Abstract
Deguelin, a natural component derived from leguminous plants, has been used as pesticide in some regions. Accumulating evidence show that deguelin has promising chemopreventive and therapeutic activities against cancer cells. This study shows that low concentrations of deguelin can lead to significant delay in zebrafish embryonic development through growth inhibition and induction of apoptosis. Furthermore, we identified fibroblast growth factor receptor 4 (FGFR4) as the putative target of deguelin. The candidate was initially identified by a microarray approach and then validated through in vitro experiments using hormone‐responsive (MCF‐7) and nonresponsive (MDA‐MB‐231) human breast cancer cell lines. The results show that deguelin suppressed cell proliferation and induced apoptosis in both cancer cell lines, but not in Hs 578Bst cells, by blocking PI3K/AKT and mitogen‐activated protein kinases (MAPK) signaling. The FGFR4 mRNA and protein level also diminished in a dose‐dependent manner. Interestingly, we found that forced FGFR4 overexpression attenuated deguelin‐induced proliferative suppression and apoptotic cell death in both zebrafish and MCF‐7 cell lines, p‐AKT and p‐ERK levels were restored upon FGFR4 overexpression. Taken together, our results strongly suggest that deguelin inhibition of PI3K/AKT and MAPK signaling in zebrafish and breast cancer cell lines is partially mediated through down‐regulation of FGFR4 activity.
Keywords: Breast cancer, deguelin, FGFR4, zebrafish
Abbreviations
- AKT
protein kinase B
- ANOVA
analysis of variance
- ATCC
American Type Culture Collection
- DMSO
dimethyl sulfoxide
- ERK
extracellular regulated protein kinases
- FBS
fetal bovine serum
- FGFR4
fibroblast growth factor receptor 4
- hpf
hours post fertilization
- MAPK
mitogen‐activated protein kinases
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- PH3
phosphorylated histone H3
- PI3K
phosphoinositide 3‐kinase
- RT‐qPCR
reverse transcription, real‐time quantitative polymerase chain reaction
- SDS
sodium dodecyl sulfate
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Introduction
Deguelin, a naturally occurring rotenoid found in leguminous plants, has ever been used as a commercial insecticide and pesticide (Fang and Casida 1999). Both in vitro and in vivo models have shown that deguelin has antitumor effects. Deguelin induces apoptosis of cancer cells (Murillo et al. 2002; Chun et al. 2003; Peng et al. 2007), and inhibits cyclooxygenase‐2 expression (Lee et al. 2004) in premalignant and malignant human bronchial epithelial cells. Furthermore, it inhibits the growth of pulmonary adenoma in A/J mice (Yan et al. 2005) and the formation of carcinogen‐induced aberrant crypt foci in mouse colon (Murillo et al. 2003). In addition, deguelin has been proven to enhance the chemosensitivity of leukemia cells (Bortul et al. 2005). More recently, deguelin has been shown to suppress pancreatic tumor growth (Boreddy and Srivastava 2013) and triple‐negative breast cancer cells proliferation (Mehta et al. 2013a,b; Suh et al. 2013).
Mechanistically, deguelin induces apoptosis, causes cell cycle arrest, restrains cell proliferation, and inhibits angiogenesis in cancers. These effects are mediated by the suppression of various molecular pathways, such as the phosphoinositide 3‐kinase (PI3K)/protein kinase B (AKT) (Chun et al. 2003; Lee 2004; Bortul et al. 2005) and nuclear factor kappa‐light‐chain‐enhancer of activated B‐cells pathways (Nair et al. 2006; Dell'Eva et al. 2007; Geeraerts et al. 2007); Blocking individual proteins including nuclear factor of kappa light polypeptide gene enhancer in B‐cells inhibitor, Alpha (Nair et al. 2006), the apoptosis inhibitors survivin and X‐linked inhibitor of apoptosis protein (Jin et al. 2007; Peng et al. 2007; Ito et al. 2010), heat shock protein 90 (Oh et al. 2007; Kim et al. 2009; Chang et al. 2012), and AMP‐activated protein kinase (Jin et al. 2007; Wang et al. 2013; Yang et al. 2013) also mediated the same suppressive effects. Among all the target pathways suggested for deguelin, the PI3K/AKT and mitogen‐activated protein kinases (MAPK) pathways have the strongest support (Chun et al. 2003; Peng et al. 2007; Hu et al. 2010). More recently, studies show that deguelin inhibits the growth of triple‐negative breast cancer cells by mediating through EGF‐p‐AKT/c‐Met p‐ERK (Mehta et al. 2013a) and suppressing metastasis of 4T1 cells with decreased p‐AKT and phospho‐extracellular regulated protein kinases (p‐ERK) levels (Mehta et al. 2013b). However, the detailed mechanisms of deguelin function and exactly how deguelin regulates multiple downstream signaling pathways remains unclear.
The zebrafish (Danio rerio) belong to freshwater fish and native to Himalayan region (Patton and Zon 2001). Zebrafish is an attractive model to use in biomedical research due to its transparency and rapid regenerative ability (Major and Poss 2007; White et al. 2008; Mione and Trede 2010). Recently, we found that low‐concentration deguelin treatment led to significant growth retardation of zebrafish embryos. So, microarray approach was undertaken with zebrafish embryos to identify the dysregulated RNA expression after deguelin treatment. We identified fibroblast growth factor receptor 4 (FGFR4) as the special target, since FGFR4 is substantially down‐regulated in the microarray data and large amounts of literatures indicate that FGFRs activate a wide range of signaling pathways including PI3K/AKT and MAPK/ERK pathways (Fernandez et al. 2002; Agazie et al. 2003; Koziczak et al. 2004; Katoh and Katoh 2006; Thomson et al. 2008).
Fibroblast growth factors and their receptors control many biological activities, including proliferation, antiapoptosis, and drug resistance (Becker et al. 1992; Segev et al. 2000; Ho et al. 2009; Katoh and Nakagama 2014; Turkington et al. 2014). FGFR4 plays a vital role in myogenic 3 differentiation and muscle regeneration after injury, but not in differentiated skeletal muscle (Zhao and Hoffman 2004; Zhao et al. 2006; Lagha et al. 2008). Moreover, aberrant FGFR signaling were found in pathogenesis of diverse cancers (Haugsten et al. 2010; Turner and Grose 2010; Katoh and Nakagama 2014), including breast (Jaakkola et al. 1993), prostate (Wang et al. 2008; Xu et al. 2011),pancreatic (Leung et al. 1994), colorectal cancer (Liu et al. 2013), and ovarian cancer (Zaid et al. 2013). Glycine (Gly388) or Arginine (Arg388) at codon 388 in the transmembrane domain of FGFR4 has been proved to have important role in cancer progression. The Arg388 allele contributes to breast cancer cell motility and drug resistance (Thussbas et al. 2006), and also to poor prognosis in colon cancer (Bange et al. 2002). Besides, the FGFR4 Gly388 polymorphism lead to prostate cancer progression (Xu et al. 2011). As the role of FGFR4 was established in multiple cancers, we investigated whether the mechanism in zebrafish is also equally applicable to breast cancer cells, which has the same rapid proliferative ability and similar FGFR4 level as zebrafish.
Taken together, our data suggest that down‐regulation of FGFR4 contributes to deguelin‐induced apoptosis in zebrafish and breast cancer cells, which results in decreased p‐AKT and p‐ERK levels.
Materials and Methods
Cell culture, reagents, and antibodies
Human breast cancer cell lines MCF‐7, MDA‐MB‐231 and normal mammary epithelial cell line Hs 578Bst were purchased from American Type Culture Collection (ATCC) and cultured as suggested. RPMI 1640, F‐15 medium, fetal bovine serum (FBS), and Penicillin Streptomycin were purchased from Gibco (Guangzhou, China). Hybri‐Care Medium was from ATCC. Deguelin (Sigma, Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) at a 1 mmol/L stock concentration and stored at −80°C. FGFR inhibitor SU5402 (Sigma) was dissolved to 1 mol/L as a stock solution. Antibody against FGFR4, Phospho‐AKT (S473), Phospho‐p44/p42 MAPK, p44/p42 MAPK, Phospho‐Histone H3, β‐actin, and HRP‐conjugated secondary antibody were all obtain from Cell Signaling Technology (Guangzhou, China).
