Abstract
Reovirus is a double-stranded RNA virus with demonstrated oncolysis or preferential replication in cancer cells. The oncolytic properties of reovirus appear to be dependent, in part, on activated Ras signaling. In addition, Ras-transformation promotes reovirus oncolysis by affecting several steps of the viral life cycle. Reovirus-mediated immune responses can present barriers to tumor targeting, serve protective functions against reovirus systemic toxicity, and contribute to therapeutic efficacy through antitumor immune-mediated effects via innate and adaptive responses. Preclinical studies have demonstrated the broad anticancer activity of wild-type, unmodified type 3 Dearing strain reovirus (Reolysin®) across a spectrum of malignancies. The development of reovirus as an anticancer agent and available clinical data reported from 22 clinical trials will be reviewed.
Keywords: Reovirus, Type 3 Dearing, Oncolytic virus, Ras, Epidermal growth factor receptor, Clinical trial, Preclinical, Immune modulation
Core tip: Reovirus has demonstrated oncolysis or preferential replication in cancer cells. The anticancer activity of reovirus has been demonstrated across a spectrum of malignancies in the preclinical setting. The relatively tolerable toxicity profile of reovirus renders it an attractive agent as part of combination therapy in cancer treatment. Reovirus-mediated immune modulation contributes to its antitumor activity via innate and adaptive immune responses and renders it an attractive component of immunotherapy. Here we compile the most extensive list of clinical trials investigating the anticancer efficacy of reovirus to date.
INTRODUCTION
Reovirus and mechanism of oncolysis
The Reoviridae family of viruses consists of six genera, three of which including rotavirus, orbivirus, and reovirus are known to infect animals and humans, while the other three are known to infect plants and insects[1,2]. In 1959, the name reovirus was given to a virus commonly isolated from the respiratory and enteric tract that seldom caused few, if any, clinical symptoms (orphan virus)[3]. However, when symptomatic, reovirus infection is characterized by mild enteric and respiratory symptoms in humans[1-5]. Wild-type reovirus is ubiquitous throughout the environment with seropositivity having been documented in as many as 70%-100% of subjects[3]. There exists several serotypes of reovirus [type 1 Lang, type 2 Jones, type 3 Abney, and type 3 Dearing (T3D)] that have been identified by antibody hemagglutination-inhibition and neutralization studies[2,3,5].
Reovirus is approximately 80 nm in diameter and comprised of a protein shell with outer and inner components that altogether create an icosahedral capsid housing ten segments of double-stranded RNA (dsRNA)[1,2,4-7]. It has been more than 30 years since wild-type reovirus was demonstrated to replicate preferentially in transformed cell lines but not in normal cells[8,9]. The means by which reovirus oncolysis occurred remained elusive until rodent cell lines transformed with genes encoding the epidermal growth factor receptor (EGFR) and a truncated form of the EGFR, possessing constitutive tyrosine kinase activity but lacking the extracellular ligand-binding domain, demonstrated increased susceptibility to reovirus infection and thereby proposing that EGFR-mediated pathways facilitated reovirus infection[10,11]. Indeed, transfection with constitutively activated Ras oncogenes or son of sevenless in NIH-3T3 fibroblasts resulted in increased vulnerability to reovirus infection and elucidated the involvement of activated Ras signaling pathways in reovirus oncolysis[12,13].
Given that approximately 30% of all cancers in humans have been linked to activating Ras mutations, subsequent studies investigated prospective downstream mediators of Ras that may be critical to reovirus oncolysis and implicated, in particular, the Ras/RalGEF/p38 pathway in promoting preferential reovirus replication[14,15]. Additionally, it was determined that dsRNA-activated protein kinase (PKR), which is normally activated in the presence of viral transcripts and inactivates eukaryotic initiation factor 2α (eIF-2α), protein synthesis, and viral replication, is kept inactivated in Ras-transformed cells thereby providing the link between PKR and an activated Ras signaling pathway in reovirus oncolysis[13,16]. Aside from viral translation, Ras-transformation has been shown to promote oncolysis by affecting other steps of the reovirus infectious life cycle including viral disassembly or uncoating, production of viral progeny with boosted infectivity, progeny release through increased apoptosis, and spread of virus in later cycles of infection[17-19].
PRECLINICAL DEVELOPMENT OF REOVIRUS
Monotherapy
Given the wide-reaching implications of activated Ras mutations in human cancers, the first proof-of-concept preclinical studies involved tumors established from v-erbB-transformed murine NIH-3T3 fibroblasts and human U87 glioblastoma cells implanted in severe combined immune deficient (SCID) mice that demonstrated marked tumor regression in approximately 80% of mice following single intratumoral injections of reovirus by day 12 and week 4, respectively[20]. However, SCID mice represented a non-ideal model for reovirus antitumor studies given that approximately 50%-60% of reovirus-treated animals experienced limb necrosis and death[20]. The “Black Foot” syndrome has been characterized by infection with live reovirus of venule endothelial cells and myocardial and musculoskeletal myocytes leading to vasculitis, localized hemorrhage, and/or thrombosis in the extremities of SCID mice[21]. Activated Ras signaling pathways are present in a majority of malignant gliomas, and accordingly, reovirus demonstrated antitumor activity in 83% of malignant glioma cells in vitro, in 2 subcutaneous and 2 intracerebral human malignant glioma models in vivo, and in 100% of glioma specimens ex vivo[22]. In medulloblastoma cell lines, reovirus translation was restricted to cell lines with higher levels of activated Ras, and intratumoral injections of reovirus prolonged survival in orthotopic in vivo animal models of medulloblastoma with spinal and leptomeningeal metastases[23].
The incidence of activated Ras mutations in colon cancer is approximately 50%[15]. The significance of Ras transformation in reovirus oncolysis of colon cancer cells has also been highlighted in K-Ras knockdown murine colorectal cancer cells that demonstrated complete nullification of reovirus-induced apoptosis compared to control[24]. Indeed, treatment with reovirus exhibited significant antitumor effects in human colorectal cancer in vitro and in vivo characterized by elevated Ras activity in colon cancer cell lines and restriction of reovirus infection to tumor cells when compared to controls[25]. Other studies also demonstrated the antitumor efficacy of reovirus in vitro in colon cancer cell lines, in vivo in rodent models of colorectal liver metastases, and notably, in fresh human colorectal tissue isolates that required the processing of virions to infectious subvirion particles (ISVPs) and proper localization and quantity of junctional adhesion molecule-1 on tumor cells for productive lysis[26-28]. Furthermore, colon cancer cell lines HEK293 and HCT116 demonstrated sensitization to reovirus-induced apoptosis by downregulation of nuclear factor-kappa B (NF-κB) through inhibition of glycogen synthase kinase-3β[29].
In adenocarcinomas of the pancreas, the incidence of K-Ras mutations is among the highest in human cancer (approximately 90%)[15]. Not surprisingly, reovirus demonstrated potent cytotoxicity in 100% of pancreatic cancer cell lines in vitro and induced regression in 100% of subcutaneous tumor mouse models in vivo[30]. Interestingly, antitumor activity was seen in BxPC3 pancreatic cancer cells, which are known to have normal K-Ras oncogenes, treated with reovirus in vitro and in vivo though the reovirus-induced cytotoxicity observed in these cells was attributed to overall increased Ras activity, a concept reintroduced below[30]. Administration of reovirus also induced regression in immunocompetent hamster models of pancreatic cancer with liver and peritoneal metastases compared to controls[31,32].
Although the incidence of H-Ras mutations has been reported as high as 17% in cases of bladder carcinoma, activated EGFR-mediated pathways are present in up to 50% of cases of transitional cell carcinoma (TCC) of the bladder[15,33]. Treatment of co-cultured spheroids established by culturing TCC of the bladder cell lines and fibroblasts with reovirus demonstrated selective killing of tumor cells by lysis or induction of apoptosis in vitro[33]. Additionally, intravesical administration of reovirus resulted in significantly higher tumor-free survival in an orthotopic rat model of bladder cancer compared to control[34]. Along similar lines of thought, the incidence of N-Ras mutations in melanoma is relatively lower (approximately 8%-19%) compared to those found in colon and pancreatic cancers[15]. Nevertheless, human melanoma cell lines and murine xenograft models of melanoma were susceptible to tumor killing by reovirus with implications towards the role of the immune system in reovirus oncolysis (which will be further discussed later)[35].
Interestingly, activating Ras mutations in breast cancer are relatively rare though unregulated stimulation of Ras signaling pathways through mediators such as human EGFR 2 (Her-2 or ErbB-2) and its homologue Neu, both tyrosine kinases of the EGFR family, and the Src family of nonreceptor tyrosine kinases can occur highlighting the concept that activated Ras signaling rather than mutations in the Ras protein itself can be important to disease pathogenesis[3-5,36]. Accordingly, reovirus demonstrated significant antitumor effects in vitro in breast cancer cell lines characterized by resistance to infection in normal cell lines, in vitro in breast cancer stem cells, and in vivo in animal tumor models including models of brain and leptomeningeal metastases[36-39]. Furthermore, the presence of replicating reovirus was confirmed in ex vivo surgical breast cancer specimens[36]. Notably, there was no observed relationship between susceptibility to reovirus infection and HER2 expression, in vitro, though levels of Ras activity were higher in breast cancer cell lines when compared to control[37]. Ovarian cancer represents another example in which activating Ras mutations are rare but increased Ras signaling via increased activation of Her-2/Neu and/or Src likely contribute to pathogenesis[4,5,25]. Treatment with reovirus resulted in potent antitumor activity, when compared to controls, in ovarian cancer cell lines in vitro highlighted by increased reovirus protein synthesis in tumor cell lines but not in normal cells, in a human ovarian SKOV3 cell line implanted in the flanks of mice in vivo, and in a murine ascites model of human ovarian cancer highlighted by prolonged survival in those treated with intraperitoneal injections of live virus every 2 wk[25]. All 3 ex vivo human ovarian tumor surgical biopsy specimens also demonstrated susceptibility to reovirus infection[25].
Similarly, marked cytopathic effects and inhibition of tumor growth were observed with reovirus treatment, in vitro and in vivo, in cancers where relatively little has been known, historically, about the involvement of Ras mutations in transformation such as head and neck cancer, prostate cancer, and sarcomas[40-42]. Intriguingly, although reovirus-induced cytotoxicity was observed in several head and neck carcinoma cell lines, correlative analyses revealed no associations between phosphorylated eIF-2α or EGFR levels and cytopathic effects suggesting that reovirus oncolysis appears to occur independently of PKR, Ras signaling, and EGFR signaling pathways[43,44].
Hematologic malignancies posed a perplexing dilemma regarding their susceptibility to reovirus infection given the near absence of N-Ras mutations particularly in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphomas (NHLs) such as follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL)[15,45]. Nevertheless, it was hypothesized that certain hematologic malignancies may still be amenable to reovirus therapy from knowledge that the break point cluster-Abelson (Bcr-Abl) nonreceptor tyrosine kinase present in 95% of chronic myelogenous leukemia is dependent on Ras activation, Myc oncogenes coordinate with Ras in B-cell transformation, specific ligand-receptor interactions in CLL lymphoid cells stimulate Ras signaling, and mutations in a proto-oncogene member of the Ras superfamily is present in up to 46% of DLBCLs[3,45]. Indeed, reovirus treatment of human lymphoma cells produced antitumor effects in all 4 DLBCL cell lines and 2 out of 5 Burkitt lymphoma cell lines in vitro highlighted by increased reovirus protein synthesis and progeny production in sensitive cell lines compared to resistant cell lines and in vivo in a Burkitt cell line sensitive to reovirus implanted in mice but not in a xenograft model of a previously determined resistant Burkitt cell line[45]. Furthermore, all ex vivo human primary CLL samples and a majority of NHL samples including Burkitt lymphoma, mantle cell lymphoma, and DLBCL were susceptible to reovirus oncolysis while a majority of FL specimens were resistant[45].
Treatment of acute myeloid leukemia (AML) with reovirus showed marked antitumor responses in 2 out 4 AML cell lines in vitro and in 8 out of 10 peripheral blood primary AML specimens ex vivo[46]. Concordant with prior findings, a FL cell line was resistant to reovirus therapy in vitro and in vivo while mantle cell lymphoma cell lines displayed a heterogenous response to reovirus that correlated with levels of activated Ras and proteolytic disassembly of reovirus into ISVPs in vitro[47]. The discrepancies in sensitivity to reovirus infection between various hematologic malignancies have been attributed, in part, to differential Ras activation and interferon sensitivities[4,5,45,47]. Reovirus induced cell death via apoptotic and autophagic pathways in a majority of multiple myeloma cell lines in vitro with sensitivity conferred to ex vivo tumor specimens as well[48]. Reovirus also showed meaningful inhibition of tumor growth in in vivo multiple myeloma models compared to control, and treatment with reovirus did not abrogate human stem cell repopulation and differentiation in vivo[48]. Earlier studies revealed that reovirus did not affect hematopoietic progenitor stem cells, and the mixture of reovirus with human monocytic and myeloma cancer cell lines in vitro and ex vivo tumor cells of DLBCL, CLL, Waldenström macroglobulinemia, and small lymphocytic lymphoma showed complete purging of disease in patient products of apheresis[49]. The use of reovirus as a purging strategy for autologous stem cell transplantations has since been an emerging concept with demonstrated efficacy in breast cancer and multiple myeloma[50,51].
