Abstract
The relaxin hormone is involved in a variety of biological functions including female reproduction and parturition, regulation of cardiovascular, renal, pulmonary, and hepatic functions. It regulates extracellular matrix remodeling, cell invasiveness, proliferation, differentiation, and overall tissue homeostasis. The G protein-coupled receptor (GPCR) RXFP1, relaxin family receptor 1, is a cognate relaxin receptor that mainly signals through cyclic AMP second messenger. While agonists of the receptor could have a wide range of pharmacological utility, up to date, there are no reported small molecule agonists for relaxin receptors. Here, we report the development of quantitative high-throughput platform for RXFP1 agonist screen based on homogenous cell-based HTRF cAMP assay technology. Two small molecules of similar structure were independently identified from a screen of more than 365,677 compounds. Neither compound showed activity in a counter screen with HEK293T cells transfected with an unrelated GPCR vasopressin 1b receptor. These small molecule agonists also demonstrated selectivity against the RXFP2 receptor, providing a basis for future medicinal chemistry optimization of selective relaxin receptor agonists.
Keywords: relaxin, GPCR, RXFP1, qHTS, agonist, small molecule
Introduction
The relaxin hormone is involved in a variety of biological functions in normal tissues and diseases. The role of relaxin is well-established in female reproduction and parturition, mammary gland and endometrial development, and maintenance of myometrial quiescence during pregnancy. Relaxin signaling through its cognate G protein-coupled receptor (GPCR), the relaxin/insulin-like family peptide receptor 1 (RXFP1), results in extracellular matrix remodeling through regulation of collagen deposition, cell invasiveness, proliferation, and overall tissue homeostasis. Significantly, the therapeutic effects of relaxin in the treatment of renal, cardiac, skin, lung fibrosis, inflammation, and wound healing in animal models are well-established 1. Other data strongly indicate the significance of relaxin in prostate, breast, thyroid and other tumorigenesis 2. Several clinical trails have been conducted with recombinant human relaxin hormone as a treatment for scleroderma, cervical ripening, fibromyalgia, preeclampsia, and congestive heart failure. The wide range of effects and applications of relaxin underscores the importance of this hormone in human physiology and diseases. Small molecule agonists would be extremely useful in furthering the understanding of relaxin hormone biology, and have the potential for therapeutic development.
The cognate relaxin receptor, RXFP1, is a member of the relaxin/insulin-like family of GPCR peptide receptors 3, 4. RXFP2 is another member of this family (previously called LGR7 and LGR8, respectively), which shares 60% sequence identity and similar structure. RXFP2 is a cognate receptor for insulin-like 3 peptide, belonging to the same group of peptide hormones as relaxin 5. Relaxin can activate cAMP production in cells transfected with RXFP2 in vitro, however with lower potency. RXFP1 was deorphanized in 2002, and was found to couple to Gas proteins, which in turn stimulate adenylate cyclase (AC) to generate cyclic AMP (cAMP) 3. In addition, RXFP1 also couples to Gi/Go proteins to further modulate the cAMP production. The activated G-α derived from Gi/Go inhibits the cAMP production by AC, while the G-βγ subunits activate the delayed cAMP response via PI3K and PKCζ 4. The RXFP1 and RXFP2 receptors contain a unique low density lipoprotein class A (LDLa) module at the amino terminus, followed by an extracellular leucine rich repeats (LRRs) and seven transmembrane (7TM) helices of the transmembrane domain 1. The LRRs and the exoloops within the transmembrane domain have been shown to participate in ligand binding 6, 7, while the LDLa modules contribute to receptor activation 8, 9.
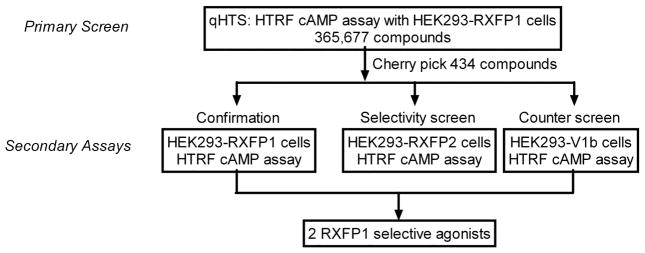
Multiple reports have indicated that expression of RXFP1 in human embryonic kidney cells (HEK293T) is able to signal through the classical Gas-coupled mechanism to activate AC, resulting in elevation of intracellular and extracellular cAMP concentrations 3, 4. We have developed a homogeneous cAMP assay using HEK293T cells stably expressing human RXFP1 receptor to screen for small molecule agonists of human RXFP1. The assay was successfully miniaturized and used to screen a 365,677-compound library in quantitative high-throughput screening (qHTS) format. Following confirmation and counter screening, we have identified small molecule agonists that are >100-fold selective towards RXFP1 over RXFP2; making this the first report of a series of small molecule agonists for the human RXFP1 receptor.
