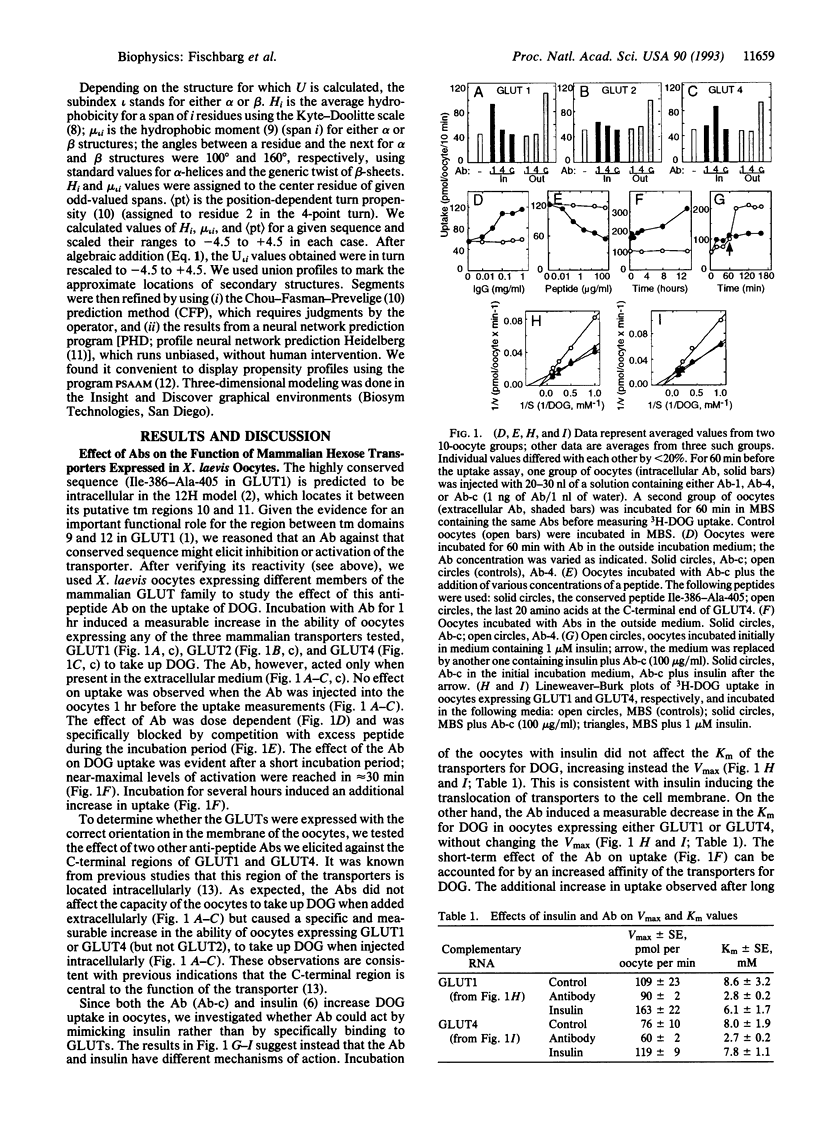
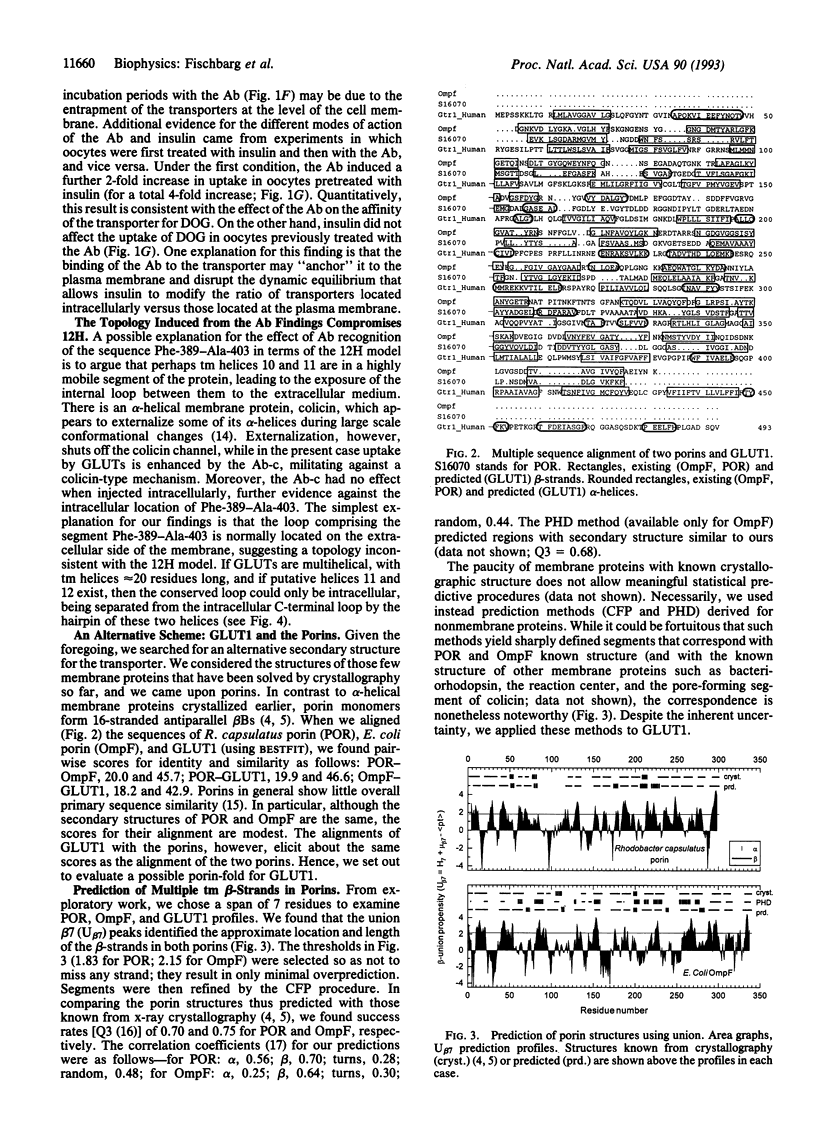
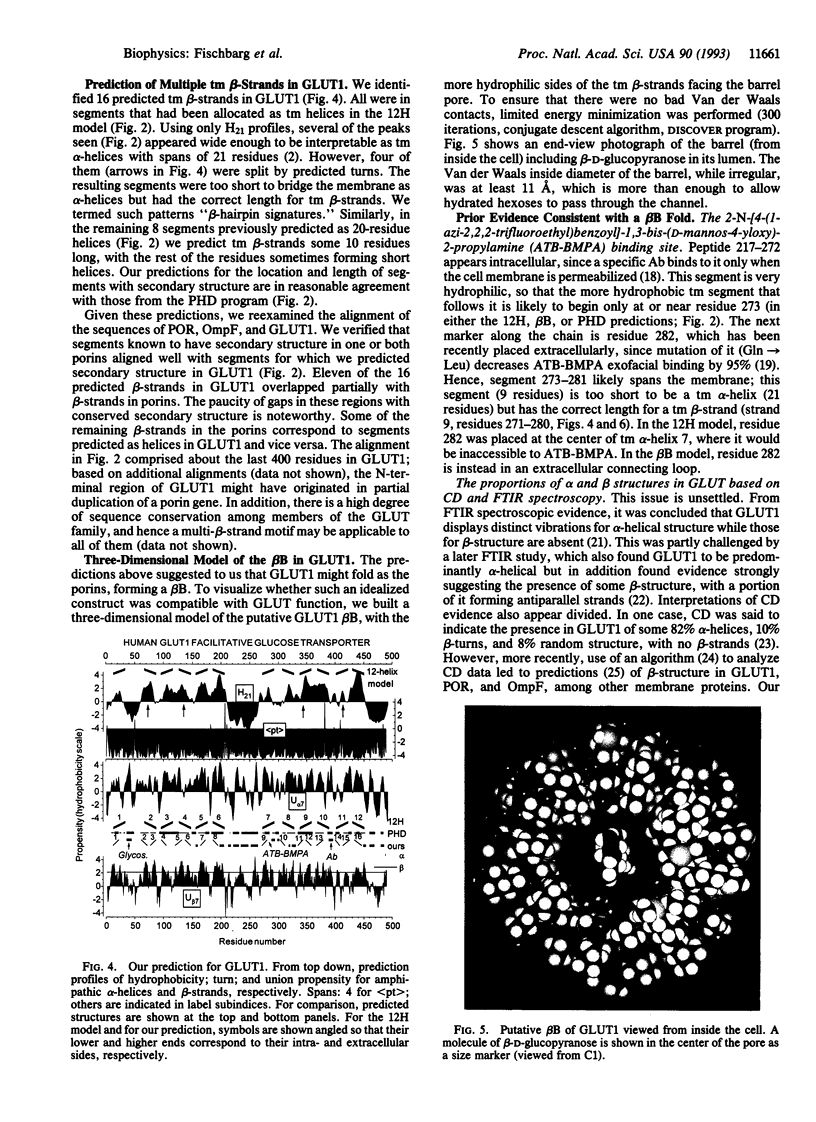
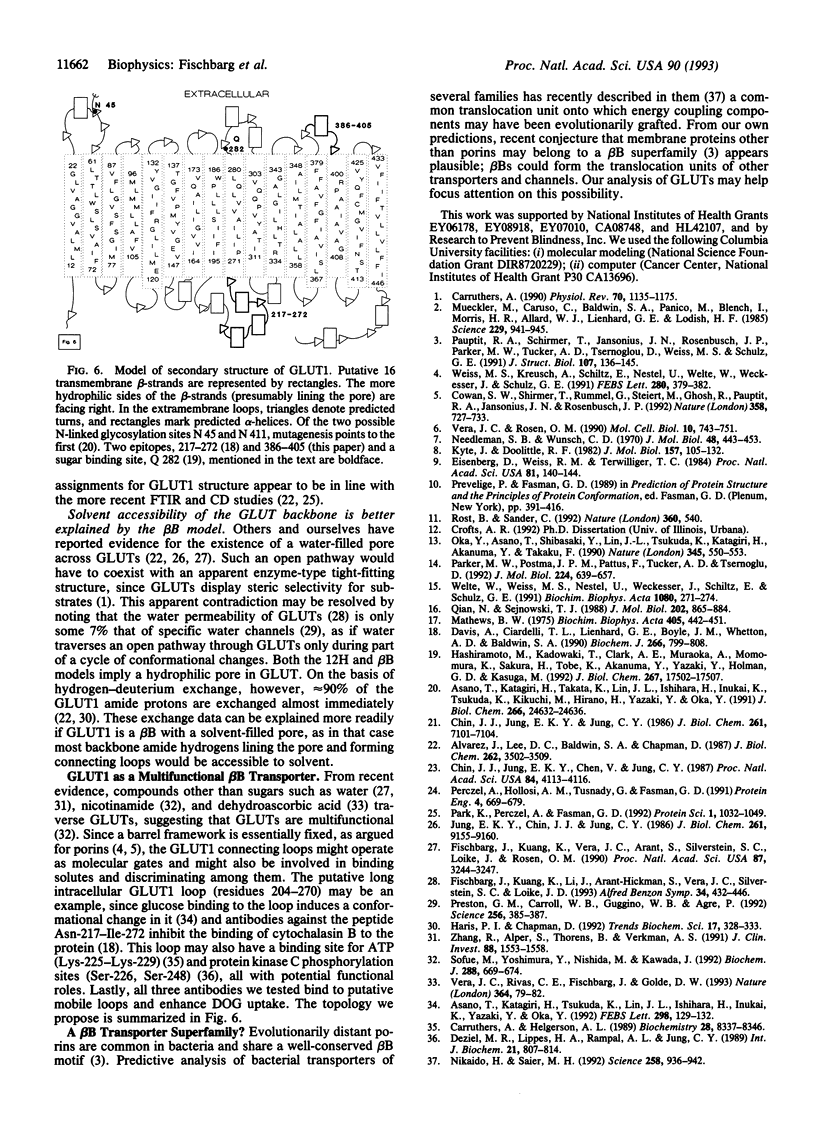
Abstract
A widely accepted model for the structure of the facilitative glucose transporters (GLUTs) predicts that they form 12 transmembrane alpha-helices and that the highly conserved sequence Ile-386-Ala-405 in GLUT1 is intracellular. We raised a polyclonal antibody against a synthetic peptide encompassing this conserved sequence and found that antibody treatment increased 2-deoxy-D-glucose (DOG) uptake in Xe-nopus oocytes expressing GLUT1, GLUT2, or GLUT4 only when applied to the extracellular side. This effect was dose dependent and was specifically blocked by competition with the peptide Ile-386-Ala-405; it was due to a decrease in the Km for the transport of DOG. To ascertain GLUT orientation, we raised anti-peptide antibodies against the last 21 and 25 C-terminal amino acids of GLUT1 and GLUT4, respectively, which were previously shown to be intracellular. These antibodies increased DOG uptake when injected into oocytes expressing GLUT1 and GLUT4, but not when added extracellularly. Prompted by the noted discrepancy, we found sequence similarity between GLUTs and porins, two of which are known from crystallography to form 16-stranded transmembrane antiparallel beta-barrels. Analysis of the hydrophobicity, amphiphilicity, and turn propensity of GLUT1 leads us to propose that GLUTs fold as porin-like transmembrane beta-barrels. This model is consistent with the results of the present antibody studies and also with previously published experimental evidence inconsistent with the 12-helix model.