Combination therapy
The earliest preclinical studies involving reovirus in combination therapy entailed L1210 murine leukemia cells and EL4 murine lymphoma cells treated with the chemotherapeutic agent 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) followed by treatment with reovirus that increased survival in ascites tumor mouse models when compared to controls and were among the first to illustrate that resistance of surviving animals to challenges with homologous tumor was orchestrated by an immune-mediated process[52-54]. Reovirus in combination with radiation therapy, when compared to controls, produced enhanced apoptosis across head and neck, colorectal, and breast cancer cell lines in vitro (independent of treatment sequence or schedule and without affecting viral replication at clinically relevant radiation doses) and delayed tumor growth in colorectal cancer and melanoma models in vivo[55]. Criteria for therapeutic enhancement were met for ewing sarcoma (ES) and osteosarcoma murine xenografts and rhabdomyosarcoma and ES murine xenografts treated with reovirus in combination with cisplatin and reovirus in combination with radiotherapy [4 gray (Gy) daily × 5 fractions], respectively[40].
In murine melanoma xenografts, metronomic dosing of high-dose cyclophosphamide with reovirus permitted access to tumors by therapeutically high levels of virus while reducing serious toxicities associated with ablation of neutralizing antibody titers, and cisplatin with reovirus significantly inhibited tumor growth compared to controls without affecting neutralizing antibody response though cisplatin reduced the inflammatory cytokine response to reovirus[56,57]. Treatment with reovirus and cyclosporin A significantly inhibited tumor growth in a murine Ras-transformed fibroblastic xenograft while reovirus with cyclosporin A or T-cell depletion significantly improved survival in a murine metastatic lung cancer model compared to controls[58]. Although reovirus alone demonstrated potent cytotoxicity in 7 of 9 non-small cell lung cancer (NSCLC) cell lines in vitro, heterogenous synergistic effects on cell killing were observed with reovirus in combination with cisplatin, gemcitabine, or vinblastine on NSCLC cancer cell lines in vitro[59]. The reovirus and paclitaxel combination, however, showed synergistic cell killing in all NSCLC cell lines in vitro characterized by enhanced apoptosis[59].
More recently, although trastuzumab and reovirus monotherapy both inhibited tumor growth in vitro, treatment with reovirus was found to sensitize gastric cancer cells that overexpressed HER2 to apoptosis when combined with trastuzumab[60]. However, in HER2 low expressing cells, reovirus monotherapy or in combination with trastuzumab increased apoptosis in vitro, but there was no reduction in growth when treated with trastuzumab alone[60]. Further analysis showed that reovirus induced expression of TRAIL, a protein implicated in promoting apoptosis, without upregulating TRAIL receptors. TRAIL expression was increased with both trastuzumab and reovirus therapy, but this effect was enhanced by combination therapy[60].
Similar synergistic antitumor effects have been established, when compared to controls, in combination regimens involving: (1) reovirus with cisplatin and paclitaxel in head and neck cancer in vitro characterized by enhanced apoptosis and cell cycle disruption (though without enhancing reovirus replication) and in vivo; (2) reovirus with bortezomib in pancreatic cancer in vitro and in vivo characterized by enhanced levels of ER stress and apoptosis; (3) reovirus with cyclosporin A in a murine model of colorectal liver metastases; (4) reovirus and gemcitabine in human colon cancer in vitro and in vivo; and (5) reovirus with paclitaxel, vincristine, cisplatin, doxorubicin, or docetaxel in prostate cancer in vitro highlighted by the greatest synergism in the reovirus and docetaxel combination with enhanced apoptosis and microtubule stabilization[27,61-64]. Reovirus and docetaxel also produced significant tumor growth retardation in a murine prostate cancer xenograft[63]. Interestingly, reovirus in combination with Newcastle disease virus or parvovirus resulted in significant synergistic antitumor responses in glioblastoma cell lines in vitro with an efficient rate of co-infection and without affecting the kinetics of viral replication among the viruses[65]. Furthermore, reovirus with Newcastle disease virus significantly inhibited tumor growth in a murine glioblastoma xenograft compared to control without significant toxicity though the experiments were terminated 12 d after virus injection[65].
In sum, preclinical studies have demonstrated the broad anticancer activity of reovirus across a spectrum of malignancies including colon, breast, ovarian, lung, skin (melanoma), neurological, hematological, prostate, bladder, and head and neck cancer which have ultimately provided the basis for human clinical trials[1,5,6,66]. The three serotypes of reovirus including type 1 Lang, type 2 Jones, type 3 Abney, and T3D all have demonstrated oncolytic properties, but the T3D strain has been most extensively studied as an anticancer agent and is the only therapeutic wild-type reovirus in clinical development under its proprietary formulation, Reolysin®, developed by Oncolytics Biotech Inc. (Calgary, Canada)[2-4,67]. Thus far, there are a total of 34 clinical trials involving reovirus in the treatment of a variety of cancers that are both completed and ongoing (http://www.oncolyticsbiotech.com/clinical-trials). Clinical data available and reported from 22 clinical trials will now be discussed (Tables 1 and 2).
Table 1.
Phase I trials involving reovirus
| Phase | Malignancy | Dosing regimen | Clinical response |
| I (REO 001) | Various advanced or refractory solid malignancies | 1 × 107 PFU to 1 × 1010 PFU intralesional injection once or 3 × weekly (dose escalation) | Out of 19 patients, best overall response ≥ 6 wk was CR in 1 with Klatskin (5.3%), PR in 2 with head and neck cancer (10.5%), SD in 4 1 with head and neck, 1 with melanoma, 1 with breast cancer, 1 with Kaposi's (21.1%) |
| I/translational (REO 002) | Localized prostate cancer | 1 × 107 PFU single intratumoral injection 3 wk prior to planned prostatectomy | Out of 6 patients, all did not exhibit significant fluctuations in PSA from baseline. Five of 6 patients showed staining for reovirus proteins localized to cancer areas but sparing of adjacent benign and remote cancer areas. Pathologic specimens showed peritumoral inflammation in 4 patients, apoptosis in 4 patients, and necrosis in 2 patients |
| I (REO 003) | Advanced or recurrent malignant gliomas | 1 × 107 TCID50 to 1 × 109 TCID50 single stereotactic intralesional injection (dose escalation) | Out of 12 patients, best overall response was SD in 1 patient with oligo-astrocytoma with a TTP of 39 wk. The overall median TTP was 4.3 wk (range 2.6-39 wk), and median OS was 21 wk (range 6-234 wk) |
| I (REO 004) | Various advanced or refractory solid malignancies | 60-min IV infusion from 1 × 108 TCID50 to 3 × 1010 TCID50 once every 28 d (dose escalation) | Out of 18 patients, best overall response was PR > 5 cycles in 1 patient with breast cancer (5.6%) and SD > 1 cycle in 7 (5 with ovarian cancer, 1 with carcinoid, 1 with STS, 38.9%); CBR of about 45% |
| I (REO 005) | Various advanced or refractory solid malignancies | 60-min IV infusion from 1 × 108 TCID50 once every 28 d to 3 × 1010 TCID50 once daily for 5 d every 28 d (dose escalation); IV reovirus 3 × 1010 TCID50 once daily for 5 d every 28 d became recommended phase II dose | Out of 33 enrolled patients, best overall response was SD > 7 wk in 10 patients (2 with colon cancer, 2 with prostate cancer, 2 with STS, 1 with lung cancer, 1 with TCC of the bladder, 1 with melanoma, 1 with endometrial cancer) |
| I (REO 006) | Various advanced or refractory solid malignancies | 1 × 108 TCID50 to 1 × 1010 TCID50 intratumoral injection on days 2 and 4 with 20 Gy local irradiation daily × 5 fractions phase 1b: 1 × 1010 TCID50 intratumoral injection twice weekly from 1-3 wk with 36 Gy local irradiation × 12 fractions over 16 d (two-stage dose escalation); intratumoral 3 × 1010 TCID50 × 2 injections with 20 Gy × 5 fractions and intratumoral 1 × 1010 TCID50 × 6 injections with 36 Gy × 12 fractions became recommended phase II doses for short and prolonged palliative regimens, respectively | Out of 7 patients in phase 1a, best overall response was PR in 2 (esophageal adenocarcinoma and SCC of skin), SD in 5 (melanoma, pancreatic adenocarcinoma, SCC of larynx, and 2 with SCC of skin); out of 7 patients in phase 1b, 5 had PR (lung adenocarcinoma, colorectal cancer, ovarian adenocarcinoma, 2 with melanoma) and 2 had SD (melanoma) up to 3 mo post-treatment |
| I (REO 007) | Recurrent malignant gliomas | 72-h intratumoral infusion from 1 × 108 TCID50 to 1 × 1010 TCID50 (dose escalation) | Out of 15 patients enrolled, best overall response was SD in 10 patients during the study period of 24 wk. The median TTP was 61 d (range 29-150 d), and median survival was 140 d (range 97-989) |
| I (REO 009) | Various advanced or refractory solid malignancies | 60-min IV infusion from 1 × 109 TCID50 to 3 × 1010 TCID50 on day 1 (dose escalation) with 30-min IV infusion of gemcitabine 1000 mg/m2 days 1 and 8 every 21 d (1 × 1010 TCID50 reovirus on day 1 became recommended phase II dose with gemcitabine) | Out of 10 patients, best overall response was PR after 4 cycles in 2 patients (1 with nasopharyngeal carcinoma, 1 with breast cancer) and SD for 4-8 cycles in 5 patients (median SD 72 d, range 36-112 d); CBR of 80% |
| I (REO 010) | Various advanced or refractory solid malignancies | 60-min IV infusion from 3 × 109 TCID50 to 3 × 1010 TCID50 days 1-5 (dose escalation) with 60-min IV infusion of docetaxel 75 mg/m2 day 1 every 21 d (3 × 1010 TCID50 reovirus days 1-5 every 21 d became recommended phase II dose with docetaxel) | Out of 16 patients, best overall response was PR ≥ 2 cycles in 4 patients (1 with breast cancer who experienced CR in liver lesion, 1 with gastric cancer, 1 with gastroesophageal cancer, 1 with ocular melanoma) and SD ≥ 2 cycles in 10 patients (cancers included prostate, mesothelioma, SCC of head and neck, unknown primary, melanoma, esophageal cancer, pancreatic cancer); CBR of 88% |
| I/translational (REO 013) | Colorectal cancer metastatic to the liver | 60-min IV infusion of 1 × 1010 TCID50 daily × 5 d between 6-28 d prior to planned radical resection of liver metastases | Out of 10 patients, 9 patients with resected tumor specimens demonstrated positive staining for reovirus that was greatest in tumor metastases compared to surrounding tumor stroma or adjacent normal liver. In addition, tissue analysis in 4 patients showed findings consistent with reovirus-associated apoptosis |
| I (REO 022) | Metastatic colorectal cancer | 60-min IV infusion from 1 × 1010 TCID50 to 3 × 1010 TCID50 days 1-5 every 28 d (dose escalation) with standard FOLFIRI doses (recommended phase II dose was irinotecan 150 mg/m2 with 3 × 1010 TCID50 IV reovirus days 1-5 every 28 d) | Out of 18 patients, best overall response was PR in 1 patient (5%) and SD in 9 (50%) with median PFS in FOLFIRI-naïve patients of 7.4 mo (95%CI: 1.9-12.9 mo) and overall median PFS of 7.4 mo (95%CI: 0.6-14.1 mo) |
| I (OSU-11148, NCI trial) | Refractory or relapsed multiple myeloma | 60-min IV infusion from 3 × 109 TCID50 to 3 × 1010 TCID50 days 1-5 every 28 d (dose escalation) | Out of 12 patients, best overall response was SD with longest duration being 8 cycles. During cycle 1, 5 patients had decreased myeloma proteins, 3 had minimal increases, and 4 had progressive disease |
PFU: Plaque forming units; CR: Complete response; PR: Partial response; SD: Stable disease; PSA: Prostate-specific antigen; TCID50: Tissue culture infectious dose-50; TTP: Time to disease progression; OS: Overall survival; IV: Intravenous; STS: Soft tissue sarcoma; CBR: Clinical benefit rate; TCC: Transitional cell carcinoma; Gy: Gray; SCC: Squamous cell carcinoma; FOLFIRI: Irinotecan/fluorouracil/leucovorin; PFS: Progression-free survival.