Morphology observation
Zebrafish embryos were treated with deguelin (0–500 nmol/L) from shield period (6 hpf [hours post fertilization]). The morphology of each group was observed at 36 hpf under a stereo microscope (ZEISS Stemi 2000‐c; Zeiss, Guangzhou, China).
Cell proliferation in zebrafish
A phosphorylation of histone H3 (PH3) assay was conducted to analyze cell proliferation in zebrafish. Zebrafish larvae were exposed to deguelin at 24 hpf for 6 h, and then fixed in 4% paraformaldehyde overnight. The larvae were treated with 3% H2O2 in carbinol, followed by incubation with 10 μg/mL Proteinase K to enhance their permeability. Afterwards, larvae were blocked in block solution (1%(V/V) Tween 20 in 1× Phosphate‐buffered saline(PBST), 2 mg/mL BSA, 10% Bovine Serum Albumin (FBS), 1% DMSO) for 1 h and incubated with Phospho‐Histone H3 antibody (1:200) overnight. Samples were incubated in secondary antibody (1:200) (Invitrogen, Guangzhou, China) at room temperature for 1 h before using for immediate microscopy (Zeiss AxioImager A1 fluorescent microscope; CarlZeiss, Guangzhou, China).
TUNEL
Cells apoptosis was examined using In Situ Cell Death Detection Kit, TMR red (Roche, Shanghai, China), which labels DNA fragments resulting from apoptotic signaling cascades. Briefly, cells/zebrafish, treated as indicated, were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X‐100/Proteinase K (10 μg/mL). After incubating in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reaction mixture for 1 h in the dark, slices of cells were treated with 4′,6‐diamidino‐2‐phenylindole for 10 min and observed under a Zeiss AxioImager A1 fluorescent microscope.
GeneChip zebrafish genome array
Zebrafish embryos at 2‐cell stage were selected and treated with 0.6 μmol/L deguelin, whereas control groups received 0.1% DMSO. Total RNAs was extracted from zebrafish at sphere stage and handed to Shanghai Biotechnology Corporation (Shanghai, China) for affymetrix zebrafish microarray analysis.
Real‐time RT‐PCR
Total RNAs from cells and zebrafish embryos was extracted with RNeasy mini Kit (Qiagen, Guangzhou, China) and reverse translated with PrimeScriptTM RT reagent Kit (TaKaRa, Dalian, China). Real‐time polymerase chain reaction (PCR) was conducted using the SYBR Premix Ex TaqTM Kit (TaKaRa) and an ABI PRISM 7300 Sequence Detection System (Applied Biosystems, Guangzhou, China). Human cell FGFR4‐specific primers: forward, 5′‐TCCGCTGGCTTAAGGATGG‐3′; reverse, 5′‐CACGAGACTCCAGTGCTGATG‐3′. Zebrafish FGFR4 primers: forward, 5′‐AGGGTGCTGGTGTCAATTTC‐3′; reverse, 5′‐TTAGGTCCATCCGAGAATGC‐3′. The validity of the analysis was evaluated by melting curve analysis.
Western Blot analysis
Cells and zebrafish embryos were cultured in the presence or absence of deguelin, and then lysed in RIPA Lysis Buffer (50 mmol/L Tris‐HCl pH7.4, 1% NP‐40, 150 mmol/L NaCl, 1 mmol/L Ethylenediaminetetraacetic acid (EDTA), 1% Triton X‐100, 0.1% sodium dodecyl sulfate (SDS), 1% Sodium deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF)) for 30 min at 4°C. After centrifugation, the supernatants were incubated at 100°C for 10 min with 6× SDS loading buffer to denature proteins. Then, samples were loaded in a 10% SDS‐polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked in 5% milk or BSA for 1 h and incubated with primary antibody (1:1000) at 4°C with gentle shaking overnight, followed by the incubation with HRP‐conjugated secondary antibody (1:3000). Lastly, the blots were visualized using an enhanced chemiluminescence (ECL) kit (keyGEN Biotech, Nanjing, China).
Cell viability assay
To determine the inhibitory action of deguelin on cell growth, the MCF‐7 (5 × 103/well), MDA‐MB‐231 (8 × 103/well) and Hs 578Bst (8 × 103/well) cell lines were plated in 96‐well plates. The following day, cells were treated with various concentrations of deguelin and further incubated for 24, 48, and 72 h. Control groups received 0.1% DMSO. The proportion of viable cells was determined by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay: MTT (Sigma) (5 mg/mL in phosphate‐buffered saline) was added to each well before culturing for 4 h at 37°C, then MTT was removed and replaced by 150 μL DMSO. Absorbance was examined by an iMark Microplate Absorbance Reader (Bio‐Rad, 168‐1130EDU, Hercules, California, USA).
Clonogenic assays
The colony formation assay was used to estimate cellular sensitivity. After culturing with or without deguelin for 48 h, cells were reseeded at a density of 1000 cells/well in six‐well plates and grow for 14 days. Cell clones were fixed with methanol and stained with 0.05% crystal violet (Santa Cruz Biotechnology, Guangzhou, China). Finally, colonies >0.2 mm in diameter were counted.
Hoechst staining
Apoptosis was further determined by nucleus morphology using Hoechst 33258 cell apoptosis staining kit (Beyotime, Jiangsu, China). MCF‐7, MDA‐MB‐231, and Hs 578Bst were cultured in six‐well plates to 70% confluence, and then treated with various concentrations of deguelin for 48 h. After being fixed and stained with Hoechst 33258, the cells were examined under a Zeiss AxioImager A1 fluorescent microscope (Carl Zeiss).
Transfection
MCF‐7 cells were transfected with empty mammalian expression vector pEGFP‐C3 (EV) or the same plasmid containing the FGFR4 gene using Lipofectamine 2000 (Invitrogen, Guangzhou, China). Next day, the transfection efficiency was detected using the Enhanced Green Fluorescent Protein (EGFP) in the vector, and then, the cells were treated with deguelin for 2 days after transfection, followed by various assays which measured proliferating and apoptotic cells as well as the protein levels of related genes.
Injection in zebrafish
Mammalian expression plasmid pEGFP‐C3 containing human or zebrafish FGFR4 gene were injected into one‐cell period zebrafish embryos. The embryos were treated with deguelin from 24 hpf for 6 h and fixed in 4% paraformaldehyde for the following experiments: PH3 proliferation assay, TUNEL assay, and western blot analysis.
Statistical analysis
Three independent experiments of each assay were performed to validate the results. Data from each experiment were analyzed using Student's t‐test and displayed as the mean ± standard deviation. A repeated‐measures analysis of variance (ANOVA) model was used for identifying the significant difference among different time points (24, 48, 72 h) after deguelin treatment in cell viability test. SPSS 23 (IBM, Chicago, Illinois, USA) was used to perform the analysis. P values <0.05 were regarded as statistically significant.
Results
Deguelin treatment leads to growth retardation and induces apoptosis in zebrafish
We first examined the effects of deguelin treatment in vivo using zebrafish embryos. We found that deguelin blocked the growth of zebrafish embryos. Growth stalled at 21‐somite stage after 200 nmol/L deguelin treatment and stopped at the six‐somite stage with 500 nmol/L deguelin treatment (Fig. 1A). We further examined these embryos for cell proliferation and apoptosis. Phospho‐histone H3 antibody labeling was performed to detect proliferating cells. PH3 labeling indicated that cell proliferation is significantly decreased after a 6‐h exposure upon 100 nmol/L deguelin and completely suppressed with 200 nmol/L deguelin treatment (Fig. 1B). In TUNEL assay, the global rate of apoptosis increased in a dose‐dependent manner. Specifically, the TUNEL‐positive cells increased slightly at low deguelin concentration and rose dramatically at 200 nmol/L (Fig. 1C).
Figure 1.

Growth repression and apoptosis induction caused by deguelin. (A) Morphological change in zebrafish with or without deguelin treatment. Significant growth retardation can be found in 200 and 500 nmol/L deguelin‐treated group. (B) Whole‐mount embryos labeled with anti‐pH3 antibody to examine proliferating cells in zebrafish larvae. The numbers of pH3‐positive cells decreased dramatically and rarely expressed with 200 nmol/L deguelin treatment (magnification 50×). (C) Phenotypic assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. There was a dose‐dependent increase of apoptotic cells in TUNEL assay. (magnification 50×).
