Abstract
Cortical spreading depression (CSD), a wave of neuronal depolarization in the cerebral cortex following traumatic brain injury or cerebral ischemia, significantly aggravates brain damage. Here, we tested whether N-palmitoylethanolamine (PEA), a substance that effectively reduces lesion volumes and neurological deficits after ischemic stroke, influences CSD. CSD was elicited chemically in adult rats and occurrence, amplitude, duration and propagation velocity of CSD was determined prior to and for 6 hours after intraperitoneal injection of PEA. The chosen systemic administration of PEA stabilized the amplitude of CSD for at least four hours and prevented the run-down of amplitudes that is typically observed and was also seen in untreated controls. The propagation velocity of the CSD waves was unaltered indicating stable neuronal excitability. The stabilization of CSD amplitudes by PEA indicates that inhibition or prevention of CSD does not underlie PEA’s profound neuroprotective effect. Rather, PEA likely inhibits proinflammatory cytokine release thereby preventing the run-down of CSD amplitudes. This contribution of PEA to the maintenance of neuronal excitability in healthy tissue during CSD potentially adds to neuroprotection outside a damaged area, while other mechanisms control PEA-mediated neuroprotection in damaged tissue resulting from traumatic brain injury or cerebral ischemia.
Stroke is the fourth leading cause of death in the United States, accounting for 1 in 20 deaths1. Approximately 795,000 stroke cases are reported every year, making it a significant health problem1.
In malignant hemispheric stroke, two areas of injury within the ischemic cerebrovascular bed can be distinguished: the ischemic core and the penumbra. The penumbra is characterized by hypoperfusion and electrical function insufficient to sustain neuronal function, but adequate to maintain cellular survival2. As such, the penumbra represents the primary target in efforts to develop novel pharmaceutical strategies against stroke and for the prevention of secondary damage after stroke3. One such sequelae of stroke is cortical spreading depression (CSD), a depolarization wave in the cerebral gray matter, originally described by Leao4. In addition, the higher prevalence of stroke in patients suffering with migraine5,6, where CSD has been identified as a characteristic hallmark7 , broadens the clinical rationale to study the role of CSD in stroke. Depolarizations resembling CSD were identified in the penumbra region of experimentally induced lesions and termed peri-infact depolarization (PID)8,9. These spontaneous depolarizations share the characteristic features of experimentally-induced CSD, including a shift in plasma membrane polarization, disturbed ion homeostasis, and altered propagation velocities7,8. Despite this preclinical evidence the pathologic role and mechanistic involvement of CSD in neurological disorders and traumatic brain injury has been ignored in clinical practice until recently, when events resembling both PID and CSD were identified in stroke patients by the Co-Operative Studies on Brain Injury Depolarizations consortium10.
We have previously described the neuroprotective effects of N-acylethanolamines in a rat model of ischemic stroke using temporary middle cerebral artery occlusion (MCAO) to generate ischemia/reperfusion injury11,12. N-Palmitoylethanolamine (PEA) belongs to the group of N-acylethanolamines (NAEs), and is an endogenous signaling lipid involved in cellular signaling and neuroprotection13,14,15,16. While several NAEs, such as anandamide, are ligands for cannabinoid receptors, we and others have shown that the neuroprotective effects of PEA are mediated by an intracellular mechanism independent of cannabinoid and vanilloid receptor activation11,12,14,16,17,18.
Here, we investigated whether the neuroprotective properties of PEA mediated by intracellular signaling pathways and resulting in reduced lesion size and improved neurological deficit score after experimental stroke, also contribute mechanistically to CSD/PID events and their deleterious impact on stroke.
Methods
Animals
The in vivo experiments in the present study were approved by the Thuringian Government (Registration Numbers 02-040/06 and 02-005/12) and performed according to the Protection of Animals Act of the Federal Republic of Germany. The animals were treated in strict adherence to the American Physiological Society’s Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training.
Surgical preparation of the rats
Adult male Wistar rats (n = 9, 350–450 grams) were deeply anesthetized with sodium thiopental (Trapanal®, Inresa GmbH, Freiburg, Germany; initially 100 mg/kg i.p.). During dissection depth of anesthesia was checked by testing the corneal blink reflex and reflexes to noxious squeezing the tail tip. During the experiments, absence of the corneal blink reflex was maintained by supplemental doses of 20 mg/kg i.p. The trachea, the right femoral vein and artery were cannulated. The mean arterial blood pressure and the electrocardiogram were continuously monitored. Body temperature was kept at 37 °C by a feedback controlled heating system.