Table 2.
Phase I/II, II, and III trials involving reovirus
| Phase | Malignancy | Dosing regimen | Clinical response |
| I/II (REO 011) | Various advanced or refractory solid malignancies | 60-min IV infusion from 3 × 109 TCID50 to 3 × 1010 TCID50 days 1-5 (dose escalation) with IV paclitaxel 175 mg/m2 over 3 h and IV carboplatin AUC5 (over 30 min) on day 1 every 21 d (3 × 1010 TCID50 IV reovirus days 1-5 every 21 d became recommended phase II dose with paclitaxel and carboplatin) | Out of 26 patients, best overall response was CR in 1 patient (3.8%,head and neck cancer), PR in 6 patients (23.1%, 3 each with SCC of head and neck and head and neck cancer), major clinical response not evaluable by RECIST criteria in 2 patients (7.7%, SCC of head and neck), and SD in 9 patients (34.6%, 3 with SCC of head and neck, 3 with head and neck cancer, 1 with gynecological cancer, 1 with melanoma, 1 with sarcoma) with median duration of SD and PR of 6 mo (range 3-10 mo). Of the 24 patients with head and neck cancer, median OS was 7.1 mo (CI: 4.2-11.5 mo) |
| I/II (OSU-07022, NCI trial) | Recurrent or refractory ovarian, peritoneal, and fallopian tube carcinomas | 60-min IV infusion 3 × 1010 TCID50 days 1-5 with daily IP administration days 2-3 beginning cycle 2 every 28 d (dose escalation with IP dosing) | Thus far 8 patients have received treatment. Biopsied ovarian and peritoneal tumor samples reveal detection of viral proteins in tumor tissues compared to control after systemic (IV) administration of reovirus and presence of reovirus replication in tumors due to overlap of reovirus protein and microtubules |
| II (REO 008) | Various advanced or refractory solid malignancies | Open-label, single-arm, multicenter: 1 × 1010 TCID50 intratumoral injection on days 2 and 4 with 4 Gy local irradiation daily × 5 (total 20 Gy) every cycle | Out of 16 patients enrolled (5 with melanoma, 4 colorectal, 1 gastric, 1 ovarian, 1 pancreatic, 1 lung, 1 cholangiocarcinoma, 1 sinus, 1 thyroid), 14 were evaluable and best overall response was SD or better in 13 patients (93%). Of these patients, 4 had PR (2 with melanoma, 1 lung, 1 gastric) and 2 had minor responses (1 thyroid and 1 ovarian) |
| II (MAYO-MC0672, NCI trial) | Metastatic melanoma | Open-label, single-arm, multicenter: 60-min IV infusion 3 × 1010 TCID50 days 1-5 every 28 d | Out of 21 evaluable patients, best overall response was SD > 8 wk in 6 patients. The median TTP was 45 d (range 13-96 d) and median OS was 165 d (range 15 d-15.8 mo). Trial was closed as did not meet previously defined efficacy criteria to proceed to second stage of accrual |
| II (REO 014) | Advanced or refractory sarcomas metastatic to lung | Open-label, single-arm, multicenter: 60-min IV infusion 3 × 1010 TCID50 days 1-5 every 28 d | Out of 53 enrolled patients, best overall response was SD ≥ 12 wk in 18 patients (34%) with a subgroup of 12 patients (3 with synovial sarcoma, 2 with leiomyosarcoma, 2 with MFH, 1 with ES, 1 with non-specified spindle cell sarcoma, 1 with chordoma, 1 with ASPS), 1 with myxoid liposarcoma) having prolonged SD > 16 wk. Three of these patients demonstrated SD > 1 yr (1 with MFH, 1 with synovial sarcoma, 1 with ES). The median TTP was 58.0 d (95%CI, 54-110, range 8-726 d). The prolonged SD demonstrated fulfilled the study criteria for consideration as an active agent |
| II (REO 015) | Refractory, recurrent, or metastatic SCC of the head and neck | Open-label, single-arm: 60-min IV infusion 3 × 1010 TCID50 days 1-5 with IV paclitaxel 175 mg/m2 over 3 h and IV carboplatin AUC5 (over 30 min) on day 1 every 21 d | Out of 13 evaluable patients (sites included 3 larynx, 6 oral cavity, 4 pharynx, 1 other), 4 had PR (31%) and 2 had SD ≥ 12 wk for a CBR of 46% |
| II (REO 016) | Recurrent or metastatic NSCLC | 60-min IV infusion 3 × 1010 TCID50 days 1-5 with IV paclitaxel 175 mg/m2 over 3 h and IV carboplatin AUC5 (over 30 min) on day 1 every 21 d | Out of 37 patients enrolled, 20 patients had detected K-Ras mutations, 3 patients had EGFR mutations, 10 patients had EGFR amplifications alone, and 4 patients had BRAF V600E mutations. Median PFS was 4 mo (95%CI: 2.9-6.1), median OS was 13.1 mo (95%CI: 9.2-21.6), and 1-yr OS rate was 57% (95%CI: 39%-72%) |
| II (REO 017) | Advanced or unresectable pancreatic cancer | 60-min IV infusion 1 × 1010 TCID50 on days 1, 2, 8 and 9 with IV infusion of gemcitabine 800 mg/m2 days 1 and 8 every 21 d | Out of 34 enrolled patients, median PFS was 4 mo and OS was 10.2 mo. One- and 2-yr survival rates were 45% and 24%, respectively |
| II (REO 021) | Recurrent or metastatic SCC of the lung | Open-label, single-arm: 60-min IV infusion 3 × 1010 TCID50 days 1-5 with IV paclitaxel 200 mg/m2 over 3 h and IV carboplatin AUC6 every 21 d | Out of 25 patients who received more than 1 cycle of therapy, best overall response was PR in 12 patients (48%) and SD in 10 patients (40%) for a CBR of 88%. Of 21 patients with > 6 mo follow-up 7 had PFS ≥ 6 mo (33.3%) |
| III (REO 018) | Advanced or metastatic head and neck cancer | Randomized, double-arm, double-blinded, multicenter: 60-min IV infusion 3 × 1010 TCID50 days 1-5 with standard doses of IV paclitaxel and IV carboplatin on day 1 only every 21 d (treatment arm) vs standard doses of IV paclitaxel and IV carboplatin alone (control arm) | Out of 167 enrolled patients, 118 patients were segregated into an intent-to-treat basis group with loco-regional head and neck cancer (with or without metastases). In this group, median PFS was 94 d (13.4 wk, n = 62) in the test arm vs 50 d (7.1 wk, n = 56) in control arm maintained through 5 cycles. In the 88 patients discontinued from the study from this group, median OS was 150 d (21.4 wk, n = 50) in the test arm vs 115 d (16.4 wk, n = 38) in the control arm. Survival analysis in the other group (distal metastases-only) has not been conducted |
IV: Intravenous; TCID50: Tissue culture infectious dose-50; AUC5/6: Area under curve-5/-6; CR: Complete response; PR: Partial response; SCC: Squamous cell carcinoma; RECIST: Response evaluation criteria in solid tumors; SD: Stable disease; OS: Overall survival; IP: Intraperitoneal; Gy: Gray; TTP: Time to disease progression; PFS: Progression-free survival; MFH: Malignant fibrous histiocytoma; ES: Ewing sarcoma; ASPS: Alveolar soft part sarcoma; CBR: Clinical benefit rate; NSCLC: Non-small cell lung cancer; EGFR: Epidermal growth factor receptor.
CLINICAL DEVELOPMENT OF REOVIRUS
Phase I trials
The first phase I trial (REO 001) involved administration of intralesional reovirus in patients with advanced solid tumors and histologically confirmed cutaneous lesions[68]. In a dose-escalation design, doses of 1 × 107 plaque forming units (PFU) once weekly up to maximum doses of 1 × 1010 PFU once weekly were used[68]. Out of 19 patients, dose-limiting toxicities (DLTs) and a maximum-tolerated dose (MTD) were not observed even at maximum dose[68]. The most common treatment-related adverse events (AEs) included nausea (79%), vomiting (58%), and local erythema of injection site (42%) while fevers/chills and transient flu-like symptoms accounted for 37% and 32%, respectively[68]. The best overall response ≥ 6 wk was complete response (CR) in 1 (5.3%), partial response (PR) in 2 (10.5%), stable disease (SD) in (21.1%)[68].
REO 002 enrolled 6 patients with localized prostate cancer who received a single intratumoral injection of 1 × 107 PFU of reovirus 3 wk prior to planned prostatectomy as definitive cancer treatment[42]. There were no DLTs or grade 3 or higher toxicities observed and the most common AE included mild flu-like illness in 4 out of 6 patients[42]. In all patients, prostate-specific antigen (PSA) levels did not significantly fluctuate from baseline, and pathologic specimens showed moderate to strong staining for reovirus proteins localized to areas of cancer but sparing of adjacent benign areas and remote areas of cancer in 5 patients[42].
Another phase I study (REO 003) involved single stereotactic intralesional injection of reovirus at doses ranging from 1 × 107 tissue culture infectious dose-50 (TCID50) to 1 × 109 TCID50 in 12 patients with progressive or recurrent malignant gliomas[69]. The MTD was not reached even at maximum doses and there were no DLTs observed with the only grade 3 or higher treatment-related AE being an elevation in γ-glutamyl transpeptidase[69]. The median time to disease progression (TTP) was 4.3 wk (range 2.6-39 wk), median overall survival (OS) was 21 wk (range 6-234 wk), and best overall response was SD in 1 patient with TTP of 39 wk[69]. REO 007, a multicenter phase I study, aimed to determine DLTs, MTD, and target lesion response rate after administering reovirus via intratumoral infusion in 15 patients with recurrent malignant gliomas[70]. Similarly to REO 003, the MTD was not achieved at maximum doses. Only three patients suffered from convulsions, a grade 3 AE, which does occur commonly in patients with intracranial tumors[70]. Additionally, only one of these three grade 3 AEs was possibly related to infusion of reovirus[70]. During the study period of 24 wk, ten patients were reported to have stable disease, four with progressive disease, and one with partial response[70]. However, ultimately 12 out of the 15 patients did have progressive disease with the median time to progression being 61 d (range 29-150) and the median survival being 140 d (range 97-989)[70]. The one patient that did achieve a partial response did receive the maximum dose[70].
REO 004 included 18 patients with advanced solid tumors treated with intravenous (IV) reovirus from 1 × 108 TCID50 to 3 × 1010 TCID50 once every 28 d in which the latter dose was declared the MTD due to protocol termination once the protocol-defined highest dose was reached[71]. No DLTs were observed and the most common AEs included myalgia, fatigue, and fever[71]. Out of 18 patients, the best overall response was PR > 5 cycles in 1 patient (5.6%) with taxane and anthracycline refractory breast cancer (whose post-treatment chest wall biopsy showed viral replication and extensive necrosis consistent with reovirus activity) and SD > 1 cycle in 7 patients (38.9%) for a clinical benefit rate (CBR) of about 45% (combined CR, PR, and SD)[71]. Of note, 5 patients had Ras mutations and 1 patient had a Braf mutation, and the formation of neutralizing anti-reovirus antibodies (NARAs) bore no relationship to clinical benefit while those with detectable viral shedding appeared to have greater benefit[71]. One phase I study (REO 005) pitted IV reovirus against various refractory or metastatic cancers, and a MTD was reached at a dose of 3 × 1010 TCID50 once daily for 5 d every 28 d by virtue of being the highest dose available for administration (this subsequently also became the recommended phase II dose)[72]. No DLTs were observed and the most common AEs were fever, fatigue, and headache[72]. Out of 33 enrolled patients, the best overall response was SD > 7 wk in 10 patients, and no relationships between SD to dose or duration of reovirus therapy were established[72].