Microarray expression profile in deguelin‐treated zebrafish embryos
To identify the molecular basis of deguelin in zebrafish embryos. We explored dysregulated gene expression after deguelin treatment by microarray analysis. We noticed the substantial down‐regulation of FGFR4 in microarray data (Fig. 2). As the down‐regulated effects of deguelin on p‐AKT and p‐ERK levels are well established and FGFRs are showed widely in activating the PI3K/AKT/MAPK pathway, we supposed FGFR4 as the potential upstream target of deguelin.
Figure 2.
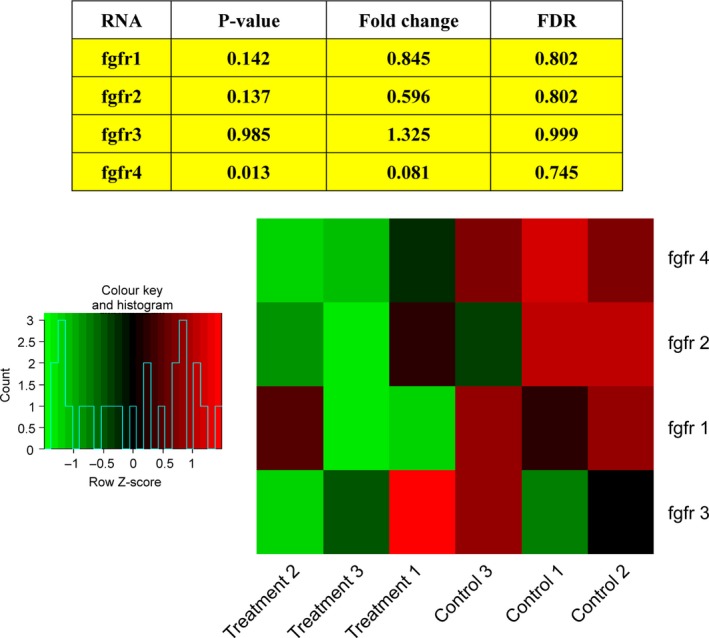
Microarray analysis. Fibroblast growth factor receptor 4 (FGFR4) is substantially down‐regulated after deguelin treatment.
Deguelin treatment significantly inhibits the expression of FGFR4 and the PI3K/AKT/MAPK pathway in zebrafish embryos
To validate and further quantify the expression of FGFR4, FGFR4 levels were profiled by real‐time RT‐PCR analysis and immunoblot (Fig. 3). We confirmed that deguelin treatment caused a dose‐dependent reduction of FGFR4 at mRNA level. Moreover, FGFR4 protein was decreased in both 200 and 500 nmol/L deguelin‐treated groups. As a positive control, an obvious reduction of FGFR4 protein was showed after SU5402 treatment. We also checked the expression levels of downstream signaling components and found that the protein levels of p‐AKT and p‐ERK were also reduced in a dose‐dependent manner. However, there is no obvious effect on the total content of ERK.
Figure 3.
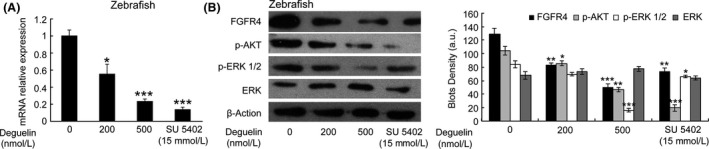
Reduced levels of FGFR4 and related downstream genes induced by deguelin. (A) Real‐time reverse transcription‐PCR for FGFR4 was conducted to examine FGFR4 mRNA expression. Deguelin dose‐dependently suppressed FGFR4 release, which was validated by positive control group. Three individual experiments were conducted. Each bar indicates the mean ± SD. *P < 0.05; **P < 0.01;***P < 0.001 (t‐test). Each drug‐treated group was compared to respective control group with DMSO treatment to decide its significance. FGFR inhibitor SU5402 was used as a positive control. (B) Immunoblot was performed with specialized antibodies for FGFR4, p‐AKT, p‐ERK, and ERK to detect the protein levels. The results showed that deguelin inhibited FGFR4 expression and constitutive phosphorylation of AKT and ERK in zebrafish. FGFR inhibitor SU5402 was used as a positive control. Bar graph, Density analysis results from each concentration in western Blot. a.u. represent arbitrary units. *P < 0.05; **P < 0.01;***P < 0.001 (t‐test). Each drug‐treated group was compared to control group with DMSO treatment to decide its significance. FGFR4, fibroblast growth factor receptor 4; PCR, polymerase chain reaction; p‐AKT, phospho‐protein kinase B; p‐ERK, phospho‐extracellular regulated protein kinases; DMSO, dimethyl sulfoxide.
Based on these results, we suggest that the deguelin‐induced apoptosis in zebrafish is mediated by the down‐regulation of FGFR4, which leads to the reduction in p‐AKT and p‐ERK levels.
The antiproliferative effects of deguelin on breast cancer cells but not on Hs 578Bst cells
To investigate whether the mechanism of deguelin is generally conserved in zebrafish, we first performed MTT to examine cell viability of hormone‐responsive (MCF‐7) and ‐unresponsive (MDA‐MB‐231) human breast cancer cell lines after deguelin treatment. The proliferation of both cell lines showed a significant decrease, which in a dose‐and time‐dependent manner. Particularly, after 72 h of deguelin treatment, we observed ~10–30% growth inhibition at 100 nmol/L and ~50–70% growth inhibition at 500 nmol/L deguelin‐treated groups (Fig. 4A). The IC50 values are 278.4 and 633.9 nmol/L for MCF‐7 and MDA‐MB‐231 after 72 h treatment. To see if the time points (24, 48, 72 h) have statistical significance from each other, a separate repeated‐measures ANOVA was performed among different time periods in three cell lines. The study revealed that MCF‐7 cells have significant interaction in three levels (three time points) even in 10 nmol/L deguelin‐treated group. MDA‐MB‐231 cells also show significance, but began with 100 nmol/L deguelin treatment. However, there is no significant difference showed in Hs 578Bst cells among each time points (Table 1). Besides, we observed that 500 nmol/L deguelin treatment led to decreased colony formation of MCF‐7 and MDA‐MB‐231 cells (Fig. 4B).
Figure 4.
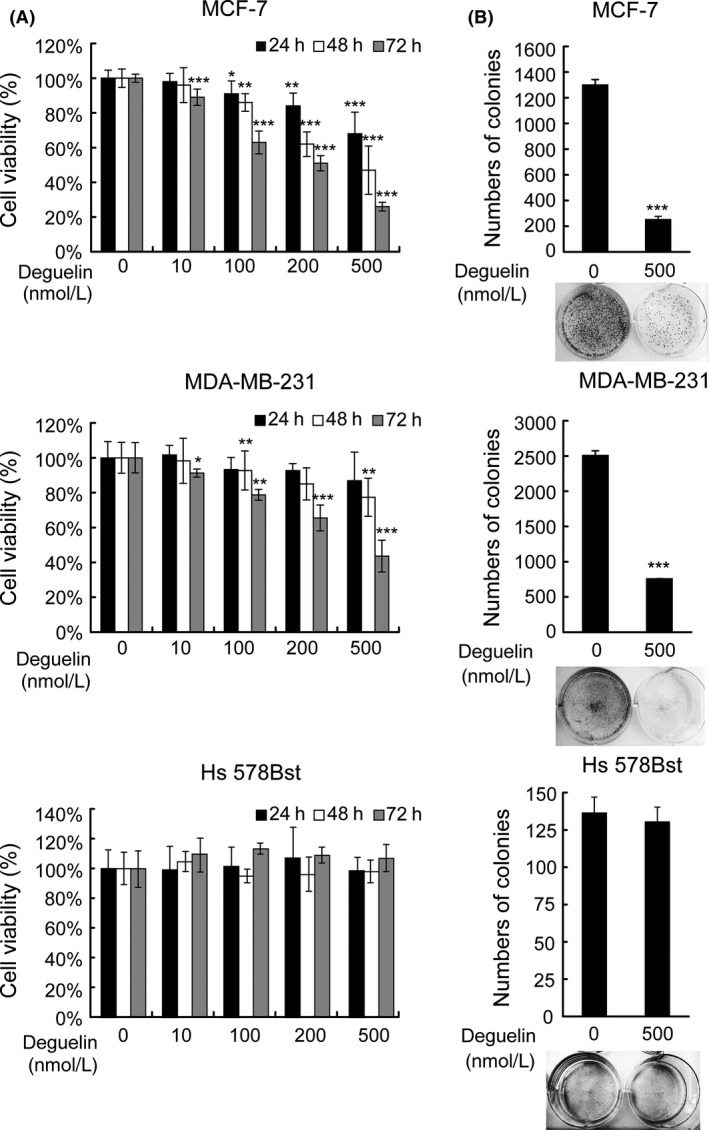
Growth inhibition of MCF‐7 and MDA‐MB‐231 induced by deguelin. (A) MTT assay. MCF‐7, MDA‐MB‐231 and Hs 578Bst were exposed to deguelin at indicated concentrations (0–500 nmol/L) for 24, 48, 72 h. Cell viability was measured by a spectrophotometer. Each spot indicates the mean ± SD of 6 samples. *P < 0.05; **P < 0.01;***P < 0.001 (t‐test). Each drug‐treated group was compared to respective control group with DMSO treatment to decide its significance. (B) colony formation assay. The breast cancer cells were treated in the presence or absence of deguelin for 14 days, cell colonies were labeled by crystal violet and counted in Image J. Each bar indicates the mean ± SD of 3 samples. *P < 0.05; **P < 0.01;***P < 0.001 (t‐test). Each drug‐treated group was compared to respective control group with DMSO treatment to decide its significance. MTT, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide; DMSO, dimethyl sulfoxide.
