Abstract
Antiangiogenic properties of thalidomide have created an interest in the use of the drug in treatment of cancer. However, thalidomide is responsible for thalidomide embryopathy (TE). A lack of knowledge regarding the mechanisms of thalidomide teratogenesis acts as a barrier in the aim to synthesize a safer analogue of thalidomide. Recently, our group detected a higher frequency of alleles that impair the pro-angiogenic mechanisms of endothelial nitric oxide synthase (eNOS), coded by the NOS3 gene. In this study we evaluated variable number tandem repeats (VNTR) functional polymorphism in intron 4 of NOS3 in individuals with TE (38) and Brazilians without congenital anomalies (136). Haplotypes were estimated for this VNTR with previously analyzed polymorphisms, rs2070744 (−786C > T) and rs1799983 (894T > G), in promoter region and exon 7, respectively. Haplotypic distribution was different between the groups (p = 0.007). Alleles −786C (rs2070744) and 4b (VNTR), associated with decreased NOS3 expression, presented in higher frequency in TE individuals (p = 0.018; OR = 2.57; IC = 1.2–5.8). This association was not identified with polymorphism 894T > G (p = 0.079), which influences eNOS enzymatic activity. These results suggest variants in NOS3, with pre-transcriptional effects as susceptibility factors, influencing the risk TE development. This finding generates insight for a new approach to research that pursues a safer analogue.
The consumption of thalidomide in early pregnancy was very common in the 1960s, as an antiemetic to control morning sickness1. The lack of awareness about the teratogenic properties of the drug led to the birth of ten thousand babies around the world affected by Thalidomide Embryopathy (TE). TE is a condition mainly characterized by limb defects1 which occur in 79–89% of the affected individuals2. Other organs frequently affected are the eyes, ears and heart1,3, occurring together with limb defects in around 19% of cases2. Severe internal organ defects were also diagnosed at the time, although the majority of the children affected by these anomalies died in the first years of life3. Nowadays, thalidomide is well known as a potent immunomodulator and antiangiogenic drug, properties that have led to its current use in treatment of many types of cancer (especially multiple myeloma) and immunological conditions4.
Many aspects of TE have been studied over the past few decades; however, its teratogenic molecular mechanism remains to be elucidated. One of the accepted hypotheses is that antiangiogenic property of thalidomide inhibits the proper development of new blood vessels, which are essential for limb development2,5.
The importance of nitric oxide in TE development has been reported in experimental studies assessing the teratogenic potential and antiangiogenic property of the drug6,7,8. Our group has recently published the first molecular study of TE in humans; we reported that the −786C (rs2070744) and 894T (rs1799983) alleles – which reduce the expression and activity of the NOS3 gene, respectively, and their derived haplotype – were associated with TE, when compared with Brazilian people without congenital anomalies9.
In order to further explore and confirm the role that polymorphic variants in the NOS3 gene result in susceptibility to TE, here we investigated the variable number tandem repeats (VNTR) of intron 4 (rs61722009) in the NOS3 gene. Similarly to the previously investigated polymorphisms (−786T > C and 894G > T), the VNTR 4a/4b is common in different populations10. It has an important functional role in regulating eNOS and was believed to function either as an enhancer or repressor of NOS3 gene transcription11,12, according to the number of repeats present in each allele13.
Results
Sample Characteristics
In total, our study involved 38 Brazilian individuals with TE, born between 1959 and 2010. The control group was comprised of 136 Brazilian individuals without congenital anomalies, born in similar regions and time periods as those TE individuals.
Analysis of Allelic and Genotypic Frequencies
The distribution of all polymorphisms in both the sample groups was in accordance with the Hardy-Weinberg equilibrium.
Allele and genotype frequencies of the three polymorphisms are listed in Table 1. The frequency of alleles C and T in the polymorphism −786T > C was statistically different between the two sample groups (p = 0.022), corroborating results detailed in our previous study with a smaller sample size. However, the intron 4 VNTR and the 894G > T polymorphism was not significantly different between the two sample groups.