After stereotactic fixation of the head a trephination was made over the left hemisphere (spanning from the coronal suture over a length of 5–8 mm, 3–4 mm wide) using a mini-drill under cooling with artificial cerebrospinal fluid (ACSF, in mmol/L: NaCl 138.4, KCl 3.0, CaCl2 1.3, MgCl2 0.5, NaH2PO4 0.5, urea 2.2, glucose 3.4, warmed to 37 °C and equilibrated with 5% CO2 in O2). The underlying dura and arachnoidea were removed; the exposed cortex was kept moist with ACSF. A wall was built with dental acrylic on the skull around the trephination thereby forming a trough in which the exposed cortex was superfused with ACSF.
Recording of intracortical DC potentials
We used an Ag/AgCl reference electrode (containing 2 M KCl) on the nasal bone. The electrode arrays for elicitation of CSD and recordings of intracortical DC potentials at sites 1–4 are displayed in Fig. 1A. Electrodes for DC recordings had tip diameters of about 5 μm, resistance <10 MΩ, and were filled with 150 mmol/L NaCl. The distance between the electrode tips was 400–500 μm. The electrode for CSD elicitation contained 1 mol/L KCl; SDs were elicited by injection of 0.5 μL KCl with a pressure of 100 kPa for 1 second using a microinjector (picoinjector PLI-100; Harvard Apparatus, Holliston, MA, USA). The signals were recorded using a four-channel high-impedance amplifier (Meyer, Munich, Germany) and stored on PC. CSDs were accepted if they had a steep onset, exceeded amplitudes ≥5 mV, and migrated over the whole recording area. At the end of an experiment the animals were euthanized by an overdose of the anesthetic (100 mg per rat, intravenously).
Figure 1. Display of experimental approach and effect of PEA on CSD.
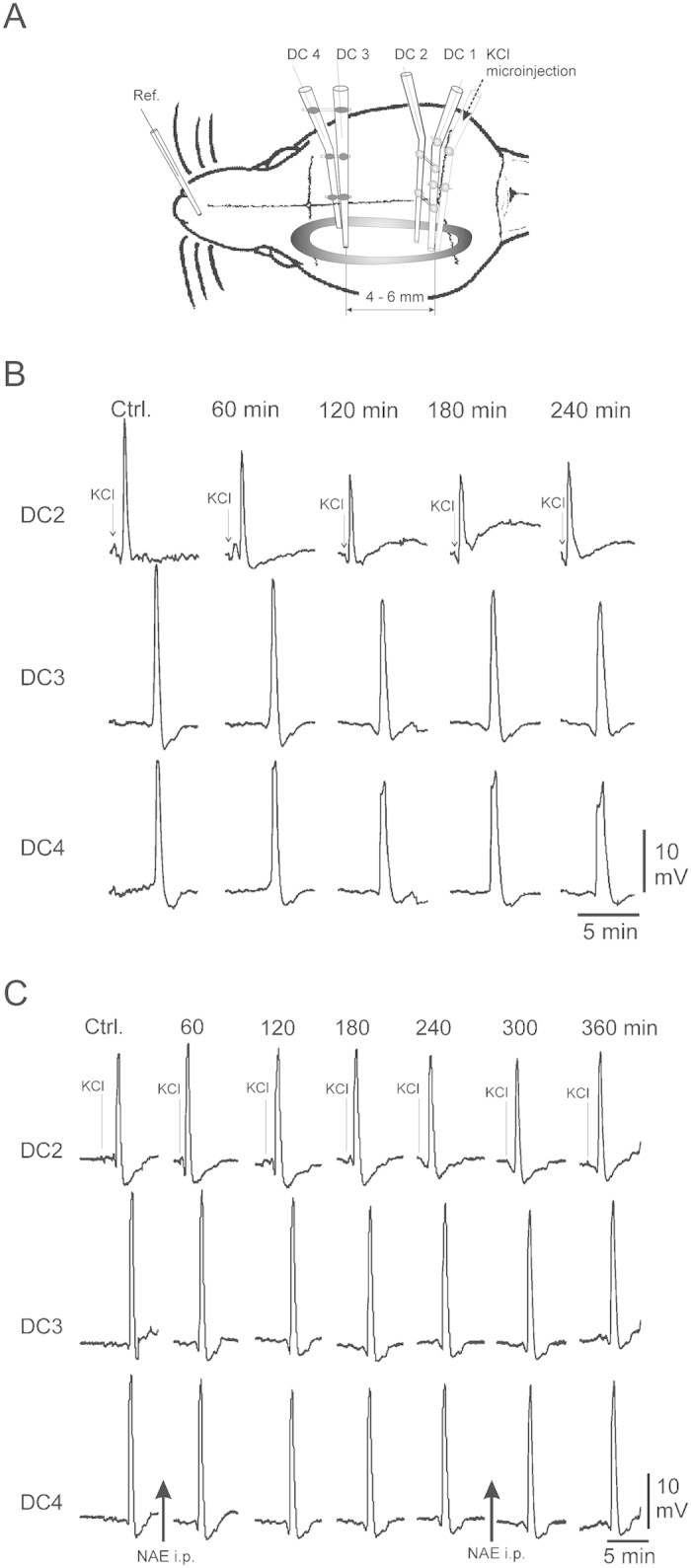
(A) Schematic view of the adult rat skull (not to scale) with the trephination that is surrounded by a wall made from dental acrylic (grey oval). Electrode arrays were lowered to a depth of 1,200–1,400 μm, approximately cortical layer V for the deepest electrode tips. Recording electrodes in an array had vertical and horizontal tip separations of 800 μm. (B) Representative samples of CSD from electrode DC2, DC3, and frontal electrode DC 4 in adult rat cortex when only ACSF was applied to the cortical surface and no PEA was given. Arrows mark the microinjection of KCl. (C) CSDs in cerebral cortex before and after intraperitoneal application of PEA. Arrows mark the microinjection of KCl.