Table 1.
Significant difference among 24, 48, and 72 h after treatment (repeated‐measures ANOVA)
| Cell lines | Concentration (nmol/L) | Type III sum of squares | df | Mean square | F value | P value |
|---|---|---|---|---|---|---|
| MCF‐7 | 10 | 0.026 | 2 | 0.013 | 4.755 | 0.035 |
| 100 | 0.279 | 2 | 0.140 | 29.314 | 0.000 | |
| 200 | 0.338 | 2 | 0.169 | 56.770 | 0.000 | |
| 500 | 0.546 | 2 | 0.273 | 76.405 | 0.000 | |
| MDA‐MB‐231 | 10 | 0.033 | 2 | 0.016 | 2.401 | 0.141 |
| 100 | 0.078 | 2 | 0.039 | 4.566 | 0.039 | |
| 200 | 0.244 | 2 | 0.122 | 15.192 | 0.001 | |
| 500 | 0.629 | 2 | 0.314 | 31.033 | 0.000 | |
| Hs 578Bst | 10 | 0.046 | 2 | 0.023 | 0.993 | 0.404 |
| 100 | 0.113 | 2 | 0.056 | 2.728 | 0.113 | |
| 200 | 0.070 | 2 | 0.035 | 2.488 | 0.133 | |
| 500 | 0.037 | 2 | 0.019 | 0.947 | 0.420 |
Based on MTT analysis, MCF‐7, MDA‐MB‐231 and Hs 578Bst were exposed to deguelin at indicated concentrations (0–500 nmol/L) for 24, 48, 72 h. Cell viability was measured by a spectrophotometer. A repeated‐measures ANOVA was used to see if the time points are significantly different to each other in three cell lines. As the P values in Mauchly's Test of Sphericity are all more than 0.05, sphericity has not been violated. The results in “sphericity assumed” in SPSS were presented in the table. ANOVA, analysis of variance; MTT, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide.
*P < 0.05; **P < 0.01; ***P < 0.001.
Hs 578Bst cells, which are derived from normal human mammary epithelial, were used to test the toxic effect of deguelin. Strikingly, treatment within 500 nmol/L deguelin had only marginal effects on cells survival (Fig. 4A). Likewise, colony formation ability of Hs 578Bst cells was not affected by deguelin treatment (Fig. 4B). These data demonstrate that deguelin selectively inhibits the viability of breast cancer cells, but not normal cells within 500 nmol/L.
Deguelin induces apoptosis in MCF‐7 and MDA‐MB‐231 but not Hs 578Bst cells
To address whether the growth inhibitory effects of deguelin are accompanied by the induction of apoptosis, in situ cell death detection and Hoechst 33258 staining were used to detect apoptosis. Both methods confirmed that deguelin induces apoptosis in MCF‐7 and MDA‐MB‐231 cell lines (Fig. 5A). In TUNEL assays, A dose‐dependent increase in apoptotic cells were observed in deguelin‐treated group, especially with 500 nmol/L deguelin treatment. In Hoechst 33258 assay, the control group showed cobblestone monolayer appearances and homogeneous color, whereas clear apoptotic morphology was observed in deguelin‐treated groups in both breast cancer cells. Specifically, MCF‐7 had hypercondensed chromatin while MDA‐MB‐231 showed apoptotic bodies and abnormal nuclear morphology after 500 nmol/L deguelin treatment.
Figure 5.
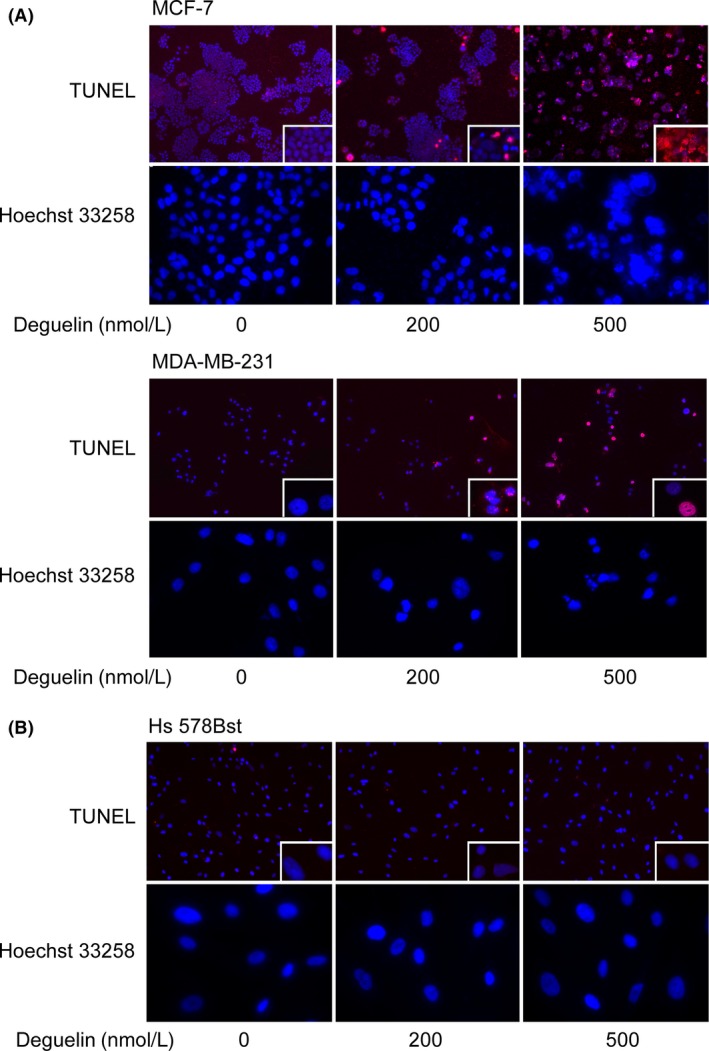
Induction of apoptosis by deguelin in MCF‐7 and MDA‐MB‐231. (A) Deguelin induced a dose‐dependent apoptosis in breast cancer cells determined by in situ cell death detection and Hoechst 33258 staining. In upper pictures of each group, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)‐positive cells increased with rising concentrations of deguelin treatment (magnification 100×). Pictures in the bottom right‐hand corner of each TUNEL image were amplified to show the morphology more clearly (magnification 400×). Likewise, all kinds of cells with the same treatment of deguelin were labeled with Hoechst 33258, condensed chromatin and apoptotic nucleus fragmentations were observed in breast cancer cells. (B) Deguelin exerted marginal toxic influence on normal cells Hs 578Bst. TUNEL and Hoechst 33258 assays were applied to Hs 578Bst as indicated, the morphology of Hs 578Bst cells were as normal as the control group after deguelin treatment. Three independent experiments were made to validate the result.