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., Lee D. C., Baldwin S. A., Chapman D. Fourier transform infrared spectroscopic study of the structure and conformational changes of the human erythrocyte glucose transporter. J Biol Chem. 1987 Mar 15;262(8):3502–3509. [PubMed] [Google Scholar]
- Asano T., Katagiri H., Takata K., Lin J. L., Ishihara H., Inukai K., Tsukuda K., Kikuchi M., Hirano H., Yazaki Y. The role of N-glycosylation of GLUT1 for glucose transport activity. J Biol Chem. 1991 Dec 25;266(36):24632–24636. [PubMed] [Google Scholar]
- Asano T., Katagiri H., Tsukuda K., Lin J. L., Ishihara H., Inukai K., Yazaki Y., Oka Y. Glucose binding enhances the papain susceptibility of the intracellular loop of the GLUT1 glucose transporter. FEBS Lett. 1992 Feb 24;298(2-3):129–132. doi: 10.1016/0014-5793(92)80038-i. [DOI] [PubMed] [Google Scholar]
- Carruthers A. Facilitated diffusion of glucose. Physiol Rev. 1990 Oct;70(4):1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- Carruthers A., Helgerson A. L. The human erythrocyte sugar transporter is also a nucleotide binding protein. Biochemistry. 1989 Oct 17;28(21):8337–8346. doi: 10.1021/bi00447a011. [DOI] [PubMed] [Google Scholar]
- Chin J. J., Jung E. K., Chen V., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in proteoliposome vesicles: circular dichroism measurements. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4113–4116. doi: 10.1073/pnas.84.12.