Abstract
The current tests of anxiety in mice and rats used in preclinical research include the elevated plus‐maze (EPM) or zero‐maze (EZM), the light/dark box (LDB), and the open‐field (OF). They are currently very popular, and despite their poor achievements, they continue to exert considerable constraints on the development of novel approaches. Hence, a novel anxiety test needs to be compared with these traditional tests, and assessed against various factors that were identified as a source of their inconsistent and contradictory results. These constraints are very costly, and they are in most cases useless as they originate from flawed methodologies. In the present report, we argue that the EPM or EZM, LDB, and OF do not provide unequivocal measures of anxiety; that there is no evidence of motivation conflict involved in these tests. They can be considered at best, tests of natural preference for unlit and/or enclosed spaces. We also argued that pharmacological validation of a behavioral test is an inappropriate approach; it stems from the confusion of animal models of human behavior with animal models of pathophysiology. A behavioral test is developed to detect not to produce symptoms, and a drug is used to validate an identified physiological target. In order to overcome the major methodological flaws in animal anxiety studies, we proposed an open space anxiety test, a 3D maze, which is described here with highlights of its various advantages over to the traditional tests.
Keywords: 3‐dimensional maze, amphetamine, diazepam, dizocilpine, fluoxetine, habituation, mice, plus‐maze, rats
Abbreviations
- EPM
elevated plus‐maze
- EZM
elevated zero‐maze
- LDB
light/dark box
- OAAI
open arms avoidance index
- OF
open‐field
- POAE
percent open arm entries
- POAT
percent open arm time
- TUA
tests of unconditioned anxiety
Introduction
Tests of unconditioned anxiety (TUA) consist mainly of the elevated plus‐maze (EPM) or zero‐maze (EZM), the light–dark box (LDB) and the open‐field (OF). These tests are all intensively used, particularly the EPM, in the study of the neurobiological basis of anxiety and in screening for novel targets and anxiolytic compounds. These TUA have been subjects of numerous reviews, which highlighted their shortcomings concerning their sensitivity and some aspects of their validity (Belzung and Griebel 2001; Belzung 2001; Crabbe et al. 1999; Cryan and Sweeney 2011; Dawson and Tricklebank 1995; Griebel and Holmes 2013; Hogg 1996; Milner and Crabbe 2008; O'Leary et al. 2013; Rodgers 1997; Rodgers and Dalvi 1997; Treit et al. 2010), followed by various recommendations and protocol improvement proposals (Bailey et al. 2006; Bouwknecht and Paylor 2008; Crawley et al. 1997; Crawley 1999; Kalueff et al. 2007; Sousa et al. 2006; van der Staay and Steckler 2001; Wahlsten et al. 2003; Wahlsten 2001; Würbel 2002). Despite their poor achievements, they remain as popular as ever (Haller and Alicki 2012; Haller et al. 2013; Herzog et al. 2000).
In most reports, there is an implicit assumption that the construct validity of TUA has been achieved with their sensitivity to benzodiazepine drugs, although limited mostly to this class of drugs (Belzung 2001; Griebel and Holmes 2013; Cryan and Sweeney 2011; Haller and Alicki 2012; Rodgers 1997). Inconsistent and conflicting results have been accounted for by differences in mice and rats innate state or trait anxiety (Andreatini and Bacellar 2000; Avgustinovich et al. 2000; Belzung and Griebel 2001; Bourin et al. 2007; Goes et al. 2009, 2015; Griebel et al. 1996) and/or by various test environment factors (Albrechet–Souza et al. 2005; Crabbe et al. 1999; Fonken et al. 2009; Violle et al. 2009; Garcia et al. 2005; Heredia et al. 2012; Abramov et al. 2008; Lewejohann et al. 2006; Chesler et al. 2002; Loss et al. 2015; Ravenelle et al. 2014). However, post hoc research studies appear unable to support these accounts (Goes et al. 2015; Jones and King 2001; Arndt et al. 2009; Augustsson et al. 2003; Becker and Grecksch 1996; Nicholson et al. 2009; Hagenbuch et al. 2006; Cohen et al. 2001; Lewejohann et al. 2006; Pellow et al. 1985; Wolfer et al. 2004). Inconsistent and conflicting results continue to occupy central stage in animal studies of anxiety. Critical analysis remains limited within the constraints of traditional approaches and methodologies. Authors of a novel test and/or methodological approach are unable to publish or secure funding support without the test having been compared with the EPM, and demonstrated positive sensitivity to benzodiazepines and 5‐HT drugs. Sensitivity to differences between strains of rats or mice is considered insufficient. In addition, a novel test needs to be assessed against various factors that were identified as a source of inconsistencies and contradictions in the traditional tests. Hence, a novel test remains viewed as an adaptive strategy, in continuity with the traditional approaches. With the above constraints, it is very difficult for a novel behavioral approach to progress and succeed.
In the present report, we examine some major issues that have been overlooked, or inadvertently misrepresented in various critical assessments of the methodologies currently in use in animal studies of anxiety. We also describe a novel open anxiety test, a 3D maze that we proposed to overcome the flaws and limitations of the current tests. We will argue that (1) the assumption of the presence of a conflict between two opposite motivational drives in the TUA remains to be verified. While the avoidance drive is apparent in these tests, the approach drive has yet to be demonstrated; (2) that a number of methodological validity concepts are incorrectly attributed to behavioral tests; this is mainly due to the lack of distinction between animals models of human behavior and animal models of human pathology. Pharmacological validity is the consequence of this poor distinction.
The review starts with a definition of anxiety and some clarifications regarding the uses and misuses of methodological concepts in animal anxiety literature reviews. A description of the main TUA, including the 3D maze, is provided. This is followed by a discussion of the differences between these while highlighting major flaws, pitfalls, and limitations. Results obtained in the 3D maze with different strains of mice, and with drugs such as diazepam, fluoxetine, and dizocilpine will be described.
Animal Models and Validity
In a recent review, Ennaceur (2014) described various methodological flaws that undermine the validity of the current TUA. He reported that these tests do not provide unequivocal measures of anxiety as the conflict hypothesis cannot be verified. He also pointed out that in numerous critical review analysis, attributes of animal models of human anxiety disorders are wrongly associated to behavioral tests of anxiety (Belzung and Lemoine 2011; Belzung and Griebel 2001; Cryan and Holmes 2005; Cryan and Sweeney 2011; Geyer and Markou 1995; Griebel and Holmes 2013; Homberg 2013; Hendriksen and Groenink 2015; Nemeroff 2002; Silverman et al. 2010; Willner 1997; Shekhar et al. 2001). We argue here, that a behavioral test provides a set of conditions under which a mental state or condition is assessed. A behavioral test does not produce a psychiatric or neurological disorder; it does not produce symptoms as requested by the authors of these critical reviews. If a behavioral test is sensitive enough, it should be able to detect symptoms. However, to achieve this sensitivity with consistency and reliability, a behavioral test needs to demonstrate that it is measuring the construct that it is meant to measure, and that it not measuring a different construct which it may be confused with. It should demonstrate discriminant validity, and provide unequivocal measures of anxiety.
An animal model of human behavior represents a theory of a cognitive or an emotional process, which is translated from humans to animals. A behavioral test is developed primarily and specifically to verify and support a theory of cognition or emotion; it can also be used to verify a theory of a psychopathology, but it is not developed for a particular psychopathology. For instance, a behavioral test can be developed to assess the effects of various factors and experimental manipulations on memory in normal subjects. It can be used to determine the presence or absence of memory impairment in animal models of schizophrenia in the same way it is used to assess memory in animal models of Alzheimer's disease, stroke, autism, asthma, or any pathophysiological condition. The same is true for an anxiety test. There is no such thing as a behavioral test suitable only for a particular class of drugs, a particular brain structure or a pathophysiology.
An animal model of human psychopathology is developed with the aim that such a model displays symptoms characteristic of a particular disorder. These can be achieved with various experimental interventions (drug administrations, genetic manipulations, lesion applications). The induction of these symptoms requires that the underlying physiological and/or neurochemical basis of these symptoms have been already determined. Up‐to‐date neuroscientists have been relying intensively on drugs from serendipitous discovery, which appear to alleviate symptoms. These drugs have been used to determine drug targets and neurochemical pathways that account for the disorders. They provide the basis upon which most animal models of a psychopathology have been developed. This pharmacological validation approach rests on a fragile assumption that a drug has specificity and efficacy in the treatment of a particular psychopathology. Pharmacological validity creates a sort of association in which a drug forms an intrinsic component of the behavioral test. Two serious risks emerge from such an association. The first one is that a behavioral test can be viewed as specific to a particular class of drugs. The second risk is dogmatization of assumptions. The fundamental basis upon which anxiolytic properties were attributed to both benzodiazepines and SSRIs, and the fundamental basis upon which the EPM, EZM, LDB, and OF are established as tests of anxiety remain almost untouchable. Hence, we witnessed over more than 30 years that a lack of consistency and reliability of the current tests of anxiety was accounted for by almost anything that a scientist can hypothesis about, except the validity of the construct that these behavioral tests were set to measure.
An animal model of a neurological or a psychiatric disorder can be achieved using a behavioral test with validated measures of the construct it intends to measure, and the determination of the physiological and/or neurochemical changes that occurred during the exposition to the test. This traditional method involves normal animals, and can be based on the use of strains of rats and mice that express differences in emotionality. The association of the measured construct to specific physiological and neurochemical changes will determine drug targets, and will facilitate the design of the type of pathological model for further investigation. This strategy provides a strong rational for the investigation of the neurobiological basis of anxiety free from the fertile constraints of pharmacological validity.
Definitions of Fear and Anxiety
Fear is defined as a negative emotional state associated with the perception of imminent or present threat to wellbeing or survival. It is a defensive reaction, which facilitates escape and avoidance of impending identifiable danger. Anxiety, on the other hand, is defined as a negative emotional state associated with the perception of potential or ambiguous threat. Like fear, it is a defensive reaction, but characterized by a feeling of apprehension, uncertainty, worry, uneasiness, or tension stemming from the anticipation of potential threat or negative outcomes. Hence, in fear conditions, humans and animals face an unambiguous situation; they can avoid the threatening stimulus or escape to safety. The aversive stimulus does not carry an incentive that diminishes or moderates the need to avoid or escape. However, in anxiety conditions, humans and animals face an ambiguous situation. They are unable to avoid/escape or approach the perceived threat stimulus. They experience a high level of uncertainty and unpredictability as the threat stimulus appears associated with both positive and negative outcomes.
Therefore, a test of unconditioned anxiety needs to demonstrate construct validity, which comprises a number subset of validity items. We are able to cover only the most important one, in this review. Construct validity originated for early validation process of psychometric tests, and therefore a note of caution is necessary when applying this to animal behavioral tests – some adjustments and adaptations are required.
Face validity, that is at face value, the test conditions and the elicited responses should conduct to a general agreement whether these two appear to involve anxiety. For instance, agreement on novelty‐ or unfamiliarity‐induced fear response, agreement on the equivalence and ambiguity of the whole test situation that evokes fear‐induced avoidance/escape and approach, and agreement on a particular response or a set of responses that are selected to measure anxiety.
Discriminant validity, that is the test evokes and provides measures of anxiety rather than fear‐induced escape or avoidance response. This should be demonstrated by comparing the behavior of animals in fear‐induced anxiety setting to animals in fear‐induced avoidance setting using the same test and manipulating a single element of the test. For instance, removing the ambiguity of fear‐evoking stimuli or the uncertainty of the response outcome so that animals can escape or avoid to terminate fear and anxiety. Another element of discriminant validity concerns the measurement of the anxiety response. The test should be able to discriminate between confounding factors, in particular when hyperactivity, impulsivity or impaired cognitive processes are manifested in the presence of an anxiogenic stimulus.
Convergent validity is often conducted to determine whether the measurements from two or more tests of the same construct converge to produce comparable, convergent results. This is only possible if at least one of these tests has already established construct validity, which in our view is not the case with the TUA. However, convergent validity is also concerned with the extent to which the different measures of the construct (anxiety) are related to each other. For instance, in the EPM, discriminant validity is concerned with various spatiotemporal and ethological parameters that are thought to measure anxiety such as open arm entries, open time entries and their respective percent values, as well as risk assessment behaviors. Unfortunately, the accumulated evidence demonstrates no convergence between these measurements (see Tables 1 and 2 on spatiotemporal parameters, and Ennaceur 2014 on ethological parameters).
Predictive validity refers to the ability of a test of anxiety to predict the performance of the same or comparable sample population in other provoking anxiety situations. However, it has been extended to refer also to the ability of a behavioral test of anxiety to predict the anxiolytic efficacy of known drugs (i.e., diazepam or fluoxetine). This assumes that a reference drug has a well‐established specificity, that its primary effect (i.e., anxiolysis) is clearly distinguishable from and not confounded with its secondary effects (i.e., sedation, relaxation, psychomotor stimulation, or impaired perceptual and cognitive processes). In some reports, predictive validity is associated with the ability of an anxiety test to predict novel drugs, which are believed to have anxiolytic properties. In this case, there are two unverified assumptions, one concerning the validity of the behavioral test itself and the other one concerning the anxiolytic properties of the drug. Failure to detect an effect on anxiety can invalidate neither the test nor the drug.
Table 1.
Sample data from various research reports illustrating the consistency between results and concordances between elevated plus‐maze (EPM) test parameters in the study of mouse strain differences
| Strains | OA | EA | Total | DIFF | POAE | POAT | OAAI | References |
|---|---|---|---|---|---|---|---|---|
| C57BL/6JOla | 12.0 | 2.8 | 14.8 | −9.2 | 81 | 61 | 29 | Mathiasen et al. 2008 (T2) |
| BALB/cByJ | 17.0 | 8.0 | 25.0 | −9.0 | 68 | 90 | 21 | Trullas and Skolnick 1993 |
| BALBc/J | 6.4 | 1.6 | 8.0 | −4.8 | 80 | 69 | 26 | Trullas and Skolnick 1993 |
| C57BL/6JOla | 10.0 | 5.3 | 15.3 | −4.7 | 65 | 46 | 44 | Mathiasen et al. 2008 (T1) |
| C3H/HeN | 10.4 | 6.6 | 17.0 | −3.7 | 61 | 52 | 44 | Trullas and Skolnick 1993 |
| CBA/J | 9.6 | 6.4 | 16.0 | −3.2 | 60 | 58 | 41 | Trullas and Skolnick 1993 |
| C3H/HeJ | 8.4 | 5.6 | 14.0 | −2.8 | 60 | 69 | 36 | Trullas and Skolnick 1993 |
| NMRI | 8.7 | 6.3 | 15.0 | −2.4 | 58 | 37 | 53 | Griebel et al. 2000 |
| NMRI | 8.3 | 6.9 | 15.2 | −1.4 | 55 | 40 | 53 | Mathiasen et al. 2008 (T2) |
| NMRI | 9.2 | 9.3 | 18.6 | 0.1 | 49 | 38 | 56 | Mathiasen et al. 2008 (T1) |
| C3H/HeJ | 1.5 | 2.5 | 4.0 | 1.0 | 37 | 29 | 67 | Griebel et al. 2000 |
| C3H/HeJ | 1.5 | 3.0 | 4.5 | 1.5 | 33 | 13 | 77 | Yilmazer–Hanke et al. 2003 |
| SJL/J | 8.7 | 10.3 | 19.0 | 1.5 | 46 | 23 | 66 | Griebel et al. 2000 |
| C57BL/6J | 3.5 | 5.5 | 9.0 | 2.0 | 39 | 1 | 80 | Yilmazer–Hanke et al. 2003 |
| CBA/J | 2.6 | 5.4 | 8.0 | 2.9 | 32 | 28 | 70 | Griebel et al. 2000 |
| BALB/cByJ | 3.6 | 7.4 | 11.0 | 3.7 | 33 | 15 | 76 | Griebel et al. 2000 |
| DBA/2Ola | 7.3 | 11.6 | 18.9 | 4.3 | 39 | 43 | 59 | Mathiasen et al. 2008 (T1) |
| BALB/cJ | 6.5 | 11.0 | 17.0 | 4.5 | 38 | 21 | 70 | Yilmazer–Hanke et al. 2003 |
| A/J | 3.0 | 8.0 | 11.0 | 5.1 | 27 | 65 | 54 | O'Leary et al. 2013 |
| DBA | 2.4 | 7.6 | 10.0 | 5.2 | 24 | 11 | 83 | Griebel et al. 2000 |
| DBA/2Ola | 4.9 | 10.3 | 15.2 | 5.4 | 32 | 66 | 51 | Mathiasen et al. 2008 (T2) |
| BALBc/J | 7.8 | 13.2 | 21.0 | 5.5 | 37 | 21 | 71 | O'Leary et al. 2013 |
| DBA/2J | 2.8 | 8.3 | 11.0 | 5.5 | 25 | 62 | 57 | Trullas and Skolnick 1993 |
| NMRI | 6.0 | 11.5 | 17.5 | 5.5 | 34 | 34 | 66 | Yilmazer–Hanke et al. 2003 |
| BALB/cByJ | 8.1 | 13.9 | 22.0 | 5.7 | 37 | 41 | 61 | O'Leary et al. 2013 |
| C3H/HeJ | 8.8 | 16.3 | 25.0 | 7.5 | 35 | 42 | 62 | O'Leary et al. 2013 |
| A/J | 0.2 | 7.8 | 8.0 | 7.7 | 2 | 27 | 86 | Trullas and Skolnick 1993 |
| C57BL/6J | 3.5 | 11.6 | 15.0 | 8.1 | 23 | 37 | 70 | Griebel et al. 2000 |
| C57BL/6ByJ | 2.2 | 10.8 | 13.0 | 8.6 | 17 | 34 | 75 | Trullas and Skolnick 1993 |
| DBA/2J | 2.5 | 13.5 | 16.0 | 11.0 | 16 | 36 | 74 | Yilmazer–Hanke et al. 2003 |
| C57BL/6J | 0.8 | 13.2 | 14.0 | 12.3 | 6 | 35 | 80 | Trullas and Skolnick 1993 |
| 129S1/SvImJ | 6.3 | 18.8 | 25.0 | 12.5 | 25 | 5 | 85 | O'Leary et al. 2013 |
| C57BL/6J | 7.2 | 22.8 | 30.0 | 15.6 | 24 | 19 | 79 | O'Leary et al. 2013 |
| SJL/J | 11.3 | 27.7 | 39.0 | 16.4 | 29 | 35 | 68 | O'Leary et al. 2013 |
| DBA/2J | 7.7 | 24.3 | 32.0 | 16.6 | 24 | 6 | 85 | O'Leary et al. 2013 |
| AKR | 5.8 | 23.2 | 29.0 | 17.4 | 20 | 36 | 72 | O'Leary et al. 2013 |
| FVB/NJ | 9.8 | 31.2 | 41.0 | 21.3 | 24 | 15 | 81 | O'Leary et al. 2013 |
| BTBR | 7.4 | 29.6 | 37.0 | 22.2 | 20 | 46 | 67 | O'Leary et al. 2013 |
The above data were mostly estimated from average group values of available test parameters in tables or graphs. They are presented in the order of the difference (DIFF) between open arm (OA) and enclosed arms (EA) entries. Negative values indicate a preference for open arms. T1 and T2 in Mathiasen et al. 2008 refer to Table 1 and table 2, respectively. The above data sample demonstrates lack of concordance between the EPM test parameters. It also demonstrates that the same strain of mice can be low anxiety in one study and high anxiety in another one. Note also that, in most research reports, the POAE and POAT are below 50%.