We next explored whether Hs 578Bst cells underwent apoptosis as well (Fig. 5B). In contrast to cancer cell lines, Hs 578Bst cells only showed a baseline level of apoptosis in all groups (Fig. 5B). Taken together, our results suggest that deguelin dramatically induces apoptosis in breast cancer cell lines without toxic effects on Hs 578Bst normal cells.
Deguelin treatment significantly inhibits the expression of FGFR4 and the PI3K/AKT/MAPK pathway in breast cancer cells
To determine if the mechanisms of deguelin in zebrafish are the same in breast cancer cell lines, we performed real‐time RT‐PCR and immunoblot to check mRNAs and protein level of FGFR4.
We found that deguelin treatment down‐regulates FGFR4 mRNA expression in a dose‐dependent manner in MCF‐7 and MDA‐MB‐231 cells (Fig. 6A). Even though no obvious effects on FGFR4 protein expression was observed in any cell lines with 100 nmol/L deguelin treatment (Fig. 6B), 500 nmol/L deguelin significantly decreased FGFR4 protein in MCF‐7. MDA‐MB‐231 cells express low levels of FGFR4, but a detectable change can also be observed after deguelin treatment. As the role of PI3K/AKT/MAPK pathway in deguelin treatment is crucial, we verified some protein levels in these two pathways as well. As expected, a dose‐dependent reduction in p‐AKT and p‐ERK levels were observed, but marginal effect on the content of ERK in both cell lines. Taken together, these data suggest that deguelin has a similar mechanism in breast‐cancer cell lines as it does in zebrafish.
Figure 6.
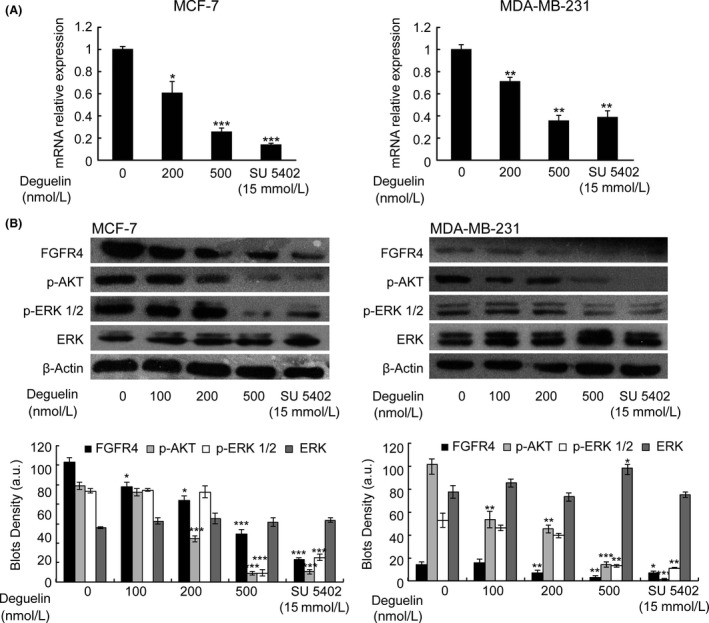
The reduced expression of FGFR4 in breast cancer cells after the treatment of deguelin. (A) Real‐time reverse transcription–PCR for FGFR4 expression in two breast cancer cells. After deguelin treatment for 48 h, the RNA was extracted and detected by real‐time RT‐PCR. FGFR4 expression gradually decreased in a dose‐dependent manner. Three individual experiments were conducted. Each bar indicates the mean ± SD. *P < 0.05; **P < 0.01;***P < 0.001 (t‐test). Each drug‐treated group was compared to respective control group with DMSO treatment to decide its significance. (B) Western blot analysis on FGFR4 and related gene protein expression in MCF‐7 and MDA‐MB‐231. The cells cultured with 0–500 nmol/L deguelin for indicated time witnessed a declined expression of FGFR4, p‐AKT, and p‐ERK without effects on total ERK expression, which was validated by the positive‐control group (SU5402). Bar graph, Density analysis results from each concentration in western Blot. a.u. represent arbitrary units. *P < 0.05; **P < 0.01;***P < 0.001 (t‐test). Each drug‐treated group was compared to control group with DMSO treatment to decide its significance. FGFR4, fibroblast growth factor receptor 4; PCR, polymerase chain reaction; DMSO, dimethyl sulfoxide.
FGFR4 is one of the pivotal elements responsible for deguelin‐induced antiproliferative and proapoptotic effect in breast cancer cells through PI3K/AKT/MAPK pathway
If FGFR4 truly is the central signaling molecule for deguelin to induce apoptosis, one would predict that artificially increasing FGFR4 expression would counteract the effects of deguelin treatment. To test this hypothesis, we evaluated the effects of deguelin on FGFR4‐overexpressed zebrafish embryos and MCF‐7 cancer cells. The injection of human and zebrafish FGFR4 into zebrafish embryos partially rescued effect of deguelin on proliferation and apoptotic cell death (Fig. 7). Similarly, MTT assay demonstrated that transfection of human FGFR4 into MCF‐7 cells attenuated the antiproliferative effect of deguelin (Fig. 8A). We also confirmed that overexpression of FGFR4 blocked deguelin‐induced apoptosis in MCF‐7 cells by TUNEL and Hoechst 33258 staining assays (Fig. 8B). Moreover, protein immunoblot of both zebrafish embryos and MCF‐7 cells revealed that up‐regulation of FGFR4 restored p‐AKT and p‐ERK levels (Fig. 9). Overall, these results illustrate that deguelin exerts antiproliferative and proapoptotic effects in zebrafish and breast cancer cells, at least in part, by down‐regulating FGFR4 signaling.
Figure 7.
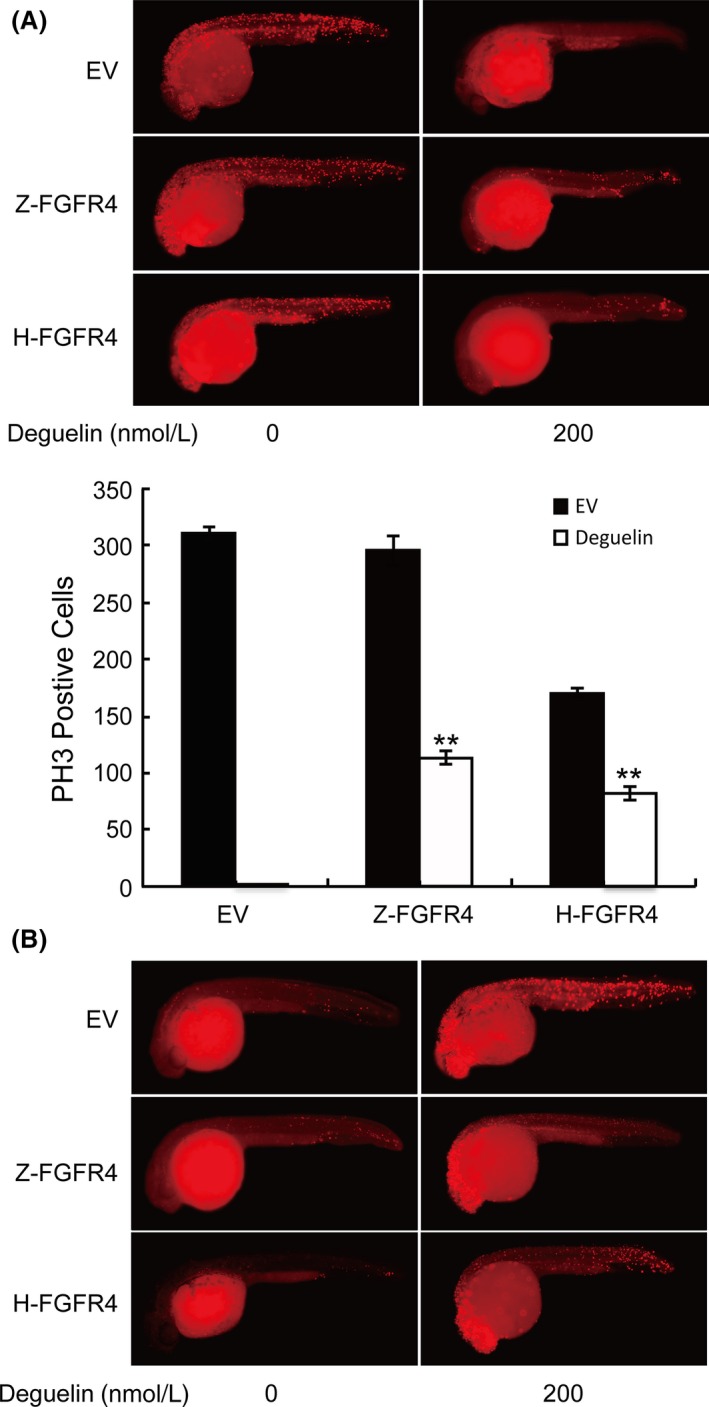
The counteractant effect of overexpressing FGFR4 in zebrafish after deguelin treatment. (A) Zebrafish embryos were labeled with anti‐pH3 antibody after injection of pEGFP‐C3 containing FGFR4 (Z‐FGFR4 and H‐FGFR4 stand for zebrafish and human FGFR4, respectively) to detect proliferating cells. DyLight 594 secondary antibody was used to avoid green fluorescence emitted by pEGFP‐C3 vector. Up‐regulation of FGFR4 has partly restored proliferating cells compared with the control group. PH3‐positive cells are counted in Image J. **P < 0.01 (t‐test) (B). TUNEL assay was conducted to analyze the apoptosis after the injection of FGFR4. Apoptotic cells were reduced in the trunk areas in injected groups. FGFR4, fibroblast growth factor receptor 4; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Figure 8.