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. J., Jung E. K., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in reconstituted vesicles. J Biol Chem. 1986 Jun 5;261(16):7101–7104. [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Davies A., Ciardelli T. L., Lienhard G. E., Boyle J. M., Whetton A. D., Baldwin S. A. Site-specific antibodies as probes of the topology and function of the human erythrocyte glucose transporter. Biochem J. 1990 Mar 15;266(3):799–808. [PMC free article] [PubMed] [Google Scholar]
- Deziel M. R., Lippes H. A., Rampal A. L., Jung C. Y. Phosphorylation of the human erythrocyte glucose transporter by protein kinase C: localization of the site of in vivo and in vitro phosphorylation. Int J Biochem. 1989;21(7):807–814. doi: 10.1016/0020-711x(89)90214-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J., Kuang K. Y., Vera J. C., Arant S., Silverstein S. C., Loike J., Rosen O. M. Glucose transporters serve as water channels. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haris P. I., Chapman D. Does Fourier-transform infrared spectroscopy provide useful information on protein structures? Trends Biochem Sci. 1992 Sep;17(9):328–333. doi: 10.1016/0968-0004(92)90305-s. [DOI] [PubMed] [Google Scholar]
- Hashiramoto M., Kadowaki T., Clark A. E., Muraoka A., Momomura K., Sakura H., Tobe K., Akanuma Y., Yazaki Y., Holman G. D. Site-directed mutagenesis of GLUT1 in helix 7 residue 282 results in perturbation of exofacial ligand binding. J Biol Chem. 1992 Sep 5;267(25):17502–17507. [PubMed] [Google Scholar]
- Jung E. K., Chin J. J., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in reconstituted system. Hydrogen exchange. J Biol Chem. 1986 Jul 15;261(20):9155–9160. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim Biophys Acta. 1975 Oct 20;405(2):442–451. doi: 10.1016/0005-2795(75)90109-9. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Saier M. H., Jr Transport proteins in bacteria: common themes in their design. Science. 1992 Nov 6;258(5084):936–942. doi: 10.1126/science.1279804. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Katagiri H., Akanuma Y., Takaku F. C-terminal truncated glucose transporter is locked into an inward-facing form without transport activity. Nature. 1990 Jun 7;345(6275):550–553. doi: 10.1038/345550a0. [DOI] [PubMed] [Google Scholar]
- Park K., Perczel A., Fasman G. D. Differentiation between transmembrane helices and peripheral helices by the deconvolution of circular dichroism spectra of membrane proteins. Protein Sci. 1992 Aug;1(8):1032–1049. doi: 10.1002/pro.5560010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Postma J. P., Pattus F., Tucker A. D., Tsernoglou D. Refined structure of the pore-forming domain of colicin A at 2.4 A resolution. J Mol Biol. 1992 Apr 5;224(3):639–657. doi: 10.1016/0022-2836(92)90550-4. [DOI] [PubMed] [Google Scholar]
- Pauptit R. A., Schirmer T., Jansonius J. N., Rosenbusch J. P., Parker M. W., Tucker A. D., Tsernoglou D., Weiss M. S., Schultz G. E. A common channel-forming motif in evolutionarily distant porins. J Struct Biol. 1991 Oct;107(2):136–145. doi: 10.1016/1047-8477(91)90017-q. [DOI] [PubMed] [Google Scholar]
- Perczel A., Hollósi M., Tusnády G., Fasman G. D. Convex constraint analysis: a natural deconvolution of circular dichroism curves of proteins. Protein Eng. 1991 Aug;4(6):669–679. doi: 10.1093/protein/4.6.669. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Qian N., Sejnowski T. J. Predicting the secondary structure of globular proteins using neural network models. J Mol Biol. 1988 Aug 20;202(4):865–884. doi: 10.1016/0022-2836(88)90564-5. [DOI] [PubMed] [Google Scholar]