Table 1. Allelic and genotypic frequencies of the polymorphisms of the NOS3 gene in individuals with thalidomide embryopathy, as well as in Brazilian individuals without congenital anomalies (unaffected group).
| Gene | Polymorphism | Genotype/Allele | Affected | Unaffected | P-Valuea | ||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| NOS3 | rs2070744 | CC | 10 | 26.3 | 17 | 12.5 | 0.060 |
| (C/T) | CT | 17 | 44.7 | 57 | 41.9 | ||
| TT | 11 | 28.9 | 62 | 45.6 | |||
| C | 37 | 48.7 | 91 | 33.5 | 0.022 | ||
| T | 39 | 51.3 | 181 | 66.5 | |||
| rs61722009 | 4b4b | 27 | 71.1 | 81 | 61.8 | 0.263 | |
| (VNTR) | 4b4a | 11 | 28.9 | 41 | 31.3 | ||
| 4a4a | 0 | 0.0 | 9 | 6.9 | |||
| 4b | 59 | 85.5 | 203 | 77.5 | 0.149 | ||
| 4a | 11 | 14.5 | 59 | 22.5 | |||
| rs1799983 | TT | 5 | 13.2 | 13 | 9.6 | 0.360 | |
| (T/G) | TG | 21 | 55.3 | 63 | 46.7 | ||
| GG | 12 | 31.6 | 59 | 43.7 | |||
| T | 31 | 40.8 | 89 | 33.0 | 0.221 | ||
| G | 45 | 59.2 | 181 | 67.0 | |||
aChi-Square Test.
Linkage Disequilibrium and Haplotype Analysis
Linkage disequilibrium analysis demonstrated that the three polymorphisms evaluated are in moderate disequilibrium (D′ > 0.3; p < 0.001).
Haplotypes and their frequencies in the two samples are described in Table 2. A comparison of the haplotype frequencies between groups revealed a statistically significant difference (p = 0.007).
Table 2. The inferred haplotypes and haplotypic frequencies in individuals with thalidomide embryopathy, as well as in Brazilian individuals without congenital anomalies (unaffected group).
| Gene | Haplotype | Affected | Unaffected | P-Valuea | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| NOS3 | T 4b G | 24 | 31.6 | 120 | 44.1 | 0.007 |
| T 4b T | 9 | 11.8 | 30 | 11.0 | ||
| T 4a G | 6 | 7.9 | 30 | 11.0 | ||
| T 4a T | 0 | 0.0 | 1 | 0.4 | ||
| C 4b G | 10 | 13.2 | 6 | 2.2 | ||
| C 4b T | 22 | 28.9 | 56 | 20.6 | ||
| C 4a G | 5 | 6.6 | 26 | 9.6 | ||
| C 4a T | 0 | 0.0 | 3 | 1.1 | ||
aChi-Square Test.
Evaluation of Risk Allele Association
Univariate logistic regression (Table 3) was performed to determine the association of NOS3 with TE susceptibility. The alleles responsible for decrease in NOS3 gene expression or eNOS enzyme activity were considered to indicate risk8. Based on this, we divided the sample in two groups: (i) individuals with at least one copy of the C, 4b, and T alleles of −786T > C polymorphism, the VNTR, and 894G > T polymorphism, respectively and (ii) individuals without such variants in at least one of the polymorphisms.
Table 3. Univariate logistic regression model to assess risk alleles in individuals with thalidomide embryopathy and in individuals of the unaffected group.
| Risk Alleles of NOS3 Gene | Presence | Absence | Odds Ratio (95% IC) | P-Value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| (−786)C + (VNTR)4b | 91 | 53.8 | 78 | 46.2 | 2.570 (1,20–5,80) | 0.018 |
| (−786)C + (VNTR)4b + (894)T | 68 | 52.6 | 101 | 36.6 | 1.921 (0,93–4,01) | 0.079 |
CI: confidence interval; Polymorphisms used in the model are rs2070744 (−786C), rs61722009 (VNTR), and rs1799983 (894T).
An association was not identified when all three polymorphisms were present together (p = 0.079). However, an analysis of the polymorphism in the promoter region (−786T > C) and the VNTR (performed in this study) showed that compared to the controls, case group showed a higher frequency of the alleles that reduce NOS3 expression (Odds ratio (OR) = 2.57; 95% IC: 1,20–5,80; p = 0.018) (Table 3).