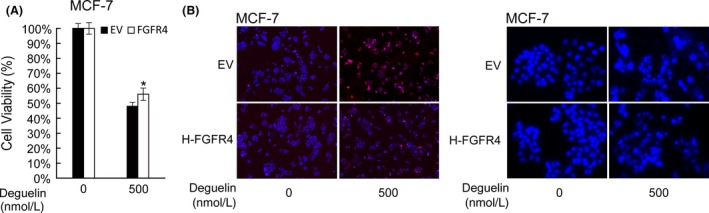
The counteractant effect of overexpressing FGFR4 in MCF‐7 cells after deguelin treatment. (A) MTT assay. MCF‐7 cancer cells transfected of pEGFP‐C3 with or without FGFR4 were treated with deguelin for 2 days. Transfected group showed better cell viability. Each bar indicates the mean ± SD of 6 samples. *P < 0.05, t‐test. (B) In situ cell death detection and Hoechst 33258 staining were performed to detect apoptotic cells. The apoptosis were dramatically decreased in the transfected group. FGFR4, fibroblast growth factor receptor 4; MTT, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide.
Figure 9.
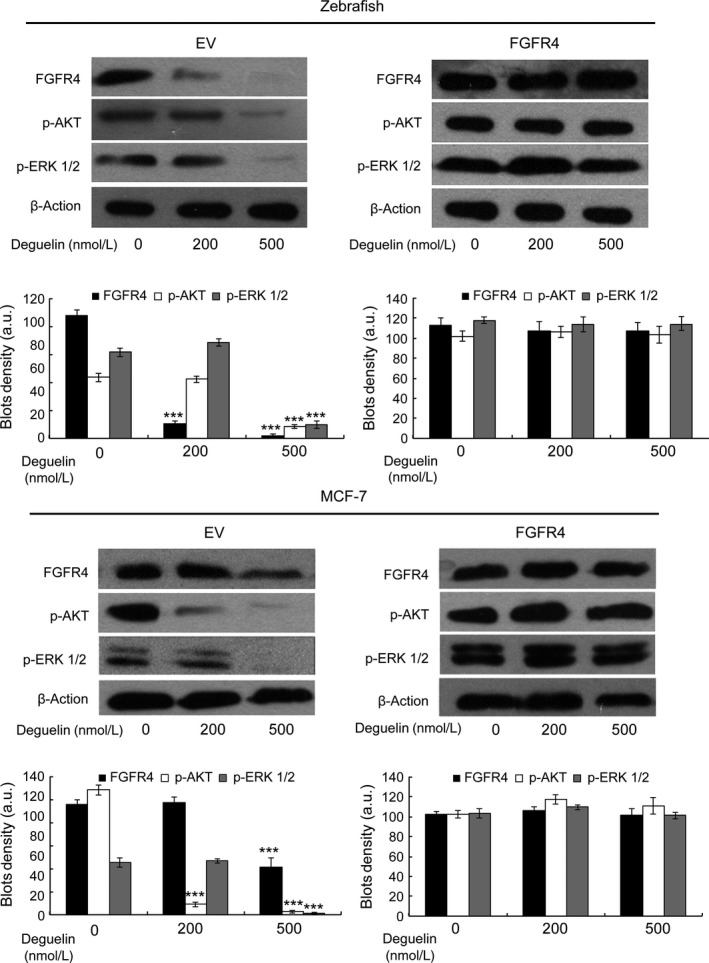
The effect of FGFR4 overexpression on PI3K/AKT/MAPK pathway. The injection in zebrafish embryos or transfected in MCF‐7 cells with human FGFR4 could regain the expression levels of p‐AKT and p‐ERK. Bar graph, Density analysis results from each concentration in western Blot. a.u. represent arbitrary units. ***P < 0.001 (t‐test). Each drug‐treated group was compared to control group with DMSO treatment to decide its significance. FGFR4, fibroblast growth factor receptor 4; PI3K, phosphoinositide 3‐kinase; AKT, protein kinase B; MAPK, mitogen‐activated protein kinases; DMSO, dimethyl sulfoxide.
Discussion
We observed obvious effects of deguelin on the morphology, proliferation, and apoptosis of zebrafish embryos. Our finding showed that this is partly due to the inhibition of FGFR4 expression, which leads to the decreases in p‐AKT and p‐ERK levels.
FGFR4 is known to be elevated in multiple forms of cancer. The FGFR4 gene was shown to be amplified in 30 primary breast tumor samples and several other gynecological cancers (Jaakkola et al. 1993). More recently, increased levels of FGFR4 signaling have been found in many human prostate cancers (Wang et al. 2008; Xu et al. 2011). Due to the long‐lasting recognition of a correlation between breast cancer cells and FGFR4, we decided to verify whether the mechanism of deguelin in zebrafish is applicable to breast cancer cells. As we expected, deguelin has shown to suppress breast cancer cells growth and induce apoptosis through inhibiting FGFR4 expression, resulting in reduced activity of the PI3K/AKT and MAPK/ERK pathways. Furthermore, western blot showed an up‐regulation of p‐AKT and p‐ERK with artificially increasing FGFR4 expression in both zebrafish and breast cancer cells.
Consistent with our observations, TKI258, an FGFR tyrosine kinase inhibitor, induced mammary tumor cell apoptosis by reducing the levels of p‐AKT, p‐ERK, and phospholipase Cγ (Dey et al. 2010). Furthermore, BGJ‐398, another FGFR inhibitor, decreased the levels of p‐ERK and p‐AKT and blocked liposarcoma cell proliferation (Zhang et al. 2013). Likewise, the same mechanism is observed in rhabdomyosarcomas cells, where decreases levels of p‐AKT and p‐ERK were observed after the mutation of FGFR4 gene (Leung et al. 1994; Taylor et al. 2009). Additionally, FGFR4 knockout mice do not seem to form liver tumors (French et al. 2012).
While the link between reduction in FGFR4 expression and decreases in p‐AKT and p‐ERK levels is well established, the correlation between the inhibition of FGFR4 expression, decreased proliferation and induction of apoptosis seems more controversial. Actually, inhibiting FGFR activity exquisitely suppressed HuH7 (high FGFR4 expression) proliferation (Ho et al. 2009). FGF19 increased hepatocyte proliferation and induced hepatocellular carcinoma formation by activating FGFR4 in transgenic mice (Wu et al. 2010). Recently, FGFR4 silencing lead to a great reduction of proliferation and an enhancement of apoptosis in ovarian cancer cells (Zaid et al. 2013). By injecting zebrafish and human FGFR4 into zebrafish, we verified that overexpression of FGFR4 partially rescued the delayed growth and increased apoptosis in zebrafish embryos after deguelin treatment. Likewise, transfection of FGFR4 into breast cancer cells was able to enhance proliferation as well as resistance to apoptosis. Overall, these data indicate that deguelin suppresses cell proliferation and induces apoptosis by down‐regulating FGFR4 levels, resulting in a down‐regulation of p‐AKT and p‐ERK levels.