OA, open arm entries; EA, enclosed arm entries; Total, OA + EA; DIFF, EA‐OA; POAE, percent open arm entries; POAT, percent open arm time; OAAI, open arm avoidance index.
Table 2.
Sample data from various research reports illustrating the consistency between results and concordances between tests of unconditioned anxiety test parameters in the study of mouse strain differences
| Plus‐maze | Light/Dark | Open field | References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OA | OA | OA | OA | OA | TT | DL | LD | LIT | LIT | LIT | TT | C | C | C | TT | |||
| Mouse strain 1 | Mouse strain 2 | lt | x | t | %x | %t | x | lt | lt | x | t | %t | x | lt | x | t | x | |
| 129P3/J | 129S6/Sv EvTac | ns | > | Bothe et al. 2004 | ||||||||||||||
| 129S2/Sv Hsd | 129/Sv Ev | < | ns | ns | ns | ns | ns | < | > | Rodgers et al. 2002b | ||||||||
| 129S1/Sv ImJ | A/J | ns | < | > | ns | > | < | > | O'Leary et al. 2013 | |||||||||
| 129S1/Sv ImJ | A/J | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| 129S1/Sv ImJ | A/J | < | < | > | > | > | Moy et al. 2007 | |||||||||||
| 129S1/Sv ImJ | A/J | ns | ns | ns | > | > | < | ns | ns | ns | Milner and Crabbe 2008 | |||||||
| 129S1/Sv ImJ | CBA/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| 129S3/Sv lmJ | CBA/J | > | < | < | Cook et al. 2001 | |||||||||||||
| 129/Sv J | CBA/J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| 129/Sv Hsd | CBA/Ca OlaHsd | < | < | < | < | < | Rogers et al. 1999 | |||||||||||
| 129S2/Sv Hsd | CBA/Ca OlaHsd | > | < | ns | Brooks et al. 2005 | |||||||||||||
| 129S1/Sv ImJ | SJL/J | ns | ns | < | ns | < | < | < | O'Leary et al. 2013 | |||||||||
| 129S1/Sv ImJ | SJL/J | < | > | > | ns | > | ns | < | > | Lad et al. 2010 | ||||||||
| 129S1/Sv ImJ | SJL/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| 129/Sv Ev | Swiss Webster | < | ns | ns | ns | > | ns | < | Rodgers et al. 2002b | |||||||||
| 129S2/Sv Hsd | Swiss Webster | ns | ns | ns | < | ns | ns | ns | < | Rodgers et al. 2002b | ||||||||
| 129S2/Sv Hsd | Swiss Webster | ns | > | ns | < | Rodgers et al. 2002a | ||||||||||||
| 129S1/Sv ImJ | SWR/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| 129S2/Sv Hsd | ICR:Hsd | ns | ns | > | < | Kulesskaya and Võikar 2014 | ||||||||||||
| BALB/c J | BALB/c ByJ | > | > | < | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c ByJ | BALB/c J | ns | ns | ns | < | ns | ns | ns | O'Leary et al. 2013 | |||||||||
| BALB/c ByJ | 129/Sv J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| BALB/c ByJ | 129S1/Sv ImJ | > | > | ns | < | > | ns | ns | O'Leary et al. 2013 | |||||||||
| BALB/c ByJ | 129S1/Sv ImJ | ns | ns | < | ns | < | Moy et al. 2007 | |||||||||||
| BALB/c ByJ | 129S1/Sv ImJ | > | < | < | > | < | < | > | < | Lad et al. 2010 | ||||||||
| BALB/c ByJ | 129S1/Sv lmJ | ns | ns | ns | < | < | ns | ns | ns | ns | Milner and Crabbe 2008 | |||||||
| BALB/c ByJ | 129S3/Sv lmJ | < | ns | > | Cook et al. 2001 | |||||||||||||
| BALB/c J | 129S1/Sv ImJ | > | > | ns | ns | > | > | ns | O'Leary et al. 2013 | |||||||||
| BALB/c OlaHsd | 129/Sv Hsd | > | > | ns | > | ns | Rogers et al. 1999 | |||||||||||
| BALB/c OlaHsd | 129S2/Sv Hsd | < | ns | ns | Brooks et al. 2005 | |||||||||||||
| BALB/c ByJ | A/J | ns | ns | > | ns | ns | ns | ns | ns | Lad et al. 2010 | ||||||||
| BALB/c ByJ | A/J | < | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| BALB/c ByJ | A/J | < | < | > | > | > | Moy et al. 2007 | |||||||||||
| BALB/c ByJ | A/J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c ByJ | A/J | > | > | > | < | > | ns | > | O'Leary et al. 2013 | |||||||||
| BALB/c J | A/J | > | > | ns | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c J | A/J | > | > | > | ns | > | ns | > | O'Leary et al. 2013 | |||||||||
| BALB/c | C3H/He | < | < | Kopp et al. 1999 | ||||||||||||||
| BALB/c ByJ | C3H/He J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| BALB/c ByJ | C3H/He J | > | < | ns | Cook et al. 2001 | |||||||||||||
| BALB/c ByJ | C3H/He J | > | ns | ns | > | < | < | > | < | Lad et al. 2010 | ||||||||
| BALB/c ByJ | C3H/He J | ns | ns | < | ns | Milner and Crabbe 2008 | ||||||||||||
| BALB/c ByJ | C3H/He J | < | < | Moy et al. 2007 | ||||||||||||||
| BALB/c ByJ | C3H/He J | ns | ns | > | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c ByJ | C3H/He J | ns | ns | ns | < | ns | ns | ns | O'Leary et al. 2013 | |||||||||
| BALB/c ByJ | C3H/He N | ns | ns | > | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c ByJ | C3H/He OuJ | ns | ns | > | ns | ns | Griebel et al. 2000 | |||||||||||
| BALB/c J | C3H/He J | > | > | ns | > | > | Yilmazer–Hanke et al. 2003 | |||||||||||
| BALB/c J | C3H/He J | > | > | < | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c J | C3H/He J | ns | ns | ns | ns | ns | ns | < | O'Leary et al. 2013 | |||||||||
| BALB/c J | C3H/He N | > | > | < | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c OlaHsd | C3H/He HNsd | > | < | ns | Brooks et al. 2005 | |||||||||||||
| BALB/c OlaHsd | C3H/He NHsd | > | > | > | ns | > | Rogers et al. 1999 | |||||||||||
| BALB/c A | CBA/N | > | ns | > | Kim et al. 2002 | |||||||||||||
| BALB/c ByJ | CBA/J | > | < | < | Cook et al. 2001 | |||||||||||||
| BALB/c ByJ | CBA/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| BALB/c ByJ | CBA/J | ns | ns | ns | ns | ns | Griebel et al. 2000 | |||||||||||
| BALB/c ByJ | CBA/J | ns | ns | > | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c ByJ | CBA/J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| BALB/c J | CBA/J | > | > | < | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c OlaHsd | CBA/Ca OlaHsd | ns | < | ns | Brooks et al. 2005 | |||||||||||||
| BALB/c OlaHsd | CBA/Ca OlaHsd | > | > | < | < | < | Rogers et al. 1999 | |||||||||||
| BALB/c ByJ | DBA/2J | > | ns | Ducottet and Belzung 2005 | ||||||||||||||
| BALB/c ByJ | DBA/2J | < | > | ns | Cook et al. 2001 | |||||||||||||
| BALB/c ByJ | DBA/2J | ns | ns | ns | > | < | < | ns | < | Lad et al. 2010 | ||||||||
| BALB/c ByJ | DB/2J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| BALB/c ByJ | DBA/2J | ns | ns | ns | ns | < | Moy et al. 2007 | |||||||||||
| BALB/c ByJ | DBA/2J | ns | ns | ns | ns | ns | Griebel et al. 2000 | |||||||||||
| BALB/c ByJ | DBA/2J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c ByJ | DBA/2J | > | > | ns | < | ns | ns | < | O'Leary et al. 2013 | |||||||||
| BALB/c J | DBA/2J | > | > | > | > | ns | Yilmazer–Hanke et al. 2003 | |||||||||||
| BALB/c J | DBA/2J | > | > | < | Trullas and Skolnick 1993 | |||||||||||||
| BALB/c J | DBA/2J | > | > | ns | ns | ns | > | < | O'Leary et al. 2013 | |||||||||
| BALB/c OlaHsd | DBA/2 OlaHsd | > | ns | ns | Brooks et al. 2005 | |||||||||||||
| BALB/c OlaHsd | DBA/2 OlaHsd | > | > | < | < | < | Rogers et al. 1999 | |||||||||||
| BALB/c A | FVB/N | < | < | < | Kim et al. 2002 | |||||||||||||
| BALB/c ByJ | FVB/N A | > | ns | Ducottet and Belzung 2005 | ||||||||||||||
| BALB/c ByJ | FVB/N J | > | ns | ns | > | < | < | > | < | Lad et al. 2010 | ||||||||
| BALB/c ByJ | FVB/N J | > | ns | ns | ns | ns | ns | < | < | < | < | < | < | Milner and Crabbe 2008 | ||||
| BALB/c ByJ | FVB/N J | < | < | Moy et al. 2007 | ||||||||||||||
| BALB/c ByJ | FVB/N J | > | > | < | < | < | ns | < | O'Leary et al. 2013 | |||||||||
| BALB/c J | FVB/N J | > | > | < | < | < | ns | < | O'Leary et al. 2013 | |||||||||
| BALB/c ByJ | SJL/J | ns | ns | ns | > | < | < | ns | ns | Lad et al. 2010 | ||||||||
| BALB/c ByJ | SJL/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| BALB/c ByJ | SJL/J | ns | ns | < | < | < | Griebel et al. 2000 | |||||||||||
| BALB/c ByJ | SJL/J | > | > | < | < | < | ns | < | O'Leary et al. 2013 | |||||||||
| BALB/c J | SJL/J | > | > | < | ns | < | > | < | O'Leary et al. 2013 | |||||||||
| BALB/c J | Swiss Webster/HSD | < | Crawley and Davis 1982 | |||||||||||||||
| BALB/c J | Swiss Webster/NIH | < | Crawley and Davis 1982 | |||||||||||||||
| BALB/c ByJ | Swiss | ns | ns | < | ns | ns | Griebel et al. 2000 | |||||||||||
| BALB/c ByJ | SWR/J | > | < | ns | < | < | ns | ns | < | ns | Milner and Crabbe 2008 | |||||||
| BALB/c | ICR | ns | ns | < | Nesher et al. 2012 | |||||||||||||
| C3H/He J | C3H/He N | ns | ns | > | Trullas and Skolnick 1993 | |||||||||||||
| C3H/He J | 129/Sv J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C3H/He J | 129S1/Sv ImJ | ns | > | ns | ns | > | > | > | O'Leary et al. 2013 | |||||||||
| C3H/He J | 129S1/Sv lmJ | > | > | Hagenbuch et al. 2006 | ||||||||||||||
| C3H/He J | 129S1/Sv ImJ | ns | < | < | ns | < | ns | ns | > | Lad et al. 2010 | ||||||||
| C3H/He J | 129S1/Sv ImJ | > | < | Moy et al. 2007 | ||||||||||||||
| C3H/He J | 129S1/Sv lmJ | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C3H/He J | 129S3/Sv lmJ | < | > | > | Cook et al. 2001 | |||||||||||||
| C3H/He HNsd | 129S2/Sv Hsd | < | > | > | Brooks et al. 2005 | |||||||||||||
| C3H/He NHsd | 129/Sv Hsd | > | < | < | > | < | Rogers et al. 1999 | |||||||||||
| C3H/He J | A/J | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| C3H/He J | A/J | < | ns | > | ns | Milner and Crabbe 2008 | ||||||||||||
| C3H/He J | A/J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| C3H/He J | A/J | ns | > | > | ns | > | ns | > | O'Leary et al. 2013 | |||||||||
| C3H/He J | A/J | > | > | Moy et al. 2007 | ||||||||||||||
| C3H/He N | A/J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| C3H/He N | CBA/J | ns | ns | ns | Trullas and Skolnick 1993 | |||||||||||||
| C3H/He J | CBA/J | ns | ns | < | Cook et al. 2001 | |||||||||||||
| C3H/He J | CBA/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C3H/He J | CBA/J | ns | ns | > | Trullas and Skolnick 1993 | |||||||||||||
| C3H/He J | CBA/J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C3H/He HNsd | CBA/Ca OlaHsd | ns | ns | > | Brooks et al. 2005 | |||||||||||||
| C3H/He NHsd | CBA/Ca OlaHsd | ns | < | < | < | < | Rogers et al. 1999 | |||||||||||
| C3H/He OuJ | CBA/J | ns | ns | < | ns | ns | Griebel et al. 2000 | |||||||||||
| C3H/He HNsd | DBA/2 OlaHsd | ns | > | ns | Brooks et al. 2005 | |||||||||||||
| C3H/He NHsd | DBA/2 OlaHsd | ns | ns | < | < | < | Rogers et al. 1999 | |||||||||||
| C3H/He J | DBA/2J | < | > | ns | Cook et al. 2001 | |||||||||||||
| C3H/He J | DBA/2J | ns | ns | > | ns | < | Yilmazer–Hanke et al. 2003 | |||||||||||
| C3H/He J | DBA/2J | < | ns | ns | ns | ns | > | < | > | Lad et al. 2010 | ||||||||
| C3H/He J | DBA/2J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C3H/He J | DBA/2J | > | > | ns | > | ns | ns | ns | O'Leary et al. 2013 | |||||||||
| C3H/He J | DBA/2J | > | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C3H/He J | DBA/2J | > | < | Moy et al. 2007 | ||||||||||||||
| C3H/He J | DBA/2J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| C3H/He N | DBA/2J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| C3H/He OuJ | DBA/2J | ns | ns | < | ns | ns | Griebel et al. 2000 | |||||||||||
| C3H/He J | FVB/N A | > | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C3H/He J | FVB/N J | < | ns | ns | ns | < | ns | < | > | Lad et al. 2010 | ||||||||
| C3H/He J | FVB/N J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C3H/He J | FVB/N J | < | < | Moy et al. 2007 | ||||||||||||||
| C3H/He J | FVB/N J | > | > | < | ns | < | ns | < | O'Leary et al. 2013 | |||||||||
| C3H/He J | SJL/J | < | ns | ns | ns | ns | ns | < | > | Lad et al. 2010 | ||||||||
| C3H/He J | SJL/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C3H/He J | SJL/J | > | > | < | ns | < | ns | ns | O'Leary et al. 2013 | |||||||||
| C3H/He OuJ | SJL/J | ns | ns | < | < | < | Griebel et al. 2000 | |||||||||||
| C3H/He J | SWR/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C3H/He OuJ | Swiss | ns | ns | < | ns | ns | Griebel et al. 2000 | |||||||||||
| C57BL/6J | 129P3/J | ns | ns | Bothe et al. 2004 | ||||||||||||||
| C57BL/6 | 129S6/Sv Ev/Tac | ns | ns | ns | Abramov et al. 2008 | |||||||||||||
| C57BL/6J | 129/Sv J | > | ns | ns | ns | ns | Homanics et al. 1999 | |||||||||||
| C57BL/6J | 129/Sv J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C57BL/6J | 129S1/Sv ImJ | ns | ns | > | > | > | > | > | O'Leary et al. 2013 | |||||||||
| C57BL/6J | 129S1/Sv ImJ | ns | ns | ns | ns | > | > | < | > | Lad et al. 2010 | ||||||||
| C57BL/6J | 129S1/Sv ImJ | > | > | > | ns | < | Moy et al. 2007 | |||||||||||
| C57BL/6J | 129S1/Sv ImJ | ns | > | Hagenbuch et al. 2006 | ||||||||||||||
| C57BL/6J | 129S1/Sv lmJ | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | 129S3/Sv lmJ | < | ns | > | Cook et al. 2001 | |||||||||||||
| C57BL/6J | 129S6/Sv EvTac | ns | ns | > | ns | > | > | Holmes et al. 2002 | ||||||||||
| C57BL/6J | 129S6/Sv EvTac | ns | > | Bothe et al. 2004 | ||||||||||||||
| C57BL/6J | 129S6/Sv EvTac | < | > | > | > | > | Bouwknecht et al. 2004a | |||||||||||
| C57BL/6J | 129S6/Sv EvTac | ns | > | > | > | > | > | Bouwknecht et al. 2004b | ||||||||||
| C57BL/6J OlaHsD | 129/Sv Ev | > | ns | > | > | ns | ns | ns | > | Rodgers et al. 2002b | ||||||||
| C57BL/6J OlaHsD | 129/Sv Hsd | > | ns | ns | > | ns | Rogers et al. 1999 | |||||||||||
| C57BL/6J OlaHsD | 129S2/Sv Hsd | < | > | > | > | < | ns | ns | > | Võikar et al. 2004 | ||||||||
| C57BL/6J OlaHsD | 129S2/Sv Hsd | < | ns | > | Brooks et al. 2005 | |||||||||||||
| C57BL/6J OlaHsD | 129S2/Sv Hsd | < | ns | > | ns | ns | > | > | > | > | Võikar et al. 2001 | |||||||
| C57BL/6J OlaHsD | 129S2/Sv Hsd | > | ns | ns | > | ns | ns | ns | > | Rodgers et al. 2002b | ||||||||
| C57BL/6J OlaHsD | 129S2/Sv Hsd | > | ns | ns | > | Rodgers et al. 2002a | ||||||||||||
| C57BL/6N Tac | 129P3/J | ns | ns | Bothe et al. 2004 | ||||||||||||||
| C57BL/6N Tac | 129S6/Sv EvTac | ns | > | Bothe et al. 2004 | ||||||||||||||
| C57BL/6N Hsd | 129S2/Sv Hsd | ns | ns | < | > | Kulesskaya and Võikar 2014 | ||||||||||||
| C57BL/6N Hsd (Hel) | 129S2/Sv Hsd | > | ns | < | > | Kulesskaya and Võikar 2014 | ||||||||||||
| C57L/J | 129S1/Sv lmJ | ns | ns | ns | ns | ns | ns | > | Milner and Crabbe 2008 | |||||||||
| C57BL/10J | A/J | ns | ns | ns | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6ByJ | A/J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | A/J | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| C57BL/6J | A/J | < | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | A/J | < | ns | > | > | > | Moy et al. 2007 | |||||||||||
| C57BL/6J | A/J | ns | ns | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | A/J | ns | ns | > | > | > | ns | > | O'Leary et al. 2013 | |||||||||
| C57L/J | A/J | < | > | ns | > | > | > | ns | Milner and Crabbe 2008 | |||||||||
| C57 | BALB/c | > | > | > | ns | Augustsson & Meyerson 2004 | ||||||||||||
| C57BL/6 | BALB/c | > | > | Kopp et al. 1999 | ||||||||||||||
| C57BL/6J | BALB/c | < | < | ns | Nesher et al. 2012 | |||||||||||||
| C57BL/6J | BALB/c | < | < | < | < | Verleye et al. 2011 | ||||||||||||
| C57BL/6J | BALB/c | < | < | Brinks et al. 2007 | ||||||||||||||