Discussion
In this study, we have corroborated, using a larger sample size, that the −786C variant in the promoter region of the eNOS gene is more frequently present in individuals affected by TE than in those without congenital abnormalities. Moreover, we also identified an interaction between the −786T > C polymorphism and a functional intronic variant, which was previously not analyzed, as a genetic susceptibility factor to TE.
Nitric oxide is responsible for the regulation of vasodilation, exerting its effect through regulating the migration of endothelial cells6,14. It is also an important pro-angiogenic factor, stimulating the synthesis of cyclic guanosine monophosphate (cGMP) through soluble guanylyl-cyclase (sGC) activation7. Experimental models utilizing thalidomide and nitric oxide are well established in literature, and have helped to understand the physiological actions of nitric oxide in presence or absence of a highly anti-angiogenic teratogen. A previous study has demonstrated the relevance of nitric oxide in teratogenesis, especially during induction of limb reduction defects in mice models15. In addition, a role for nitric oxide in TE development has been suggested in thalidomide experimental models6,7,8. Thalidomide appears to attenuate nitric-oxide-induced migration of endothelial cells through suppression of cGMP levels, inhibiting angiogenesis at a cellular level through interaction with sGC6,7,8. It has not been determined whether a polymorphic eNOS protein would respond differently to the downstream effects of sGC-thalidomide interaction, and what consequences this polymorphic protein might have in terms of intensifying or decreasing the teratogenic potential of thalidomide. Nonetheless, it has been demonstrated that nitric oxide is able to rescue thalidomide induced teratogenicity in chicken and zebrafish embryos, reinforcing vasculogenesis and blocking apoptosis through modulating redox levels8.
Thalidomide is known as a multi-target drug with different therapeutic proprieties. It is not known whether these therapeutic proprieties overlap with its teratogenic ones. Anti-inflammatory actions of thalidomide occur mainly through the reduction of TNF-alpha mRNA levels16. TNF-alpha is a pro-inflammatory cytokine, stimulated by nuclear factor kappa-B (NF-KB), that able to reduce the half-life of eNOS mRNA17. NF-KB activates TNF-alpha to increase the expression of inducible oxide nitric synthase (iNOS) during inflammatory processes18. Thus, iNOS is principally responsible for high level of nitric oxide seen during inflammatory reactions, while eNOS has little activity in this context19. Overall, eNOS plays a fundamental role during limb outgrowth in the context of angiogenesis, while iNOS is irrelevant.
In regards to the effect of thalidomide upon oxidative stress, ROS levels are increased on drug exposure leading to a misbalance in the NF-KB pathway20. The consequences of this effect on NF-KB, its control of TNF-alpha, iNOS activation and eNOS repression remain unknown. Nonetheless, the high levels of ROS generated by thalidomide are also suggested to contribute to the drug’s teratogenic effect. Thus, it is possible that for carriers of alleles with reduced production of NO, the risks are not only for altered angiogenesis as well as for increased oxidative stress (due to thalidomide effect per se) causing synergistic effect susceptibility. Clearly these hypotheses should be further tested by in vitro and in vivo assays. As a complicating factor in this scenario, thalidomide is well known as an immunomodulatory drug, acting on interleukin (IL) and cytokines levels20. Hence, an individual’s response to thalidomide will also depend on their immunological status and developmental stage. Taking all this together, we believe that the role played by eNOS during thalidomide teratogenesis could be through multiple functions.
The fact that thalidomide is non-teratogenic in mouse and rats21, highlights the need of molecular studies in humans in order for us to fully understand the species-specific teratogenic properties of thalidomide. It has been reported that a human’s capacity of responding to an angiogenic stimulus is affected by genetic variants22, reflecting a variable susceptibility to develop a disease. The extent of thalidomide-induced teratogenesis is also dependent on genetic variability, and so must be considered in the context of TE.