PEA was dissolved in ethyl alcohol and administered intraperitoneally at a dose of 20 mg/kg body weight. The recording protocol consisted of at least two CSD elicitations at intervals of 30 min prior to the injection of PEA forming a baseline. After injection of PEA, CSD were elicited at intervals of 30 min for two hours and then every hour. Four hours after the injection the rats received a second dose of PEA (20 mg/kg body weight), and CSD elicitations were continued 30 min, one hour and two hours after the second injection.
In a subset of three adult rats, two instead of one trephinations were made over the left hemisphere (1 frontal, spanning from 2 mm in front of bregma over a length of 5–6 mm, 3–4 mm wide, and 1 caudal, with a diameter of 3–4 mm in front of lambda), and a wall was built with dental acrylic on the skull around the frontal trephination thereby forming a trough with a capacity of 150 to 200 μL. The same sets of recording electrodes (DC 1 and DC 2 in the untreated area; DC 3 and DC 4 in the treated area) and the same stimulation device as in the experiments with PEA were used.
After testing for CSD under control conditions (both threphinations were superfused with warmed equilibrated ACSF), in the trough around the frontal trephination 1 μg of the IL-1ß receptor antagonist Anakinra (recombinant N2-L-methionyl-interleukin 1 receptor antagonist; Sobi, Stockholm, Sweden) was applied until the end of the experiment. CSD were elicited following the same schedule as in the experiments with PEA. CSD amplitudes in untreated and treated areas were evaluated and CSD propagation velocity was calculated from the time interval between microinjection of KCl and the peak of the CSD in the treated area.
Data are reported as means ± S.E.M. Statistical analysis was performed using the Wilcoxon matched pairs signed rank test. Bonferroni adjustment was performed as necessary. Significance was accepted at P < 0.05.
Results
In vivo CSD recordings
Microinjection of KCl elicited single propagating CSD with typical parameters (cf. Somjen)19 in all rats at all recording electrodes. Typically observed delays between the DC potential peaks (as shown in Fig. 1B,C, traces DC2 and DC3/DC4), are due to the distance between the recording electrode arrays.