| C57BL6/J | BALB/c J | > | Crawley and Davis 1982 | |||||||||||||||
| C57BL/6J | BALB/c J | < | ns | ns | ns | < | Yilmazer–Hanke et al. 2003 | |||||||||||
| C57BL/6J | BALB/c J | < | < | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | BALB/c J | > | > | > | < | Norcross et al. 2008 | ||||||||||||
| C57BL/6J | BALB/c J | < | < | > | > | > | < | > | O'Leary et al. 2013 | |||||||||
| C57BL/6J | BALB/c J | < | < | > | > | > | An et al. 2011 | |||||||||||
| C57BL/6J | BALB/c J | ns | < | ns | Brinks et al. 2007 | |||||||||||||
| C57BL/6J | BALB/c ByJ | > | > | ns | > | > | > | Lepicard et al. 2000 | ||||||||||
| C57BL/6J | BALB/c ByJ | < | ns | > | Cook et al. 2001 | |||||||||||||
| C57BL/6J | BALB/c ByJ | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| C57BL/6J | BALB/c ByJ | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | BALB/c ByJ | > | > | < | > | > | Verleye et al. 2011 | |||||||||||
| C57BL/6J | BALB/c ByJ | > | > | > | > | > | > | Akillioglu et al. 2012 | ||||||||||
| C57BL/6J | BALB/c ByJ | ns | < | ns | > | > | > | Post et al. 2011 | ||||||||||
| C57BL/6J | BALB/c ByJ | > | > | > | ns | > | Moy et al. 2007 | |||||||||||
| C57BL/6J | BALB/c ByJ | ns | ns | ns | > | > | Griebel et al. 2000 | |||||||||||
| C57BL/6J | BALB/c ByJ | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | BALB/c ByJ | < | ns | > | > | > | ns | > | < | ns | > | O'Leary et al. 2013 | ||||||
| C57BL/6J | BALB/c ByJ | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C57BL/6J | BALB/c A | ns | > | ns | Kim et al. 2002 | |||||||||||||
| C57BL/6J | BALB/c AnN | > | ns | ns | ns | Lalonde and Strazielle 2008 | ||||||||||||
| C57BL/6ByJ | BALB/c J | < | < | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6ByJ | BALB/c ByJ | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J Ico | BALB/c AnNIco | > | ns | ns | ns | Lalonde and Strazielle 2008 | ||||||||||||
| C57BL/6J OlaHsD | BALB/c OlaHsd | < | ns | ns | Brooks et al. 2005 | |||||||||||||
| C57BL/6J OlaHsD | BALB/c OlaHsd | < | < | ns | ns | ns | Rogers et al. 1999 | |||||||||||
| C57BL/6N CrlBR | BALB/c AnNCrlBR | > | ns | > | > | > | Carola et al. 2002 | |||||||||||
| C57BL/10J | BALB/c ByJ | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/10J | BALB/c J | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57L/J | BALB/c ByJ | ns | ns | ns | > | > | > | > | Milner and Crabbe 2008 | |||||||||
| C57BL/6J | CBA/J | ns | < | ns | Cook et al. 2001 | |||||||||||||
| C57BL/6J | CBA/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | CBA/J | ns | ns | > | ns | ns | Griebel et al. 2000 | |||||||||||
| C57BL/6J | CBA/J | < | < | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | CBA/J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C57BL/6ByJ | CBA/J | < | < | ns | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | CBA/N | > | > | > | Kim et al. 2002 | |||||||||||||
| C57BL/6J OlaHsD | CBA/Ca OlaHsd | ns | ns | ns | Brooks et al. 2005 | |||||||||||||
| C57BL/6J OlaHsD | CBA/Ca OlaHsd | ns | < | < | < | < | Rogers et al. 1999 | |||||||||||
| C57BL/10J | CBA/J | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57L/J | CBA/J | ns | ns | ns | ns | > | > | ns | Milner and Crabbe 2008 | |||||||||
| C57BL/6J | C57L/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | C57BL/6ByJ | < | < | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | C57BL/6N Tac | ns | ns | Bothe et al. 2004 | ||||||||||||||
| C57BL/6J | C57BSW/6 CrlBR | ns | ns | ns | ns | ns | > | ns | ns | ns | ns | van Gaalen and Steckler 2000 | ||||||
| C57BL/6J | C57BL/10J | ns | ns | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6N Hsd | C57BL/6N Hsd (Hel) | < | ns | ns | ns | Kulesskaya and Võikar 2014 | ||||||||||||
| C57BL/10J | C57BL/6ByJ | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6 | C3H/He | > | > | Kopp et al. 1999 | ||||||||||||||
| C57BL/6J | C3H/He J | ns | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C57BL/6J | C3H/He J | > | > | ns | > | > | Yilmazer–Hanke et al. 2003 | |||||||||||
| C57BL/6J | C3H/He J | ns | < | > | Cook et al. 2001 | |||||||||||||
| C57BL/6J | C3H/He J | ns | > | ns | < | > | > | ns | ns | Lad et al. 2010 | ||||||||
| C57BL/6J | C3H/He J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | C3H/He J | < | > | Moy et al. 2007 | ||||||||||||||
| C57BL/6J | C3H/He J | < | < | ns | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | C3H/He J | < | < | > | > | > | < | ns | O'Leary et al. 2013 | |||||||||
| C57BL/6J | C3H/He J | ns | > | Hagenbuch et al. 2006 | ||||||||||||||
| C57BL/6J | C3H/He N | < | < | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | C3H/e OuJ | ns | ns | > | ns | ns | Griebel et al. 2000 | |||||||||||
| C57BL/6ByJ | C3H/He J | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6ByJ | C3H/He N | < | < | ns | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J OlaHsD | C3H/He HNsd | ns | < | ns | Brooks et al. 2005 | |||||||||||||
| C57BL/6J OlaHsD | C3H/He NHsd | ns | > | > | ns | > | Rogers et al. 1999 | |||||||||||
| C57BL/10J | C3H/He J | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/10J | C3H/He N | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57L/J | C3H/He J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6 | DBA/2 | ns | ns | ns | > | ns | ns | Gard et al. 2001 | ||||||||||
| C57BL/6ByJ | DBA/2J | ns | ns | ns | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | DBA/2J | < | > | > | Cook et al. 2001 | |||||||||||||
| C57BL/6J | DBA/2J | ns | > | > | > | < | Yilmazer–Hanke et al. 2003 | |||||||||||
| C57BL/6J | DBA/2J | ns | ns | > | ns | > | > | Holmes et al. 2002 | ||||||||||
| C57BL/6J | DBA/2J | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| C57BL/6J | DBA/2J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | DBA/2J | > | > | > | > | ns | Moy et al. 2007 | |||||||||||
| C57BL/6J | DBA/2J | ns | ns | > | ns | ns | Griebel et al. 2000 | |||||||||||
| C57BL/6J | DBA/2J | < | < | > | Trullas and Skolnick 1993 | |||||||||||||
| C57BL/6J | DBA/2J | ns | ns | < | > | > | ns | ns | O'Leary et al. 2013 | |||||||||
| C57BL/6J | DBA/2J | > | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C57BL/6J OlaHsD | DBA/2 OlaHsd | ns | ns | ns | Brooks et al. 2005 | |||||||||||||
| C57BL/6J OlaHsD | DBA/2 OlaHsd | < | > | > | ns | < | ns | Võikar et al. 2005 | ||||||||||
| C57BL/6J OlaHsD | DBA/2 OlaHsd | ns | > | < | < | < | Rogers et al. 1999 | |||||||||||
| C57BL/6J OlaHsD | DBA/2 OlaHsd | > | ns | > | > | ns | > | > | ns | Mathiasen et al. 2008 | ||||||||
| C57BL/6J OlaHsD | DBA/2 OlaHsd | < | > | > | Mathiasen et al. 2008 T4 | |||||||||||||
| C57BL/6J OlaHsD | DBA/2 OlaHsd | > | < | ns | Mathiasen et al. 2008 T3 | |||||||||||||
| C57BL/6N CrlBR | DBA/2NCrlBR | < | < | < | < | ns | ns | ns | < | < | Podhorna and Brown 2002 | |||||||
| C57BL/6N Hsd | DBA/2 OlaHsd | < | ns | ns | > | Kulesskaya and Võikar 2014 | ||||||||||||
| C57BL/6N Hsd (Hel) | DBA/2 OlaHsd | ns | ns | ns | > | Kulesskaya and Võikar 2014 | ||||||||||||
| C57BL/10J | DBA/2J | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| C57L/J | DBA/2J | ns | > | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | FVB/N | < | ns | < | Kim et al. 2002 | |||||||||||||
| C57BL/6J | FVB/N A | > | ns | Ducottet and Belzung 2005 | ||||||||||||||
| C57BL/6J | FVB/N J | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| C57BL/6J | FVB/N J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | FVB/N J | < | < | Moy et al. 2007 | ||||||||||||||
| C57BL/6J | FVB/N J | ns | ns | < | > | ns | ns | < | O'Leary et al. 2013 | |||||||||
| C57BL/6J | FVB/N Tac | ns | < | Bothe et al. 2004 | ||||||||||||||
| C57BL/6J OlaHsD | FVB/N Hsd | ns | ns | ns | ns | ns | < | ns | < | < | Võikar et al. 2001 | |||||||
| C57BL/6N Tac | FVB/N Tac | < | < | Bothe et al. 2004 | ||||||||||||||
| C57L/J | FVB/N J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | SJL/J | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| C57BL/6J | SJL/J | ns | > | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | SJL/J | ns | < | ns | ns | < | Griebel et al. 2000 | |||||||||||
| C57BL/6J | SJL/J | < | < | ns | > | > | ns | ns | O'Leary et al. 2013 | |||||||||
| C57L/J | SJL/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57BL/6J | Swiss | ns | ns | ns | ns | ns | Griebel et al. 2000 | |||||||||||
| C57BL6/J | Swiss Webster/HSD | > | Crawley and Davis 1982 | |||||||||||||||
| C57BL6/J | Swiss Webster/NIH | < | Crawley and Davis 1982 | |||||||||||||||
| C57BL/6J | Swiss Webster | ns | ns | > | < | ns | ns | ns | ns | ns | ns | van Gaalen and Steckler 2000 | ||||||
| C57BL/6J OlaHsD | Swiss Webster | ns | ns | ns | < | ns | ns | ns | < | Rodgers et al. 2002b | ||||||||
| C57BL/6J OlaHsD | Swiss Webster | > | > | > | ns | Rodgers et al. 2002a | ||||||||||||
| C57BSW/6 CrlBR | Swiss Webster | ns | ns | ns | < | ns | ns | ns | ns | ns | < | van Gaalen and Steckler 2000 | ||||||
| C57BL/6N Hsd | ICR:Hsd | ns | ns | > | ns | Kulesskaya and Võikar 2014 | ||||||||||||
| C57BL/6N Hsd (Hel) | ICR:Hsd | > | ns | ns | ns | Kulesskaya and Võikar 2014 | ||||||||||||
| C57BL/6J | ICR | < | < | < | Nesher et al. 2012 | |||||||||||||
| C57BL/6J | CD1 | < | < | ns | ns | ns | Benatti et al. 2011 | |||||||||||
| C57BL/6J | CD1 | < | < | ns | ns | Benatti et al. 2011 | ||||||||||||
| C57BL/6J | SWR/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| C57L/J | SWR/J | ns | < | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| DBA/2J | 129/Sv J | < | ns | Ducottet and Belzung 2005 | ||||||||||||||
| DBA/2J | 129S1/Sv ImJ | ns | ns | ns | < | > | > | > | O'Leary et al. 2013 | |||||||||
| DBA/2J | 129S1/Sv ImJ | > | < | < | > | < | < | ns | ns | Lad et al. 2010 | ||||||||
| DBA/2J | 129S1/Sv ImJ | ns | ns | < | ns | ns | Moy et al. 2007 | |||||||||||
| DBA/2J | 129S1/Sv lmJ | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| DBA/2J | 129S3/Sv lmJ | ns | < | > | Cook et al. 2001 | |||||||||||||
| DBA/2J | 129S6 | < | ns | ns | ns | ns | > | Holmes et al. 2002 | ||||||||||
| DBA/2 OlaHsd | 129/Sv Hsd | > | < | > | > | > | Rogers et al. 1999 | |||||||||||
| DBA/2 OlaHsd | 129S2/Sv Hsd | > | ns | < | ns | Kulesskaya and Võikar 2014 | ||||||||||||
| DBA/2 OlaHsd | 129S2/Sv Hsd | < | ns | > | Brooks et al. 2005 | |||||||||||||
| DBA/2J | A/J | < | > | ns | < | > | ns | < | > | Lad et al. 2010 | ||||||||
| DBA/2J | A/J | < | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| DBA/2J | A/J | < | < | ns | > | > | Moy et al. 2007 | |||||||||||
| DBA/2J | A/J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| DBA/2J | A/J | ns | < | > | ns | > | ns | > | O'Leary et al. 2013 | |||||||||
| DBA/2J | CBA/J | > | < | < | Cook et al. 2001 | |||||||||||||
| DBA/2J | CBA/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| DBA/2J | CBA/J | ns | ns | ns | ns | ns | Griebel et al. 2000 | |||||||||||
| DBA/2J | CBA/J | < | < | < | Trullas and Skolnick 1993 | |||||||||||||
| DBA/2J | CBA/J | < | ns | Ducottet and Belzung 2005 | ||||||||||||||
| DBA/2 OlaHsd | CBA/Ca OlaHsd | ns | < | ns | Brooks et al. 2005 | |||||||||||||
| DBA/2 OlaHsd | CBA/Ca OlaHsd | ns | < | ns | ns | ns | Rogers et al. 1999 | |||||||||||
| DBA/2J | FVB/N A | > | ns | Ducottet and Belzung 2005 | ||||||||||||||
| DBA/2J | FVB/N J | ns | ns | ns | ns | < | < | ns | ns | Lad et al. 2010 | ||||||||
| DBA/2J | FVB/N J | ns | < | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| DBA/2J | FVB/N J | < | < | Moy et al. 2007 | ||||||||||||||
| DBA/2J | FVB/N J | ns | ns | < | < | < | ns | < | O'Leary et al. 2013 | |||||||||
| DBA/2J | SJL/J | ns | ns | ns | ns | ns | > | < | > | Lad et al. 2010 | ||||||||
| DBA/2J | SJL/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| DBA/2J | SJL/J | ns | < | < | < | < | Griebel et al. 2000 | |||||||||||
| DBA/2J | SJL/J | ns | < | < | < | < | ns | ns | O'Leary et al. 2013 | |||||||||
| DBA/2J | Swiss | ns | ns | < | ns | ns | Griebel et al. 2000 | |||||||||||
| DBA/2J | SWR/J | ns | < | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| DBA/2 OlaHsd | ICR:Hsd | > | ns | < | > | Kulesskaya and Võikar 2014 | ||||||||||||
| FVB/N J | 129S1/Sv ImJ | ns | ns | > | ns | > | > | > | O'Leary et al. 2013 | |||||||||
| FVB/N J | 129S1/Sv ImJ | > | < | < | ns | ns | ns | ns | ns | Lad et al. 2010 | ||||||||
| FVB/N J | 129S1/Sv ImJ | > | > | Moy et al. 2007 | ||||||||||||||
| FVB/N J | 129S1/Sv lmJ | ns | ns | ns | ns | ns | ns | > | Milner and Crabbe 2008 | |||||||||
| FVB/N Hsd | 129S2/Sv Hsd | < | ns | ns | ns | ns | > | > | > | > | Võikar et al. 2001 | |||||||
| FVB/N A | 129/Sv J | < | ns | Ducottet and Belzung 2005 | ||||||||||||||
| FVB/N Tac | 129S6/Sv EvTac | > | > | Bothe et al. 2004 | ||||||||||||||
| FVB/N Tac | 129P3/J | ns | ns | Bothe et al. 2004 | ||||||||||||||
| FVB/N J | A/J | < | > | > | < | > | > | < | > | Lad et al. 2010 | ||||||||
| FVB/N J | A/J | < | ns | ns | ns | ns | < | > | > | > | > | > | ns | Milner and Crabbe 2008 | ||||
| FVB/N J | A/J | ns | < | > | ns | > | ns | > | O'Leary et al. 2013 | |||||||||
| FVB/N J | A/J | > | > | Moy et al. 2007 | ||||||||||||||
| FVB/N A | CBA/J | < | ns | Ducottet and Belzung 2005 | ||||||||||||||
| FVB/N J | CBA/J | ns | ns | ns | ns | > | > | ns | Milner and Crabbe 2008 | |||||||||
| FVB/N | CBA/N | > | > | > | Kim et al. 2002 | |||||||||||||
| SJL/J | A/J | < | > | > | < | > | > | ns | ns | Lad et al. 2010 | ||||||||
| SJL/J | A/J | ns | ns | > | ns | > | ns | > | O'Leary et al. 2013 | |||||||||
| SJL/J | A/J | < | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| SJL/J | CBA/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| SJL/J | CBA/J | ns | ns | > | > | > | Griebel et al. 2000 | |||||||||||
| SJL/J | FVB/N J | ns | ns | ns | ns | < | ns | > | < | Lad et al. 2010 | ||||||||
| SJL/J | FVB/N J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| SJL/J | FVB/N J | ns | > | ns | ns | < | ns | < | O'Leary et al. 2013 | |||||||||
| SJL/J | SWR/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| SJL/J | Swiss | ns | ns | ns | > | > | Griebel et al. 2000 | |||||||||||
| CBA/J | A/J | < | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
| CBA/J | A/J | > | > | > | Trullas and Skolnick 1993 | |||||||||||||
| CBA/J | Swiss | ns | ns | < | ns | ns | Griebel et al. 2000 | |||||||||||
| CBA/J | SWR/J | ns | ns | ns | ns | Milner and Crabbe 2008 | ||||||||||||
Most data were estimated from average group values of available test parameters in tables or graphs. Difference between strains were reported from each selected research paper when available, otherwise we used tables and figures and estimated a difference between two group based on the mean and standard error to the mean (s.e.m.). A difference between strains was considered to be present when we observed no overlap between mean and between s.e.m. of a group pair. Mathiasen et al. 2008 T3 and T4, refers to table 3 and table 4, respectively.