Materials and methods
Materials
Ro 20-1724 and forskolin were purchased from Sigma-Aldrich (St. Louis, MO). HTRF cAMP HiRange kit was purchased from CisBio (Bedford, MA), and the cAMP-Screen ELISA assay kit from Life Technologies (Carlsbad, CA). Porcine relaxin 10 was a gift from Dr. O. David Sherwood (University of Illinois at Urbana-Champaign).
Cell line generation
The HEK293T cells stably transfected with human RXFP1 (HEK293-RXFP1) 11, or RXFP2 (HEK293-RXFP2) 3 expression constructs were kindly provided by Drs. András Kern and Gillian D. Bryant-Greenwood (University of Hawaii), and Dr. Sheau Yu Teddy Hsu (Stanford University). HEK293T cells stably transfected with V1b (HEK293-V1b) was purchased from PerkinElmer (Waltham, MA).
Cell culture
HEK293-V1b cells were grown in DMEM medium with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in 5% CO2. HEK293-RXFP1 and HEK293-RXFP2 cells were grown in the same conditions with the addition of 500 μg/mL geneticin for HEK293-RXFP1 cells and 100 μg/mL zeocin for HEK293-RXFP2 cells. All cell lines were grown at 37 °C in the presence of 5% CO2 and 95% humidity. Cells were passaged once they reach 80% confluence at a split ratio of 1:8.
HTRF cAMP assay
The initial assay optimization and all follow up assays were conducted in 384-well format. For 384-well format assays, cells were seeded as 8,000 cells/well in 30 μL/well media with a MultiDrop Combi dispenser (Thermo Scientific, Waltham, MA), and allowed to attach overnight at 37 °C in 5% CO2. Following the incubation, 2 μL/well of 1.6 mM Ro 20-1724 in PBS+ (DPBS, 1 mM CaCl2, 0.5 mM MgCl2, 0.05% BSA, 0.005% Tween 20) was dispensed using a BioRAPTR FRD dispenser (Beckman Coulter, Brea, CA). Subsequently, 0.25 μL/well of compound solutions in DMSO was dispensed with CyBi-well dispenser (CyBio, Jena, Germany). The cells were stimulated with compounds for 30 min at 37 °C in 5% CO2, after which, 8 μL/well of each HTRF cAMP HiRange kit (CisBio, Bedford, MA) detection reagent was dispensed with a BioRAPTR FRD dispenser. The detection reagents were diluted as such: K-anti-cAMP antibody at 1:20 and cAMP-d2 at 1:18 in HTRF lysis buffer (supplied by the assay kit). The plates were incubated for 30 min at room temperature before the signal was read on an Envision plate reader (PerkinElmer, Waltham, MA).
The primary screen was done in a 1,536-well format. Briefly, HEK293-RXFP1 cells were seeded at 2,000 cells/well in 3 μL/well growth media with a MultiDrop Combi dispenser (Thermo Scientific, Waltham, MA), and allowed to attach overnight at 37 °C in 5% CO2. Following the incubation, 1 μL/well 400 μM Ro 20-1724 in PBS+ (DPBS, 1 mM CaCl2, 0.5 mM MgCl2, 0.05% BSA, 0.005% Tween 20) was dispensed using a BioRAPTR FRD dispenser (Beckman Coulter, Brea, CA). Then, 23 nL/well of compound solutions in DMSO was dispensed via pintool transfer (Kalypsys, San Diego, CA). The cells were stimulated with compounds for 30 min at 37 °C in 5% CO2, after which, 1 μL/well of each HTRF detection reagent was dispensed with a BioRAPTR FRD dispenser. The detection reagents were diluted as such: K-anti-cAMP antibody at 1:20 and cAMP-d2 at 1:18 in HTRF lysis buffer (supplied by the assay kit). The plates were incubated for 30 min at room temperature, and then the signal was read on a ViewLux plate reader (PerkinElmer, Waltham, MA).