Breast cancer is considered as a heterogeneous disease, since diverse types of tumors exist at the molecular level. The majority of breast cancers, which express estrogen receptor, progesterone receptor (Bange et al.), and/or human epidermal growth factor receptor 2, are generally hormone treatment sensitive. Approximately, 15–20% breast carcinomas lack these receptors and are thus referred as triple‐negative breast cancer. The latter has poor prognostics due to the absence of effective treatment targets (Brouckaert et al. 2012; Griffiths and Olin 2012). In our study, we confirmed that deguelin is capable of suppressing the proliferation of both MCF‐7 and MDA‐MB‐231 cell lines (triple‐positive and triple‐negative mammary cancer cell lines, respectively). Furthermore, we have verified, to the best of our knowledge for the first time, FGFR4 as potential target for both types of breast cancer treatment.
Deregulated FGFR4 activity is generally considered oncogenic, as excessive FGFR4 signaling is found in dozens of carcinomas (Haugsten et al. 2010). Because of the significant effect of deguelin on zebrafish, we found FGFR4 as the potential cancer therapeutic target for breast cancer. Even though small‐molecule FGFR4 inhibitors have been developed to attenuate FGFR4 signaling (Dey et al. 2010; Zhang et al. 2013), the effect of selective inhibitor of FGFR4 is limited toward clinical treatment. To date, only one research reporting BLU9931, a small‐molecule inhibitor of FGFR4, have successfully inhibit FGFR4 activity in vitro and suppress tumor growth in hepatocellular carcinomas xenograft mice model (Hagel et al. 2015). In this study, we demonstrated that deguelin, a naturally occurring rotenoid, is capable of suppressing growth and inducing apoptosis in rapidly proliferating zebrafish embryos as well as breast cancer cells, but not in Hs 578Bst cells, by down‐regulating FGFR4 levels, which suggest that aberrant FGFR4 signaling molecules may be a central component in mastocarcinoma pathogenesis, affecting patient prognosis.
Conclusion
FGFR4 is a highly conserved tyrosine kinase receptor, which is able to regulate proliferation, differentiation, and survival. FGFR4 overexpression enhances the proliferation and survival in both breast cancer cells and zebrafish, suggesting that FGFR4 could be a new therapeutic target in breast cancer. The role of FGFR4 signaling is worth further investigating in cancer development.
Authorship Contributions
Participated in research design: Wei Wu, Lu Chen, Yu‐Xiang Han, Xin‐Rong Wu. Conducted experiments: Wei Wu, Yang Hai, Rui‐Jin Liu, Wen‐Hao Li. Contributed new reagents or analytic tools: Song Li, Shuo Lin, Xin‐Rong Wu. Performed data analysis: Wei Wu, Yang Hai. Wrote or contributed to the writing of the manuscript: Wei Wu, Shuo Lin, Xin‐Rong Wu.
Disclosures
None declared.
Wu W., Hai Y., Chen L., Liu R.‐J., Han Y.‐X., Li W.‐H., Li S., Lin S., Wu X.‐R., Deguelin induced blockade of PI3K/protein kinase B/MAP kinase signaling in zebrafish and breast cancer cell lines is mediated by down‐regulation of fibroblast growth factor receptor 4 activity, Pharma Res Per, 4(2), 2016, e00212, doi: 10.1002/prp2.212
References
- Agazie YM, Movilla N, Ischenko I, Hayman MJ (2003). The phosphotyrosine phosphatase SHP2 is a critical mediator of transformation induced by the oncogenic fibroblast growth factor receptor 3. Oncogene 22: 6909–6918. [DOI] [PubMed] [Google Scholar]
- Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, Schmitt M, et al. (2002). Cancer progression and tumor cell motility are associated with the FGFR4 Arg(388) allele. Cancer Res 62: 840–847. [PubMed] [Google Scholar]
- Becker D, Lee PL, Rodeck U, Herlyn M (1992). Inhibition of the fibroblast growth factor receptor 1 (FGFR‐1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene 7: 2303–2313. [PubMed] [Google Scholar]
- Boreddy SR, Srivastava SK (2013). Deguelin suppresses pancreatic tumor growth and metastasis by inhibiting epithelial‐to‐mesenchymal transition in an orthotopic model. Oncogene 32: 3980–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortul R, Tazzari PL, Billi AM, Tabellini G, Mantovani I, Cappellini A, et al. (2005). Deguelin, A PI3K/AKT inhibitor, enhances chemosensitivity of leukaemia cells with an active PI3K/AKT pathway. Br J Haematol 129: 677–686. [DOI] [PubMed] [Google Scholar]
- Brouckaert O, Wildiers H, Floris G, Neven P (2012). Update on triple‐negative breast cancer: prognosis and management strategies. Int J Womens Health 4: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DJ, An H, Kim KS, Kim HH, Jung J, Lee JM, et al. (2012). Design, synthesis, and biological evaluation of novel deguelin‐based heat shock protein 90 (HSP90) inhibitors targeting proliferation and angiogenesis. J Med Chem 55: 10863–10884. [DOI] [PubMed] [Google Scholar]
- Chun KH, Kosmeder JW II, Sun S, Pezzuto JM, Lotan R, Hong WK, et al. (2003). Effects of deguelin on the phosphatidylinositol 3‐kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst 95: 291–302. [DOI] [PubMed] [Google Scholar]
- Dell'Eva R, Ambrosini C, Minghelli S, Noonan DM, Albini A, Ferrari N (2007). The Akt inhibitor deguelin, is an angiopreventive agent also acting on the NF‐kappaB pathway. Carcinogenesis 28: 404–413. [DOI] [PubMed] [Google Scholar]
- Dey JH, Bianchi F, Voshol J, Bonenfant D, Oakeley EJ, Hynes NE (2010). Targeting fibroblast growth factor receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs mammary tumor outgrowth and metastasis. Cancer Res 70: 4151–4162. [DOI] [PubMed] [Google Scholar]
- Fang N, Casida JE (1999). Cube resin insecticide: identification and biological activity of 29 rotenoid constituents. J Agric Food Chem 47: 2130–2136. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Serraino D, Rezza G, Lence J, Ortiz RM, Cruz T, et al. (2002). Infection with human herpesvirus type 8 and human T‐cell leukaemia virus type 1 among individuals participating in a case‐control study in Havana City, Cuba. Br J Cancer 87: 1253–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French DM, Lin BC, Wang M, Adams C, Shek T, Hotzel K, et al. (2012). Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One 7: e36713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraerts B, Vanhoecke B, Vanden Berghe W, Philippe J, Offner F, Deforce D (2007). Deguelin inhibits expression of IkappaBalpha protein and induces apoptosis of B‐CLL cells in vitro. Leukemia 21: 1610–1618. [DOI] [PubMed] [Google Scholar]
- Griffiths CL, Olin JL (2012). Triple negative breast cancer: a brief review of its characteristics and treatment options. J Pharm Pract 25: 319–323. [DOI] [PubMed] [Google Scholar]
- Hagel M, Miduturu C, Sheets M, Rubin N, Weng W, Stransky N, et al. (2015). First selective small molecule inhibitor of FGFR4 for the treatment of hepatocellular carcinomas with an activated FGFR4 signaling pathway. Cancer Discov 5: 424–437. [DOI] [PubMed] [Google Scholar]
- Haugsten EM, Wiedlocha A, Olsnes S, Wesche J (2010). Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res 8: 1439–1452. [DOI] [PubMed] [Google Scholar]
- Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, et al. (2009). Fibroblast growth factor receptor 4 regulates proliferation, anti‐apoptosis and alpha‐fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol 50: 118–127. [DOI] [PubMed] [Google Scholar]
- Hu J, Ye H, Fu A, Chen X, Wang Y, Chen X, et al. (2010). Deguelin–an inhibitor to tumor lymphangiogenesis and lymphatic metastasis by downregulation of vascular endothelial cell growth factor‐D in lung tumor model. Int J Cancer 127: 2455–2466. [DOI] [PubMed] [Google Scholar]
- Ito S, Oyake T, Murai K, Ishida Y (2010). Deguelin suppresses cell proliferation via the inhibition of survivin expression and STAT3 phosphorylation in HTLV‐1‐transformed T cells. Leuk Res 34: 352–357. [DOI] [PubMed] [Google Scholar]
- Jaakkola S, Salmikangas P, Nylund S, Partanen J, Armstrong E, Pyrhonen S, et al. (1993). Amplification of fgfr4 gene in human breast and gynecological cancers. Int J Cancer 54: 378–382. [DOI] [PubMed] [Google Scholar]