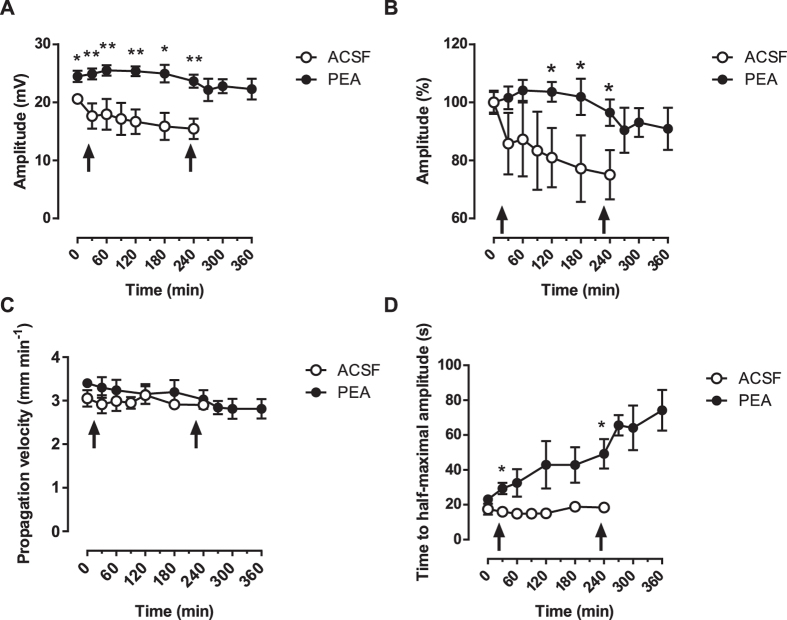
In rats that were not dosed with PEA (vehicle control animals), we observed a continuous decline of CSD amplitudes at all three recording sites (Fig. 1B). At the end of the experiments after four hours, we were still able to elicit CSD with similar slopes, but CSD amplitudes were reduced significantly to 79.5 ± 2.7% of amplitudes at the beginning of the experiments (Fig. 2A,B; n = 3; P < 0.05). In parallel, the propagation velocity of the experimentally induced CSD remained unaltered (2.3 ± 0.1 mm/min at the beginning versus 2.4 ± 0.2 mm/min at the end of experiments (Fig. 2C; n = 3; P = 0.664), i.e. a decrease of 4.3 ± 8.1%), while CSD duration at half maximal amplitude increased by 27.7 ± 35.6%, from 19.5 ± 3.7 s to 24.9 ± 8.6 s (Fig. 2D; n = 3, P = 0.240).
Figure 2. Comparison of the effect of PEA on different parameters of CSD.
Empty circles represent vehicle-treated animals, filled circles animals treated with PEA. The arrows on the abscissae indicate i.p. injections of either vehicle or PEA. Data are presented as mean values ± S.E.M. (A) Comparison of amplitudes of CSD in untreated animals vs. treated animals (*p < 0.05; **p < 0.01); (B) Normalized change in CSD amplitudes in untreated animals vs. treated animals (*p < 0.05); (C) Comparison of propagation velocity of CSD in untreated animals vs. treated animals; (D) Comparison of CSD duration at half maximal amplitude in untreated animals vs. treated animals (*p < 0.05).
In contrast in PEA-treated rats, amplitudes of the experimentally induced CSD remained constant at all recording sites over the entire experimental observation period (Fig. 1C) with a statistically not significant decline to 92.1 ± 2.0% of baseline after four hours (Fig. 2A,B; n = 5; P = 0.262). After a second systemic dosing with PEA this statistically not significant decrease continued, but reached only 83.6 ± 6.4% of the amplitudes at the beginning of the experiment two hours after that (Fig. 2A,B; n = 5; P = 0.283). In the same time, CSD propagation velocity was unchanged statistically with a decline from 3.4 ± 0.3 mm/min to 3.0 ± 0.5 mm/min in the first four hours after the first application of PEA (Fig. 2C; n = 5; P = 0.321) and to 2.8 ± 0.5 mm/min in the two hours after the second injection with PEA (Fig. 2C; n = 5; P = 0.167). CSD duration at half maximal amplitude increased significantly from 23.12 ± 1.2 s to 49.2 ± 8.5 s (Fig. 2D; n = 5; P < 0.05) in the first four hours. In the two hours after the second injection with PEA, CSD duration at half maximal amplitude further increased significantly to 74.3 ± 11.8 s (Fig. 2D).