REO 006 enrolled 25 patients with various refractory or progressive solid cancers in a two-stage dose-escalation design where phase Ia treated patients with 1 × 108 TCID50 to 1 × 1010 TCID50 intratumoral injection of reovirus on days 2 and 4 with 20 Gy local irradiation daily × 5 fractions while phase 1b treated patients with 1 × 1010 TCID50 intratumoral injection of reovirus twice weekly for 1-3 wk with 36 Gy local irradiation × 12 fractions over 16 d[73]. There were no DLTs observed, a MTD was not reached, and the most common treatment-related AEs included pyrexia (43.5%), lymphopenia (26.1%), and influenza-like symptoms (17.4%)[73]. The best overall response was PR in 2 patients and SD in 5 patients (out of 7 patients in phase Ia) and PR in 5 patients and SD in 2 patients (out of 7 patients in phase Ib) up to 3 mo post-treatment[73]. The recommended phase II doses were 1 × 1010 TCID50 of reovirus × 2 intratumoral injections with 20 Gy of radiation × 5 fractions and 1 × 1010 TCID50 of reovirus × 6 intratumoral injections with 36 Gy of radiation × 12 fractions for short and prolonged palliative regimens, respectively[73]. Another phase I study (REO 009) originally used 3 × 109 TCID50 days 1-5 of IV reovirus with gemcitabine in the treatment of advanced solid tumors but the dosing of reovirus was amended to 1 × 109 TCID50 to 3 × 1010 TCID50 IV reovirus on day 1 only with 30-min IV infusion of gemcitabine 1000 mg/m2 on days 1 and 8 every 21 d when DLTs of grade 3 transaminitis and grade 3 elevation in troponin I occurred[74]. A MTD was not reached, but a third DLT of grade 3 transaminitis also occurred at the amended 3 × 1010 TCID50 day 1 dose[74]. Interestingly, the elevation in liver enzymes was associated with concomitant acetaminophen use and prompted the recommendation of avoidance of acetaminophen during reovirus clinical trials[74]. The most common treatment-related AEs were pyrexia (68.8%), nausea (43.8%), and diarrhea (37.5%), and 1 × 1010 TCID50 IV reovirus on day 1 became the recommended phase II dose in combination with gemcitabine[74]. Out of 10 patients, the best overall response was PR after 4 cycles in 2 patients and SD from 4-8 cycles in 5 patients (median SD of 72 d, range 36-112 d) for a CBR of 80%[74].
In REO 010, a MTD was not reached though a DLT of grade 4 neutropenia resulted in a 20% reduction of the docetaxel dose in refractory or metastatic solid cancers treated with 3 × 109 TCID50 to 3 × 1010 TCID50 days 1-5 of IV reovirus (the last being the recommended phase II dose with docetaxel) with IV docetaxel 75 mg/m2 on day 1 every 21 d[75]. Four AEs of grade 4 neutropenia were felt to be due to docetaxel alone and an additional grade 4 lymphopenia also occurred; the most common AEs were flu-like symptoms, diarrhea, and fatigue[75]. Out of 16 patients, the best overall response was PR ≥ 2 cycles in 4 patients and SD ≥ 2 cycles in 10 patients for a CBR of 88%[75]. REO 013 enrolled 10 patients with metastatic colorectal cancer to the liver to be treated with 1 × 1010 TCID50 of IV reovirus daily × 5 d between 6-28 d prior to planned radical resection of liver metastases[76]. There were no grade 3 or higher toxicities and the most common AEs were flu-like symptoms[76]. Resected tumor specimens from 9 patients showed staining for reovirus protein greatest in tumor when compared to surrounding tumor stroma and normal liver[76].
Preliminary results of REO 022 included PR in 1 patient (5%) and SD in 9 patients (50%) with a median progression-free survival (PFS) in irinotecan/fluorouracil/leucovorin (FOLFIRI)-naïve patients of 7.4 mo (95%CI: 1.9-12.9 mo) and overall median PFS of 7.4 mo (95%CI: 0.6-14.1 mo) in 18 patients with metastatic colorectal cancer treated with 60-min IV infusion of reovirus from 1 × 1010 TCID50 to 3 × 1010 TCID50 days 1-5 every 28 d with standard FOLFIRI[77]. Irinotecan 150 mg/m2 with 3 × 1010 TCID50 IV reovirus days 1-5 every 28 d became the recommended phase II dose[77]. The most common (> 10%) grade 3 or higher toxicities were neutropenia, anemia, and thrombocytopenia, and DLTs of neutropenia were observed[77].
Results from a National Cancer Institute (NCI)-sponsored phase I study (OSU-11148) included SD in 5 of 12 patients (42%) with relapsed multiple myeloma treated with 60-min IV infusion of reovirus from 3 × 109 TCID50 to 3 × 1010 TCID50 days 1-5 every 28 d[78]. A MTD was not reached, no DLTs were observed, and grade 3 toxicities included neutropenia, leukopenia, thrombocytopenia, and hypophosphatemia (Table 1)[78]. From this study, combination therapy is presumed to be more beneficial than oncolytic reovirus therapy alone in patients with multiple myeloma. Overall, phase I trials did demonstrate that treatment with reovirus via various methods of administration was well tolerated by patients with minimal adverse effects.
Phase I/II trials
A phase I/II trial (REO 011) involved 60-min IV infusion of reovirus from 3 × 109 TCID50 to 3 × 1010 TCID50 days 1-5 (the latter being the recommended phase II dose) with IV paclitaxel 175 mg/m2 over 3 h and IV carboplatin area under curve-5 (AUC5) over 30 min on day 1 every 21 d in untreatable, relapsed, or metastatic solid cancers[79]. A MTD was not reached even at ceiling doses and there were no DLTs observed though a total of 8 patients required dose reductions in paclitaxel and carboplatin[79]. The most common treatment-related AEs were alopecia (64.5%), fever (58.1%), and fatigue (58.1%); no relationships between reovirus dose and incidence or grade of symptoms were observed[79]. Out of 26 patients, the best overall response was CR in 1 patient (3.8%), PR in 6 patients (23.1%), major clinical response not evaluable by standard criteria in 2 patients (7.7%), and SD in 9 patients (34.6%) with a median duration of SD and PR of 6 mo (range 3-10 mo)[79]. Of the 24 patients with head and neck cancer, the median OS was 7.1 mo (CI: 4.2-11.5 mo)[79]. Preliminary results from a NCI-sponsored trial (OSU-07022) showed penetration and detection of replicating reovirus in tumor tissues thus far in 8 patients with recurrent or refractory ovarian, peritoneal, and fallopian tube carcinomas treated with IV reovirus at a fixed dose of 3 × 1010 TCID50 days 1-5 with dose-escalation of daily intraperitoneal (IP) reovirus every 28 d (Table 2)[80,81].
Pharmacokinetics and pharmacodynamics
In keeping with the wide range of historically observed seropositivities to reovirus, baseline seropositivity for NARAs was 37% in one phase I study and more than 90% in another phase I trial[68,82]. In general, phase I trials demonstrated a wide time to induction and time to peak levels of NARA titers from baseline though both more or less occurred within 1-4 wk with a median time to induction of 1.4 wk (range 1-3 wk) and median time to peak of 3.8 wk (range 1-10 wk) in one study[42,68,69,71-76,78,79]. Maximum NARA titers also varied considerably from 1/512 in one study to greater than 1/531441 in another (expressed as last dilution causing < 80% cytotoxicity) with a median increase from baseline of 250-fold (range 9- to 6437-fold)[42,72,82]. The neutralizing antibody response appeared to be blunted in cohorts with leukopenia from high-dose systemic reovirus therapy and myelosuppression from prior lumbosacral or pelvic radiotherapy[82]. Interestingly, reovirus in combination with gemcitabine or paclitaxel/carboplatin resulted in an attenuation in the time to induction and peak levels of NARA titers compared to prior phase I results while co-administration with docetaxel had no such effects[74,75,79]. Pharmacokinetic parameters, however, of gemcitabine, docetaxel, or paclitaxel/carboplatin, when co-administered with reovirus, were not appreciably different compared to receiving those agents alone[74,75,79]. Phase I data also illustrated that reverse transcription-polymerase chain reaction (RT-PCR) analysis of specimens including serum, stool, urine, saliva, and sputum for post-treatment viral shedding were negative in a majority of cases highlighting that reovirus administration in the outpatient setting is relatively safe[42,68,69,71-73,75,79]. When post-treatment viral shedding RT-PCR analyses were positive, they generally occurred within a few weeks (range 1-149 d) with some exceptions[42,68,69,71-73,75,79]. REO 013 showed that viral genome, though replication-incompetent, was present in plasma in 80% of patients at 1 h after the first dose of reovirus[76]. However, replication-competent reovirus was detected in peripheral blood mononuclear cells (PBMCs), granulocytes, and platelets, but not in plasma and red blood cells, at 1-h post-infusion and as late as 5-d post-infusion in PBMCs highlighting the idea of reovirus “hitchhiking” on such cells to evade the NARA response[76].
With respect to pharmacodynamics, available pathologic specimens have demonstrated positive detection of reovirus proteins localized to areas of cancer (occasionally with less involvement of surrounding tumor stroma and adjacent areas of normal tissue) with evidence of reovirus replication, apoptosis, and necrosis consistent with cytopathic effects[42,71-73,75,76,78,80,81]. In REO 005, 3 patients had reductions in cancer markers (carcinoembryonic antigen and PSA) consistent with clinical benefit, and 3 patients with biopsies showed the presence of viable reovirus post-treatment whose recovered titers correlated with doses of reovirus administered[72]. Similarly, in REO 013, replicating virus was recovered from lysates from surgical specimens in all 4 patients tested[76]. Interestingly, patients with 100% co-expression of reovirus RNA and CD138 showed greatest reductions in percent of myeloma cells with treatment in a NCI-sponsored phase I study (OSU-11148)[78].
Pathologic specimens in REO 002 showed peritumoral inflammation in 4 patients while REO 003 demonstrated focal collections of plasma cells not present previously during pathologic tumor examination in 3 of 6 patients[42,69]. Indeed, these observations have been somewhat corroborated in a separate phase I trial (REO 005) that revealed increases in CD3+CD4+ T-lymphocytes in 47.6% of patients, CD3+CD8+ T-lymphocytes in 33% of patients, CD8+ perforin/granzyme+ T-lymphocytes in 23.8% of patients, CD3-CD56+ natural killer (NK) cells in 28.6% of patients, and combined T-cell helper 1 and 2 (Th1 and Th2) cytokines in 38% of patients after reovirus therapy highlighting the potential significance of immune-mediated responses as a facilitator of reovirus anticancer efficacy[82]. Of note, there were no clear relationships between immune responses and reovirus dose, clinical response, or toxicity[82].
Phase II and III trials
An early multicenter, single-arm, open-label, phase II trial (REO 008) involved 1 × 1010 TCID50 intratumoral injections of reovirus on days 2 and 4 with 4 Gy of local irradiation daily × 5 fractions (total 20 Gy per cycle) in the treatment of refractory or metastatic solid tumors[83]. Out of 14 evaluable patients, the best overall response was SD or better in 13 patients (93%)[83]. Of these 13 patients, 4 experienced PR (2 with melanoma, 1 with lung cancer, and 1 with gastric cancer) and 2 experienced minor response (1 with thyroid cancer and 1 with ovarian cancer)[83]. The most common treatment-related AEs were grade 1 or 2 chills, pyrexia, headache, lethargy, anorexia, vomiting, shivering, nausea, and mild pain at injection site[83]. The NCI-sponsored MAYO-MC0672 was a multicenter, single-arm, open-label, phase II trial pitting 60-min IV infusion of reovirus 3 × 1010 TCID50 days 1-5 every 28 d against metastatic melanomas[84]. Out of 21 evaluable patients, the best overall response was SD > 8 wk in 6 patients with a median TTP of 45 d (range 13-96 d) and median OS of 165 d (range 15 d-15.8 mo)[84]. The study was ultimately closed due to failure to meet previously defined efficacy criteria to proceed to second stage of accrual, but 1 patient with 2 surgically removed metastatic cutaneous lesions demonstrated treatment effect as 75%-90% necrosis of these lesions were present on sampling[84]. Of note, out of 13 biopsies with metastatic tumor, productive reovirus replication was detected in 2 patients who had longer PFS of 80 and 87 d, respectively[84]. No dose reductions occurred, and the most common treatment-related grade 1 or 2 AEs were fatigue (66.7%), nausea (57.1%), and fever (52.4%)[84]. The most common treatment-related grade 3 or 4 AEs were fatigue (9.5%), hyponatremia (9.5%), and lymphopenia (9.5%)[84].
REO 014 enrolled 53 patients with refractory or untreatable soft tissue and bone sarcomas metastatic to the lung in a multicenter, single-arm, open-label phase II trial with 60-min IV infusions of reovirus 3 × 1010 TCID50 administered on days 1-5 every and given 28 d (personal communication). The best overall response was SD ≥ 12 wk in 18 patients (34%) with a subgroup of 12 patients having prolonged SD > 16 wk. Of these 12 patients, 3 patients demonstrated SD > 1 year (1 with malignant fibrous histiocytoma, 1 with synovial sarcoma, and 1 with ES). The median TTP was 58.0 d (95%CI: 54-110, range 8-726 d). The prolonged SD demonstrated fulfilled study criteria for consideration as an active agent. No dose reductions occurred, and the most common treatment-related AEs were pyrexia (81.1%), chills (66.4%), fatigue (47.2%), myalgia (37.7%), and nausea (37.7%). Of note, the first case of optic neuritis related to reovirus therapy was reported as a serious AE. Results from a single-arm, open-label, phase II study (REO 015) were PR in 4 patients (31%) and SD ≥ 12 wk in 2 patients for a CBR of 46% in 13 patients with refractory, recurrent, or metastatic SCC of the head and neck treated with 60-min IV infusion of reovirus 3 × 1010 TCID50 days 1-5 with IV paclitaxel 175 mg/m2 over 3 h and IV carboplatin AUC5 over 30 min on day 1 every 21 d[85]. Grade 1 or 2 AEs included fevers, chills, fatigue while grade 3 or 4 AEs were hypokalemia, fatigue, nausea, aspartate aminotransferase elevation, neutropenia, and anemia[85].