- Jin Q, Feng L, Behrens C, Bekele BN, Wistuba II, Hong WK, et al. (2007). Implication of AMP‐activated protein kinase and Akt‐regulated survivin in lung cancer chemopreventive activities of deguelin. Cancer Res 67: 11630–11639. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M (2006). FGF signaling network in the gastrointestinal tract (review). Int J Oncol 29: 163–168. [PubMed] [Google Scholar]
- Katoh M, Nakagama H (2014). FGF receptors: cancer biology and therapeutics. Med Res Rev 34: 280–300. [DOI] [PubMed] [Google Scholar]
- Kim WY, Oh SH, Woo JK, Hong WK, Lee HY (2009). Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation‐induced stabilization of hypoxia‐inducible factor‐1alpha. Cancer Res 69: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziczak M, Holbro T, Hynes NE (2004). Blocking of FGFR signaling inhibits breast cancer cell proliferation through downregulation of D‐type cyclins. Oncogene 23: 3501–3508. [DOI] [PubMed] [Google Scholar]
- Lagha M, Kormish JD, Rocancourt D, Manceau M, Epstein JA, Zaret KS, et al. (2008). Pax3 regulation of FGF signaling affects the progression of embryonic progenitor cells into the myogenic program. Genes Dev 22: 1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY (2004). Molecular mechanisms of deguelin‐induced apoptosis in transformed human bronchial epithelial cells. Biochem Pharmacol 68: 1119–1124. [DOI] [PubMed] [Google Scholar]
- Lee HY, Suh YA, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM (2004). Deguelin‐induced inhibition of cyclooxygenase‐2 expression in human bronchial epithelial cells. Clin Cancer Res 10: 1074–1079. [DOI] [PubMed] [Google Scholar]
- Leung HY, Gullick WJ, Lemoine NR (1994). Expression and functional activity of fibroblast growth factors and their receptors in human pancreatic cancer. Int J Cancer 59: 667–675. [DOI] [PubMed] [Google Scholar]
- Liu XM, Yang FF, Yuan YF, Zhai R, Huo LJ (2013). SUMOylation of mouse p53b by SUMO‐1 promotes its pro‐apoptotic function in ovarian granulosa cells. PLoS One 8: e63680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Major RJ, Poss KD (2007). Zebrafish heart regeneration as a model for cardiac tissue repair. Drug Discov Today Dis Models 4: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Katta H, Alimirah F, Patel R, Murillo G, Peng X, et al. (2013a). Deguelin action involves c‐Met and EGFR signaling pathways in triple negative breast cancer cells. PLoS One 8: e65113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RR, Katta H, Kalra A, Patel R, Gupta A, Alimirah F, et al. (2013b). Efficacy and mechanism of action of deguelin in suppressing metastasis of 4T1 cells. Clin Exp Metastasis 30: 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mione MC, Trede NS (2010). The zebrafish as a model for cancer. Dis Model Mech 3: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo G, Salti GI, Kosmeder JW II, Pezzuto JM, Mehta RG (2002). Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur J Cancer 38: 2446–2454. [DOI] [PubMed] [Google Scholar]
- Murillo G, Kosmeder JW II, Pezzuto JM, Mehta RG (2003). Deguelin suppresses the formation of carcinogen‐induced aberrant crypt foci in the colon of CF‐1 mice. Int J Cancer 104: 7–11. [DOI] [PubMed] [Google Scholar]
- Nair AS, Shishodia S, Ahn KS, Kunnumakkara AB, Sethi G, Aggarwal BB (2006). Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF‐kappaB‐regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J Immunol 177: 5612–5622. [DOI] [PubMed] [Google Scholar]
- Oh SH, Woo JK, Yazici YD, Myers JN, Kim WY, Jin Q, et al. (2007). Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J Natl Cancer Inst 99: 949–961. [DOI] [PubMed] [Google Scholar]
- Patton EE, Zon LI (2001). The art and design of genetic screens: zebrafish. Nat Rev Genet 2: 956–966. [DOI] [PubMed] [Google Scholar]
- Peng XH, Karna P, O'Regan RM, Liu X, Naithani R, Moriarty RM, et al. (2007). Down‐regulation of inhibitor of apoptosis proteins by deguelin selectively induces apoptosis in breast cancer cells. Mol Pharmacol 71: 101–111. [DOI] [PubMed] [Google Scholar]
- Segev O, Chumakov I, Nevo Z, Givol D, Madar‐Shapiro L, Sheinin Y, et al. (2000). Restrained chondrocyte proliferation and maturation with abnormal growth plate vascularization and ossification in human FGFR‐3(G380R) transgenic mice. Hum Mol Genet 9: 249–258. [DOI] [PubMed] [Google Scholar]
- Suh YA, Kim JH, Sung MA, Boo HJ, Yun HJ, Lee SH, et al. (2013). A novel antitumor activity of deguelin targeting the insulin‐like growth factor (IGF) receptor pathway via up‐regulation of IGF‐binding protein‐3 expression in breast cancer. Cancer Lett 332: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JG VI, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, et al. (2009) Identification of FGFR4‐activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest 119: 3395–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Petti F, Sujka‐Kwok I, Epstein D, Haley JD (2008). Kinase switching in mesenchymal‐like non‐small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis 25: 843–854. [DOI] [PubMed] [Google Scholar]
- Thussbas C, Nahrig J, Streit S, Bange J, Kriner M, Kates R, et al. (2006). FGFR4 Arg388 allele is associated with resistance to adjuvant therapy in primary breast cancer. J Clin Oncol 24: 3747–3755. [DOI] [PubMed] [Google Scholar]
- Turkington RC, Longley DB, Allen WL, Stevenson L, McLaughlin K, Dunne PD, et al. (2014). Fibroblast growth factor receptor 4 (FGFR4): a targetable regulator of drug resistance in colorectal cancer. Cell Death Dis 5: e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R (2010). Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10: 116–129. [DOI] [PubMed] [Google Scholar]
- Wang J, Yu W, Cai Y, Ren C, Ittmann MM (2008). Altered fibroblast growth factor receptor 4 stability promotes prostate cancer progression. Neoplasia 10: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma W, Zheng W (2013). Deguelin, a novel anti‐tumorigenic agent targeting apoptosis, cell cycle arrest and anti‐angiogenesis for cancer chemoprevention. Mol Clin Oncol 1: 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. (2008). Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ge H, Lemon B, Vonderfecht S, Weiszmann J, Hecht R, et al. (2010). FGF19‐induced hepatocyte proliferation is mediated through FGFR4 activation. J Biol Chem 285: 5165–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Tong N, Chen SQ, Hua LX, Wang ZJ, Zhang ZD, et al. (2011). FGFR4 Gly388Arg polymorphism contributes to prostate cancer development and progression: a meta‐analysis of 2618 cases and 2305 controls. BMC Cancer 11: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Wang Y, Tan Q, Lubet RA, You M (2005). Efficacy of deguelin and silibinin on benzo(a)pyrene‐induced lung tumorigenesis in A/J mice. Neoplasia 7: 1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Ji C, Bi ZG, Lu CC, Wang R, Gu B, et al. (2013). Deguelin induces both apoptosis and autophagy in cultured head and neck squamous cell carcinoma cells. PLoS One 8: e54736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid TM, Yeung TL, Thompson MS, Leung CS, Harding T, Co NN, et al. (2013). Identification of FGFR4 as a potential therapeutic target for advanced‐stage, high‐grade serous ovarian cancer. Clin Cancer Res 19: 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Chu K, Wu X, Gao H, Wang J, Yuan YC, et al. (2013). Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high‐grade liposarcoma. Cancer Res 73: 1298–1307. [DOI] [PubMed] [Google Scholar]
- Zhao P, Hoffman EP (2004). Embryonic myogenesis pathways in muscle regeneration. Dev Dyn 229: 380–392. [DOI] [PubMed] [Google Scholar]
- Zhao P, Caretti G, Mitchell S, McKeehan WL, Boskey AL, Pachman LM, et al. (2006). Fgfr4 is required for effective muscle regeneration in vivo. Delineation of a MyoD‐Tead2‐Fgfr4 transcriptional pathway. J Biol Chem 281: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]