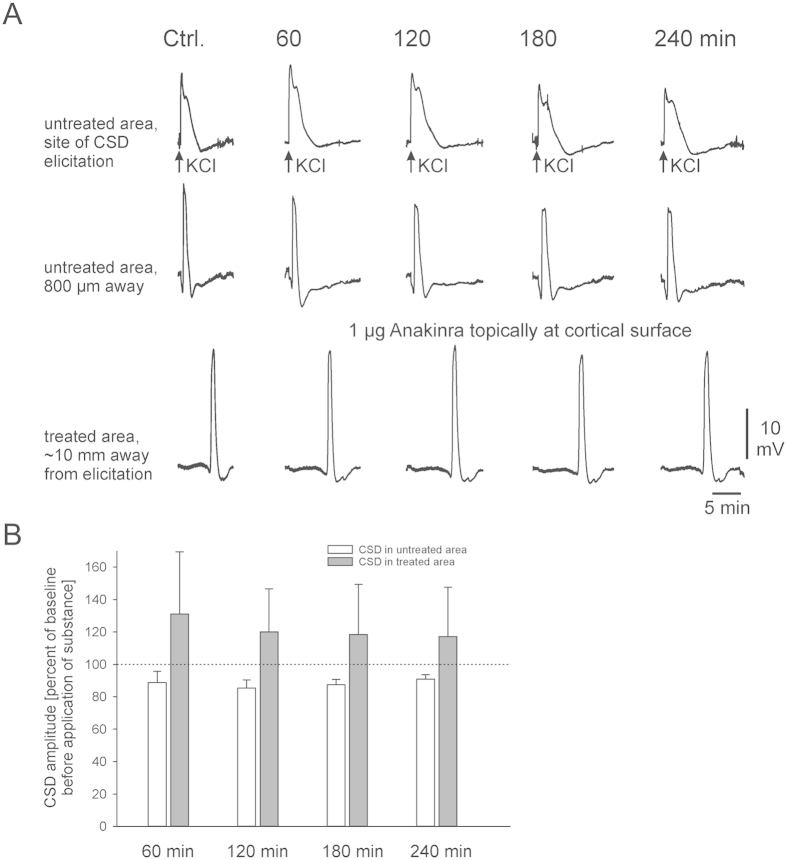
In a subset of experiments, we tested the effects of the IL-1β blocker, Anakinra. As evident from the sample traces (Fig. 3A), CSD amplitudes slowly decreased to 90.1 ± 2.8% of control in the untreated cortical area. In the treated area, however, such decrease did not occur and CSD amplitudes were stable. Summary data (Fig. 3B) indicate that blockade of IL-1ß receptors stabilizes CSD amplitudes, confirming that IL-1ß contributes to the run-down of CSD amplitudes in the cortical area superfused with ACSF. The slight increase in CSD amplitudes in the untreated area 240 min after beginning of the treatment might be due to diffusion of the drug below the skull and a drug effect in remote brain areas as well.
Figure 3. Blockade of IL-1β receptors prevents CSD amplitude rundown.
(A) Representative samples of CSD in adult rat cortex in the untreated area from electrode DC 1 at the site of KCl microinjection, and electrode DC 2 (about 800 μm away) and from electrode DC 3 in the treated area (about 10 mm away from CSD elicitation) before and after application of 1 μg Anakinra. (B) Superfusion of a brain area with Anakinra prevents the effect of IL-1ß on CSD, stabilizing CSD amplitudes. Bars represent mean CSD amplitudes ± S.E.M. at different time points with topical application of Anakinra (n = 3).
Discussion
The in vivo study showed that systemically applied PEA is able to keep the amplitude of single experimentally induced CSD events at a stable level for at least four hours, whereas in untreated controls, we measured a significant run-down of CSD amplitudes in the same time interval. In parallel, CSD propagation velocity was unaltered and the lengthening of CSD duration at half maximal amplitude was the same in controls and PEA-treated animals.
This novel finding that dosing with PEA prevents the run-down of CSD amplitudes differs from all observations on CSD in untreated control animals or untreated cortical areas that were used for example to determine the effects of topically applied cytokines20.
An inflammatory process in the exposed cortex of the healthy rat could be a reason for the rundown of the CSD amplitudes in the native control animals. Though the cortex surface in our experiments was superfused with warmed, equilibrated ACSF, this cannot prevent an activation of the microglia as a source of cytokines. In previous work we have shown, that the proinflammatory cytokine TNFα dose-dependently diminishes amplitudes of CSD20. TNFα is released after brain trauma (TBI) or after stroke21,22,23,24,25. Similarly, the proinflammatory cytokine IL-1ß is able to diminish CSD amplitudes26 and also released after TBI and/or stroke22,27.