OA, open arms; TT, total crossings; DL, dark to lit; LD, Lit to dark; LIT, lit compartment; C, central area of the open field; lt, latency; x, crossings; t, time; %x, percent entries; %t, percent time; Inferior (<), superior (>) signs and nonsignificant (ns) refer to difference obtained by a mouse strain in column 1 compared to a mouse strain in column 2 of the same row.
The Tests of Unconditioned of Anxiety
The EPM consists of four arms radiating from a central platform forming a plus sign shape; it is elevated from the ground with two opposed walled arms and two opposed open arms (Fernandes and File 1996; Handley and Mithani 1984). Another variant of this test is the EZM, which consists of a circular runway divided in two enclosed quadrants opposite to two open quadrants (Shepherd et al. 1994; Weiss et al. 1998). In the EPM, a mouse or a rat is released in the central area (Griebel et al. 2000; Holmes et al. 2003; Rodgers et al. 2002a,b), whereas in the EZM a mouse or a rat is released in one of the enclosed quadrants (Heredia et al. 2013; Holmes et al. 2003). The LDB consists of two chambers one lit and the other dark connected through a small opening or a tunnel (Aulich 1976; Crawley and Goodwin 1980; Hascoët and Bourin 1998). Animals are placed either in the middle of the lit chamber (Bourin and Hascoët 2003; Costall et al. 1989; Holmes et al. 2003) or the dark chamber (Heredia et al. 2014; Müller et al. 2003; Oitzl et al. 2001). The OF consists of either a cylindrical, rectangular, or a square box with open top, and with (van Gaalen and Steckler 2000) or without (Heredia et al. 2014; Lalonde and Strazielle 2008; van Gaalen and Steckler 2000) an object in the center of the field. In the OF without object, animals are released from the central arena (Heredia et al. 2014; Hall et al. 2000; Lalonde and Strazielle 2008) or from one of the corners (Kelley et al. 2003; Kulesskaya and Võikar 2014). In the OF with object, animals are released from one of the corner of the arena (Hall et al. 2000; Kelley et al. 2003). In all these tests, mice or rats mice are left to explore the mazes for 5–10 min. In the case of the OF, animals can be exposed for more than 10 min.
The 3D Maze Open Space Anxiety Test
The 3D maze is a modified version of the radial arm maze (Ennaceur et al. 2008). It was originally developed for assessing spatial navigation from different view perspectives (Mostafa et al. 2002). It consists of nine arms. Each arm is attached to a bridge, which radiates from a nonagonal shaped central hub. Mice can access an arm only by crossing a bridge. The bridges can be level with the arms providing a standard radial maze configuration. They can also be tilted upward or downward providing a maze with raised or lowered arm configurations, respectively (Fig. 1). All parts of the maze apparatus are unprotected; hence, mice are exposed to a complete open space. In our anxiety experiments, we used the raised arms configuration; the bridge to each arm formed a slope, which was inclined upward by about 40°. A mouse is transported in a small beaker; this is tilted gently over the center platform of the maze for the release of the mouse, which is then let free to explore for 12 min.
Figure 1.

Picture of the three‐dimensional 9 arms maze.
The validity of the open space anxiety tests, which include the 3D maze and the elevated platform with attached slopes, and the validity of the TUA were discussed in a recent review (Ennaceur 2014). The 3D maze offers a completely open space. It is based on the view that in anxiety conditions, humans and animals face an ambiguous situation. They are (or feel) unable to avoid/escape or approach the perceived threat stimulus. Therefore, a test of anxiety needs to expose animals to conditions which involve uninformative or ambiguous stimuli, and that the outcomes from the choice between these stimuli are uncertain. When exposed to an open space, animals try to escape or explore to find a refuge. This motivation to escape is exploited in the 3D maze to provide measures of anxiety. Hence, apparent escape routes are made available, but the distant segments of these routes are left inaccessible to immediate or direct sensory perception. The experience of fear from the unfamiliar and open space is therefore complicated by the ambiguity of the choices and the uncertainty of the choice outcomes. Entries into the distal segments of the test environment are used to determine anxiety in animals. A low level of anxiety or a reduction in anxiety is reflected by an increase in the number of entries into the arms of the maze.
Natural Preference Versus Security and Safety Versus Conflict motivations
In the TUA, untreated animals have been reported to show a natural preference for the protected/unlit space and a natural aversion of the unprotected/lit space. For most authors, TUA set into play a conflict between these two natural tendencies. The motivation to stay in a protected/unlit space, which is naturally associated with safety and security, opposes the motivation to explore an unprotected/lit space, which is naturally associated with possible threat and danger. Diazepam and other benzodiazepine drugs appear to moderate and lessen this conflict.
In the EPM, animals are reported to display an aversion of the open arms from the second minute of a test session and, this aversion is increased further throughout the test session and, in subsequent sessions (Arabo et al. 2014; Casarrubea et al. 2013; Espejo 1997; Holmes and Rodgers 1998; Rosa et al. 2000; Treit et al. 1993). In addition, a single previous experience of the EPM or LDB has been reported to reduce or abolish the effects of both anxiolytic and anxiogenic drugs (Dawson et al. 1994; Escarabajal et al. 2003; Holmes et al. 2001; Holmes and Rodgers 2003; Rodgers and Shepherd 1993). Furthermore, this persistent aversion of the open arms and this “one‐trial tolerance” has been reported for various strains of mice and rats (Cook et al. 2002; Izídio et al. 2005; Rodgers and Cole 1993). Numerous interpretations have been provided to account for these behaviors, but none has considered the possibility that the current TUA promotes a natural preference for a protected and/or an unlit space over risk taking (see Ennaceur 2014). A number of studies suggest that, in a natural or experimental open field environment, the primary function of the behavior of mice and rats is to optimize security (Alstott and Timberlake 2009; Whishaw et al. 2006; Yaski and Eilam 2007). Hence, whether impulsivity, curiosity or attempt to find an escape route would have led animals initially to make a few entries into the open and/or lit space, these entries can only decline within and between sessions. The prevalence of security and safety provided by the enclosed spaces is likely to reduce or eliminate the incentive to explore other parts of a test apparatus, which are lit and/or unprotected. Indeed, in our previous studies, when a refuge was provided during the test, both anxious (BALB/c) and less anxious (C57/BL6J and CD‐1) strains of mice did not venture into the arms of the 3D maze (Ennaceur et al. 2008) and into the steep slopes attached to an elevated platform (Michalikova et al. 2010); they spent most of the time inside the refuge. These results are supported by other studies, which suggest that the behavior of rats and mice in a novel environment is directed toward optimizing safety (Alstott and Timberlake 2009; Whishaw et al. 2006; Yaski and Eilam 2007). Rats and mice, like other animals of prey in the wild, are most likely to experience anxiety when they are in the open than when they are hiding in a burrow. The interpretation of the behavior of rodents in the current TUA suggests the opposite; avoidance of the open/lit space is considered indicative of high anxiety though most, if not all, authors describe the selection of the protected/unlit space as a natural preference response. It has been difficult to challenge this paradox. The anxiety construct validity of the current TUA is defended on the basis that these tests involve a conflict, though no objective evidence has been provided to support the view that animals are intent on visiting the open/lit space. It is not clear why the selection and preference of the protected/unlit space indicates anxiety rather than a sense of safety and security. In fact, avoidance and escape responses that terminates the occurrence or experience of an aversive stimulus is rewarding, and would reinforce the repetition of these responses (see, Kim et al. 2006). Hence, a mouse or a rat exposed to EPM, EZP, LDB, or OF escapes to or avoids from the protected/unlit space, and these responses are consolidated further with repeated exposures to these tests (Arabo et al. 2014; Casarrubea et al. 2013; Espejo 1997; Holmes and Rodgers 1998; Rosa et al. 2000; Treit et al. 1993).
Stretch‐attend posture is one of the ethological parameters that is presented as indicative of the conflict experienced by animals in the TUA. Decreased open arm entries and increased stretch‐attend postures are considered indicative of increased anxiety in the EPM. We argue here that stretch‐attend posture does not provide objective and unequivocal measures of the ‘hidden motivation’ of animals to explore the open/lit space, and less likely an indicator of anxiety. In fact, it proved inconsistent and unreliable in a number of studies. In the EPM, diazepam was reported to increase the percent open arm entries (POAE) (Dalvi and Rodgers 1999; Mechan et al. 2002) and percent open arm time (POAT) (Mechan et al. 2002) without producing any effect on stretch‐attend posture (Dalvi and Rodgers 1999; Mechan et al. 2002). Gepirone, a 5‐HT partial agonist, was also reported to increase POAE and POAT without any effect on SAP (Silva and Brandão 2000). In the EZM, both amphetamine and chlordiazepoxide were reported to increase the amount of time in the open areas of the maze and decreased the occurrence of stretched‐attend postures (Weiss et al. 1998). This anxiolytic‐like effect of amphetamine contrasts with the anxiogenic‐like effect of this same drug observed in the EPM in another study in which chronic treatment with AMPH produced a significant decrease in POAT and no effect on SAP (Cancela et al. 2001). In addition, acute treatment with fluoxetine was reported to decrease POAE and POAT while chronic treatment had no effect, and both treatments did not affect SAP (Silva and Brandão 2000). The above studies highlight the inconsistency of the results obtained in the EPM or EZM, and illustrate the poor utility of stretch‐attend posture. There is no concordance between this ethological parameter and the traditional measures of anxiety.
In the 3D maze, animals that express high anxiety through avoidance of the arms in the first sessions do visit the arms after a number of exposures to the test (Ennaceur 2011). The motivation to explore the arms is evident with both low and high anxiety strains as the number of entries increases, and exceeds 8 arm visits with further exposures. In the EPM, however, the number of open arm entries decline to a floor level in a subsequent exposure whether animals were low or high anxiety strain (Arabo et al. 2014; Cook et al. 2002; Espejo 1997; Holmes and Rodgers 1998; Rodgers and Shepherd 1993; Treit et al. 1993), and whether they received saline or anxiolytic treatments (Dawson et al. 1994; Bertoglio and Carobrez 2003; Escarabajal et al. 2003; File et al. 1992; Holmes and Rodgers 1998; Rodgers and Shepherd 1993). These results from repeated exposures to the EPM underlie furthermore animals’ lack of motivation to explore the open/lit space.
Single Versus Multiple Test Sessions
One of the major limitations of the EPM is that it cannot be used for more than one session in screening for potential anxiolytic candidate drugs. Numerous studies reported that animals exposed for more than one session to the EPM demonstrate further avoidance of the open arms. Benzodiazepines and other drugs proved ineffective in a second exposure to the test (Bertoglio and Carobrez 2003; Dawson et al. 1994; Escarabajal et al. 2003; File et al. 1992; Holmes and Rodgers 1998; Rodgers and Shepherd 1993).This lack of sensitivity makes it very difficult to predict the therapeutic potential of a drug, especially for chronic use, as it is possible that an initial reaction to a drug differs from its effects on subsequent uses (Abuhamdah et al. 2015; Cole and Pieper 1973; de Wit and Phillips 2012).
When exposed to an unfamiliar radial arm maze, rats and mice enter frequently into the proximal segment of an arm of the maze and do not continue into the distal segment. In the 3D maze, these proximal (bridges) and distal (arms) segments are clearly delineated. Animals are observed to reach the end of the first segment, then withdraw and return to the central platform. They seem unable to take a risk and venture far away from the central platform. This avoidance of the distal segment is used as an indicator of fear and anxiety in mice. In previous studies (Ennaceur et al. 2006, 2008; Ennaceur 2011), we demonstrated that BALB/c mice, unlike C57BL/6J and CD‐1 mice, did not venture into the arms of the maze when left to explore for the first time. C57BL/6J and CD‐1 mice visited a number of arms on the first and second exposure, respectively, whereas BALB/c required more exposures (Fig. 3). Hence, unlike in the EPM and the other anxiety tests, in which subsequent exposures lead to a reduction in motor activity and further avoidance of the open/lit space in both anxious and nonanxious strains of mice, in the 3D maze there is no decrease in motor activity but there is rather a decrease in avoidance responses. When a mouse starts visiting an arm or a few arms in a session, it continues visiting more arms in subsequent sessions (i.e., becomes less anxious with experience).
The 3D maze anxiety test can be run in a single 10–12 min session, or in multiple sessions with or without food deprivation. Repeated visits, each initiated from the central platform, to the same arms are counted as separate individual visits whereas repeated back and forth visits between a bridge and an arm are counted as a single visit. It is possible to set a criterion of 8 or 9 arm visits in a session that lasts 10–12 min. BALB/c mice reached this criterion in five sessions, whereas C57 and CD‐1 required 1 to 2 sessions, respectively (Fig. 3). Consistent differences were observed between these three strains of mice in a number of experiments conducted in our laboratory.
The 3D maze offers a large window of opportunity to observe the effects of an experimental manipulation on anxiety. Using a high anxiety strain, the effect of an anxiolytic drug can be detected within a few number of sessions, whereas using a low anxiety strain an anxiogenic effect can be detected in the first session and can last over a number of sessions.
Anxiety Indices and Measurements
The TUA are further complicated by the availability of a variety of spatio‐temporal and ethological parameters, among which only a few and sometimes a single parameter (not always the same one) is reported to indicate a change in anxiety response (Crawley and Davis 1982; Drapier et al. 2007; Ducottet and Belzung 2004, 2005; Kulesskaya and Võikar 2014; Lalonde and Strazielle 2008; Lin et al. 1999; Rodgers et al. 2002a,b; Võikar et al. 2004). In addition, in the EPM, the majority of authors prefer reporting percent instead of absolute values (Dawson et al. 1995; Silva and Brandão 2000; Rodgers et al. 2002a) while it is apparent that, in some cases, differences between strains or drug treatment and doses are observed in animals with low exploratory activity and/or with a small difference between open arm and enclosed arm entries. In addition, POAT is obtained from time spent in the open arms divided by test duration (Rodgers et al. 1997, 2002a,b; Dalvi and Rodgers 1999; Jones and King 2001; Mathiasen et al. 2008) or time spent divided by the total time spent in both arms (Bertoglio and Carobrez 2002; Lin et al. 1999; Fernandes and File 1996; Trullas and Skolnick 1993). The former includes a significant amount of time spent in the central area of the maze.
In the EPM, changes in anxiety are often determined by one selected index, and in most cases it is the time spent in the open arms or POAT (Cook et al. 2001; Hendrie et al. 1997; Harada et al. 2006; Heredia et al. 2012; Rodgers and Dalvi 1997; Wilson et al. 2004; Popik et al. 2006). However, a large amount of time spent in open arms can sometimes refer to a single or very few open arm entries. In addition, a mouse strain is determined as low or high anxiety irrespective of the number of entries and amount of time spent in open arms, which are often below 50% of the total entries or the total test duration (Chaouloff et al. 1997; Dalvi and Rodgers 1999, 2001; Griebel et al. 2000; Hagenbuch et al. 2006; Harada et al. 2006; Mechan et al. 2002; Menard and Treit 1996; O'Leary et al. 2013; Rodgers et al. 1997; Shepherd et al. 1994). There is no criterion that determines when avoidance of open arms ceases to be avoidance. A place preference parameter can be derived from the difference between open and closed arm entries or time, but we are not aware that it has ever been exploited. However, whichever the selected anxiety parameter, most studies were unable to demonstrate any concordance between measurements (File et al. 1998; Harada et al. 2006; Mathiasen et al. 2008; O'Leary et al. 2013; Rodgers et al. 2002a; Smith et al. 2012; see Table 2). Hence, there is not a single measure of anxiety that is commonly used to account for changes in rodents’ anxiety response, and that one can rely on to compare anxiety test results between research studies (see Tables 1 and 2). Looking at the first four rows in table 1, DIFF (preference index) suggests that the strains of mice in the first and second row are less anxious than the two strains from the rows below, whereas the POAE suggests that strains of mice in the first and third row are the least anxious. However, POAT suggests that mice on the second row are less anxious than all other strains, and those in the fourth row are the most anxious. It is also possible to argue that mice with 90% open time show either strong preference for the open arms or strong avoidance of the closed arms.