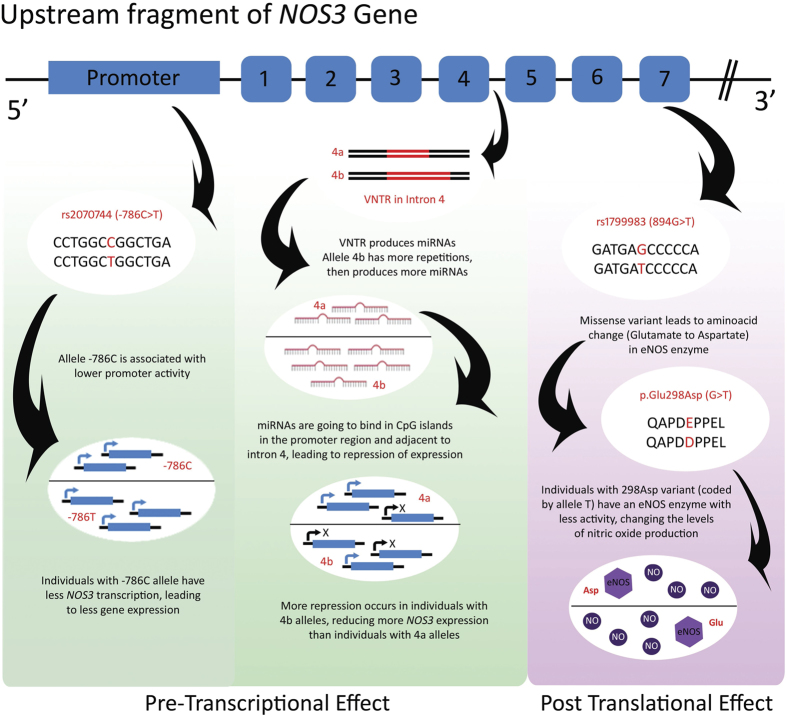
As described in Fig. 1, experimental evidence suggests that the 894G > T polymorphism influences eNOS enzyme activity, altering production of nitric oxide25. On the other hand, the 786T > C polymorphism and the 4a/4b VNTR have been observed to regulate nitric oxide activity at the level of gene expression24,26. Absence of interactions between the 894G > T polymorphism and the two other polymorphisms in our univariate logistic regression model appears to indicate that thalidomide regulates NOS3 at a pre-transcriptional level; this needs to be further investigated in studies evaluating additional gene regulation mechanisms. Regardless, the presence of alleles that result in reduced NOS3 expression in thalidomide-affected individuals, as well as in other members of the population, results in a higher risk of developing the cited vascular conditions. The haplotype C/4b/T has previously been associated with lower levels of circulating nitric oxide27; many studies report C/4b/T as the susceptibility haplotype to conditions with impaired vascular function, such as hypertension28,29, preeclampsia30, and cardiomyopathies31.
Figure 1. Pre-transcriptional and post translational effects of polymorphisms rs2070744, VNTR in intron 4 (rs61722009) and rs1799983.
Furthermore, the 27pb VNTR, at intron 4 of the NOS3 gene, plays an important role in controlling NOS3 gene expression. It is responsible for producing microRNA capable of altering DNA methylation and histone acetylation in the promoter region of the NOS3 gene, and in regions adjacent to the VNTR itself23,24. It is believed that alleles with larger repeats, such as 4b, produce more microRNAs, therefore resulting in reduced NOS3 gene expression compared to 4a13. The polymorphism 894G > T has been previously associated with congenital heart defects (CHD), especially when together with tobacco exposure in pregnancy33. Recent research has evaluated this polymorphism, with −786C > T in a Chinese sample, although an association was not identified34. In our study, it was not possible to determine an association between NOS3 risk haplotypes and congenital anomalies (data not showed), possibly due to the evaluated sample size”.
It was reported in the 1960’s that TE occurs in 10–50% of all babies exposed to thalidomide in utero32. Genetic variability may help to explain why some individuals are apparently resistant to the drug’s teratogenic effects while others are more susceptible. The best approach in a case-control study would be to access people who were exposed to thalidomide, although did not develop TE, as a control group. Unfortunately, such data does not exist since the thalidomide tragedy occurred more than fifty years ago and unaffected individuals were not identified at the time. The control group in the current study is composed of individuals born at the same time as individuals affected with TE and in the same Brazilian regions. In these regions, thalidomide was highly available and could be bought without prescription. However, it is not possible to confirm if any individuals in the control group were exposed to the drug. Clearly, not only this variable was lost, but many other variables needed to evaluate susceptibility or phenotypes characteristic of individuals affected by TE.