It has been shown previously that induction of repetitive CSD by KCl results in a 24-fold increase in IL-1β mRNA ipsilaterally within a 4 hr time window28. Importantly, such increase could not be induced by other stimuli, and was predominantly localized to ramified microglia in the cortex28. Our results provide evidence that blockade of the IL-1β receptor using Anakinra prevents CSD amplitude rundown in our experimental paradigm (Fig. 3), directly implicating cytokine signaling as the underlying physiological mechanism. There is evidence from the literature, that activation and secretion of IL-1ß is mediated via the inflammasome29,30 which is formed from intracellular protein complexes that activate caspases needed to convert pro IL-1ß into bioactive IL-1ß31,32. The activators of the inflammasome are potassium efflux33 and/or decrease of the intracellular potassium concentration either caused by the potassium efflux itself or by the influx of water from the extracellular space together with an influx of sodium ions34. All three elements needed for activation of the inflammasome occur during CSD: potassium ions transiently leave the neurons and glial cells, and in the same time sodium ions and water enter the cells35, leading to shrinkage of the extracellular space down to 35% of the normal values36. It is likely therefore, that activation of the inflammasome by CSD is the reason for a release of IL-1ß causing the decline in CSD-amplitudes. Indeed, this decrease was prevented, when the IL-1ß pathway was blocked by Anakinra.
Several previous reports have shown reduced levels of IL-1β following PEA administration. For example, Di Paola and colleagues measured significantly reduced IL-1β levels after PEA in their splanchnic artery occlusion (SAO) shock model37. Similarly, intrathecal administration of PEA attenuated the increase of IL-1β levels in the rat spinal cord38, and analogously, the fatty acid amide hydrolase (FAAH) inhibitor URB597 resulted in an up-regulation of endogenous PEA and an attenuation of induction of IL-1β in a model for Toll-like receptor 3-mediated neuroinflammation39. Altogether, it appears logical to speculate that PEA treatment counteracts the upregulation of IL-1β during CSD, thereby stabilizing CSD amplitudes.
Our previous studies have excluded cannabinoid receptors as mediators of PEA’s neuroprotective properties in a preclinical model for stroke11,12,14,16. It has been established that activation of CB1 receptors by WIN 55212-2 dose-dependently suppressed CSD amplitude, duration and propagation velocity40. The same compound also decreased the spatial spread of neocortical excitation by interfering with glutamatergic neurotransmission in an in vitro assay41. By contrast, local application of WIN 55212-2 together with the AMPA receptor blocker CNQX facilitated the propagation of CSD in rat brain slices from neocortex into the hippocampus42. Therefore, a CB1 receptor-mediated mechanism underlying the prevention of a run-down of CSD amplitudes by PEA is unlikely.
Another potential mechanism for the action of PEA is a reduction of CSD-induced astrogliosis and reduced astrocyte-mediated cytokine release. It has previously been shown that potassium concentrations, similar to those occurring during CSD, result in reactive astrogliosis in the rat cortex43, hippocampus44, and spinal cord45. This research served as the basis for our own previous work, in which we concluded that changes in the extracellular space volume and geometry after CSD are the result of astrogliosis36. Similarly, astrogliosis has more recently been described after status epilepticus46, which shares many aspects of hyperexcitability with acute CSD. Ahmad and colleagues47 have reported that PEA blocked the infiltration of astrocytes after ischemic brain injury in mice and inhibited the expression of pJNK, NF-κB and degradation of IκB-α47. Signaling pathways controlled by these three mediators are linked to the release of proinflammatory cytokines and/or to the control of their actions after receptor binding.
In summary, our data support the notion that systemic application of PEA in our experimental paradigm prevented or reduced the release of proinflammatory cytokines. Such cytokines are released in our experimental model as a consequence of trephination of the skull and exposure of the brain surface, leading to a slow rundown of the CSD amplitude.
Additional Information
How to cite this article: Richter, F. et al. N-Palmitoylethanolamine Prevents the Run-down of Amplitudes in Cortical Spreading Depression Possibly Implicating Proinflammatory Cytokine Release. Sci. Rep. 6, 23481; doi: 10.1038/srep23481 (2016).
Acknowledgments
Research reported in this publication was supported by grants from the National Eye Institute (EY022774), the National Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This material is the result of work supported with resources and the use of facilities at the Edward Hines Jr. VA Hospital, Hines, IL. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Additional support by the Dr. John P. and Therese E. Mulcahy Endowed Professor in Ophthalmology (SK), the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research (PK), a Challenge Grant from Research to Prevent Blindness (PK) and the Vision Research Foundation of Kansas City (PK) is gratefully acknowledged. The authors would like to thank Ms. Konstanze Ernst for technical and scientific support.