The use of open arms avoidance index (OAAI = 100–(% time + % entries in the open arms) / 2) proposed by Trullas and Skolnick (1993) can complicate the matter further. O'Leary et al. (2013) reported that POAE and POAT were significantly high in BALB/cBy compared to all other mouse strains, except BALB/cJ and C3H on POAE; these two mouse strains were not different from each other. POAE and POAT were also significantly high in BALB/cJ compared to AKR and BTBR. However, the OAAI, which has been used by this group in other studies (Brown et al. 1999; Podhorna and Brown 2002) seems low in A/J mice compared to any other mouse strain, and it seems high in BALB/cJ compared to BALB/cBy and C3H mice. There were no differences between BALB/cJ mice and AKR, BTBR or SJL mice. Based on this index, one can reach a different conclusion from that reported by the authors. Contrary to POAE and POAT, this index suggests that A/J is the least anxious mice and not BALB/cBy mice, and that BALB/cJ mice are more anxious than BALB/cBy and C3H mice, and they are not less anxious than AKR, BTBR or SJL mice.
In the 3D maze, a number of parameters are recorded such as latency of first crossing into a bridge and an arm, number of crossings and time spent on the bridges and arms, but only the number of crossings into the arms is used as the main index of anxiety. In addition, a criterion of 8 or 9 arm visits in a session that lasts 10–12 min is used to determine differences in anxiety between mouse strains and between treatments. Mice that achieve the criterion earlier than others are deemed to present low level of anxiety. The latency of first entry onto an arm is another specific index of anxiety, but it can be influenced by the handling expertise of the experimenter. We recommend that a small beaker is used to transport a mouse to the maze. The beaker is then tilted gently over the floor of the central platform to release a mouse.
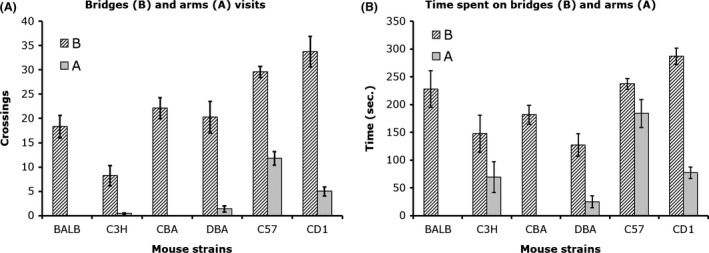
It has been suggested that risk‐avoidant decision making is specifically associated to anxiety (Maner et al. 2007; Giorgetta et al. 2012; Paulus and Yu 2012). This behavior implies that, in anxiety situation, there is a time spent to evaluate a risk, which may or may not be followed by the execution of a risky decision. Hence, the time it takes to approach a threatening stimulus (latency) and the number of approaches of this stimulus can be used as specific measures of anxiety. However, it is not possible to rely on the latency to approach as well as the time spent in contact with the threatening stimulus unless more than a single approach is recorded, for a mouse or a rat may approach and spend a long time in contact with a potentially threatening stimulus then demonstrates a systematic avoidance response afterward. For instance, a mouse can run into an arm and freezes there. This mouse may record longer time than a mouse that moved freely in the maze and recorded a high number of arm entries. We observed this behavior with some C3H mice, which did not differ significantly from CD‐1 in the time spent in the arms (see Fig. 2). However, C3H mice did not visit more than one arm, whereas CD‐1 mice made at least three arm visits each.
Figure 2.
In this experiment, different strains of mice were exposed to 8 arms maze in a single 12 min session. (A) The number of crossings into the bridges was significantly high in C57 and CD1 mice and significantly low in C3H mice compared to the other strains of mice. BALB/c, C3H, CBA/J, and DBA mice made generally no entries into the arms (80% made zero visits), whereas C57 and CD1 mice did cross into the arms with a group average of 12 and 5 arm visits, respectively. (B) The time spent on the arms is significantly high in C57 mice compared to the other groups. The time spent by C3H on the arms represents a single arm visit made by half of the group, the other half made no visit.
It is important to note here that, in the 3D maze, nonspecific effects of a treatment are determined from entries and time spent on the bridges. Strictly, a treatment is deemed anxiolytic if a high anxiety strain makes at least 8 arm visits, and that arm/bridge entries ratio approaches 1. A treatment is deemed anxiogenic if a low anxiety strain of mice demonstrates a reduction in the number of arm entries and the index arm bridge ratio is inferior to 50%. The reduction in arm entries must be below the minimum 8 arm visits.
Among the most commonly used mouse strains in anxiety studies, C3H/J, CBA/J, FVB/NJ, and SJL/J have been reported to present retinal degeneration (Mrosovsky et al. 1999; Chang et al. 2002; Clapcote et al. 2005). Inconsistencies between reports do not allow us determine whether such handicap could account for differences in anxiety response between any of these and other strains of mice in TUA. These inconsistencies are not limited to anxiety indices but extend to locomotor and exploratory activity as well. Each of these mouse strains has been shown to demonstrate either high or low anxiety in different reports (Table 2). In a number of studies, C3H mice appear to spend longer time in the open arms (expressed in percent) than some other mouse strains (Brooks et al. 2005; Cook et al. 2001; Griebel et al. 2000; Hagenbuch et al. 2006; Lad et al. 2010; O'Leary et al. 2013). These studies did not indicate whether these visits were limited to the proximal or distal segments of the open arms, and some authors did not disclose the actual number of entries into the open or enclosed arms. In the 3D maze, C3H mice appear to differ from all other mouse strains by their low number of bridge entries (8.23 ± 2.04). This is not the case with CBA mice, which suffer from the same retinal degeneration. The number of crossings in CBA (22.13 ± 2.16) was not different from that of BALB/c (18.31 ± 2.31) and DBA (20.25 ± 3.24). In the present experiment, C3H mice appear to demonstrate high anxiety comparable to that of BALB/c, CBA, and DBA mice. They may require a number of exposures to the test to make eight or more arm visits as it was demonstrated in BALB/c mice.
Sensitivity of the 3D Maze to Strains of Mice and Drug Treatments
Strains of mice
Assessment of the effects of an experimental intervention requires either the selection of a strain of rats or mice that allows bidirectional changes in anxiety responses, or the selection of two strains of rats or mice that show opposite anxiety responses. In the latter, an anxiolytic treatment will be expected to bring the level of high anxiety strain (experimental) close to that of the low anxiety strain (control) and an anxiogenic treatment will increase anxiety to the level of the high anxiety strain (control).
We examined a number of mouse strains for differences in response to exposure to the 3D maze in a single 12 min test sessions. These strains comprise five inbred strains (BALB/cByJ, C3H/HeJ, C57BL/6J, CBA/J, DBA/2J), and one outbred strain (CD1‐ICR). We did also examine difference between BALB/cByJ, C57BL/6J, and CD1‐ICR mice in three or more test sessions; these are either food or nonfood deprived. The single test session study indicated that BALB/c, C3H, CBA/J, and DBA mice made generally no entries into the arms (80% made 0 visit), whereas C57 and CD1 mice did cross into the arms with a group average of 12 and 5 arm visits, respectively (Fig. 2). This study indicates that C57 and CD‐1 presented a low level of anxiety compared to the other strains of mice. However, if we introduce 8‐arm visits criterion, then only C57 qualifies as a low anxiety strain. This criterion is necessary to determine when animals are no longer avoiding the arms. Its relevance is more evident when animals are exposed to the test for more than a single session.
In a multiple test sessions, we examined the behavior of food deprived BALB/c, C57, and CD‐1 mice, and we observed that BALB/c mice required about five sessions to make 8 arm visits, whereas C57 and CD1 mice made this number of arm visits after one or two sessions, respectively (Fig. 3). C57BL/6J mice treated with dizocilpine, an NMDA receptor antagonist, demonstrated an increase in anxiety which was maintained over more than seven sessions (Ennaceur et al. 2011). These mice made more bridge entries than saline‐treated mice, which preclude psychomotor deficits (see section Anxiety Indices and Measurements).
Figure 3.
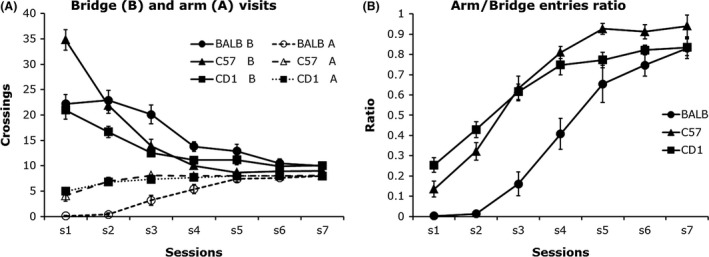
In this experiment, mice were food deprived, and exposed to 8 arms maze until 8 arm visits were made or 10 min elapsed. (A) C57 made 8 arm choices with a high number of bridge visits; it was followed by CD‐1 on the third sessions and BALB/c in the fifth session. (B) With repeated exposures to the maze, the number of bridge visits decreased until arm/bridge entries ratio got close to 1. The arm/bridge ratio is over 0.6 in the third session for C57 and CD1 mice and in the fifth session for BALB/c mice.
We explored food deprivation because this was reported to increase exploratory activity (Carr et al. 1959; De Lorge and Bolles 1961; File and Day 1972; Timberlake and Birch 1967; Levay et al. 2007) and affect anxiety responses (Levay et al. 2007; Inoue et al. 2004; Jahng et al. 2007) in rodents. In addition, anxiety as well as both anxiolytic and anxiogenic interventions can affect learning and memory performance (Macbeth and Luine 2010; Mintzer and Griffiths 2007; Nakamura–Palacios and Roelke 1997; Ohl et al. 2003; Packard 2009; Salomons et al. 2012). Screening for novel anxiolytics needs to exclude any deleterious drug effect on cognition. For instance, benzodiazepines’ anxiolytic effect is undermined by its negative action on some cognitive processes (Coull et al. 1995; Herzog et al. 2000; Mintzer and Griffiths 2007; Nakamura–Palacios and Roelke 1997; Soto et al. 2013; Tiplady et al. 2005).
It has been suggested to us that such differences between strains have been demonstrated with the current TUA, and therefore the present 3D maze open space anxiety test does not provide anything new. Indeed, numerous studies investigated the behavior of various strains of mice in the current TUA, and over 30 years since these tests were proposed, there is not a single strain of mice that is consistently reported to present either low or high anxiety within the same anxiety test or between anxiety tests (Cook et al. 2001; DuBois et al. 2006; Griebel et al. 2000; Holmes et al. 2002; Livneh et al. 2010; Podhorna and Brown 2002; Võikar et al. 2005).
BALB/c and C57BL/6 mice are the most commonly used in anxiety studies. Some studies reported that the former are more anxious than the latter in the LDB (Kopp et al. 1999; Lepicard et al. 2000; Griebel et al. 2000; Verleye et al. 2011) and in the EPM (Lepicard et al. 2000; Verleye et al. 2011), whereas other studies demonstrated lower anxiety in BALB/c mice in the EPM (An et al. 2011; Avgustinovich et al. 2000; Griebel et al. 2000; Livneh et al. 2010; Nesher et al. 2012; Trullas and Skolnick 1993) or no difference between the two mouse strains in the EPM (Brooks et al. 2005; Griebel et al. 2000; Lalonde and Strazielle 2008; Keum et al. 2016; Yilmazer–Hanke et al. 2003), the OF (Keum et al. 2016; Kim et al. 2002) and the LDB (Kim et al. 2002).
Inconsistent reports were observed in other strains of mice. For instance, DBA/2 mice were reported to present high anxiety in the OF (DuBois et al. 2006; Holmes et al. 2002; Lad et al. 2010) and the LDB (Võikar et al. 2005; DuBois et al. 2006; Holmes et al. 2002; Lad et al. 2010) compared to C57 mice, and in the EPM compared to C57 (Lad et al. 2010; Võikar et al. 2005) and BALB/c mice (Rogers et al. 1999). They were also reported to present low anxiety compared to C57 in the EPM (Gard et al. 2001; Podhorna and Brown 2002; Trullas and Skolnick 1993) and the OF (Podhorna and Brown 2002; Trullas and Skolnick 1993). Other results indicate no differences between DBA and C57 in the LDB (Gard et al. 2001; Griebel et al. 2000) and in the EPM (Brooks et al. 2005; Griebel et al. 2000; Holmes et al. 2002).
Additional examples of inconsistencies are observed in C3H mice. This strain of mice was reported to display low anxiety in the EPM compared to DBA, C57 (Brooks et al. 2005; Cook et al. 2001; Griebel et al. 2000; Trullas and Skolnick 1993; Livneh et al. 2010) and BALB/c (Brooks et al. 2005; Cook et al. 2001; Griebel et al. 2000). It was also shown to display low anxiety in the LDB compared to BALB/c (Bouwknecht and Paylor 2002; Griebel et al. 2000; Kopp et al. 1999; Lad et al. 2010), and in an OF compared to BALB/c and C57 (Kopp et al. 1999; Lad et al. 2010). However, other studies reported that C3H display high anxiety compared to BALB/c in the EPM (Rogers et al. 1999; Trullas and Skolnick 1993; Yilmazer–Hanke et al. 2003) and compared to C57 in the EPM (Yilmazer–Hanke et al. 2003) and EZM (Tarantino et al. 2000; Wilking et al. 2012). They were also reported to display high anxiety compared to BALB/c and C57 in the LDB (Kopp et al. 1999). In contrast, other studies reported no difference between C3H and C57 (Ducottet and Belzung 2005; Hagenbuch et al. 2006) and between C3H and both DBA and BALB/c in the EPM (Ducottet and Belzung 2005; Griebel et al. 200; Lad et al. 2010), the LDB (Bouwknecht and Paylor 2002; Griebel et al. 2000; Lad et al. 2010) and the OF (Lad et al. 2010).
Comparable inconsistent and conflicting results have been reported in various publications, but their authors fell short to question the construct validity of the TUA. They suggested instead various contributing factors. These include animal suppliers (Parra et al. 2013; Palm et al. 2011), the handling experimenter (Heredia et al. 2012; Crabbe et al. 1999; Lewejohann et al. 2006; Chesler et al. 2002), apparatus structure and color (Fernandes and File 1996; Violle et al. 2009; Horii and Kawaguchi 2015; Filgueiras et al. 2014; Albrechet–Souza et al. 2005; Lamberty and Gower 1996), or illumination and light/dark cycle (Fonken et al. 2009; Violle et al. 2009; Garcia et al. 2005), cage color (Sherwin and Glen 2003) and cage group size (Heredia et al. 2012; Botelho et al. 2007), enrichment (Abramov et al. 2008; Loss et al. 2015; Ravenelle et al. 2014), and bottle drinking size orifice (Dotson and Spector 2005). In fact, anything from the laboratory environment, even an allergic experimenter wearing a respirator (Crabbe et al. 1999), has been presented to justify the appalling state of affairs of the TUA. While evidence in support of the contribution of a number of these factors has been provided, subsequent reports appear to contradict these lines of evidence (Goes et al. 2015; Jones and King 2001; Arndt et al. 2009; Augustsson et al. 2003; Becker and Grecksch 1996; Nicholson et al. 2009; Hagenbuch et al. 2006; Cohen et al. 2001; Lewejohann et al. 2006; Pellow et al. 1985; Wolfer et al. 2004).
Diazepam
Diazepam, chlordiazepoxide, and other benzodiazepine drugs have been reported to demonstrate anxiolytic effects in the EPM, the LDB and the OF (Chaouloff et al. 1997; Costall et al. 1989; Crawley 1985; Crawley and Goodwin 1980; Pellow et al. 1985; Lepicard et al. 2000; Hascoët and Bourin 1998; Mechan et al. 2002). This sensitivity to the anxiolytic effects of benzodiazepines seems to vary between strains of mice, and between anxiety tests, and it is neither with the same strain of mice nor with the same anxiety test between reports (Belzung et al. 2000; Crabbe et al. 1999; Griebel et al. 2000; Rodgers et al. 2002a; Mechan et al. 2002; Lepicard et al. 2000; Hascoët and Bourin 1998). In addition, prior experience was found to abolish the effect of benzodiazepines on anxiety indices (Bertoglio and Carobrez 2002; Cruz–Morales et al. 2002; Dawson et al. 1994; File and Zangrossi 1993; Holmes et al. 2001; Rodgers and Shepherd 1993; Treit et al. 1993).
In the 3D maze, we examined the effect of different doses of diazepam in BALB/c, C57BL/6J, and CD‐1 (Ennaceur et al. 2008). The results did not produce the expected anxiolytic effects in BALB/c mice, but demonstrated rather a dose‐dependent decrease in the number of bridge and arm visits in C57BL6/J mice. The number of bridge and arm entries was also decreased in CD‐1 and appears unaffected in BALB/c mice (Fig. 4). The effect of diazepam in the 3D maze contrasts with results obtained with the same doses in another open space anxiety test, the elevated platform with steep slopes attached on two opposite sides (Ennaceur et al. 2010). In this test, all BALB/c mice that were injected with different doses of diazepam were able to cross into the slopes from the first test session, and continued to do so in subsequent two sessions, whereas BALB/c mice that were injected with saline or different doses of amphetamine remained on the platform. The effects diazepam in the 3D maze can be accounted for its impairing effects on some cognitive functions, and in particular spatial working memory, which are not necessary in the elevated platform (Coull et al. 1995; Herzog et al. 2000; Nakamura–Palacios and Roelke 1997; Soto et al. 2013; Tiplady et al. 2005). The choice of a slope in the elevated platform is less cognitively challenging than the choice between eight or nine arms of a radial maze. Hence, one would predict that an anxiolytic drug that has no impairing effect on cognition would facilitate crossings into the arms of the 3D‐maze.