ELISA cAMP assay
The ELISA cAMP assay was performed in a 96-well format. Cells were seeded at 16,000 cells/well in 95 μL/well media, and allowed to attach overnight at 37 °C in 5% CO2. Following the incubation, 30 μL/well of 417 μM Ro 20-1724 in PBS+ was added, and immediately following that, 1.6 μL/well of compound solutions in DMSO was added with a multichannel pipette. The cells were stimulated with compounds for 30 min at 37 °C in 5% CO2, after which, the cells were lysed and cAMP levels in the lysates were assayed according to the cAMP-Screen ELISA assay kit (Life Technologies, Carlsbad, CA). The luminescence signal was detected with a ViewLux plate reader (PerkinElmer, Waltham, MA).
Compound library preparation and qHTS
The library of pharmacologically active compounds (LOPAC1280) consists of a collection of small molecules with characterized biological activities. The LOPAC1280 library has been extensively used for HTS assay validations 12, 13, and was purchased from Sigma-Aldrich. The Molecular Libraries-Small Molecule Repository (ML-SMR) includes a library of structurally diverse compounds (http://mli.nih.gov/mli/compound-repository/mlsmr-compounds/), which consisted of 365,677 compounds at the time of screening. Compounds from both libraries were serially diluted 1:5 in DMSO in 384-well plates to yield 4 concentrations (from 0.64 μM to 10 mM) and formatted into 1,536-well plates at 7 μL/well 14. A qHTS was performed using a fully automated robotic screening system (Wako, San Diego, CA), as described previously 15. Final compound concentrations during cell incubation ranged from 0.46 μM to 57.5 μM.
Data analysis
Primary screen data was analyzed with customized software developed internally. The maximal response (100% activity) was determined by the response of 28.75 nM relaxin, and the basal signal (0% activity) was measured by the 0.58% DMSO control in the HEK293-RXFP1 cells. All follow-up assays were conducted with three cell lines (HEK293-RXFP1, HEK293-RXFP2 and HEK293-V1b). Because relaxin less potent in stimulating cAMP production in the HEK293-RXFP2 cell and does not stimulate HEK293-V1b cells, 57.5 μM forskolin treatment was used as the maximal response for data normalization. The concentration responses of all the compounds were analyzed using methods described by Inglese et al 15, and structural clustering of active compounds was performed using Leadscope Hosted Client (Leadscope Inc., Columbus, OH). The EC50 values of compounds in the confirmation and follow-up experiments were calculated from the dose-response curves by nonlinear regression analysis using Prism software (GraphPad Software, San Diego, CA).
Results
Assay miniaturization and validation
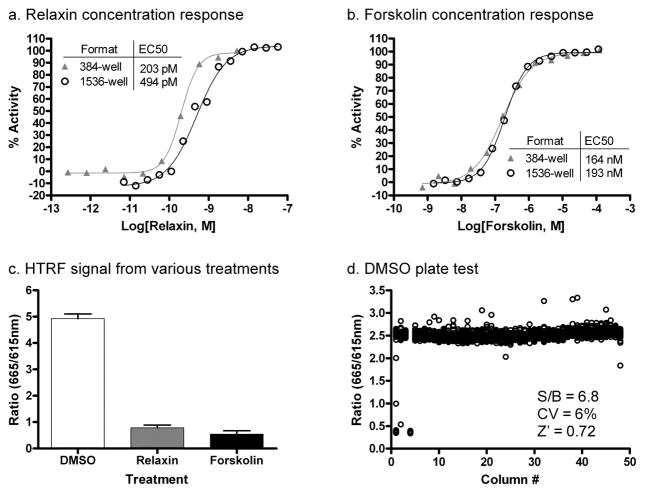
The RXFP1 receptor signals through cAMP second messenger. To screen for agonists of the receptor, we used human embryonic kidney cells stably transfected with human RXFP1 receptor (HEK293-RXFP1), which have previously been show to constitute a functional cell-based model for the RXFP1-cAMP signaling pathway 3, 4, 16. To amplify the cAMP signal, the assay was conducted in the presence of a PDE4 inhibitor Ro 20-1724 17. Cyclic AMP levels were detected using the HTRF cAMP assay kit 18. The detection kit consists of a europium cryptate labeled anti-cAMP antibody (K-anti-cAMP) and d2 dye labeled cAMP (cAMP-d2) as tracers in a time-resolved fluorescence energy transfer (TR-FRET) detection system. The TR-FRET between the K-anti-cAMP and cAMP-d2 is disrupted by cAMP in cell lysates, thus allowing detection of the second messenger in a homogenous format that is suitable for qHTS. The HTRF cAMP assay was initially developed in 384-well format, then miniaturized to 1,536-well format with an assay volume of 4 μL. The functionality of the HEK293-RXFP1 cells was tested by examining the cAMP response to porcine relaxin, which has previously been shown to activate RXFP1 with similar