In the present study we suggest that individuals with alleles −786C and (VNTR) 4b may be more sensitive to thalidomide than those without these variants. This may help to explain why some individuals were exposed to thalidomide but did not develop TE. Further analysis with a larger sample size should help evaluate endophenotypes of TE and vascular conditions in both groups. Studies focusing on thalidomide-induced susceptibility to embryopathy in angiogenesis (as well as in other pathways) might help elucidate the molecular mechanisms underlying thalidomide teratogenesis. This may contribute to our understanding and treatment of diseases in which these alleles are also considered risk factors. The alleles investigated have high frequency across different populations (−786C varying from 12–44%, and VNTR 4a with a 12–20% frequency)10. Hence, it is also relevant to look at presented results with focus and view on personalized medicine strategies. Pharmacogenetic studies, especially in Erithema Nodosum Leprosum and Multiple Myeloma (the main conditions treated by thalidomide and its analogues), can help not only to identify genetic profiles with a better response to the drug, but also to look for alternative drugs when the therapeutic benefits of thalidomide are not reached for these people. Further, in vitro and in vivo research is essential to understand the role of the NOS3 polymorphisms analyzed in thalidomide teratogenesis as well as in therapeutics settings and pharmacogenetics approaches. We believe this is a first step that can put forward different therapeutic strategies involving thalidomide.
Materials and Methods
Ethical Aspects
This study was approved by the Research Ethics Committee of the Clinical Hospital of Porto Alegre (number 10-0244). The methods were carried out in accordance with the approved guidelines.
Recruitment and Sample Collection
Individuals with TE were recruited through the Brazilian Association of People with Thalidomide Syndrome; all subjects signed the informed consent. The congenital anomalies in these individuals were assessed through the TE diagnostic guideline previously published9. Only individuals displaying phenotypes compatible with TE were included in this study. Twenty-eight individuals completed a clinical questionnaire that characterized their congenital anomalies according to organs and systems affected35.
Saliva collection was performed through Oragene-DNA OG-500 (DNA Genotek®) and DNA was obtained according to the manufacturer’s instructions.
The control group consisted of Brazilians without any congenital anomalies. DNA samples from the control subjects were stored in the Genetics Department of our institution; they have already been used in previous epidemiological studies36,37. Gender, age, date, and place of birth information were also collected from subjects.
SNPs and VNTR Genotyping
The 27bp VNTR of intron 4 (rs61722009) was genotyped according to Marroni38. A small percentage (10%) of the sample was selected randomly to confirm the result by Sanger sequencing in equipment ABI 3730XL (Applied Biosystems®).
The polymorphisms rs2070744 and rs1799983 (−786T > C and 894G > T, respectively) were also genotyped in the new cases and controls; these samples were genotyped by TaqMan® Genotyping Assay (Applied Biosystems) as previously described39.
Evaluation of Linkage Disequilibrium and Haplotypes
Linkage disequilibrium between variants was estimated using the MLocus program40, and the haplotypes were derived using the PHASE v.2.1 software (University of Chicago, Chicago, IL, USA).
Statistical Analysis
All statistical analyses were evaluated in SPSS® program, version 20 (SPSS, IBM, USA). All tests were two-tailed and the Bonferroni correction was applied in all sample groups.
Additional Information
How to cite this article: Kowalski, T. W. et al. New Findings in eNOS gene and Thalidomide Embryopathy Suggest pre-transcriptional effect variants as susceptibility factors. Sci. Rep. 6, 23404; doi: 10.1038/srep23404 (2016).