Figure 4.
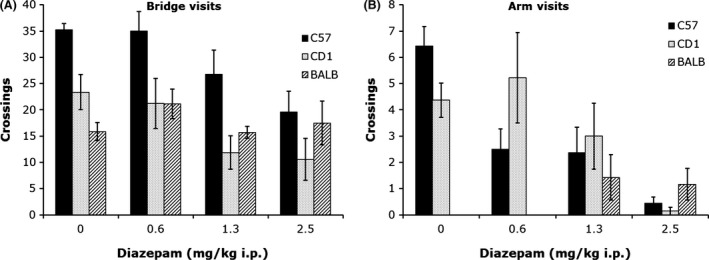
In this experiment, different strains of mice (c57BL/6J, CD‐1 and BALB/c) were introduced to 8 arms maze, and left to explore for 12 min. Each strain of mice was constituted of four groups, each receiving either saline or a single injection of one dose of diazepam 30 min before the test. Diazepam had no effect on BALB/c mice but significantly decreased the number of bridge (A) and arm (B) entries in C57 and CD‐1 mice.
Fluoxetine
Animal studies demonstrated mixed results with the use of SSRIs on anxiety. Some studies reported anxiogenic effect with acute (Birkett et al. 2011; Drapier et al. 2007; Gomes et al. 2009; Kurt et al. 2000; Robert et al. 2011; Silva et al. 1999; Silva and Brandão 2000) and anxiolytic effect with chronic (Gomes et al. 2009; Kurt et al. 2000; Nowakowska et al. 2000) treatments, whereas other studies reported anxiolytic (Griebel et al. 1999; Nowakowska et al. 1996, 2000; Rogóz and Skuza 2011) or no effect (Durand et al. 1999; Knoll et al. 2007; Takeuchi et al. 2010) with acute treatments. Some studies reported also anxiogenic (Robert et al. 2011; Silva et al. 1999) or no effect (Durand et al. 1999; Silva and Brandão 2000; Griebel et al. 1999; Takeuchi et al. 2010) with chronic treatments. These conflicting results were mostly obtained in TUA which have been reported to produce inconsistent results with a wide range of psychoactive compounds (Cryan and Sweeney 2011; Griebel and Holmes 2013; Miczek and de Wit 2008; Rodgers et al. 1995, 2002a; Thompson et al. 2015). One of the major limitations of these tests, mentioned earlier, is that they cannot be used for more than one session in screening for potential anxiolytic candidate drugs. In addition, examination of the effect of SSRIs on anxiety involves administration of the drugs for several days; this implies that animals are repeatedly handled when drugs are given by direct administration. This manipulation could affect animal response to the anxiety test as reported in a number of studies (Andrews and File 1993; Brett and Pratt 1990; Robert et al. 2011; Schmitt and Hiemke 1998).
In a recent study (Abuhamdah et al. 2015), we used the 3D maze to assess the effects of fluoxetine (20 mg/kg, i.p.) on anxiety in BALB/c mice. We examined whether the anxiolytic effects of fluoxetine can be detected over three test sessions. We examined also, whether repeated handling associated with a chronic treatment interferes with the effects of fluoxetine on anxiety responses. Two separate groups received once a day either saline (S chronic) or fluoxetine (F chronic) for 14 days, and continued to be injected before the test during the subsequent 3 days. The third group received saline (S acute) before the test, once a day for 3 days. Saline‐acute‐treated mice did not cross into the arms, and continued to do so over three sessions. Saline‐chronic‐treated mice avoided the arms in session 1, whereas fluoxetine‐chronic‐treated mice did cross into the arms. In subsequent sessions, the number of crossings into and time spent in the arms increased in these two chronic treated groups (Fig. 5). Fluoxetine appears to have produced an anxiolytic effect but this was evident only in the first session. These results suggest that repeated handling experience during the chronic treatment period did affect anxiety responses; it decreased fear and anxiety in mice, and this may have masked the anxiolytic effect of fluoxetine in the second and third test sessions. Handling experience, however, did not prevent an initial spontaneous anxiety response in chronic‐saline‐treated mice. Exposure to novelty (3D maze) appears to facilitate the “return of fear” which can be accounted for by the dishabituation phenomenon (Rachman 1989; Thompson and Spencer 1966).
Figure 5.
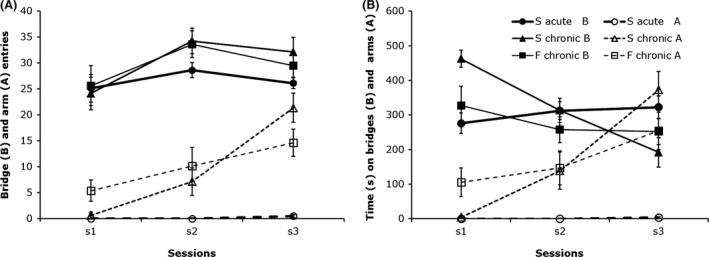
BALB/c mice were introduced to 9 arms maze and left to explore for 12 min in each test session. Number of entries into and time spent on bridges (B) and arms (A). Two separate groups received once a day either saline (S chronic, n = 8) or fluoxetine (F chronic, n = 8) for 14 days, and up to 30 min before the test during the subsequent 3 days. A third group received saline (S acute, n = 8) 30 min before the test, once a day for 3 days. (A) S acute mice did not cross into the arms in the three test sessions, whereas S chronic mice did cross into the arms in sessions 2 and 3. F chronic did cross into the arms in all three sessions. The arm/bridge entries ratio in session 3 was 0.02 ± 0.1 for S acute, 0.70 ± 0.1 for S chronic and 0.50 ± 0.1 for F chronic. (B) The arm/bridge duration ratio in session 3 was 0.01 ± 0.01 for S acute, 2.81 ± 0.56 for S chronic and 1.54 ± 0.43 for F chronic.
Dizocilpine
A number of studies suggest that NMDA antagonists may have potential anxiolytic properties (Criswell et al. 1994; Dunn et al. 1989; Engin et al. 2009; Wieronska et al. 2003; see, Cryan and Dev 2008). However, their anxiolytic effects is subject to conflicting reports (Criswell et al. 1994; Mansbach et al. 1991; Sanger and Joly 1991; Solati 2011; Solati and Salari 2011; Yagi et al. 1998). NMDA antagonists were reported to induce hyperactivity (Bardgett et al. 2003; Carey et al. 1998; Hargreaves and Cain 1992; Martin et al. 1997; Whishaw and Auer 1989). This hyperactivity is a confounding factor in the current animal tests of anxiety (Dawson and Tricklebank 1995; Dawson et al. 1995). Hence, in some studies their apparent anxiolytic effect was attributed to drug‐induced hyperactivity (Wiley et al. 1995), whereas in other studies hyperactivity was observed without evidence of reduced anxiety (Bardgett et al. 2003; Criswell et al. 1994; Mansbach et al. 1991; Sanger and Joly 1991; Silvestre et al. 1997). Furthermore, in spatial navigation tasks, familiarization with the test environment appears to prevent the impairing effects of NMDA antagonists on learning and memory (Cain 1997; Caramanos and Shapiro 1994; Roesler and Vianna 1998; Saucier et al. 1996; Saucier and Cain 1995; Shapiro and O'Connor 1992). This familiarization effect raised the issue of whether NMDA receptor antagonists do increase anxiety, which is confounded with learning and memory performance, particularly in a stressful environment such as in the water maze.
In a previous study (Ennaceur et al. 2011) conducted in the 3D maze, mice treated with dizocilpine, demonstrated avoidance of the arms despite a significant large increase in bridge entries. This translated to impaired acquisition of a working memory task. We suggested that this impairment could be due to dizocilpine producing an increased and sustained anxiety. In a recent study, we examined whether, in pretrained mice, dizocilpine will still produce increased anxiety. C57BL/6J mice, which display low anxiety in the 3D maze, were treated with saline or dizocilpine (0.1 mg/kg i.p.) and exposed to the maze in seven consecutive sessions, one session per day. The experiment involved three groups of mice. One group received a single daily injection of saline (SAL d1), whereas a second group received a single daily injection of dizocilpine (DIZ d1). A third group (DIZ d4) received saline in the first three sessions and dizocilpine in each subsequent session. All saline‐treated mice made numerous visits to the arms, whereas mice treated with dizocilpine for 7 days showed reduced entry onto the arms. Dizocilpine had no effect on arm entries in mice treated on the fourth day onward. These mice demonstrated instead a steady large increase in the number of bridge (Fig. 6A) and arm (Fig. 6B) entries, which suggests impaired habituation to the test environment. It produced sustained nonhabituating hyperactivity; a phenomenon that have been reported for NMDA receptor antagonists (Klamer et al. 2004; Réus et al. 2008; Venâncio et al. 2011) and genetic models of NMDA hypo‐function (Ballard et al. 2002; Bickel et al. 2008; Duncan et al. 2006). Mice treated with saline from day 1 and those treated with dizocilpine from day 4 reached a bridge/arm entries ratio superior to 0.9 in session 5 (Fig. 6C) which indicates that they were moving from bridges to arms without hesitations.
Figure 6.
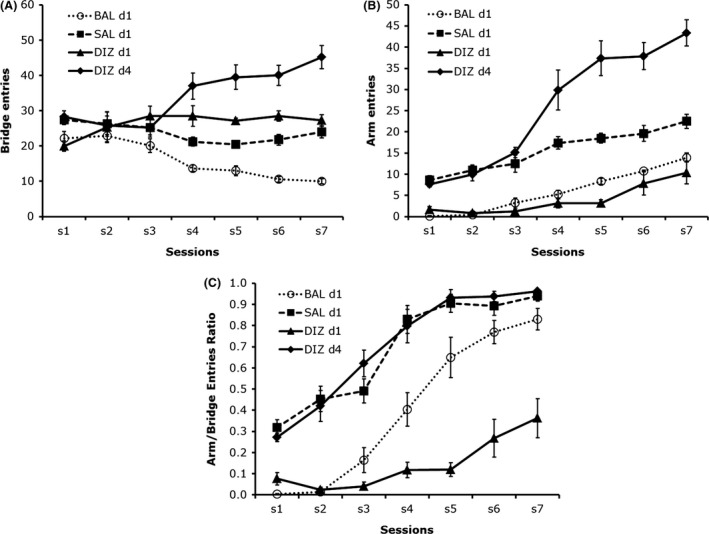
C57BL/6J mice were introduced to 9 arms maze, and left to explore for 10 min in each test session. The experiment consists of three groups of C57BL/6J mice, which received a single daily injection of either saline or dizocilpine 30 min before the test. The first group received saline each test day (SAL d1). The second group received saline on day 1 to 3, then dizocilpine from day 4 (DIZ d4). The third group received dizocilpine each day (DIZ d1). The results of BALB/c saline‐treated mice were part of a separate experiment. They are included here for illustration only. (A) Dizocilpine increased the number of bridge entries in DIZ d1 and DIZ d4 groups compared to SAL d1 group. (B) the number of arm entries was decreased in DIZ d1 group and increased in DIZ d4 group; (C) Arm/bridge entries ratio was significantly low in DIZ d1 group compared to SAL d1 group and DIZ d4 group; the latter two were not different from each other.
Dizocilpine and other NMDA antagonists have been reported to increase locomotor activity and impulsive response (Amitai and Markou 2010; Higgins et al. 2003; Scott and Taylor 2014; Smith et al. 2012). In the 3D maze, unlike in the TUA, the psychomotor‐stimulating effect of a drug is detected by the number of bridge entries and cannot be confounded with anxiolysis, which is detected, by the number of arm entries. In DIZ d1 mice, the increase in bridge entries, though not as high as in DIZ d4 mice, was opposed by a high level of anxiety, which prevented mice from crossing onto the arms. This behavior compares to that observed with amphetamine in another open space anxiety test, the elevated platform with steep slopes attached on two opposite sides (Ennaceur et al. 2010). Amphetamine produced a dose‐dependent hyperactivity in BALB/c mice without producing a single crossing onto a slope. In DIZ d4 mice, the psychomotor‐stimulating effect of dizocilpine may account for the high number of crossings into the arms. However, these mice had a low basal level of anxiety at the start of the test – a low anxiety strain, and prior experience with the maze and saline injection. It remains unlikely that such level of anxiety could be decreased further. However, this psychomotor stimulation may have impaired habituation as these mice demonstrated concurrent increase in bridge and arm entries after each exposure to the maze. NMDA antagonists have been reported to impair habituation in various behavioral tests (Ballard et al. 2002; Bickel et al. 2008; Duncan et al. 2006; Klamer et al. 2004; Réus et al. 2008; Rosat et al. 1992; Venâncio et al. 2011). The present results suggest that dizocilpine exacerbates anxiety; this contrasts with results obtained with dizocilpine and other NMDA antagonists in the EPM which suggested an anxiolytic effect (Bertoglio and Carobrez 2003; Bergink et al. 2004; Dunn et al. 1989; Wiley et al. 1995). However, NMDA antagonists increased locomotor activity, which is a confounding factor in the determination of the anxiolytic effect of drug treatments in the EPM. In the 3D maze, an increase in motor activity or hyperactivity in high anxiety mice does not facilitate crossing into the arms and remains limited to the bridges. As indicated above, dizocilpine‐treated mice made more crossings into the bridges than saline‐treated mice but they were unable to cross into the arms in the first three sessions; their number of arm entries remained significantly low in subsequent sessions compared to saline‐treated mice.
The ability to test animals for a number of sessions is an important advantage over the EPM and other TUA. In the latter, exploratory and locomotor activities decrease significantly and approach a floor level in subsequent exposures. This decrease is associated with habituation (Cook et al. 2002; Dawson et al. 1994; Espejo 1997; Holmes and Rodgers 1998; Treit et al. 1993) but it cannot be discriminated from an increase in anxiety, and it is observed in both high and low anxiety mouse and rat strains. In the 3D maze, high anxiety is observed in some mouse strains, and this does decrease in subsequent exposures to the test. This corresponds to what is generally expected in normal human subjects as well as in animals. High anxiety mouse and rat strains do not represent a model of pathological anxiety. They represent differences between individuals or group of individuals in coping strategies with threat and stress. In the 3D maze, both high and low anxiety mice demonstrate an increase in arm entries with repeated exposures, and this could be due to habituation. Therefore, it is expected that animal models of anxiety produced with drugs, lesions or genetic manipulations will demonstrate reduced or delayed habituation, and may remain unable to reach the criterion of a minimum 8 arm visits, and arm bridge entries ratio close to 1.
The present results suggest that dizocilpine exacerbates anxiety. It remains to be demonstrated whether, a comparable or an opposite effect, is observed with BALB/c mice, a high anxiety strain, and whether an anxiogenic intervention would affect habituation and anxiety response.
Conclusion
In summary, the current TUA suffer from a major initial flaw in their conception, which has been overlooked and complicated over at least 3 decades by subsequent pharmacological validation. The flaw resides in the fact that animals demonstrate escape to or avoidance from the protected and/or unlit space of these test apparatus. While one may view that an open space evokes anxiety in mice and rats, though it is apparent that generally these rodents did not explore these spaces, another may view either that animals avoided the unprotected/lit space, hence diminishing or terminating the fear response, or that they demonstrated a natural preference for the protected/unlit space which promotes a feeling of safety and security. These equivocal interpretations of the same behavioral response undermine entirely the validity of the TUA.
The TUA validity is further undermined by the diversity and inconsistencies of their measurements. Up to date, there is not a single index, commonly agreed upon, which provides a specific and/or reliable measure of anxiety. Number of crossings, time spent, percent number, and percent time in the unprotected/lit space are rarely concordant (Table 1). Anxiety is determined, in most cases, by a change to any one of these measurements. The same is true for measurement of locomotor activity, which is represented by either the number of crossings or distance travelled. In the EPM, locomotor activity is also represented by the total number of crossings into all arms in some reports, and by the number of crossings into the enclosed arms in other reports. Furthermore, measurements of anxiety and locomotor activity appear to be determined a‐posteriori. Hence, only one measurement or a subset of measurements are selected and reported in a particular study (Table 2). These measurements vary between studies, which explain their diversity and the difficulty to compare between research reports (Table 2). In addition, the lack of reliability of the primary indices of anxiety (open entries, open time and percent of these two, see Table 1) promoted a desperate need for other types and forms of measurements; these contributed to further diversity and complexity. In some studies, spatio‐temporal parameters were either complemented or supplanted with ethological parameters, whereas in other studies either one of these is selected as it seems fit.
It has been pointed out over the years that the current TUA suffer major limitations, which concern the design of the test conditions and test parameters. Various suggestions have been proposed and numerous attempts have been made to circumvent these limitations, but there is yet no evidence demonstrating any improvement in the reliability and consistency of the results obtained in these tests. As argued in this, and in a previous report (Ennaceur 2014), the current TUA do not provide unequivocal measures of anxiety; these are sine qua non for the validity of a behavioral test. This primary concern cannot be resolved with some modifications to the layouts of the test apparatus or some changes to the test procedures. There is an urgent need for a complete radical overhaul approach for the development of behavioral assays of anxiety in animal research. Such behavioral assays need to demonstrate that the measured construct, anxiety, is unequivocally discriminated from measures of other constructs that it may be confounded with, such as fear‐induced avoidance or escape. To achieve this, a novel test of anxiety needs to expose animals to an aversive situation, which involves uninformative or ambiguous stimuli, and that the outcomes from the choice between these stimuli are uncertain. Hence, an unfamiliar open space, such as the 3D maze, can provide an aversive situation that evokes fear, which motivates escape and avoidance responses of threatening situations. In anxiety conditions, fear cannot be diminished or terminated by an escape or an avoidance response. This is simply because fear is generalized to the entire situation that evoked such fear. Unlike in the current TUA, any part of the test situation can be perceived as a source of threat. Animals will try to escape the whole situation if possible but to do so they must explore to find out whether there is an escape route. This escape response has been used to determine anxiety in animals. Our studies demonstrate that some mice do not cross into the distal segments of the mazes, hence they are deemed more anxious than the one that venture on the arms. The number of crossings into the arms and the arm/bridge entries ratio are the only indices, which are considered specific to anxiety. These proved consistently reliable and concordant in all our studies.