REO 016 enrolled 37 patients with recurrent or metastatic NSCLC originally treated with IV reovirus in combination with IV paclitaxel 200 mg/m2 and IV carboplatin AUC6, but due to grade 3 diarrhea and febrile neutropenia (1 each), the dosing regimen was amended to 60-min IV infusion of reovirus 3 × 1010 TCID50 days 1-5 with IV paclitaxel 175 mg/m2 over 3 h and IV carboplatin AUC5 (over 30 min) on day 1 every 21 d[86]. Of note, 20 patients had detected K-Ras mutations, 3 patients had EGFR mutations, 10 patients had EGFR amplifications alone, and 4 patients had BRAF V600E mutations[86]. Updated results have shown a median PFS of 4 mo (95%CI: 2.9-6.1), median OS of 13.1 mo (95%CI: 9.2-21.6), and 1-year OS rate of 57% (95%CI: 39%-72%)[86]. The most common AEs were fatigue, diarrhea, nausea, arthralgia/myalgia, and anorexia[86]. Results from REO 017 have thus far included a median PFS of 4 mo and OS of 10.2 mo in 34 enrolled patients with advanced or unresectable pancreatic cancer treated with 60-min IV infusion of reovirus 1 × 1010 TCID50 on days 1, 2, 8 and 9 with IV gemcitabine 800 mg/m2 days 1 and 8 every 21 d[87]. Treatment was well tolerated with manageable non-hematologic toxicities including grade 3-4 asthenia (38%), fever (12%), diarrhea (9%), chills (3%), flu-like syndrome (3%), and nausea/vomiting (3%). Intriguingly, upregulation of immune checkpoint markers including programmed death-ligand 1 (PD-L1) on immunohistochemistry (IHC) was demonstrated following treatment with reovirus[87].
The open-label, single-arm phase II trial (REO 021) involved 60-min IV infusion of reovirus 3 × 1010 TCID50 days 1-5 with IV paclitaxel 200 mg/m2 over 3 h and IV carboplatin AUC6 every 21 d in the treatment of recurrent or metastatic SCC of the lung[88]. Out of 25 patients who received more than 1 cycle of therapy, the best overall response was PR in 12 patients (48%) and SD in 10 patients (40%) for a CBR of 88%[88]. Of the 21 patients with > 6 mo follow-up, seven patients experienced PFS ≥ 6 mo (33.3%)[88]. The most common AEs were those expected of paclitaxel/carboplatin including neutropenia and thrombocytopenia and those expected of reovirus such as fever and fatigue[88]. The only treatment-related serious AE was reversible grade 2 elevation in creatinine and blood urea nitrogen[88].
On November 21, 2013, Oncolytics Biotech® Inc. reported preliminary top-line data from the randomized, double-arm, double-blinded, multicenter phase III trial involving 60-min IV infusion of reovirus 3 × 1010 TCID50 days 1-5 with standard doses of IV paclitaxel and IV carboplatin on day 1 only every 21 d (test arm) vs standard doses of IV paclitaxel and IV carboplatin alone (control arm) in the treatment of advanced or metastatic head and neck cancers (http://www.oncolyticsbiotech.com/clinical-trials). Per their report, 167 patients were enrolled and divided into an intent-to-treat group of 118 patients with loco-regional head and neck cancer (with or without metastases) and another group with distal metastases only. In the group of 118 patients, the median PFS was 94 d (13.4 wk, n = 62) in the test arm vs 50 d (7.1 wk, n = 56) in control arm maintained through 5 cycles. In the 88 patients discontinued from the study from this group, median OS was 150 d (21.4 wk, n = 50) in the test arm vs 115 d (16.4 wk, n = 38) in the control arm. Survival analysis in the distal metastases-only group has not been conducted. Of note, at the time of the first post-treatment scan (post-cycle 2 of therapy), 62 patients in the test arm experienced PD (32.3%) vs 56 patients on the control arm (51.8%, P = 0.04), and of the 86 patients with measurable disease at the first post-treatment scan, 48 patients demonstrated tumor reduction in the test arm vs 38 patients in the control arm (P = 0.049). There was a statistically significant increase in AEs of fever, chills, nausea, and diarrhea in the test arm vs control arm though there were no statistical differences in hematologic parameters in both arms. Nine patients in each arm experienced serious AEs of neutropenia with or without demonstrated infection. Interestingly, there were no dose reductions of paclitaxel for neuropathy or neurotoxicity in the test arm vs 6 dose reductions in the control arm (P = 0.028, Table 2).
IMMUNE RESPONSES TO REOVIRUS
Neutralization by the host immune system
Early preclinical evidence showed that prior exposure to reovirus did not significantly limit the antitumor activity of locally administered (intratumoral) reovirus in immune-competent C3H mice implanted with Ras-transformed fibroblasts and previously challenged with intramuscular injection of reovirus (detection of reovirus antibodies occurred after 2 wk in all challenged animals)[20]. Neutralizing antibodies similarly did not affect the efficacy of intratumoral reovirus in immune-competent rodent models of subcutaneous and intracranial glioblastoma[89]. However, systemic administration (IV) of reovirus is of therapeutic importance in advanced cancers, and phase I data illustrated that even heavily pretreated patients were capable of mounting brisk and dynamic immune responses to IV reovirus characterized by peak NARA titers reached by day 7 in 37.5% of patients and by day 14 in 62.5% of patients[82]. Indeed, neutralization of systemic reovirus by the host immune system was demonstrated when immune-competent C3H mice bearing Ras-transformed fibroblastic tumor allografts treated with IV reovirus (via tail vein injections) exhibited significant inhibition of tumor growth compared to controls at first, but tumor regrowth occured by 3 wk of IV reovirus therapy which coincided with rising serum NARA titers[58]. The ability of systemic reovirus to suppress tumor growth in immunized mice, however, was restored when co-administered with immunosuppressive agents such as cyclosporin A or cyclophosphamide which correlated with significantly decreased production of NARAs comparable to levels in mice without previous exposure to reovirus[58].
Systemic reovirus carries an innate ability to evade the NARA response by “hitchhiking” in PBMCs, granulocytes, and platelets; this process is detectable within a few hours post-infusion[82]. However, to further counteract the significant barrier to efficacy imposed by the neutralizing antibody response, it has been recommended that systemic reovirus be administered in rapid, repeated, and high doses within the first week of treatment when the NARA response has yet to become amplified[82]. Another strategy has involved the combination of reovirus with chemotherapeutic, particularly immunosuppressive, agents that attenuate the NARA response and therefore enhance tumor seeding of the virus, as previously suggested and described[66]. Importantly, early phase clinical trials have demonstrated that reovirus in combination with gemcitabine or paclitaxel/carboplatin resulted in attenuation of the NARA response while co-administration with docetaxel had no such effects though this finding was inconsistent with preclinical data[66,74,75,79]. All 3 combination regimens, however, have produced promising findings of clinical efficacy in various advanced malignancies and await further investigation in later trials[74,75,79].
Protective function against reovirus toxicities
High-dose systemic reovirus therapy is not without inherent risks as mice killed by viral overdose showed pathologic changes among several organs including liver and heart[58]. The role of the immune system in protecting against reovirus toxicity was highlighted when reovirus co-administered with high-dose cyclophosphamide resulted in both undetectable levels of NARA titers and severe systemic toxicities characterized by myocarditis, liver necrosis, tail detachment, and death compared to controls[56]. Furthermore, reovirus has been associated with limb necrosis and death in approximately 50%-60% of reovirus-treated SCID mice[20]. Upon metronomic dosing of high-dose cyclophosphamide with reovirus, however, systemic toxicities were markedly reduced in the presence of detectable NARA titers while preserving high levels of tumor access to virus and antitumor efficacy[56].
In a phase I, dose escalation trial cyclophosphamide was co-administered with reovirus in 36 patients with various solid tumors that had received prior therapies. The dose of cyclophosphamide ranged from 25-1000 mg/m2 with at least 3 patients per cohort with a consistent dose of reovirus dose of 3 × 1010 TCID50/d[90]. The combination of cyclophosphamide and reovirus was well tolerated with few grade 3 toxicities including fever, diarrhea, neutropenia, and anemia[90]. However, cyclophosphamide did not have an effect at stimulating an antiviral response as NARA titers rose > 50 fold in all but one patient[90]. Interestingly, reoviral RNA was detected via RT-PCR in PBMCs despite the significant rise in NARA titers, suggesting that PBMCs play a role in viral delivery to tumor cells[90].
Immune-mediated antitumor activity of reovirus
It has long been postulated that oncolytic virotherapy stimulates antitumor immune responses through innate and adaptive pathways[91]. Accordingly, several investigations have shown that reovirus infection: (1) induces the release of a host of pro-inflammatory mediators including interleukin (IL)-1α, IL-1β, IL-2, IL-6, IL-8, IL-12p40/70, IL-17, regulated on activation, normal T cell expressed and secreted, macrophage inflammatory protein-1α/β, granulocyte macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-α, IFN-γ, and tumor necrosis factor-α; (2) suppresses the release of the immunosuppressive IL-10; and (3) increases activation of dendritic cells (DCs) and recruits effectors from both innate and adaptive immunity including cytotoxic CD8+ T-lymphocytes (CTLs) and NK cells to facilitate tumor cell killing[35,92,93]. Furthermore, reovirus-infected melanoma cells released eotaxin, interferon gamma-induced protein 10, and IFN-β, in a NF-κB and PKR-dependent manner, and recruited NK cells, DCs, and CTLs to altogether promote bystander immune-mediated cytotoxicity in the tumor microenvironment[94].
Although reovirus infection has been shown to induce DC maturation in a dose-dependent manner, the immune-mediated antitumor activity of reovirus appears to occur independent of direct viral oncolysis or replication[95,96]. Nevertheless, reovirus therapy is capable of stimulating pro-inflammatory responses, enhancing tumor antigen presentation and exposing inaccessible tumor antigens for processing by DCs and CTLs, overcoming tumor evasion strategies and priming adaptive tumor-specific T-cells in vitro and in vivo, and initiating antitumor immunity to protect against subsequent tumor challenges in an antigen-dependent but reovirus-independent manner[92,96]. These processes that orchestrate reovirus-mediated antitumor immune responses have been demonstrated, in part, across several cancers including melanoma, lung cancer, AML, and prostate cancer[46,92,97]. Importantly, further support has been offered in early clinical trials when phase I data showed increases in CD3+CD4+ T-lymphocytes in 47.6% of patients, CD3+CD8+ T-lymphocytes in 33% of patients, CD8+perforin/granzyme+ T-lymphocytes in 23.8% of patients, CD3-CD56+ NK cells in 28.6% of patients, and combined Th1 and Th2 cytokines in 38% of patients after reovirus therapy[82].
Interestingly, administration of reovirus with tumor-specific DCs or OT-1 T-cells in melanoma-bearing mice resulted in significantly higher survival rates compared to controls and highlighted the synergistic potential of reovirus with immunotherapy[92]. Intratumoral reovirus co-administered with intraperitoneal genetically modified cells expressing IL-2, IL-12, or GM-CSF in mice inoculated with TC-1 cancer cells failed to demonstrate significant synergistic effects with respect to tumor suppression though the combination of reovirus with cyclophosphamide (administered at specific time points) produced synergistic inhibition of tumor growth[98]. Preconditioning of mice bearing subcutaneous melanomas with regulatory T-cell (Treg) depletion and IL-2 significantly enhanced the delivery of IV reovirus to tumors and increased antitumor efficacy compared to controls though with severe systemic toxicities such as shortness of breath, inactivity, and tail necrosis/detachment[99]. Instead, preconditioning with cyclophosphamide and IL-2, which mimicked Treg depletion, induced “hyperactivated” NK cells and similarly enhanced antitumor efficacy with IV reovirus though without detectable toxicities[99]. Alternatively, reovirus in combination with gemcitabine in mice implanted with ovarian cancer cells demonstrated greater survival and postponement of peritoneal carcinomatosis by inhibiting myeloid-derived suppressor cells (MDSCs), downregulating pro-MDSC factors, and accelerating tumor-specific T-cell responses[100].