- Rost B., Sander C. Jury returns on structure prediction. Nature. 1992 Dec 10;360(6404):540–540. doi: 10.1038/360540b0. [DOI] [PubMed] [Google Scholar]
- Sofue M., Yoshimura Y., Nishida M., Kawada J. Possible multifunction of glucose transporter. Transport of nicotinamide by reconstituted liposomes. Biochem J. 1992 Dec 1;288(Pt 2):669–674. doi: 10.1042/bj2880669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rivas C. I., Fischbarg J., Golde D. W. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993 Jul 1;364(6432):79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Mol Cell Biol. 1990 Feb;10(2):743–751. doi: 10.1128/mcb.10.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Kreusch A., Schiltz E., Nestel U., Welte W., Weckesser J., Schulz G. E. The structure of porin from Rhodobacter capsulatus at 1.8 A resolution. FEBS Lett. 1991 Mar 25;280(2):379–382. doi: 10.1016/0014-5793(91)80336-2. [DOI] [PubMed] [Google Scholar]
- Welte W., Weiss M. S., Nestel U., Weckesser J., Schiltz E., Schulz G. E. Prediction of the general structure of OmpF and PhoE from the sequence and structure of porin from Rhodobacter capsulatus. Orientation of porin in the membrane. Biochim Biophys Acta. 1991 Nov 15;1080(3):271–274. doi: 10.1016/0167-4838(91)90013-p. [DOI] [PubMed] [Google Scholar]
- Zhang R., Alper S. L., Thorens B., Verkman A. S. Evidence from oocyte expression that the erythrocyte water channel is distinct from band 3 and the glucose transporter. J Clin Invest. 1991 Nov;88(5):1553–1558. doi: 10.1172/JCI115466. [DOI] [PMC free article] [PubMed] [Google Scholar]