The current tests of unconditioned tests of anxiety exert an undue influence on the development of novel approaches despite the accumulated evidence against their validity, which is demonstrated through their inconsistent and conflicting results. In this report, we argued that these are based on flawed methodologies; they do not provide unequivocal evidence of the presence of motivation conflict. They were adopted, and promoted based on reports of their sensitivity to diazepam and chlordiazepoxide. This sensitivity has been challenged when these tests proved insensitive to benzodiazepine drugs in a second test exposure, and demonstrated insensitivity to nonbenzodiazepine drugs. Numerous reviews have been published each year to highlight their achievements with some notes about their shortcomings, and a list of improvement proposals to consolidate their status in animal anxiety research. One of these proposals is to introduce ethological parameters, which would complement the TUA spatiotemporal parameters, as the latter were unable to capture the construct they were meant to measure (Griebel et al. 1997; Rodgers and Dalvi 1997). The second proposal is the use of a battery of behavioral tests, in which results would hopefully converge and determine the construct specificity (van Gaalen and Steckler 2000; Võikar et al. 2004). A third proposal is standardization, which would establish consistency and improve interlaboratory comparisons (Crabbe et al. 1999; Wahlsten 2001; Würbel 2002). The more recent proposal is endophenotyping, which would use multidisciplinary methodologies to characterize the traits of individuals with anxiety and its disorders (Bakshi and Kalin 2002; Jacobson and Cryan 2010). Simplicity in science research investigation is lost to very complex and expensive strategies, which are no more than impressive correlations between databases. All the above propositions look like desperate attempts to salvage fundamentally flawed behavioral tests that would continue to serve leading theories at the expense of novel and daring approaches.
The decline in funding for basic research, particularly in preclinical studies of anxiety, and the withdrawal of industry from investing in such research is an issue of concern for the future of animal research. “Is it poor research the cause of the declining productivity of the pharmaceutical industry” (Sams–Dodd 2013) or a “funding crisis in psychopharmacology” (Hendrie 2010)? The complexity of the brain and the complexity of human and animal behavior cannot be used to justify a long lasting failure. Researchers in other fields of science face similar complexities, and the secret of their success is that exploration is not constrained by a‐priori hypothesis and established theories, and that popularity is the least of their concern in the choice of a methodology. The introduction of compulsory hypotheses and theories in research grant applications prevent innovations; worse of all, a vast majority of scientists are constrained to remain aligned with the established views. The consequence of this policy was evident when animal behavior research proved unable to provide any satisfactory answer to the emerging demand of molecular biology and genetic manipulations. The limitations and the flaws of the classic tests of anxiety were apparent from the start, but instead of encouraging alternative and innovative approaches, the established theories and hypothesis were left in control and to self‐perpetuate.
In this report, we exposed some major flaws that undermine the validity of the current TUA, and we described a novel open space anxiety test, a 3D maze, which provides more reliable measures of anxiety. It is not expected that the findings from the 3D maze would replicate the findings obtained in the TUA. The advantage of this novel open space anxiety test over the current one is that (1) Fear‐induced avoidance is not confused with fear‐induced anxiety response; it is possible to demonstrate the difference between these two by introducing a refuge on the central platform; (2) Anxiety response is determined by the number of crossings into the arms and not by the time spent in the arms. Two measurements are set to indicate low or high anxiety mouse or rat strains: number of arm entries and arm/bridge entries ratio; (3) A criterion of a minimum of 8 arm visits and arm/bridge ratio close to 1 are required to determine an anxiolytic effect of a drug treatment or an experimental intervention. An anxiogenic effect is indicated by a number of arm visits lower than 8 and arm/bridge entries ratio lower than 50%; (4) mice and rats can be tested in a number of sessions which provides the chance to examine slow acting drugs and habituation processes; (5) The bridges have been useful in providing measure of locomotor activity, and they proved to be a barrier that psychomotor stimulation cannot overcome without a reduction in anxiety.
The results presented in this review originate from a single laboratory, and are based on limited number of animals and replications. They remain to be challenged in independent laboratories, and it remains to be seen whether the 3D maze can be used to predict the anxiolytic effects of novel drug compounds.
Disclosures
None declared.
Ennaceur A., Chazot P. L., Preclinical animal anxiety research – flaws and prejudices, Pharma Res Per,4(2), 2016, e00223, doi: 10.1002/prp2.223
References
- Abramov U, Raud S, Innos J, Lasner H, Kurrikoff K, Türna T, et al. (2008). Different housing conditions alter the behavioural phenotype of CCK(2) receptor‐deficient mice. Behav Brain Res 193: 108–116. [DOI] [PubMed] [Google Scholar]
- Abuhamdah RM, Hussain MD, Chazot PL, Ennaceur A (2015). Effects of chronic fluoxetine treatment on anxious behaviour of BALB/c mice in a 3‐dimensional maze. Stress 18: 677–685. [DOI] [PubMed] [Google Scholar]
- Akillioglu K, Melik EB, Melik E, Boga A (2012). Effect of ketamine on exploratory behaviour in BALB/c and C57BL/6 mice. Pharmacol Biochem Behav. 100:513–517. [DOI] [PubMed] [Google Scholar]
- Albrechet‐Souza L, Oliveira AR, De Luca MC, Tomazini FM, Santos NR, Brandão ML. (2005). A comparative study with two types of elevated plus‐maze (transparent vs. opaque walls) on the anxiolytic effects of midazolam, one‐trial tolerance and fear‐induced analgesia. Prog Neuropsychopharmacol Biol Psychiatry 29: 571–579. [DOI] [PubMed] [Google Scholar]
- Alstott J, Timberlake W (2009). Effects of rat sex differences and lighting on locomotor exploration of a circular open field with free‐standing central corners and without peripheral walls. Behav Brain Res 96: 214–219. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A (2010). Disruption of performance in the five‐choice serial reaction time task induced by administration of N‐methyl‐D‐aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 68:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, et al. (2011). Strain and sex differences in anxiety‐like and social behaviors in C57BL/6J and BALB/cJ mice. Exp Anim 60: 111–123. [DOI] [PubMed] [Google Scholar]
- Andreatini R, Bacellar LFS (2000). Animal models: trait or state measure? The test‐retest reliability of the elevated plus‐maze and behavioral despair. Prog Neuro‐Psychopharmacol Biol Psychiatry 24: 549–560. [DOI] [PubMed] [Google Scholar]
- Andrews N, File SE (1993). Handling history of rats modifies behavioural effects of drugs in the elevated plus‐maze test of anxiety. Eur J Pharmacol 235: 109–112. [DOI] [PubMed] [Google Scholar]
- Arabo A, Potier C, Ollivier G, Lorivel T, Roy V (2014). Temporal analysis of free exploration of an elevated plus‐maze in mice. J Exp Psychol Anim Learn Cogn 40: 457–466. [DOI] [PubMed] [Google Scholar]
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, et al. (2009). Individual housing of mice–impact on behaviour and stress responses. Physiol Behav 97: 385–393. [DOI] [PubMed] [Google Scholar]
- Augustsson H, van de Weerd HA, Kruitwagen CL, Baumans V (2003). Effect of enrichment on variation and results in the light/dark test. Lab Anim 37: 328–340. [DOI] [PubMed] [Google Scholar]
- Augustsson H, Meyerson BJ (2004). Exploration and risk assessment: a comparative study of male house mice (Mus musculus musculus) and two laboratory strains. Physiol Behav 81:685–698. [DOI] [PubMed] [Google Scholar]
- Aulich D (1976). Escape versus exploratory activity: an interpretation of rats’ behaviour in the open field and a light‐dark preference test. Behav Processes 1: 153–164. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Lipina TV, Bondar NP, Alekseyenko OV, Kudryavtseva NN (2000). Features of the genetically defined anxiety in mice. Behav Genet 30: 101–109. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Rustay NR, Crawley JN (2006). Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J 47: 124–131. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kalin NH (2002). Animal models and endophenotypes of anxiety and stress disorders Pp. 884–901 in Davis K. L., Charney D., Coyle J. T. and Nemeroff C., eds. Neuropsychopharmacology: the fifth generation of progress (5th ed.), vol. 62, Lippincott Williams & Wilkins, Philadelphia (PA). [Google Scholar]
- Ballard TM, Pauly‐Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, et al. (2002). Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug‐resistant nonhabituating hyperactivity. J Neurosci 22: 6713–6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Boeckman R, Krochmal D, Fernando H, Ahrens R, Csernansky JG (2003). NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Res Bull 60: 131–142. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G (1996). Illumination has no effect on rats’ behavior in the elevated plus‐maze. Physiol Behav 59: 1175–1177. [DOI] [PubMed] [Google Scholar]
- Belzung C (2001). Rodent models of anxiety‐like behaviors: are they predictive for compounds acting via non‐benzodiazepine mechanisms? Curr Opin Investig Drugs 2: 1108–1111. [PubMed] [Google Scholar]
- Belzung C, Griebel G (2001). Measuring normal and pathological anxiety‐like behaviour in mice: a review. Behav Brain Res 125: 141–149. [DOI] [PubMed] [Google Scholar]
- Belzung C, Lemoine M (2011). Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Crestani F (2000). Flumazenil induces benzodiazepine partial agonist‐like effects in BALB/c but not C57BL/6 mice. Psychopharmacology 148: 24–32. [DOI] [PubMed] [Google Scholar]
- Benatti C, Alboni S, Montanari C, Caggia F, Tascedda F, Brunello N, Blom JM (2011). Central effects of a local inflammation in three commonly used mouse strains with a different anxious phenotype. Behav Brain Res. 224:23–34. [DOI] [PubMed] [Google Scholar]
- Bergink V, van Megen HJ, Westenberg HG (2004). Glutamate and anxiety. Eur Neuropsychopharmacol 14: 175–183. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP (2002). Prior maze experience required to alter midazolam effects in rats submitted to the elevated plus‐maze. Pharmacol Biochem Behav 72: 449–455. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP (2003). Anxiolytic‐like effects of NMDA/glycine‐B receptor ligands are abolished during the elevated plus‐maze trial 2 in rats. Psychopharmacology 170: 335–342. [DOI] [PubMed] [Google Scholar]
- Bickel S, Lipp HP, Umbricht D (2008). Early auditory sensory processing deficits in mouse mutants with reduced NMDA receptor function. Neuropsychopharmacology 33: 1680–1689. [DOI] [PubMed] [Google Scholar]
- Birkett MA, Shinday NM, Kessler EJ, Meyer JS, Ritchie S, Rowlett JK (2011). Acute anxiogenic‐like effects of selective serotonin reuptake inhibitors are attenuated by the benzodiazepine diazepam in BALB/c mice. Pharmacol Biochem Behav 98: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG (2004). Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 3:149–157. [DOI] [PubMed] [Google Scholar]
- Botelho S, Estanislau C, Morato S (2007). Effects of under‐ and overcrowding on exploratory behavior in the elevated plus‐maze. Behav Processes 74: 357–362. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoët M (2003). The mouse light/dark box test. Eur J Pharmacol 463: 55–65. [DOI] [PubMed] [Google Scholar]
- Bourin M, Petit‐Demoulière B, Dhonnchadha BN, Hascöet M (2007). Animal models of anxiety in mice. Fundam Clin Pharmacol 21: 567–574. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R (2002). Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res 136: 489–501. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R (2008). Pitfalls in the interpretation of genetic and pharmacological effects on anxiety‐like behaviour in rodents. Behav Pharmacol 19: 385–402. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, van der Gugten J, Groenink L, Olivier B, Paylor RE (2004a). Effects of repeated testing in two inbred strains on flesinoxan dose‐response curves in three mouse models for anxiety. Eur J Pharmacol. 494:35–44. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, van der Gugten J, Groenink L, Olivier B, Paylor RE (2004b). Behavioral and physiological mouse models for anxiety: effects of flesinoxan in 129S6/SvEvTac and C57BL/6J mice. Eur J Pharmacol. 494:45–53. [DOI] [PubMed] [Google Scholar]
- Brett RR, Pratt JA (1990). Chronic handling modifies the anxiolytic effect of diazepam in the elevated plus‐maze. Eur J Pharmacol 178: 135–138. [DOI] [PubMed] [Google Scholar]
- Brinks V, van der Mark M, de Kloet R, Oitzl M (2007). Emotion and cognition in high and low stress sensitive mouse strains: a combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front Behav Neurosci. 2007; 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Pask T, Jones L, Dunnett SB (2005). Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes Brain Behav 4: 307–317. [DOI] [PubMed] [Google Scholar]
- Brown RE, Corey SC, Moore AK (1999). Differences in measures of exploration and fear in MHC‐congenic C57BL/6J and B6‐H‐2K mice. Behav Genet 29: 263–271. [Google Scholar]
- Cain DP (1997). Prior non‐spatial pretraining eliminates sensorimotor disturbances and impairments in water maze learning caused by diazepam. Psychopharmacology 130: 313–319. [DOI] [PubMed] [Google Scholar]
- Cancela LM, Basso AM, Martijena ID, Capriles NR, Molina VA (2001). A dopaminergic mechanism is involved in the ‘anxiogenic‐like’ response induced by chronic amphetamine treatment: a behavioral and neurochemical study. Brain Res 909: 179–186. [DOI] [PubMed] [Google Scholar]
- Caramanos Z, Shapiro ML (1994). Spatial memory and N‐methyl‐D‐aspartate receptor antagonists APV and MK‐801: memory impairments depend on familiarity with the environment, drug dose, and training duration. Behav Neurosci 108: 30–43. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Dai H, Gui J (1998). Effects of dizocilpine (MK‐801) on motor activity and memory. Psychopharmacology 137: 241–246. [DOI] [PubMed] [Google Scholar]
- Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P (2002). Evaluation of the elevated plus‐maze and open‐field tests for the assessment of anxiety‐related behaviour in inbred mice. Behav Brain Res 134: 49–57. [DOI] [PubMed] [Google Scholar]
- Carr RM, Overall JE, White RK, Brown WL (1959). The effects of food deprivation and restricted activity upon exploratory behavior of the rat. J Genet Psychol 95: 321–328. [DOI] [PubMed] [Google Scholar]
- Casarrubea M, Roy V, Sorbera F, Magnusson MS, Santangelo A, Arabo A (2013). Temporal structure of the rat's behavior in elevated plus maze test. Behav Brain Res 237: 290–299. [DOI] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR (2002). Retinal degeneration mutants in the mouse. Vis Res 42: 517–525. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Durand M, Mormède P (1997). Anxiety‐ and activity‐related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behav Brain Res 85: 27–35. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez‐Zas SL, Mogil JS (2002). Influences of laboratory environment on behavior. Nat Neurosci 5: 1101–1102. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lazar NL, Bechard AR, Wood GA, Roder JC (2005). NIH Swiss and Black Swiss mice have retinal degeneration and performance deficits in cognitive tests. Comp Med 55: 310–316. [PubMed] [Google Scholar]
- Cohen RM, Kang A, Gulick C (2001). Quantitative trait loci affecting the behavior of A/J and CBA/J intercross mice in the elevated plus maze. Mamm Genome 12: 501–507. [DOI] [PubMed] [Google Scholar]
- Cole JM, Pieper WA (1973). The effects of N, N‐dimethyltryptamine on operant behavior in squirrel monkeys. Psychopharmacologia 29: 107–112. [DOI] [PubMed] [Google Scholar]
- Cook MN, Williams RW, Flaherty L (2001). Anxiety‐related behaviors in the elevated zero‐maze are affected by genetic factors and retinal degeneration. Behav Neurosci 115: 468–476. [PubMed] [Google Scholar]
- Cook MN, Crounse M, Flaherty L (2002). Anxiety in the elevated zero‐maze is augmented in mice after repeated daily exposure. Behav Genet 32: 113–118. [DOI] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM (1989). Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav 32: 777–785. [DOI] [PubMed] [Google Scholar]
- Coull JT, Middleton HC, Robbins TW, Sahakian BJ (1995). Contrasting effects of clonidine and diazepam on tests of working memory and planning. Psychopharmacology 120: 311–321. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC (1999). Genetics of mouse behavior: interactions with laboratory environment. Science 284: 1670–1672. [DOI] [PubMed] [Google Scholar]
- Crawley JN (1985). Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev 9: 37–44. [DOI] [PubMed] [Google Scholar]
- Crawley JN (1999). Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835: 18–26. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Davis LG (1982). Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res Bull 8: 609–612. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Goodwin FK (1980). Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13: 167–170. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. (1997). Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology 132: 107–124. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Knapp DJ, Overstreet DH, Breese GR (1994). Effects of ethanol, chlordiazepoxide, and MK‐801 on performance in the elevated‐plus maze and on locomotor activity. Alcohol Clin Exp Res 18: 596–601. [DOI] [PubMed] [Google Scholar]
- Cruz‐Morales SE, Santos NR, Brandão ML (2002). One‐trial tolerance to midazolam is due to enhancement of fear and reduction of anxiolytic‐sensitive behaviors in the elevated plus‐maze retest in the rat. Pharmacol Biochem Behav 72: 973–978. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dev KK (2008). The glutamatergic system as a potential therapeutic target for the treatment of anxiety disorders Pp. 269–301 in Blanchard R. J., Blanchard D. C., Griebel G. and Nutt D., eds. Handbook of anxiety and fear. Elsevier, Elsevier: vol. 17. [Google Scholar]
- Cryan JF, Holmes A (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discovery 4: 775–790. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF (2011). The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 164: 1129–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi A, Rodgers RJ (1999). Behavioral effects of diazepam in the murine plus‐maze: flumazenil antagonism of enhanced head dipping but not the disinhibition of open‐arm avoidance. Pharmacol Biochem Behav 62: 727–734. [DOI] [PubMed] [Google Scholar]