Also of relevance, recent phase II trials have identified prolonged OS with reovirus in combination with conventional chemotherapy in advanced NSCLC and pancreatic cancer suggestive of an immunomodulatory influence on outcomes[86,87]. Upregulation of the immune checkpoint marker PD-L1 on IHC was observed following treatment with reovirus in REO 017. Although immune checkpoint inhibition and boosting of the immune response may be counterintuitive and detrimental to the efficacy of oncolytic reovirus by restricting viral replication, reovirus therapy in combination with anti-PD-1 therapy demonstrated improved survival in mouse models of melanoma, in vivo, compared to reovirus or anti-PD-1 therapy alone[101]. Checkpoint inhibition improved the ability of NK cells to kill reovirus-infected tumor cells and enhanced the CD8+ Th1 antitumor response primed by reovirus therapy in vitro. Furthermore, PD-1 blockade enhanced antiviral immune responses but through mechanisms that may differ from those affecting the antitumor response and thus offering a novel platform for combining immune modulation and reovirus in anticancer therapy.
DISCUSSION, PERSPECTIVES AND THE FUTURE
At the time of this review, there are a total of 34 clinical trials (both ongoing and completed) involving wild-type, unmodified T3D reovirus (Reolysin®) in the treatment of a variety of cancers (http://www.oncolyticsbiotech.com/clinical-trials). Nineteen of these clinical trials are early phase trials (phase I and I/II) or translational studies, and 10 of these 19 trials (53%) have investigated reovirus as monotherapy. Although not the primary objectives of these early trials, several phase I trials investigating single-agent reovirus produced promising results with a CBR as high as 45% in one study (though with a smaller and limited cohort of patients) when antitumor responses were evaluated by conventional criteria and reported (Table 1)[68,69,71,72,78]. However, of the remaining 15 clinical trials (phase II and III), only 2 of these investigated reovirus as monotherapy (13%). In an attempt to carry over the clinical efficacy observed in earlier trials, one phase II trial investigating single-agent reovirus in metastatic melanomas (the NCI-sponsored MAYO-MC0672) failed to meet previously defined efficacy criteria to advance to second stage of accrual and was ultimately closed[84]. However, REO 014 is the only phase II trial in which single-agent reovirus fulfilled study criteria for consideration as an active agent in untreatable, refractory, or metastatic sarcomas; further trials involving reovirus as montherapy in advanced sarcomas are warranted (personal communication). Nevertheless, this trend is likely a reflection of a growing consensus that single-agent reovirus is unlikely to have sufficient clinical efficacy to be used alone as an anticancer agent[2,4,6,7].
The delivery of viruses to target tissues in sufficient numbers to produce a meaningful therapeutic effect has been a longstanding tenet of virotherapy[2]. Early investigations into the anticancer potential of reovirus demonstrated that the neutralizing antibody response to the virus may pose a dilemma to its therapeutic efficacy given its ubiquitous nature and high seropositivity within the population. The effect of the neutralizing antibody response was most profound with systemic (IV) reovirus, which is of therapeutic importance in advanced cancers, when repeated IV delivery of reovirus in immune-competent mice bearing Ras-transformed tumor allografts demonstrated tumor regrowth within a few weeks that coincided with rising NARA titers[58]. Furthermore, phase I data showed that even heavily pretreated patients experienced a brisk induction of NARA titers from baseline with a time to peak levels within a few weeks after systemic reovirus.
In an attempt to circumvent this barrier to efficacy, systemic reovirus has been administered in rapid, repeated, high doses within the first week of treatment before the NARA response is boosted. Another strategy involves improving tumor cell killing by including reovirus in combination with other anticancer therapies; this appears to be the avenue in which the majority of ongoing and future trials involving reovirus are headed. Reovirus offers an excellent toxicity profile with the most common treatment-related AEs being mild respiratory/enteric and constitutional symptoms characteristic of its viral pathophysiology. As a result, reovirus becomes an attractive agent to use in combination with other therapies and, overall, makes combination clinical trials much more feasible. Furthermore, the mechanism of reovirus oncolysis offers synergistic potential when used with other agents due to differing pathways of inducing cancer cell death. These reasons have formed, in part, the rationale for late phase clinical trials, and so far, very promising preliminary results have been produced in several phase II trials involving reovirus in combination with chemotherapy and radiotherapy with CBRs achieved as high as greater than 90% in one study (Table 2). Recently, updated results from REO 016 and 017 have demonstrated discordance between PFS and OS in advanced NSCLC and pancreatic cancer treated with reovirus in combination with conventional chemotherapy. The reported PFS in these trials are comparable to historical controls, but OS is substantially longer than what has ever been reported in the literature for both cancers[86,87]. The clear OS benefit in the face of apparently limited impact on PFS is often characteristic of immune involvement in outcomes and may suggest further immunomodulatory anticancer effects from reovirus therapy (see below). Preliminary results from the phase III trial involving reovirus with paclitaxel and carboplatin in advanced head and neck cancer are also promising with improved median PFS and median OS when compared to control arms; the results of this trial are highly anticipated.
Independent of direct viral oncolysis and replication, reovirus offers further anticancer potential by promoting antitumor immune-mediated responses characterized by stimulation of pro-inflammatory cascades, activation of DCs, and recruitment of NK cells and CTLs that altogether contribute to bystander cytotoxicity within the tumor microenvironment. In addition, reovirus infection primes adaptive tumor-specific T-cell responses that can provide further tumor immunity and protection against subsequent challenges with tumor. Aside from the added cytotoxic effects offered with chemotherapy, certain agents may also enhance tumor seeding of reovirus due to attenuation of the NARA response as shown by gemcitabine, paclitaxel, and carboplatin. Immunomodulation with immunosuppressive agents such as cyclosporin A and cyclophosphamide also enhanced reovirus antitumor efficacy by attenuating NARAs but consequently revealed the protective function of the NARA response against reovirus systemic toxicity. The immune responses to reovirus, therefore, represent a double-edged sword in that they can pose a significant barrier to tumor seeding of virus and antitumor efficacy but also serve to protect against severe reovirus toxicity and promote antitumor cytotoxicity through innate and adaptive responses.
Despite the promising development of reovirus as an anticancer agent, there remain several key areas warranting further investigation in order to maximize the anticancer potential of reovirus. Firstly, a few reports have argued that reovirus oncolysis can occur independently of activated Ras and EGFR signaling pathways[43,44]. Despite the coordination between Ras-transformation, PKR, and viral translational inhibition, which remains one of the best characterized hypotheses in explaining the mechanism of reovirus oncolysis, greater understanding of the infectious life cycle of reovirus has uncovered that multiple steps of the oncolytic cycle including viral uncoating, production of viral progeny, progeny release through increased apoptosis, and spread of virus in later rounds of infection are influenced by Ras-transformation. Other studies have demonstrated potential ties between reovirus oncolysis and cell cycle phase[102]. These new insights have presented potential opportunities to enhance reovirus antitumor efficacy such as adding exogenous proteases to enhance reovirus infectivity, using Nutlin-3a to enhance reovirus-induced apoptosis and virus spread through p53-dependent NF-κB activation, using hydroxyurea to affect cell cycle synchronization and enhance sensitivity to reovirus, and avoiding agents that inhibit microtubules as functional microtubules are required for reovirus endocytic processing and infectivity[102-105]. Recent studies demonstrated that cancer-upregulated gene 2 inhibits PKR activation but is still dependent on p38 and Ras activation for permissiveness to reovirus replication, which highlights the increasing complexity and degree of crosstalk evident between mediators in coordinating sensitivity to reovirus oncolysis[106]. Undoubtedly, the mechanism of reovirus oncolysis in relation to EGFR/Ras activated signaling pathways (both upstream and downstream), PKR, the reovirus life cycle, cell cycle phase, and pathways of cell death warrant further investigation.
Future studies will also need to elucidate methods to promote antitumor immune responses while suppressing immune responses against tumor seeding of reovirus without severe systemic toxicities. Immunomodulation with preconditioning with cyclophosphamide and IL-2 has shown to enhance systemic delivery of reovirus and antitumor efficacy with reduced toxicities[99]. Future trials involving reovirus in combination with immunotherapy are warranted and likely to grow in number. Phase I data involving reovirus and cyclophosphamide in advanced malignancies will likely provide greater insight in how to safely maximize reovirus-mediated antitumor immune responses while minimizing the immune responses against tumor targeting. Checkpoint inhibition represents an alternative, but increasingly popular, means for combining immunomodulation with reovirus as anticancer therapy. Preclinical studies have demonstrated improved anticancer efficacy with the combination of PD-1 blockade and reovirus therapy compared to either therapy alone. Although antiviral responses were enhanced with the addition of anti-PD-1 therapy, they appear to occur through pathways that may differ from those affecting the antitumor response. Furthermore, checkpoint inhibition improved T-cell antitumor responses primed by reovirus therapy and the ability to locally clear reovirus-infected tumor cells. Indeed, with the growing popularity of checkpoint inhibitors in the treatment of advanced cancers, clinical trials with immunomodulation and reovirus should be a focus of future studies. Upregulation of the immune checkpoint marker PD-L1 on IHC has also been observed following treatment with reovirus in REO 017. Whether increased levels of PD-L1 affect response to checkpoint inhibitors and reovirus therapy represents another issue in need of further investigation.
Recent developments highlight that reverse genetics and classical genetics have allowed for the engineering of genetically modified variants of reovirus that maintain or even enhance selective oncolytic potency while reducing toxicity[107-110]. Lastly, immune resistance to one particular oncolytic virus may not necessarily confer resistance to others, and combination therapies including multiple oncolytic viruses are possible as exemplified by the preclinical success of reovirus in combination with Newcastle disease virus or parvovirus in glioblastomas[65].
CONCLUSION
Reovirus is a dsRNA virus with demonstrated preferential replication in cancer cells, or oncolysis. The mechanism of reovirus oncolysis is still poorly understood though Ras-transformation and activated Ras signaling, appears central for sensitivity to reovirus replication. Ras-transformation modulates several steps of the viral life cycle in promoting reovirus oncolysis: (1) virus disassembly and uncoating; (2) releasing translational inhibition by PKR; (3) generation of infectious progeny; (4) enhanced apoptosis and progeny release; and (5) spread of virus in subsequent cycles of infection (Figure 1). The antitumor efficacy of reovirus is also largely dependent on immune-mediated antitumor effects involving both innate and adaptive responses. Wild-type, unmodified, replication-competent T3D reovirus (Reolysin®) has demonstrated anticancer activity across a spectrum of malignancies. Early clinical trials have shown a safe and tolerable toxicity profile of reovirus with a predictable NARA response, minimal viral shedding, and localization, replication, and cytotoxic effects in pathologic specimens consistent with activity. Phase II and III trials involving reovirus have demonstrated promising results of clinical efficacy and reinforce its potential as an anticancer agent. Future trials will likely take advantage of its excellent toxicity profile in combination therapies for synergistic tumor cell killing.
Figure 1.
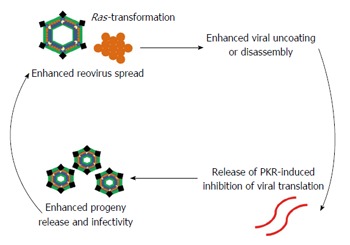
Ras-transformation promotes reovirus preferential replication in cancer cells or oncolysis by affecting several key steps of the viral infectious life cycle. Ras-transformation enhances viral uncoating or dissembly. dsRNA-activated protein kinase (PKR), which in the presence of viral transcripts, normally phosphorylates eukaryotic initiation factor 2α rendering it inactive and thereby leading to the inhibition of protein synthesis and viral replication, remains inactivated in Ras-transformed cells. Lastly, Ras-transformation enhances generation of viral progeny with increased infectivity, enhances release of progeny through apoptosis, and enhances viral spread in subsequent rounds of infection.