Footnotes
Author Contributions F.R., P.K. and S.K. jointly wrote the manuscript, prepared the figures and reviewed the manuscript.
References
- Mozaffarian D. et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–322 (2015). [DOI] [PubMed] [Google Scholar]
- Astrup J., Siesjo B. K. & Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 12, 723–725 (1981). [DOI] [PubMed] [Google Scholar]
- Liu R., Yuan H., Yuan F. & Yang S. H. Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke. Neurol Res 34, 331–337 (2012). [DOI] [PubMed] [Google Scholar]
- Leão A. A. Spreading depression of activity in the cerebral cortex. J Neurophysiol 7, 359–390 (1944). [DOI] [PubMed] [Google Scholar]
- Sacco S. et al. Migraine and risk of ischaemic heart disease: a systematic review and meta-analysis of observational studies. European journal of neurology: the official journal of the European Federation of Neurological Societies 22, 1001–1011 (2015). [DOI] [PubMed] [Google Scholar]
- Dorfman L. J., Marshall W. H. & Enzmann D. R. Cerebral infarction and migraine: clinical and radiologic correlations. Neurology 29, 317–322 (1979). [DOI] [PubMed] [Google Scholar]
- Lauritzen M. et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 31, 17–35 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mies G., Iijima T. & Hossmann K. A. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport 4, 709–711 (1993). [DOI] [PubMed] [Google Scholar]
- Hossmann K. A. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 8, 195–208 (1996). [PubMed] [Google Scholar]
- Dohmen C. et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 63, 720–728 (2008). [DOI] [PubMed] [Google Scholar]
- Garg P., Duncan R. S., Kaja S. & Koulen P. Intracellular mechanisms of N-acylethanolamine-mediated neuroprotection in a rat model of stroke. Neuroscience 166, 252–262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P. et al. Lauroylethanolamide and linoleoylethanolamide improve functional outcome in a rodent model for stroke. Neurosci Lett. 492, 134–138 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E. Endocannabinoids in the central nervous system–an overview. Prostaglandins Leukot Essent Fatty Acids 66, 221–233 (2002). [DOI] [PubMed] [Google Scholar]
- Duncan R. S., Chapman K. D. & Koulen P. The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line. Molecular neurodegeneration 4, 50 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. S. et al. Control of intracellular calcium signaling as a neuroprotective strategy. Molecules 15, 1168–1195 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. S., Xin H., Goad D. L., Chapman K. D. & Koulen P. Protection of neurons in the retinal ganglion cell layer against excitotoxicity by the N-acylethanolamine, N-linoleoylethanolamine. Clinical ophthalmology 5, 543–548 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R. et al. Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br J Pharmacol. 134, 945–950 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B., Comelli F., Bettoni I., Colleoni M. & Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain 139, 541–550 (2008). [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 81, 1065–1096 (2001). [DOI] [PubMed] [Google Scholar]
- Richter F. et al. Tumor necrosis factor reduces the amplitude of rat cortical spreading depression in vivo. Ann Neurol. 76, 43–53 (2014). [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A., Di Raimondo D., di Sciacca R., Pinto A. & Licata G. Inflammatory cytokines in acute ischemic stroke. Current pharmaceutical design 14, 3574–3589 (2008). [DOI] [PubMed] [Google Scholar]
- Mathiesen T., Edner G., Ulfarsson E. & Andersson B. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. Journal of neurosurgery 87, 215–220 (1997). [DOI] [PubMed] [Google Scholar]
- Ross S. A., Halliday M. I., Campbell G. C., Byrnes D. P. & Rowlands B. J. The presence of tumour necrosis factor in CSF and plasma after severe head injury. British journal of neurosurgery 8, 419–425 (1994). [DOI] [PubMed] [Google Scholar]
- Liu T. et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 25, 1481–1488 (1994). [DOI] [PubMed] [Google Scholar]
- Goodman J. C., Robertson C. S., Grossman R. G. & Narayan R. K. Elevation of tumor necrosis factor in head injury. Journal of neuroimmunology 30, 213–217 (1990). [DOI] [PubMed] [Google Scholar]