- Dalvi A, Rodgers RJ (2001). Anxiolytic effects of valproate and diazepam in mice are differentially sensitive to picrotoxin antagonism. Pharmacol Biochem Behav 68: 23–32. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Tricklebank MD (1995). Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16: 33–36. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Crawford SP, Stanhope KJ, Iversen SD, Tricklebank MD (1994). One‐trial tolerance to the effects of chlordiazepoxide on the elevated plus‐maze may be due to locomotor habituation, not repeated drug exposure. Psychopharmacology (Berl.) 113: 570–572. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Crawford SP, Collinson N, Iversen SD, Tricklebank MD (1995). Evidence that the anxiolytic‐like effects of chlordiazepoxide on the elevated plus maze are confounded by increases in locomotor activity. Psychopharmacology 118: 316–323. [DOI] [PubMed] [Google Scholar]
- De Lorge J, Bolles RC (1961). Effects of food deprivation on exploratory behavior in a novel situation. Psychol Rep 9: 599–606. [Google Scholar]
- Dotson CD, Spector AC (2005). Drinking spout orifice size affects licking behavior in inbred mice. Physiol Behav 85: 655–661. [DOI] [PubMed] [Google Scholar]
- Drapier D, Bentué‐Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, et al. (2007). Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus‐maze. Behav Brain Res 176: 202–209. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Perlegas A, Floyd DW, Weiner JL, McCool BA (2006). Distinct functional characteristics of the lateral/basolateral amygdala GABAergic system in C57BL/6J and DBA/2J mice. J Pharmacol Exp Ther 318: 629–640. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Belzung C (2004). Behaviour in the elevated plus‐maze predicts coping after subchronic mild stress in mice. Physiol Behav 81: 417–426. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Belzung C (2005). Correlations between behaviours in the elevated plus‐maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res 156(153): 162. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH (2006). Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav 85: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RW, Corbett R, Fielding S (1989). Effects of 5‐HT1A receptor agonists and NMDA receptor antagonists in the social interaction test and the elevated plus‐maze. Eur J Pharmacol 169: 1–10. [DOI] [PubMed] [Google Scholar]
- Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormède P, et al. (1999). Effects of repeated fluoxetine on anxiety‐related behaviours, central serotonergic systems, and the corticotropic axis in SHR and WKY rats. Neuropharmacology 38: 893–907. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT (2009). Anxiolytic‐ and antidepressant‐like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience 161: 359–369. [DOI] [PubMed] [Google Scholar]
- Ennaceur A (2011). Omission of the habituation procedure in the acquisition of a working memory task ‐ evidence from Balb/c, C57/BL6J, and CD‐1 mice. Behav Brain Res 223: 203–210. [DOI] [PubMed] [Google Scholar]
- Ennaceur A (2014). Tests of unconditioned anxiety ‐ pitfalls and disappointments. Physiol Behav 135: 55–71. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL (2006). Models of anxiety: responses of mice to novelty and open spaces in a 3D maze. Behav Brain Res 174: 9–38. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL (2008). Are benzodiazepines really anxiolytic? Evidence from a 3D maze spatial navigation task. Behav Brain Res 188: 136–153. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL (2010). Distinguishing anxiolysis and hyperactivity in an open space behavioral test. Behav Brain Res 207: 84–98. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL (2011). MK‐801 increases the baseline level of anxiety in mice introduced to a spatial memory task without prior habituation. Neuropharmacology 61: 981–991. [DOI] [PubMed] [Google Scholar]
- Escarabajal MD, Torres C, Flaherty CF (2003). The phenomenon of one‐trial tolerance to the anxiolytic effect of chlordiazepoxide in the elevated plus‐maze test is abolished by previous administration of chlordiazepoxide or buspirone. Life Sci 73: 1063–1074. [DOI] [PubMed] [Google Scholar]
- Espejo EF (1997). Effects of weekly or daily exposure to the elevated plus‐maze in male mice. Behav Brain Res 87: 233–238. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE (1996). The influence of open arm ledges and maze experience in the elevated plus‐maze. Pharmacol Biochem Behav 54: 31–40. [DOI] [PubMed] [Google Scholar]
- File SE, Day S (1972). Effects of time of day and food deprivation on exploratory activity in the rat. Anim Behav 20: 758–762. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H Jr (1993). “One‐trial tolerance” to the anxiolytic actions of benzodiazepines in the elevated plus‐maze, or the development of a phobic state? Psychopharmacology 110: 240–244. [DOI] [PubMed] [Google Scholar]
- File SE, Andrews N, Wu PY, Zharkovsky A, Zangrossi H (1992). Modification of chlordiazepoxide's behavioural and neurochemical effects by handling and plus‐maze experience. Eur J Pharmacol 218: 9–14. [DOI] [PubMed] [Google Scholar]
- File SE, Amarbirpal M, Mangiarini L, Bates GP (1998). Striking changes in anxiety in Huntington's disease transgenic mice. Brain Res. 805:234–240. [DOI] [PubMed] [Google Scholar]
- Filgueiras GB, Carvalho‐Netto EF, Estanislau C (2014). Aversion in the elevated plus‐maze: role of visual and tactile cues. Behav Processes 107: 106–111. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, et al. (2009). Influence of light at night on murine anxiety‐ and depressive‐like responses. Behav Brain Res 205: 349–354. [DOI] [PubMed] [Google Scholar]
- Garcia AMB, Cardenas FP, Morato S (2005). Effect of different illumination levelson rat behavior in the elevated plus‐maze. Physiol. Behav 85:265–270. [DOI] [PubMed] [Google Scholar]
- Gard PR, Haigh SJ, Cambursano PT, Warrington CA (2001). Strain differences in the anxiolytic effects of losartan in the mouse. Pharmacol Biochem Behav 69: 35–40. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A (1995). Animal models of psychiatric disorders Pp. 787–798 in Bloom F. E. and Kupfer D. J., eds. Psychopharmacology: the fourth generation of progress. Raven Press Ltd, New York. [Google Scholar]
- Giorgetta C, Grecucci A, Zuanon S, Perini L, Balestrieri M, Bonini N, et al. (2012). Reduced risk‐taking behavior as a trait feature of anxiety. Emotion 12: 1373. [DOI] [PubMed] [Google Scholar]
- Goes TC, Antunes FD, Teixeira‐Silva F (2009). Trait and state anxiety in animal models: is there correlation? Neurosci Lett 450: 266–269. [DOI] [PubMed] [Google Scholar]
- Goes TC, Antunes FD, Teixeira‐Silva F (2015). Environmental enrichment for adult rats: effects on trait and state anxiety. Neurosci Lett 584: 93–96. [DOI] [PubMed] [Google Scholar]
- Gomes KS, de Carvalho‐Netto EF, Monte KC, Acco B, Nogueira PJ, Nunes‐de‐Souza RL (2009). Contrasting effects of acute and chronic treatment with imipramine and fluoxetine on inhibitory avoidance and escape responses in mice exposed to the elevated T‐maze. Brain Res Bull 78: 323–327. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holmes A (2013). 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 12: 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Sanger DJ, Perrault G (1996). Further evidence for differences between non‐selective and BZ‐1 (ω1) selective, benzodiazepine receptor ligands in murine models of “state” and “trait” anxiety. Neuropharmacology 35: 1081–1091. [DOI] [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger DJ (1997). Risk assessment behaviour: evaluation of utility in the study of 5‐HT‐related drugs in the rat elevated plus‐maze test. Pharmacol Biochem Behav 57: 817–827. [DOI] [PubMed] [Google Scholar]
- Griebel G, Cohen C, Perrault G, Sanger DJ (1999). Behavioral effects of acute and chronic fluoxetine in Wistar‐Kyoto rats. Physiol Behav 67: 315–320. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ (2000). Differences in anxiety‐related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology 148: 164–170. [DOI] [PubMed] [Google Scholar]
- Hagenbuch N, Feldon J, Yee BK (2006). Use of the elevated plus‐maze test with opaque or transparent walls in the detection of mouse strain differences and the anxiolytic effects of diazepam. Behav Pharmacol 17: 31–41. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Sundstrom JM, Pert A (2000). Differential basis of strain and rearing effects on open‐field behavior in fawn hooded and wistar rats. Physiol Behav 71: 525–532. [DOI] [PubMed] [Google Scholar]
- Haller J, Alicki M (2012). Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Curr Opin Psychiatry 25: 59–64. [DOI] [PubMed] [Google Scholar]
- Haller J, Aliczki M, Gyimesine Pelczer K (2013). Classical and novel approaches to the preclinical testing of anxiolytics: a critical evaluation. Neurosci Biobehav Rev 37: 2318–2330. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S (1984). Effects of alpha‐adrenoceptor agonists and antagonists in a maze‐exploration model of ‘fear’‐motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol 327: 1–5. [DOI] [PubMed] [Google Scholar]
- Harada K, Aota M, Inoue T, Matsuda R, Mihara T, Yamaji T, et al. (2006). Anxiolytic activity of a novel potent serotonin 5‐HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol 553: 171–184. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Cain DP (1992). Hyperactivity, hyper‐reactivity, and sensorimotor deficits induced by low doses of the N‐methyl‐D‐aspartate non‐competitive channel blocker MK801. Behav Brain Res 47: 23–33. [DOI] [PubMed] [Google Scholar]
- Hascoët M, Bourin M (1998). A new approach to the light/dark procedure in mice. Pharmacol Biochem Behav 60: 645–653. [DOI] [PubMed] [Google Scholar]
- Hendrie CA (2010). The funding crisis in psychopharmacology: an historical perspective. J Psychopharmacol 24: 439–440. [DOI] [PubMed] [Google Scholar]
- Hendrie CA, Eilam D, Weiss SM (1997). Effects of diazepam and buspirone on the behaviour of wild voles (microtus socialis) in two models of anxiety. Pharmacol Biochem Behav 58: 573–576. [DOI] [PubMed] [Google Scholar]
- Hendriksen H, Groenink L (2015). Back to the future of psychopharmacology: a perspective on animal models in drug discovery. Eur J Pharmacol 759: 30–41. [DOI] [PubMed] [Google Scholar]
- Heredia L, Torrente M, Domingo JL, Colomina MT (2012). Individual housing and handling procedures modify anxiety levels of Tg2576 mice assessed in the zero maze test. Physiol Behav 107: 187–191. [DOI] [PubMed] [Google Scholar]
- Heredia L, Torrente M, Colomina MT, Domingo JL (2013). Assessing anxiety in C57BL/6J mice: a pharmacological characterization of the zero maze test. J Pharmacol Toxicol Methods 68: 275–283. [DOI] [PubMed] [Google Scholar]
- Heredia L, Torrente M, Colomina MT, Domingo JL (2014). Assessing anxiety in C57BL/6J mice: a pharmacological characterization of the open‐field and light/dark tests. J Pharmacol Toxicol Methods 69: 108–114. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Gandhi C, Bhattacharya P, Walsh TJ (2000). Effects of intraseptal zolpidem and chlordiazepoxide on spatial working memory and high‐affinity choline uptake in the hippocampus. Neurobiol Learn Mem 73: 168–179. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ (2003). The 5‐HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive‐type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl). 170:309–319. [DOI] [PubMed] [Google Scholar]
- Hogg S (1996). A review of the validity and variability of the elevated plus‐maze as an animal model of anxiety. Pharmacol Biochem Behav 54: 21–30. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ (1998). Responses of Swiss‐Webster mice to repeated plus‐maze experience: further evidence for a qualitative shift in emotional state? Pharmacol Biochem Behav 60: 473–488. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ (2003). Prior exposure to the elevated plus‐maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol 459: 221–230. [DOI] [PubMed] [Google Scholar]
- Holmes A, Iles JP, Mayell SJ, Rodgers RJ (2001). Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behav Brain Res 122: 159–167. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN (2002). Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav 1: 55–69. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL (2003). Mice lacking the serotonin transporter exhibit 5‐HT1A receptor‐mediated abnormalities in tests for anxiety‐like behavior. Neuropsychopharmacology 28: 2077–2088. [DOI] [PubMed] [Google Scholar]
- Homberg JR (2013). Measuring behaviour in rodents: towards translational neuropsychiatric research. Behav Brain Res 236: 295–306. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL (1999). Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol Biochem Behav. 63:21–26. [DOI] [PubMed] [Google Scholar]
- Horii Y, Kawaguchi M (2015). Higher detection sensitivity of anxiolytic effects of diazepam by ledge‐free open arm with opaque walled closed arm elevated plus maze in male rats. Behav Brain Res 294: 131–140. [DOI] [PubMed] [Google Scholar]
- Inoue K, Zorrilla EP, Tabarin A, Valdez GR, Iwasaki S, Kiriike N, et al. (2004). Reduction of anxiety after restricted feeding in the rat: implication for eating disorders. Biol Psychiatry 55: 1075–1081. [DOI] [PubMed] [Google Scholar]
- Izídio GS, Spricigo L Jr, Ramos A (2005). Genetic differences in the elevated plus‐maze persist after first exposure of inbred rats to the test apparatus. Behav Processes 68: 129–134. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF (2010). Genetic approaches to modeling anxiety in animals 161–201 in Stein M. B. and Steckler T., eds. Behavioral neurobiology of anxiety and its treatment. Springer Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Kim JG, Kim HJ, Kim BT, Kang DW, Lee JH (2007). Chronic food restriction in young rats results in depression‐ and anxiety‐like behaviors with decreased expression of serotonin reuptake transporter. Brain Res 1150: 100–107. [DOI] [PubMed] [Google Scholar]
- Jones N, King SM (2001). Influence of circadian phase and test illumination on pre‐clinical models of anxiety. Physiol Behav 72: 99–106. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, Murphy DL (2007). What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res 179: 1–18. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Bratt AM, Hodge CW (2003). Targeted gene deletion of the 5‐HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol 461: 19–25. [DOI] [PubMed] [Google Scholar]
- Keum S, Park J, Kim A, Kim KK, Jeong J, Shin HS (2016). Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav 15: 231–242 doi: 10.1111/gbb.12278 [DOI] [PubMed] [Google Scholar]
- Kim S, Lee S, Ryu S, Suk J, Park C (2002). Comparative analysis of the anxiety‐related behaviors in four inbred mice. Behav Processes 60: 181–190. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP (2006). Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol 4: e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamer D, Pålsson E, Revesz A, Engel JA, Svensson L (2004). Habituation of acoustic startle is disrupted by psychotomimetic drugs: differential dependence on dopaminergic and nitric oxide modulatory mechanisms. Psychopharmacology 176: 440–450. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA Jr (2007). Anxiolytic‐like effects of kappa‐opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther 323: 838–845. [DOI] [PubMed] [Google Scholar]
- Kopp C, Vogel E, Misslin R (1999). Comparative study of emotional behaviour in three inbred strains of mice. Behav Processes 47: 161–174. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N, Võikar V (2014). Assessment of mouse anxiety‐like behavior in the light–dark box and open‐field arena: role of equipment and procedure. Physiol Behav 133: 30–38. [DOI] [PubMed] [Google Scholar]
- Kurt M, Arik AC, Celik S (2000). The effects of sertraline and fluoxetine on anxiety in the elevated plus‐maze test in mice. J Basic Clin Physiol Pharmacol 11: 173–180. [DOI] [PubMed] [Google Scholar]
- Lad HV, Liu L, Paya‐Cano JL, Parsons MJ, Kember R, Fernandes C, et al. (2010). Behavioural battery testing: evaluation and behavioural outcomes in 8 inbred mouse strains. Physiol Behav 99: 301–316. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C (2008). Relations between open‐field, elevated plus‐maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J Neurosci Methods 171: 48–52. [DOI] [PubMed] [Google Scholar]
- Lamberty Y, Gower AJ (1996). Arm width and brightness modulation of spontaneous behaviour of two strains of mice tested in the elevated plus‐maze. Physiol Behav 59: 439–444. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez‐Diaz F, Chapouthier G (2000). Differences in anxiety‐related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav 67: 739–748. [DOI] [PubMed] [Google Scholar]
- Levay EA, Govic A, Penman J, Paolini AG, Kent S (2007). Effects of adult‐onset calorie restriction on anxiety‐like behavior in rats. Physiol Behav 92: 889–896. [DOI] [PubMed] [Google Scholar]
- Lewejohann L, Reinhard C, Schrewe A, Brandewiede J, Haemisch A, Görtz N, et al. (2006). Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes Brain Behav 5: 64–72. [DOI] [PubMed] [Google Scholar]
- Lin HQ, Burden PM, Christie MJ, Johnston GA (1999). The anxiogenic‐like and anxiolytic‐like effects of MDMA on mice in the elevated plus‐maze: a comparison with amphetamine. Pharmacol Biochem Behav 62: 403–408. [DOI] [PubMed] [Google Scholar]
- Livneh U, Dori A, Katzav A, Kofman O (2010). Strain and regional dependence of alternate splicing of acetylcholinesterase in the murine brain following stress or treatment with diisopropyl fluorophosphate. Behav Brain Res 210: 107–115. [DOI] [PubMed] [Google Scholar]
- Loss CM, Binder LB, Muccini E, Martins WC, de Oliveira PA, Vandresen‐Filho S, et al. (2015). Influence of environmental enrichment vs. time‐of‐day on behavioral repertoire of male albino Swiss mice. Neurobiol Learn Mem 125: 63–72. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Luine VN (2010). Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev 34: 452–467. [DOI] [PubMed] [Google Scholar]
- Maner JK, Richey JA, Cromer K, Mallott M, Lejuez CW, Joiner TE, et al. (2007). Dispositional anxiety and risk‐avoidant decision‐making. Pers Individ Dif 42: 665–675. [Google Scholar]
- Mansbach RS, Willetts J, Jortani SA, Balster RL (1991). NMDA antagonists: lack of antipunishment effect in squirrel monkeys. Pharmacol Biochem Behav 39: 977–981. [DOI] [PubMed] [Google Scholar]
- Martin P, Waters N, Waters S, Carlsson A, Carlsson ML (1997). MK‐801‐induced hyperlocomotion: differential effects of M100907, SDZ PSD 958 and raclopride. Eur J Pharmacol 24(335): 107–116. [DOI] [PubMed] [Google Scholar]
- