMV infection can range from normal appearing mucosa to mucosal erosion or ulceration, which can be difficult to distinguish from active UC unrelated to CMV infection. In our study, punched-out ulceration was significantly more frequent in UC patients with CMV infection, consistent with reports that CMV tends to localize to the colon mucosa and granulation tissue in deep ulcers[2,9,40,41]. Regardless of etiology, we suggest that a finding of punched-out ulceration may facilitate the rapid and accurate diagnosis of CMV-associated colitis in UC patients.
The limitations of this study include its retrospective nature and evaluation of patients at a single institution. This study also involved a relatively small number of patients, which limits its statistical power.
In conclusion, this study suggests that a total corticosteroid dose > 400 mg for 4 wk and extensive colitis are associated with an increased risk of CMV-associated colitis in patients with moderate to severe UC. In addition, punched-out ulceration appears predictive of CMV-associated colitis associated with UC. These clinical predictors and specific endoscopic findings may facilitate rapid diagnosis and antiviral treatment.
COMMENTS
Background
Although it has been reported that cytomegalovirus (CMV) infection can be associated with steroid resistance and be an exacerbating factor in ulcerative colitis (UC), the relationship between CMV and UC is not well studied.
Research frontiers
The aim of this study was to identify characteristic endoscopic findings and risk factors for CMV-associated colitis in patients with active UC.
Innovations and breakthroughs
This is one of a few retrospective studies focused on important information regarding characteristic endoscopic findings and risk factors for CMV-associated colitis in patients with active UC.
Applications
This study suggests that a total corticosteroid dose > 400 mg for 4 wk and extensive colitis are associated with an increased risk of CMV-associated colitis in patients with moderate to severe UC. In addition, punched-out ulceration appears predictive of CMV-associated colitis associated with UC. These clinical predictors and specific endoscopic findings may facilitate rapid diagnosis and antiviral treatment.
Peer-review
An interesting article dealing with clinically relevant subject of risk factors in ulcerative colitis. There is a solid number of patients and good experimental and clinical design. Data are good and discussion is a good representation of the problem.
Footnotes
Institutional review board statement: The study protocol was reviewed and approved by the institutional review board of Nagoya University Graduate School of Medicine.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to examination, treatment, and data sharing by written consent.
Conflict-of-interest statement: No conflict of interest exists for any authors with regard to the content of this study.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 25, 2015
First decision: August 31, 2015
Article in press: January 19, 2016
P- Reviewer: Landsman MJ, Ma XP, Vetvicka V S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
References
- 1.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924–935. doi: 10.7326/0003-4819-119-9-199311010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Hommes DW, Sterringa G, van Deventer SJ, Tytgat GN, Weel J. The pathogenicity of cytomegalovirus in inflammatory bowel disease: a systematic review and evidence-based recommendations for future research. Inflamm Bowel Dis. 2004;10:245–250. doi: 10.1097/00054725-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Surawicz CM, Myerson D. Self-limited cytomegalovirus colitis in immunocompetent individuals. Gastroenterology. 1988;94:194–199. doi: 10.1016/0016-5085(88)90630-0. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich DT, Rahmin M. Cytomegalovirus colitis in AIDS: presentation in 44 patients and a review of the literature. J Acquir Immune Defic Syndr. 1991;4 Suppl 1:S29–S35. [PubMed] [Google Scholar]
- 5.Papadakis KA, Tung JK, Binder SW, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:2137–2142. doi: 10.1111/j.1572-0241.2001.03949.x. [DOI] [PubMed] [Google Scholar]
- 6.de la Hoz RE, Stephens G, Sherlock C. Diagnosis and treatment approaches of CMV infections in adult patients. J Clin Virol. 2002;25 Suppl 2:S1–12. doi: 10.1016/s1386-6532(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 7.Pereyra F, Rubin RH. Prevention and treatment of cytomegalovirus infection in solid organ transplant recipients. Curr Opin Infect Dis. 2004;17:357–361. doi: 10.1097/01.qco.0000136933.67920.dd. [DOI] [PubMed] [Google Scholar]
- 8.Powell RD, Warner NE, Levine RS, Kirsner JB. Cytomegalic inclusion disease and ulcerative colitis; report of a case in a young adult. Am J Med. 1961;30:334–340. doi: 10.1016/0002-9343(61)90105-x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper HS, Raffensperger EC, Jonas L, Fitts WT. Cytomegalovirus inclusions in patients with ulcerative colitis and toxic dilation requiring colonic resection. Gastroenterology. 1977;72:1253–1256. [PubMed] [Google Scholar]
- 10.Orvar K, Murray J, Carmen G, Conklin J. Cytomegalovirus infection associated with onset of inflammatory bowel disease. Dig Dis Sci. 1993;38:2307–2310. doi: 10.1007/BF01299914. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, Okamoto S, Morohoshi Y, Izumiya M, Ichikawa H, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331–337. doi: 10.1111/j.1572-0241.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16:1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 13.Cottone M, Pietrosi G, Martorana G, Casà A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A, Pagliaro L. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol. 2001;96:773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman HS, Kahn AC, Iacobuzio-Donahue C, Talamini MA, Lillemoe KD, Hamilton SR. Cytomegaloviral enterocolitis: clinical associations and outcome. Dis Colon Rectum. 1999;42:24–30. doi: 10.1007/BF02235178. [DOI] [PubMed] [Google Scholar]
- 15.Berk T, Gordon SJ, Choi HY, Cooper HS. Cytomegalovirus infection of the colon: a possible role in exacerbations of inflammatory bowel disease. Am J Gastroenterol. 1985;80:355–360. [PubMed] [Google Scholar]
- 16.Wada Y, Matsui T, Matake H, Sakurai T, Yamamoto J, Kikuchi Y, Yorioka M, Tsuda S, Yao T, Yao S, et al. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003;46:S59–S65. doi: 10.1097/01.DCR.0000087486.21981.C6. [DOI] [PubMed] [Google Scholar]
- 17.Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004;28:365–373. doi: 10.1097/00000478-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kuwabara A, Okamoto H, Suda T, Ajioka Y, Hatakeyama K. Clinicopathologic characteristics of clinically relevant cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol. 2007;42:823–829. doi: 10.1007/s00535-007-2103-3. [DOI] [PubMed] [Google Scholar]
- 19.Domènech E, Vega R, Ojanguren I, Hernández A, Garcia-Planella E, Bernal I, Rosinach M, Boix J, Cabré E, Gassull MA. Cytomegalovirus infection in ulcerative colitis: a prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008;14:1373–1379. doi: 10.1002/ibd.20498. [DOI] [PubMed] [Google Scholar]
- 20.Roblin X, Pillet S, Oussalah A, Berthelot P, Del Tedesco E, Phelip JM, Chambonnière ML, Garraud O, Peyrin-Biroulet L, Pozzetto B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001–2008. doi: 10.1038/ajg.2011.202. [DOI] [PubMed] [Google Scholar]
- 21.Kojima T, Watanabe T, Hata K, Shinozaki M, Yokoyama T, Nagawa H. Cytomegalovirus infection in ulcerative colitis. Scand J Gastroenterol. 2006;41:706–711. doi: 10.1080/00365520500408584. [DOI] [PubMed] [Google Scholar]
- 22.Nakase H, Matsumura K, Yoshino T, Chiba T. Systematic review: cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol. 2008;43:735–740. doi: 10.1007/s00535-008-2246-x. [DOI] [PubMed] [Google Scholar]
- 23.Nakase H, Yoshino T, Ueno S, Uza N, Mikami S, Matsuura M, Chiba T. Importance of early detection of cytomegalovirus infection in refractory inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:364. doi: 10.1002/ibd.20033. [DOI] [PubMed] [Google Scholar]
- 24.Franzin G, Muolo A, Griminelli T. Cytomegalovirus inclusions in the gastroduodenal mucosa of patients after renal transplantation. Gut. 1981;22:698–701. doi: 10.1136/gut.22.9.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battaglino MP, Rockey DC. Cytomegalovirus colitis presenting with the endoscopic appearance of pseudomembranous colitis. Gastrointest Endosc. 1999;50:697–700. doi: 10.1016/s0016-5107(99)80025-x. [DOI] [PubMed] [Google Scholar]
- 26.Nishimoto Y, Matsumoto T, Suekane H, Shimizu M, Mikami Y, Iida M. Cytomegalovirus infection in a patient with ulcerative colitis: colonoscopic findings. Gastrointest Endosc. 2001;53:816–818. doi: 10.1067/mge.2001.114955. [DOI] [PubMed] [Google Scholar]
- 27.Falagas ME, Griffiths J, Prekezes J, Worthington M. Cytomegalovirus colitis mimicking colon carcinoma in an HIV-negative patient with chronic renal failure. Am J Gastroenterol. 1996;91:168–169. [PubMed] [Google Scholar]
- 28.Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol. 2010;16:1245–1251. doi: 10.3748/wjg.v16.i10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omiya M, Matsushita M, Tanaka T, Kawamata S, Okazaki K. The absence of large ulcer predicts latent cytomegalovirus infection in ulcerative colitis with positive mucosal viral assay. Intern Med. 2010;49:2277–2282. doi: 10.2169/internalmedicine.49.3657. [DOI] [PubMed] [Google Scholar]
- 30.Iida T, Ikeya K, Watanabe F, Abe J, Maruyama Y, Ohata A, Teruyuki S, Sugimoto K, Hanai H. Looking for endoscopic features of cytomegalovirus colitis: a study of 187 patients with active ulcerative colitis, positive and negative for cytomegalovirus. Inflamm Bowel Dis. 2013;19:1156–1163. doi: 10.1097/MIB.0b013e31828075ce. [DOI] [PubMed] [Google Scholar]
- 31.Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Fléjou JF, Herfarth H, Hommes DW, et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Mazzulli T, Drew LW, Yen-Lieberman B, Jekic-McMullen D, Kohn DJ, Isada C, Moussa G, Chua R, Walmsley S. Multicenter comparison of the digene hybrid capture CMV DNA assay (version 2.0), the pp65 antigenemia assay, and cell culture for detection of cytomegalovirus viremia. J Clin Microbiol. 1999;37:958–963. doi: 10.1128/jcm.37.4.958-963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori T, Mori S, Kanda Y, Yakushiji K, Mineishi S, Takaue Y, Gondo H, Harada M, Sakamaki H, Yajima T, et al. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:431–434. doi: 10.1038/sj.bmt.1704369. [DOI] [PubMed] [Google Scholar]
- 36.Nagata N, Kobayakawa M, Shimbo T, Hoshimoto K, Yada T, Gotoda T, Akiyama J, Oka S, Uemura N. Diagnostic value of antigenemia assay for cytomegalovirus gastrointestinal disease in immunocompromised patients. World J Gastroenterol. 2011;17:1185–1191. doi: 10.3748/wjg.v17.i9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaugerie L, Cywiner-Golenzer C, Monfort L, Girard PM, Carbonnel F, Ngô Y, Cosnes J, Rozenbaum W, Nicolas JC, Châtelet FP, et al. Definition and diagnosis of cytomegalovirus colitis in patients infected by human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:423–429. doi: 10.1097/00042560-199704150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, Ordás I, Repici A, Rosa B, Sebastian S, Kucharzik T, Eliakim R; European Crohn's and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Nakase H, Chiba T. TNF-alpha is an important pathogenic factor contributing to reactivation of cytomegalovirus in inflamed mucosa of colon in patients with ulcerative colitis: lesson from clinical experience. Inflamm Bowel Dis. 2010;16:550–551. doi: 10.1002/ibd.21047. [DOI] [PubMed] [Google Scholar]
- 40.Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516–1521. doi: 10.1002/ibd.20253. [DOI] [PubMed] [Google Scholar]
- 41.Pfau P, Kochman ML, Furth EE, Lichtenstein GR. Cytomegalovirus colitis complicating ulcerative colitis in the steroid-naive patient. Am J Gastroenterol. 2001;96:895–899. doi: 10.1111/j.1572-0241.2001.03672.x. [DOI] [PubMed] [Google Scholar]
- 42.Kishore J, Ghoshal U, Ghoshal UC, Krishnani N, Kumar S, Singh M, Ayyagari A. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155–1160. doi: 10.1099/jmm.0.45629-0. [DOI] [PubMed] [Google Scholar]
- 43.Bernabeu-Wittel M, Pachón-Ibáñez J, Cisneros JM, Cañas E, Sánchez M, Gómez MA, Gentil MA, Pachón J. Quantitative pp65 antigenemia in the diagnosis of cytomegalovirus disease: prospective assessment in a cohort of solid organ transplant recipients. J Infect. 2005;51:188–194. doi: 10.1016/j.jinf.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Manteiga R, Martino R, Sureda A, Labeaga R, Brunet S, Sierra J, Rabella N. Cytomegalovirus pp65 antigenemia-guided pre-emptive treatment with ganciclovir after allogeneic stem transplantation: a single-center experience. Bone Marrow Transplant. 1998;22:899–904. doi: 10.1038/sj.bmt.1701439. [DOI] [PubMed] [Google Scholar]
- 45.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]