- Richter F., Eitner A., Leuchtweis J., Lehmenkuhler A. & Schaible H. G. The cytokine IL-1ß is able to diminish amplitudes of cortical spreading depression (CSD) in adult rats. Society for Neuroscience Meetings Abstracts 760, 12 (2015). [Google Scholar]
- Murray K. N., Parry-Jones A. R. & Allan S. M. Interleukin-1 and acute brain injury. Frontiers in cellular neuroscience 9, 18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S., Schroeter M., Peters O., Witte O. W. & Stoll G. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J Cereb Blood Flow Metab 21, 218–225 (2001). [DOI] [PubMed] [Google Scholar]
- Walsh J. G., Muruve D. A. & Power C. Inflammasomes in the CNS. Nature Rev Neurosci. 15, 84–96 (2014). [DOI] [PubMed] [Google Scholar]
- Barker B. R., Taxman D. J. & Ting J. P. Y. Cross-regulation between the IL-1β/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol. 23, 591–597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M. V. et al. Ion efflux and influenza infection trigger NLRP3 inflammasome signaling in human dendritic cells. Journal of Leukocyte Biology doi: 10.1189/jlb.3A0614-313RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zeng M. Y., Yang D., Motro B. & Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature doi: 10.1038/nature16959 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsnelson M. A., Rucker L. G., Russo H. M. & Dubyak G. R. K+ Efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol. 194, 3937–3952 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorn C. et al. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem. 286, 35–41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig R. P. & Nicholson C. Extracellular ionic variations during spreading depression. Neuroscience 3, 1045–1059 (1978). [DOI] [PubMed] [Google Scholar]
- Mazel T., Richter F., Vargova L. & Sykova E. Changes in extracellular space volume and geometry induced by cortical spreading depression in immature and adult rats. Physiological research/Academia Scientiarum Bohemoslovaca 51 Suppl 1, S85–93 (2002). [PubMed] [Google Scholar]
- Di Paola R. et al. Effects of palmitoylethanolamide on intestinal injury and inflammation caused by ischemia-reperfusion in mice. Journal of leukocyte biology 91, 911–920 (2012). [DOI] [PubMed] [Google Scholar]
- Naderi N. et al. The interaction between intrathecal administration of low doses of palmitoylethanolamide and AM251 in formalin-induced pain related behavior and spinal cord IL1-beta expression in rats. Neurochem Res. 37, 778–785 (2012). [DOI] [PubMed] [Google Scholar]
- Henry R. J., Kerr D. M., Finn D. P. & Roche M. FAAH-mediated modulation of TLR3-induced neuroinflammation in the rat hippocampus. Journal of neuroimmunology 276, 126–134 (2014). [DOI] [PubMed] [Google Scholar]
- Kazemi H., Rahgozar M., Speckmann E. J. & Gorji A. Effect of cannabinoid receptor activation on spreading depression. Iranian journal of basic medical sciences 15, 926–936 (2012). [PMC free article] [PubMed] [Google Scholar]
- Becker K., Eder M., Zieglgansberger W. & Dodt H. U. WIN 55, 212-2 decreases the spatial spread of neocortical excitation in vitro. Neuroreport 16, 993–996 (2005). [DOI] [PubMed] [Google Scholar]
- Martens-Mantai T., Speckmann E. J. & Gorji A. Propagation of cortical spreading depression into the hippocampus: The role of the entorhinal cortex. Synapse 68, 574–584 (2014). [DOI] [PubMed] [Google Scholar]
- Roitbak T. & Sykova E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia 28, 40–48 (1999). [DOI] [PubMed] [Google Scholar]
- Abraham H., Losonczy A., Czeh G. & Lazar G. Rapid activation of microglial cells by hypoxia, kainic acid, and potassium ions in slice preparations of the rat hippocampus. Brain Res. 906, 115–126 (2001). [DOI] [PubMed] [Google Scholar]
- Sykova E., Vargova L., Prokopova S. & Simonova Z. Glial swelling and astrogliosis produce diffusion barriers in the rat spinal cord. Glia 25, 56–70 (1999). [DOI] [PubMed] [Google Scholar]
- Eyo U. B. et al. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci 34, 10528–10540 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A. et al. Reduction of ischemic brain injury by administration of palmitoylethanolamide after transient middle cerebral artery occlusion in rats. Brain Res 1477, 45–58 (2012). [DOI] [PubMed] [Google Scholar]