Footnotes
Conflict-of-interest statement: All authors declare no potential conflicts of interests and no financial support.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 23, 2015
First decision: January 18, 2016
Article in press: February 17, 2016
P- Reviewer: Chung YH, Ramírez M S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Comins C, Heinemann L, Harrington K, Melcher A, De Bono J, Pandha H. Reovirus: viral therapy for cancer ‘as nature intended’. Clin Oncol (R Coll Radiol) 2008;20:548–554. doi: 10.1016/j.clon.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Vidal L, Yap TA, White CL, Twigger K, Hingorani M, Agrawal V, Kaye SB, Harrington KJ, de Bono JS. Reovirus and other oncolytic viruses for the targeted treatment of cancer. Target Oncol. 2006;1:130–150. [Google Scholar]
- 3.Norman KL, Lee PW. Reovirus as a novel oncolytic agent. J Clin Invest. 2000;105:1035–1038. doi: 10.1172/JCI9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly K, Nawrocki S, Mita A, Coffey M, Giles FJ, Mita M. Reovirus-based therapy for cancer. Expert Opin Biol Ther. 2009;9:817–830. doi: 10.1517/14712590903002039. [DOI] [PubMed] [Google Scholar]
- 5.Norman KL, Lee PW. Not all viruses are bad guys: the case for reovirus in cancer therapy. Drug Discov Today. 2005;10:847–855. doi: 10.1016/S1359-6446(05)03483-5. [DOI] [PubMed] [Google Scholar]
- 6.Harrington KJ, Vile RG, Melcher A, Chester J, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–98. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin E, Egger ME, McMasters KM, Zhou HS. Development of oncolytic reovirus for cancer therapy. J Cancer Ther. 2013;4:16. [Google Scholar]
- 8.Duncan MR, Stanish SM, Cox DC. Differential sensitivity of normal and transformed human cells to reovirus infection. J Virol. 1978;28:444–449. doi: 10.1128/jvi.28.2.444-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashiro G, Loh PC, Yau JT. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch Virol. 1977;54:307–315. doi: 10.1007/BF01314776. [DOI] [PubMed] [Google Scholar]
- 10.Strong JE, Tang D, Lee PW. Evidence that the epidermal growth factor receptor on host cells confers reovirus infection efficiency. Virology. 1993;197:405–411. doi: 10.1006/viro.1993.1602. [DOI] [PubMed] [Google Scholar]
- 11.Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shmulevitz M, Marcato P, Lee PW. Unshackling the links between reovirus oncolysis, Ras signaling, translational control and cancer. Oncogene. 2005;24:7720–7728. doi: 10.1038/sj.onc.1209041. [DOI] [PubMed] [Google Scholar]
- 13.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci USA. 2004;101:11099–11104. doi: 10.1073/pnas.0404310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 16.Clemens MJ. PKR--a protein kinase regulated by double-stranded RNA. Int J Biochem Cell Biol. 1997;29:945–949. doi: 10.1016/s1357-2725(96)00169-0. [DOI] [PubMed] [Google Scholar]
- 17.Shmulevitz M, Pan LZ, Garant K, Pan D, Lee PW. Oncogenic Ras promotes reovirus spread by suppressing IFN-beta production through negative regulation of RIG-I signaling. Cancer Res. 2010;70:4912–4921. doi: 10.1158/0008-5472.CAN-09-4676. [DOI] [PubMed] [Google Scholar]
- 18.Alain T, Kim TS, Lun X, Liacini A, Schiff LA, Senger DL, Forsyth PA. Proteolytic disassembly is a critical determinant for reovirus oncolysis. Mol Ther. 2007;15:1512–1521. doi: 10.1038/sj.mt.6300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15:1522–1530. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 20.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 21.Loken SD, Norman K, Hirasawa K, Nodwell M, Lester WM, Demetrick DJ. Morbidity in immunosuppressed (SCID/NOD) mice treated with reovirus (dearing 3) as an anti-cancer biotherapeutic. Cancer Biol Ther. 2004;3:734–738. doi: 10.4161/cbt.3.8.963. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox ME, Yang W, Senger D, Rewcastle NB, Morris DG, Brasher PM, Shi ZQ, Johnston RN, Nishikawa S, Lee PW, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 23.Yang WQ, Senger D, Muzik H, Shi ZQ, Johnson D, Brasher PM, Rewcastle NB, Hamilton M, Rutka J, Wolff J, et al. Reovirus prolongs survival and reduces the frequency of spinal and leptomeningeal metastases from medulloblastoma. Cancer Res. 2003;63:3162–3172. [PubMed] [Google Scholar]
- 24.Smakman N, van den Wollenberg DJ, Borel Rinkes IH, Hoeben RC, Kranenburg O. Sensitization to apoptosis underlies KrasD12-dependent oncolysis of murine C26 colorectal carcinoma cells by reovirus T3D. J Virol. 2005;79:14981–14985. doi: 10.1128/JVI.79.23.14981-14985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–1701. [PubMed] [Google Scholar]
- 26.Alain T, Wong JF, Endersby R, Urbanski SJ, Lee PW, Muruve DA, Johnston RN, Forsyth PA, Beck PL. Reovirus decreases azoxymethane-induced aberrant crypt foci and colon cancer in a rodent model. Cancer Gene Ther. 2007;14:867–872. doi: 10.1038/sj.cgt.7701068. [DOI] [PubMed] [Google Scholar]
- 27.Smakman N, van der Bilt JD, van den Wollenberg DJ, Hoeben RC, Borel Rinkes IH, Kranenburg O. Immunosuppression promotes reovirus therapy of colorectal liver metastases. Cancer Gene Ther. 2006;13:815–818. doi: 10.1038/sj.cgt.7700949. [DOI] [PubMed] [Google Scholar]
- 28.van Houdt WJ, Smakman N, van den Wollenberg DJ, Emmink BL, Veenendaal LM, van Diest PJ, Hoeben RC, Borel Rinkes IH, Kranenburg O. Transient infection of freshly isolated human colorectal tumor cells by reovirus T3D intermediate subviral particles. Cancer Gene Ther. 2008;15:284–292. doi: 10.1038/cgt.2008.2. [DOI] [PubMed] [Google Scholar]
- 29.Min HJ, Koh SS, Cho IR, Srisuttee R, Park EH, Jhun BH, Kim YG, Oh S, Kwak JE, Johnston RN, et al. Inhibition of GSK-3beta enhances reovirus-induced apoptosis in colon cancer cells. Int J Oncol. 2009;35:617–624. [PubMed] [Google Scholar]
- 30.Etoh T, Himeno Y, Matsumoto T, Aramaki M, Kawano K, Nishizono A, Kitano S. Oncolytic viral therapy for human pancreatic cancer cells by reovirus. Clin Cancer Res. 2003;9:1218–1223. [PubMed] [Google Scholar]
- 31.Himeno Y, Etoh T, Matsumoto T, Ohta M, Nishizono A, Kitano S. Efficacy of oncolytic reovirus against liver metastasis from pancreatic cancer in immunocompetent models. Int J Oncol. 2005;27:901–906. [PubMed] [Google Scholar]
- 32.Hirano S, Etoh T, Okunaga R, Shibata K, Ohta M, Nishizono A, Kitano S. Reovirus inhibits the peritoneal dissemination of pancreatic cancer cells in an immunocompetent animal model. Oncol Rep. 2009;21:1381–1384. doi: 10.3892/or_00000364. [DOI] [PubMed] [Google Scholar]
- 33.Kilani RT, Tamimi Y, Hanel EG, Wong KK, Karmali S, Lee PW, Moore RB. Selective reovirus killing of bladder cancer in a co-culture spheroid model. Virus Res. 2003;93:1–12. doi: 10.1016/s0168-1702(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 34.Hanel EG, Xiao Z, Wong KK, Lee PW, Britten RA, Moore RB. A novel intravesical therapy for superficial bladder cancer in an orthotopic model: oncolytic reovirus therapy. J Urol. 2004;172:2018–2022. doi: 10.1097/01.ju.0000142657.62689.f6. [DOI] [PubMed] [Google Scholar]
- 35.Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L, Ilett LJ, Prestwich R, Pandha HS, Coffey M, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15:1257–1270. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norman KL, Coffey MC, Hirasawa K, Demetrick DJ, Nishikawa SG, DiFrancesco LM, Strong JE, Lee PW. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13:641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- 37.Hata Y, Etoh T, Inomata M, Shiraishi N, Nishizono A, Kitano S. Efficacy of oncolytic reovirus against human breast cancer cells. Oncol Rep. 2008;19:1395–1398. [PubMed] [Google Scholar]
- 38.Marcato P, Dean CA, Giacomantonio CA, Lee PW. Oncolytic reovirus effectively targets breast cancer stem cells. Mol Ther. 2009;17:972–979. doi: 10.1038/mt.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang WQ, Senger DL, Lun XQ, Muzik H, Shi ZQ, Dyck RH, Norman K, Brasher PM, Rewcastle NB, George D, et al. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene Ther. 2004;11:1579–1589. doi: 10.1038/sj.gt.3302319. [DOI] [PubMed] [Google Scholar]
- 40.Hingorani P, Zhang W, Lin J, Liu L, Guha C, Kolb EA. Systemic administration of reovirus (Reolysin) inhibits growth of human sarcoma xenografts. Cancer. 2011;117:1764–1774. doi: 10.1002/cncr.25741. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda Y, Nishimura G, Yanoma S, Kubota A, Furukawa M, Tsukuda M. Reovirus oncolysis in human head and neck squamous carcinoma cells. Auris Nasus Larynx. 2004;31:407–412. doi: 10.1016/j.anl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Thirukkumaran CM, Nodwell MJ, Hirasawa K, Shi ZQ, Diaz R, Luider J, Johnston RN, Forsyth PA, Magliocco AM, Lee P, et al. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res. 2010;70:2435–2444. doi: 10.1158/0008-5472.CAN-09-2408. [DOI] [PubMed] [Google Scholar]
- 43.Song L, Ohnuma T, Gelman IH, Holland JF. Reovirus infection of cancer cells is not due to activated Ras pathway. Cancer Gene Ther. 2009;16:382. doi: 10.1038/cgt.2008.84. [DOI] [PubMed] [Google Scholar]
- 44.Twigger K, Roulstone V, Kyula J, Karapanagiotou EM, Syrigos KN, Morgan R, White C, Bhide S, Nuovo G, Coffey M, et al. Reovirus exerts potent oncolytic effects in head and neck cancer cell lines that are independent of signalling in the EGFR pathway. BMC Cancer. 2012;12:368. doi: 10.1186/1471-2407-12-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alain T, Hirasawa K, Pon KJ, Nishikawa SG, Urbanski SJ, Auer Y, Luider J, Martin A, Johnston RN, Janowska-Wieczorek A, et al. Reovirus therapy of lymphoid malignancies. Blood. 2002;100:4146–4153. doi: 10.1182/blood-2002-02-0503. [DOI] [PubMed] [Google Scholar]
- 46.Hall K, Scott KJ, Rose A, Desborough M, Harrington K, Pandha H, Parrish C, Vile R, Coffey M, Bowen D, et al. Reovirus-mediated cytotoxicity and enhancement of innate immune responses against acute myeloid leukemia. Biores Open Access. 2012;1:3–15. doi: 10.1089/biores.2012.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alain T, Muzik H, Otsuka S, Chan S, Magliocco T, Diaz R, Forsyth PA, Morris D, Bebb G. Susceptibility of mantle cell lymphomas to reovirus oncolysis. Leuk Res. 2010;34:100–108. doi: 10.1016/j.leukres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Thirukkumaran CM, Shi ZQ, Luider J, Kopciuk K, Gao H, Bahlis N, Neri P, Pho M, Stewart D, Mansoor A, et al. Reovirus as a viable therapeutic option for the treatment of multiple myeloma. Clin Cancer Res. 2012;18:4962–4972. doi: 10.1158/1078-0432.CCR-11-3085. [DOI] [PubMed] [Google Scholar]
- 49.Thirukkumaran CM, Luider JM, Stewart DA, Cheng T, Lupichuk SM, Nodwell MJ, Russell JA, Auer IA, Morris DG. Reovirus oncolysis as a novel purging strategy for autologous stem cell transplantation. Blood. 2003;102:377–387. doi: 10.1182/blood-2002-08-2508. [DOI] [PubMed] [Google Scholar]
- 50.Thirukkumaran CM, Shi ZQ, Luider J, Kopciuk K, Bahlis N, Neri P, Pho M, Stewart D, Mansoor A, Morris DG. Reovirus as a successful ex vivo purging modality for multiple myeloma. Bone Marrow Transplant. 2014;49:80–86. doi: 10.1038/bmt.2013.130. [DOI] [PubMed] [Google Scholar]
- 51.Thirukkumaran CM, Luider JM, Stewart DA, Alain T, Russell JA, Auer IA, Forsyth P, Morris DG. Biological purging of breast cancer cell lines using a replication-competent oncolytic virus in human stem cell autografts. Bone Marrow Transplant. 2005;35:1055–1064. doi: 10.1038/sj.bmt.1704931. [DOI] [PubMed] [Google Scholar]
- 52.Williams ME, Cox DC, Stevenson JR. Rejection of reovirus-treated L1210 leukemia cells by mice. Cancer Immunol Immunother. 1986;23:87–92. doi: 10.1007/BF00199812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steele TA, Cox DC. Reovirus type 3 chemoimmunotherapy of murine lymphoma is abrogated by cyclosporine. Cancer Biother. 1995;10:307–315. doi: 10.1089/cbr.1995.10.307. [DOI] [PubMed] [Google Scholar]
- 54.Kollmorgen G, Cox DC, Killion JJ, Cantrell JL, Sansing WA. Immunotherapy of EL4 lymphoma with reovirus. Cancer Immunol Immun. 1976;1:239–244. [Google Scholar]