Acknowledgments
We are indebted to Claudia Marques Maximino (Brazilian Association of People with Thalidomide Syndrome) who helped identify and monitor the TE patients for the purpose of this study. We also deeply thank Dr. Neil Vargesson of University of Aberdeen for all the helpful discussions and critical analyses and Alexandra Diamond for comments and review. The authors would like to acknowledge INAGEMP (National Institute of Population Medical Genetics; Grant CNPq 573993/2008-4) and FIPE/HCPA (GPPG #10-0244) for the financial support provided for this project.
Footnotes
Author Contributions T.W.K., L.R.F. and F.S.L.V. devised the concept, designed the molecular assays and performed statistical analysis. T.W.K. and L.T.R. performed the experiments and analyzed the haplotypic data. T.W.K. and F.S.L.V. wrote the manuscript. M.T.V.S., M.H.H. and L.S.F. supervised the project. All authors discussed the results and contributed in this manuscript.
References
- Lenz W. A short history of thalidomide embryopathy. Teratology 38, 203–15 (1988). [DOI] [PubMed] [Google Scholar]
- Vargesson N. Thalidomide-induced limb defects: resolving a 50-year-old puzzle. Bioessays 31, 1327–36 (2009). [DOI] [PubMed] [Google Scholar]
- Smithells R. W. & Newman C. G. Recognition of thalidomide defects. J Med Genet 29, 716–23 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumgartten F. J. & de Souza N. R. Clinical use and control of the dispensing of thalidomide in Brasilia-Federal District, Brazil, from 2001 to 2012. Cien Saude Colet 18, 3401–8 (2013). [DOI] [PubMed] [Google Scholar]
- Therapontos C., Erskine L., Gardner E. R., Figg W. D. & Vargesson N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc Natl Acad Sci USA 106, 8573–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamilarasan K. P. et al. Thalidomide attenuates nitric oxide mediated angiogenesis by blocking migration of endothelial cells. BMC Cell Biol 7, 17 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S. et al. Thalidomide attenuates nitric oxide-driven angiogenesis by interacting with soluble guanylyl cyclase. Br J Pharmacol 158, 1720–34 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siamwala J. H. et al. Nitric oxide rescues thalidomide mediated teratogenicity. Sci Rep 2, 679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna F. S. et al. Recognition of the phenotype of thalidomide embryopathy in countries endemic for leprosy: new cases and review of the main dysmorphological findings. Clin Dysmorphol 22, 59–63 (2013). [DOI] [PubMed] [Google Scholar]
- Abecasis G. R. et al. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. L., Sim A. S., Badenhop R. F., McCredie R. M. & Wilcken D. E. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat Med 2, 41–5 (1996). [DOI] [PubMed] [Google Scholar]
- Wang J., Dudley D. & Wang X. L. Haplotype-specific effects on endothelial NO synthase promoter efficiency: modifiable by cigarette smoking. Arterioscler Thromb Vasc Biol 22, e1–4 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlFadhli S. Influence of endothelial nitric oxide synthase gene intron-4 27bp repeat polymorphism on its expression in autoimmune diseases. Dis Markers 34, 349–56 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden P. A. et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem 268, 17478–88 (1993). [PubMed] [Google Scholar]
- Tiboni G. M. & Clementini E. Teratological consequences of nitric oxide synthesis inhibition. Curr Pharm Des 10, 2759–67 (2004). [DOI] [PubMed] [Google Scholar]
- Sampaio E. P., Sarno E. N., Galilly R., Cohn Z. A. & Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 173, 699–703 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi M., Perrella M. A., Burnett J. C. & Lee M. E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res 73, 205–9 (1993). [DOI] [PubMed] [Google Scholar]
- Taylor B. S. et al. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem 273, 15148–56 (1998). [DOI] [PubMed] [Google Scholar]
- Lee K. S. et al. Functional role of NF-κB in expression of human endothelial nitric oxide synthase. Biochem Biophys Res Commun 448, 101–7 (2014). [DOI] [PubMed] [Google Scholar]