potency of H2 human relaxin 10, and forskolin, an adenylate cyclase activator. The concentration response curves of relaxin and forskolin in 384-well and 1,536-well formats showed good correlation, with EC50 values of 203 pM and 494 pM for relaxin and 164 nM and 193 nM for forskolin in 384-well and 1,536-well formats respectively (Fig. 1a, b). The tight correlation between the two plate formats, along with the fact that the potency of relaxin in the HTRF assay also matches that of previously published results in HEK293-RXFP1 cells 7, 16, 19, indicates that this assay adequately detects RXFP1 activation in the miniaturized format. The signal for maximal stimulation was found to be similar between relaxin and forskolin in the HTRF cAMP assay, with mean ratios from 12 treatment wells in 384-well plate format being 0.78 for relaxin and 0.54 for forskolin treatments, compared with 4.9 for DMSO treatment (Fig. 1c). While previous reports have noted that maximal relaxin stimulation is lower than that of maximal forskolin stimulation 4, differences in RXFP1 expression levels and/or inclusion of PDE4 inhibitor Ro 20-1724 might have resulted in the more robust cAMP response.
Figure 1. Assay miniaturization and validation.
Concentration responses for (a) relaxin and (b) forskolin in HEK293-RXFP1 cells. Assays were performed in 384-well and 1,536-well formats using the HTRF cAMP assay kit. 100% activity was normalized as 57.7 μM forskolin stimulation and 0% activity was normalized as 0.58% DMSO controls. (c) HTRF signal from 0.57% DMSO, 28.75 nM relaxin and 57.5 μM forskolin treatments in HEK293-RXFP1 cells. Mean and standard deviations were calculated from 12 treatment wells in 384-well assay format. (d) Scatter plot of the ratiometric signal from a 1,536-well DMSO plate to establish assay performance parameters. Column 1 was treated with a relaxin titration (24.9 pM – 1.47 μM), columns 2 and 3 received DMSO, and column 4 received 125 nM relaxin.
Assay parameters were then assessed in miniaturized plate format against a DMSO control plate, as a solvent control for the compound library. The signal-to-basal ratio (S/B), the coefficient of variation (CV) and Z factor values were 6.8, 6% and 0.72, respectively (Fig. 1d). As a further validation, the assay was used to screen against the LOPAC1280 library of bioactive compounds. The LOPAC1280 library was screened in qHTS format at 5 concentrations ranging from 92 nM to 57.5 μM. The assay showed robust and clean performance with average S/B, CV and Z factor values of 6.8, 8.3% and 0.78, respectively. This preliminary screen identified 13 compounds that showed EC50 values less than 10 μM with efficacy greater than 50%. Upon closer examination, these compounds included the positive control compound, forskolin, as well as various agonists of Gs-coupled GPCRs, such as adenosine and adrenergic receptors, that are known to be expressed in HEK293T cells. The fact that the validation screen was able to isolate known GPCR agonists and forskolin indicated that the assay was effective at identifying upregulators of cAMP. Expectantly, when these 13 hits were counter screened in an HEK293T cell line stably expressing the vasopressin 1b receptor (HEK293-V1b), they were found to be active at similar potencies and efficacies. Therefore, though the LOPAC1280 screen failed to find true agonists of human RXFP1 receptor, the results validated both the primary assay and counter screening strategy.
Quantitative high throughput screen
Upon failing to isolate human RXFP1 agonists from the LOPAC1280 library, the HTRF cAMP assay was used to screen the entire ML-SMR collection of 365,677 compounds using a fully automated screening system in qHTS format 15. All compounds were assayed at four concentrations of 57.5 μM, 11.5 μM, 2.3 μM and 92 nM. The response to control agonists (relaxin and forskolin) was measured in each plate and was stable across the entire screen.