- 55.Twigger K, Vidal L, White CL, De Bono JS, Bhide S, Coffey M, Thompson B, Vile RG, Heinemann L, Pandha HS, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008;14:912–923. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- 56.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, Thompson J, Selby P, de Bono J, Melcher A, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandha HS, Heinemann L, Simpson GR, Melcher A, Prestwich R, Errington F, Coffey M, Harrington KJ, Morgan R. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res. 2009;15:6158–6166. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- 58.Hirasawa K, Nishikawa SG, Norman KL, Coffey MC, Thompson BG, Yoon CS, Waisman DM, Lee PW. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 59.Sei S, Mussio JK, Yang QE, Nagashima K, Parchment RE, Coffey MC, Shoemaker RH, Tomaszewski JE. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamano S, Mori Y, Aoyama M, Kataoka H, Tanaka M, Ebi M, Kubota E, Mizoshita T, Tanida S, Johnston RN, et al. Oncolytic reovirus combined with trastuzumab enhances antitumor efficacy through TRAIL signaling in human HER2-positive gastric cancer cells. Cancer Lett. 2015;356:846–854. doi: 10.1016/j.canlet.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 61.Carew JS, Espitia CM, Zhao W, Kelly KR, Coffey M, Freeman JW, Nawrocki ST. Reolysin is a novel reovirus-based agent that induces endoplasmic reticular stress-mediated apoptosis in pancreatic cancer. Cell Death Dis. 2013;4:e728. doi: 10.1038/cddis.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roulstone V, Twigger K, Zaidi S, Pencavel T, Kyula JN, White C, McLaughlin M, Seth R, Karapanagiotou EM, Mansfield D, et al. Synergistic cytotoxicity of oncolytic reovirus in combination with cisplatin-paclitaxel doublet chemotherapy. Gene Ther. 2013;20:521–528. doi: 10.1038/gt.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinemann L, Simpson GR, Boxall A, Kottke T, Relph KL, Vile R, Melcher A, Prestwich R, Harrington KJ, Morgan R, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer. 2011;11:221. doi: 10.1186/1471-2407-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lane M, Fahey JM, Besanceney C, Hamel N, Yu B, Thompson B, Coffey M, Wadler S. In vivo synergy between oncolytic reovirus and gemcitibane in ras-mutated human HCT116 xenografts. Cancer Res. 2007;67:4812. [Google Scholar]
- 65.Alkassar M, Gärtner B, Roemer K, Graesser F, Rommelaere J, Kaestner L, Haeckel I, Graf N. The combined effects of oncolytic reovirus plus Newcastle disease virus and reovirus plus parvovirus on U87 and U373 cells in vitro and in vivo. J Neurooncol. 2011;104:715–727. doi: 10.1007/s11060-011-0606-5. [DOI] [PubMed] [Google Scholar]
- 66.Maitra R, Ghalib MH, Goel S. Reovirus: a targeted therapeutic--progress and potential. Mol Cancer Res. 2012;10:1514–1525. doi: 10.1158/1541-7786.MCR-12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alloussi SH, Alkassar M, Urbschat S, Graf N, Gärtner B. All reovirus subtypes show oncolytic potential in primary cells of human high-grade glioma. Oncol Rep. 2011;26:645–649. doi: 10.3892/or.2011.1331. [DOI] [PubMed] [Google Scholar]
- 68.Morris DG, Feng X, DiFrancesco LM, Fonseca K, Forsyth PA, Paterson AH, Coffey MC, Thompson B. REO-001: A phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs. 2013;31:696–706. doi: 10.1007/s10637-012-9865-z. [DOI] [PubMed] [Google Scholar]
- 69.Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, Cairncross G, Matthews MV, Markert J, Gillespie Y, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 70.Kicielinski KP, Chiocca EA, Yu JS, Gill GM, Coffey M, Markert JM. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther. 2014;22:1056–1062. doi: 10.1038/mt.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, Coffey M, Gill GM, Mettinger K, Mariadason JM, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–649. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, Melcher A, Coffey M, Harrington KJ, DeBono JS. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 73.Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, Beirne D, Prestwich R, Newbold K, Ahmed M, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. 2010;16:3067–3077. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V, Twigger K, Coffey M, Mettinger K, Gill G, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581–588. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 75.Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, Vile R, Melcher A, Coffey MC, Mettinger KL, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–5572. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, Beirne D, West EJ, Jennings VA, Rose A, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ocean AJ, Bekaii-Saab TS, Chaudhary I, Palmer R, Christos PJ, Mercado A, Florendo EO, Rosales VA, Ruggiero JT, Popa EC, et al. A multicenter phase I study of intravenous administration of reolysin in combination with irinotecan/fluorouracil/leucovorin (FOLFIRI) in patients (pts) with oxaliplatin-refractory/intolerant KRAS-mutant metastatic colorectal cancer (mCRC) J Clin Oncol. 2013;31:2318. [Google Scholar]
- 78.Sborov DW, Nuovo GJ, Stiff A, Mace T, Lesinski GB, Benson DM, Efebera YA, Rosko AE, Pichiorri F, Grever MR, et al. A phase I trial of single-agent reolysin in patients with relapsed multiple myeloma. Clin Cancer Res. 2014;20:5946–5955. doi: 10.1158/1078-0432.CCR-14-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, Newbold K, Gore ME, Larkin J, Syrigos KN, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–2089. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phelps M, Cohn DE, O’Malley DM, Wei L, Wilkins D, Campbell A, Schaaf LJ, Coffey MC, Villalona-Calero MA, Grever MR, et al. Reovirus replication in ovarian and peritoneal tumors after intravenous administration. Cancer Res. 2010;70:2594. [Google Scholar]
- 81.Cohn DE, Nuovo G, Coffey MC, O’Malley D, Villalona-Calero MA, Grever MR, Deam D, Zwiebel JA, Phelps MA. Phase I/II trial of reovirus serotype 3-Dearing strain in patients with recurrent ovarian cancer. J Clin Oncol. 2010;28:TPS253. [Google Scholar]
- 82.White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, Morgan R, Merrick A, Errington F, Vile RG, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 83.Saunders M, Anthoney A, Coffey M, Mettinger K, Thompson B, Melcher A, Nutting CM, Harrington K. Results of a phase II study to evaluate the biological effects of intratumoral (ITu) reolysin in combination with low dose radiotherapy (RT) in patients (Pts) with advanced cancers. J Clin Oncol. 2009;27:e14514. [Google Scholar]
- 84.Galanis E, Markovic SN, Suman VJ, Nuovo GJ, Vile RG, Kottke TJ, Nevala WK, Thompson MA, Lewis JE, Rumilla KM, et al. Phase II trial of intravenous administration of Reolysin(®) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012;20:1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karnad A, Haigentz M, Miley T, Coffey M, Gill G, Mita M. A phase II study of intravenous wild-type reovirus (Reolysin) in combination with paclitaxel plus carboplatin in patients with platinum refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. Mol Cancer Ther. 2011;10:C22. [Google Scholar]
- 86.Villalona MA, Lam E, Otterson G, Zhao WQ, Timmons M, Subramaniam D, Hade E, Bertino E, Chao B, Selvaggi G, et al. Oncolytic reovirus in combination with paclitaxel/carboplatin in NSCLC patients with Ras activated malignancies, long term results. International Association for the Study of Lung Cancer (IASLC) 16th World Conference on Lung Cancer: 2015. p. MINI30.01. [Google Scholar]
- 87.Mahalingam D, Goel S, Coffey M, Noronha N, Selvaggi G, Nawrocki SN, Nuovo G, Mita M. Oncolytic virus therapy in pancreatic cancer: Clinical efficacy and pharmacodynamic analysis of REOLYSIN in combination with gemcitabine in patients with advanced pancreatic adenocarcinoma. Ann Oncol. 2015;26:P–175. [Google Scholar]
- 88.Mita AC, Argiris A, Coffey M, Gill GM, Mita M. A Phase 2 Study of Intravenous Administration of Reolysin (R) (Reovirus Type 3 Dearing) in Combination with Paclitaxel (P) and Carboplatin (C) in Patients with Squamous Cell Carcinoma of the Lung. J Thorac Oncol. 2013;8:S617–S618. [Google Scholar]
- 89.Yang WQ, Lun X, Palmer CA, Wilcox ME, Muzik H, Shi ZQ, Dyck R, Coffey M, Thompson B, Hamilton M, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 90.Roulstone V, Khan K, Pandha HS, Rudman S, Coffey M, Gill GM, Melcher AA, Vile R, Harrington KJ, de Bono J, et al. Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors. Clin Cancer Res. 2015;21:1305–1312. doi: 10.1158/1078-0432.CCR-14-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, Vile RG. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gujar SA, Marcato P, Pan D, Lee PW. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol Cancer Ther. 2010;9:2924–2933. doi: 10.1158/1535-7163.MCT-10-0590. [DOI] [PubMed] [Google Scholar]
- 93.Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, de Bono J, Selby P, Coffey M, Vile R, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 94.Steele L, Errington F, Prestwich R, Ilett E, Harrington K, Pandha H, Coffey M, Selby P, Vile R, Melcher A. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-κB mediated and supports innate and adaptive anti-tumour immune priming. Mol Cancer. 2011;10:20. doi: 10.1186/1476-4598-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, Thompson J, Galivo F, Harrington KJ, Pandha HS, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T, Thompson J, Morrison EE, Harrington KJ, Pandha HS, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14:7358–7366. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gujar SA, Pan DA, Marcato P, Garant KA, Lee PW. Oncolytic virus-initiated protective immunity against prostate cancer. Mol Ther. 2011;19:797–804. doi: 10.1038/mt.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sobotkova E, Duskova M, Eckschlager T, Vonka V. Efficacy of reovirus therapy combined with cyclophosphamide and gene-modified cell vaccines on tumors induced in mice by HPV16-transformed cells. Int J Oncol. 2008;33:421–426. [PubMed] [Google Scholar]
- 99.Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M, Selby P, Melcher A, Harrington K, Vile RG. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin Cancer Res. 2009;15:561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gujar SA, Clements D, Dielschneider R, Helson E, Marcato P, Lee PW. Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms. Br J Cancer. 2014;110:83–93. doi: 10.1038/bjc.2013.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, Shim KG, Diaz RM, Pandha H, Harrington K, et al. Combination Therapy With Reovirus and Anti-PD-1 Blockade Controls Tumor Growth Through Innate and Adaptive Immune Responses. Mol Ther. 2016;24:166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinemann L, Simpson GR, Annels NE, Vile R, Melcher A, Prestwich R, Harrington KJ, Pandha HS. The effect of cell cycle synchronization on tumor sensitivity to reovirus oncolysis. Mol Ther. 2010;18:2085–2093. doi: 10.1038/mt.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Golden JW, Linke J, Schmechel S, Thoemke K, Schiff LA. Addition of exogenous protease facilitates reovirus infection in many restrictive cells. J Virol. 2002;76:7430–7443. doi: 10.1128/JVI.76.15.7430-7443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mainou BA, Zamora PF, Ashbrook AW, Dorset DC, Kim KS, Dermody TS. Reovirus cell entry requires functional microtubules. MBio. 2013;4 doi: 10.1128/mBio.00405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan D, Pan LZ, Hill R, Marcato P, Shmulevitz M, Vassilev LT, Lee PW. Stabilisation of p53 enhances reovirus-induced apoptosis and virus spread through p53-dependent NF-κB activation. Br J Cancer. 2011;105:1012–1022. doi: 10.1038/bjc.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park EH, Park EH, Cho IR, Srisuttee R, Min HJ, Oh MJ, Jeong YJ, Jhun BH, Johnston RN, Lee S, et al. CUG2, a novel oncogene confers reoviral replication through Ras and p38 signaling pathway. Cancer Gene Ther. 2010;17:307–314. doi: 10.1038/cgt.2009.83. [DOI] [PubMed] [Google Scholar]
- 107.van den Wollenberg DJ, van den Hengel SK, Dautzenberg IJ, Cramer SJ, Kranenburg O, Hoeben RC. A strategy for genetic modification of the spike-encoding segment of human reovirus T3D for reovirus targeting. Gene Ther. 2008;15:1567–1578. doi: 10.1038/gt.2008.118. [DOI] [PubMed] [Google Scholar]
- 108.Boehme KW, Ikizler M, Kobayashi T, Dermody TS. Reverse genetics for mammalian reovirus. Methods. 2011;55:109–113. doi: 10.1016/j.ymeth.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim M, Garant KA, zur Nieden NI, Alain T, Loken SD, Urbanski SJ, Forsyth PA, Rancourt DE, Lee PW, Johnston RN. Attenuated reovirus displays oncolysis with reduced host toxicity. Br J Cancer. 2011;104:290–299. doi: 10.1038/sj.bjc.6606053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shmulevitz M, Gujar SA, Ahn DG, Mohamed A, Lee PW. Reovirus variants with mutations in genome segments S1 and L2 exhibit enhanced virion infectivity and superior oncolysis. J Virol. 2012;86:7403–7413. doi: 10.1128/JVI.00304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