- Hansen J. M. & Harris C. A novel hypothesis for thalidomide-induced limb teratogenesis: redox misregulation of the NF-kappaB pathway. Antioxid Redox Signal 6, 1–14 (2004). [DOI] [PubMed] [Google Scholar]
- Fratta I. D., Sigg E. B. & Maiorana K. Teratogenic effects of thalidomide in rabbits, rats, hamsters, and mice. Toxicol Appl Pharmacol 7, 268–86 (1965). [DOI] [PubMed] [Google Scholar]
- Rogers M. S. & D’Amato R. J. Common polymorphisms in angiogenesis. Cold Spring Harb Perspect Med 2, doi: 10.1101/cshperspect.a006510 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. X. et al. Regulation of endothelial nitric oxide synthase by small RNA. Proc Natl Acad Sci USA 102, 16967–72 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. X. et al. Effect of 27nt small RNA on endothelial nitric-oxide synthase expression. Mol Biol Cell 19, 3997–4005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanus-Santos J. E. et al. Effects of endothelial nitric oxide synthase gene polymorphisms on platelet function, nitric oxide release, and interactions with estradiol. Pharmacogenetics 12, 407–13 (2002). [DOI] [PubMed] [Google Scholar]
- Doshi A. A. et al. A promoter polymorphism of the endothelial nitric oxide synthase gene is associated with reduced mRNA and protein expression in failing human myocardium. J Card Fail 16, 314–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger I. F. et al. Endothelial nitric oxide synthase gene haplotypes associated with circulating concentrations of nitric oxide products in healthy men. Pharmacogenet Genomics 15, 565–70 (2005). [DOI] [PubMed] [Google Scholar]
- Kitsios G. D. & Zintzaras E. An NOS3 Haplotype is Protective against Hypertension in a Caucasian Population. Int J Hypertens 2010, 865031 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Costa D. C. et al. eNOS haplotype associated with hypertension in obese children and adolescents. Int J Obes (Lond) 35, 387–92 (2011). [DOI] [PubMed] [Google Scholar]
- Díaz-Olguín L. et al. Endothelial nitric oxide synthase haplotypes are associated with preeclampsia in Maya mestizo women. Dis Markers 31, 83–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsa L. S. et al. Haplotypes of NOS3 gene polymorphisms in dilated cardiomyopathy. PLoS One 8, e70523 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. G. The thalidomide syndrome: risks of exposure and spectrum of malformations. Clin Perinatol 13, 555–73 (1986). [PubMed] [Google Scholar]
- van Beynum I. M. et al. Common 894G > T single nucleotide polymorphism in the gene coding for endothelial nitric oxide synthase (eNOS) and risk of congenital heart defects. Clin Chem Lab Med 46, 1369–75 (2008). [DOI] [PubMed] [Google Scholar]
- Zhou K. et al. Genetic variants of the endothelial NO synthase gene (eNOS) may confer increased risk of sporadic congenital heart disease. Genet Mol Res 13, 3805–11 (2014). [DOI] [PubMed] [Google Scholar]
- Kowalski T. W., Sanseverino M. T., Schuler-Faccini L. & Vianna F. S. Thalidomide embryopathy: Follow-up of cases born between 1959 and 2010. Birth Defects Res A Clin Mol Teratol 103, 794–803 (2015). [DOI] [PubMed] [Google Scholar]
- Fiegenbaum M., de Andrade F. M. & Hutz M. H. Association between plasma lipid parameters and APOC3 genotypes in Brazilian subjects: effect of gender, smoking and APOE genotypes. Clin Chim Acta 380, 175–81 (2007). [DOI] [PubMed] [Google Scholar]
- Friedrich D. C. et al. The lactase persistence genotype is a protective factor for the metabolic syndrome. Genet Mol Biol 37, 611–5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroni A. S. et al. Consistent interethnic differences in the distribution of clinically relevant endothelial nitric oxide synthase genetic polymorphisms. Nitric Oxide 12, 177–82 (2005). [DOI] [PubMed] [Google Scholar]
- Vianna F. S. et al. Polymorphisms in the endothelial nitric oxide synthase gene in thalidomide embryopathy. Nitric Oxide 35, 89–92 (2013). [DOI] [PubMed] [Google Scholar]
- Long J. C., Williams R. C. & Urbanek M. An E-M algorithm and testing strategy for multiple-locus haplotypes. Am J Hum Genet 56, 799–810 (1995). [PMC free article] [PubMed] [Google Scholar]