During screening, freshly prepared cells were dispensed into 1,536-well assay plates as 3 μL/well at a rate of 100 plates per hour and incubated at 37 °C with 5% CO2 for 16–24 h prior to the robotic screening. The screen was initiated by addition of 1 μL/well PDE inhibitor Ro 20-1724 17, followed by 23 nL/well compound or control addition via pin transfer. Subsequently, the cells were incubated for 30 min at 37 °C before 1 μL/well of each HTRF detection reagent was dispensed, and signal was read on a ViewLux plate reader. The assay showed robust performance with an average S/B of 3.1 and Z′ factor of 0.66 over 1,140 plates screened. Concentration response of compounds and structure-activity relationship were analyzed based on primary screen data. A total of 434 compounds were cherry picked for follow up studies, for an initial hit rate of 0.1%. Compounds were considered hits if they showed high potency (< 10 μM EC50) and efficacy (> 60% of forskolin activation) irrespective of donor channel effects, or low potency (> 50% activation at 57.5 μM) without donor channel effects.
Follow up studies
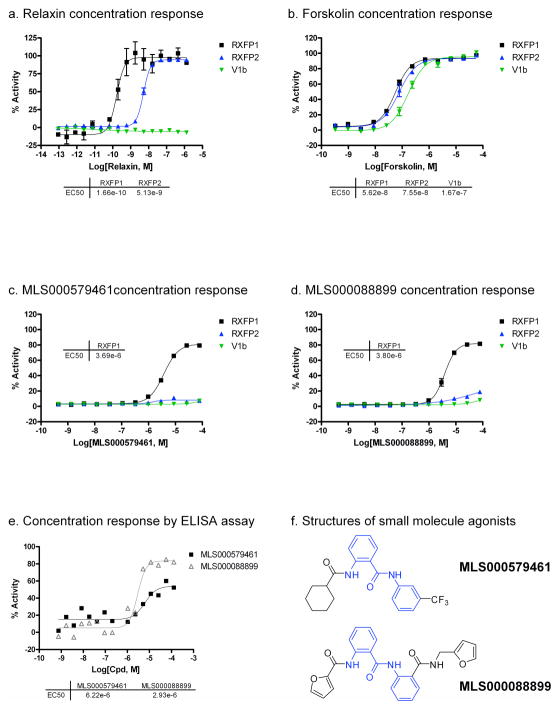
The cherry picked compounds were tested in 12-point intra-plate 1:3 titration in 384-well plate format to generate more detailed concentration response curves. Hit confirmation was carried out with HEK293-RXFP1 cells, counter screening was carried out with HEK293-V1b cells, and hit selectivity was tested with HEK293T cells stably expressing human relaxin family receptor 2 (HEK293-RXFP2). While forskolin was able to stimulate cAMP production in all three cell lines with similar potencies (EC50 of 56.2 nM – 167 nM), relaxin showed >30-fold selectivity towards the RXFP1 over the RXFP2 receptor and showed no activity towards the V1b receptor (Fig. 3a, b). The activity of relaxin against RXFP1 and RXFP2 agrees with previously published values 3, 4, 16.
Figure 3. Concentration response of human RXFP1 agonists.
HTRF cAMP response in HEK293-RXFP1 (black), HEK293-RXFP2 (blue) and HEK293-V1b (green) cell lines and per cent viability (gray) for (a) relaxin, (b), forskolin, (c) MLS000579461 and (d) MLS000088899. (e) Concentration response of MLS000579461 and MLS000088899 in ELISA cAMP assay. (f) Structures of the two lead compounds. Cyclic AMP activities of compounds were normalized to the maximal response of forskolin (57.5 μM) as 100% activity and DMSO control (0.58% DMSO) as 0% activity.
Of the 434 hits, only two compounds, MLS000579461 and MLS000088899, were found to be selective towards the RXFP1 receptor (Fig. 3c, d). The two compounds show similar potencies (3.7 μM and 3.8 μM) and efficacies (80%). Furthermore, both compounds belong to the same chemical scaffold as they contain a 2-acetamido-N-phenylbenzamide core motif (Fig. 3f, in blue).
The activities of the two hits were further confirmed with an ELISA assay for cAMP detection as an orthogonal assay that is not subjected to the same detection format. MLS000579461 and MLS000088899 showed EC50 values of 6.2 μM and 2.9 μM and efficacies of 60% and 80%, respectively (Fig. 3e). These values correlate well with those obtained from the HTRF cAMP assays.
Discussion
We report here the first series of small molecule agonists of the relaxin receptor RXFP1. The miniaturized HTRF cAMP assay performed well in both assay validation and qHTS. The validation screen against the LOPAC1280 library of bioactive compounds did not find any RXFP1 agonists. Based on this, a low hit rate was expected from qHTS. Thus, a relatively lenient cherry picking criterion was implemented. Compounds were considered a hit if they showed high potency (< 10 μM EC50) and efficacy (> 60% maximal activation) irrespective of donor channel effects, or had lower potency (> 50% activation at 57.5 μM) without donor channel effects. Because the HTRF cAMP assay utilizes a ratiometric measurement, compounds that affect the donor channel fluorescence can appear as false positives. However, potent compounds affecting donor channel fluorescence were cherry picked because the false positives could be screened out with the counter screen assay.
Upon filtering the 434 hits through the confirmation, counter screen and selectivity assays, only two compounds showed selective cAMP activation in HEK293-RXFP1 cells. It is perhaps not surprising that the screen yielded so few true hits since the natural physiological agonist is a peptide hormone that is 6 kDa in size. Full receptor binding and activation requires agonist binding at two distinct sites on the receptor, with the A chain of the hormone binding to transmembrane exoloops of the receptor and the B chain binding to the several sites of extracellular LRR domain 7, 16. Furthermore, the amino-terminal LDLa domain has also been shown to be essential to receptor activation, while the mechanism is still unclear 8, 9. Therefore, it would be difficult for a small molecule to fully mimic the large size of the natural agonist and multi-binding site mode of activation. Nevertheless, we have identified the first novel series of small molecule RXFP1 agonists with >100-fold selectivity over RXFP2 receptor through screening of a 365,677-compound library in qHTS format. The fact that the two lead compounds share a common molecular structure indicates that this scaffold is an ideal starting point for initiation of medicinal chemistry to improve potency towards RXFP1.
Figure 2.
Summary of qHTS and follow up assays.
Table 1.
qHTS protocol.
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Reagent | 3 μL | HEK293-RXFP1 cells at 2,000 cells/well |
| 2 | Incubation | 16 – 24 h | 37 °C, 5% CO2 standard cell culture conditions |
| 3 | Reagent | 1 μL | 400 μM Ro 20-1724 in PBS+ |
| 4a | Library compounds | 23 nL | Dispense library compounds via pintool transfer |
| 4b | Controls | 23 nL | Dispense control compounds via pintool transfer |
| 6 | Incubation | 30 min | 37 °C, 5% CO2 standard cell culture conditions |
| 7 | Reagent | 1 μL | cAMP-d2 diluted 1:18 in HTRF lysis buffer |
| 8 | Reagent | 1 μL | K-anti-cAMP antibody diluted 1:20 in HTRF lysis buffer |
| 9 | Incubation | 30 min | Room temperature |
| 10 | Detector | EnVision (HTRF Eu/d2 protocl). ViewLux reader. Standard HTRF protocol |
Acknowledgments
This research was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research U54MH084681 and R03 MH085705 (A.I.A.) and the Intramural Research Program of the National Human Genome Research Institute and National Center for Advancing Translational Sciences, National Institutes of Health. The authors thank Drs. András Kern and Gillian D. Bryant-Greenwood (University of Hawaii) and Dr. Sheau Yu Teddy Hsu (Stanford University) for HEK293-RXFP1 and HEK293-RXFP2 cell lines and Dr. O. David Sherwood (University of Illinois at Urbana-Champaign) for porcine relaxin.
Abbreviations
- GPCR
G protein-coupled receptor
- AC
adenylate cyclase
- LDLa
low density lipoprotein class A
- LRR
leucine rich repeat
- HEK293T
human embryonic kidney cell line 293T
- qHTS
quantitative high-throughput screening
- DMEM
Dulbecco’s modified Eagle medium
- FBS
fetal bovine serum
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kong RC, Shilling PJ, Lobb DK, Gooley PR, Bathgate RA. Membrane receptors: structure and function of the relaxin family peptide receptors. Mol Cell Endocrinol. 2010;320(1–2):1–15. doi: 10.1016/j.mce.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Van Der Westhuizen ET, Summers RJ, Halls ML, Bathgate RA, Sexton PM. Relaxin receptors--new drug targets for multiple disease states. Curr Drug Targets. 2007;8(1):91–104. doi: 10.2174/138945007779315650. [DOI] [PubMed] [Google Scholar]
- 3.Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295(5555):671–4. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 4.Halls ML, Bathgate RA, Summers RJ. Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms. Mol Pharmacol. 2006;70(1):214–26. doi: 10.1124/mol.105.021691. [DOI] [PubMed] [Google Scholar]
- 5.Bogatcheva NV, Truong A, Feng S, Engel W, Adham IM, Agoulnik AI. GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol. 2003;17(12):2639–46. doi: 10.1210/me.2003-0096. [DOI] [PubMed] [Google Scholar]
- 6.Bullesbach EE, Schwabe C. The trap-like relaxin-binding site of the leucine-rich G-protein-coupled receptor 7. J Biol Chem. 2005;280(14):14051–6. doi: 10.1074/jbc.M500030200. [DOI] [PubMed] [Google Scholar]
- 7.Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA, et al. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem. 2003;278(10):7855–62. doi: 10.1074/jbc.M212457200. [DOI] [PubMed] [Google Scholar]
- 8.Kern A, Agoulnik AI, Bryant-Greenwood GD. The low-density lipoprotein class A module of the relaxin receptor (leucine-rich repeat containing G-protein coupled receptor 7): its role in signaling and trafficking to the cell membrane. Endocrinology. 2007;148(3):1181–94. doi: 10.1210/en.2006-1086. [DOI] [PubMed] [Google Scholar]
- 9.Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJ, Tregear GW, et al. Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem. 2006;281(46):34942–54. doi: 10.1074/jbc.M602728200. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood CD, O’Byrne EM. Purification and characterization of porcine relaxin. Arch Biochem Biophys. 1974;160(1):185–96. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- 11.Kern A, Hubbard D, Amano A, Bryant-Greenwood GD. Cloning, expression, and functional characterization of relaxin receptor (leucine-rich repeat-containing g protein-coupled receptor 7) splice variants from human fetal membranes. Endocrinology. 2008;149(3):1277–94. doi: 10.1210/en.2007-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CZ, Kulakova L, Southall N, Marugan JJ, Galkin A, Austin CP, et al. High-throughput Giardia lamblia viability assay using bioluminescent ATP content measurements. Antimicrob Agents Chemother. 2011;55(2):667–75. doi: 10.1128/AAC.00618-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CZ, Sobczak K, Hoskins J, Southall N, Marugan JJ, Zheng W, et al. Two high-throughput screening assays for aberrant RNA-protein interactions in myotonic dystrophy type 1. Anal Bioanal Chem. 2012;402(5):1889–98. doi: 10.1007/s00216-011-5604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasgar A, Shinn P, Jadhav A, Auld D, Michael S, Zheng W, et al. Compound Management for Quantitative High-Throughput Screening. JALA Charlottesv Va. 2008;13(2):79–89. doi: 10.1016/j.jala.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, et al. Quantitative high-throughput screening, a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103(31):11473–8. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halls ML, Bond CP, Sudo S, Kumagai J, Ferraro T, Layfield S, et al. Multiple binding sites revealed by interaction of relaxin family peptides with native and chimeric relaxin family peptide receptors 1 and 2 (LGR7 and LGR8) J Pharmacol Exp Ther. 2005;313(2):677–87. doi: 10.1124/jpet.104.080655. [DOI] [PubMed] [Google Scholar]
- 17.Titus SA, Li X, Southall N, Lu J, Inglese J, Brasch M, et al. A cell-based PDE4 assay in 1536-well plate format for high-throughput screening. J Biomol Screen. 2008;13(7):609–18. doi: 10.1177/1087057108319977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriel D, Vernier M, Pfeifer MJ, Dasen B, Tenaillon L, Bouhelal R. High throughput screening technologies for direct cyclic AMP measurement. Assay Drug Dev Technol. 2003;1(2):291–303. doi: 10.1089/15406580360545107. [DOI] [PubMed] [Google Scholar]
- 19.Kern A, Bryant-Greenwood GD. Characterization of relaxin receptor (RXFP1) desensitization and internalization in primary human decidual cells and RXFP1-transfected HEK293 cells. Endocrinology. 2009;150(5):2419–28. doi: 10.1210/en.2008-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]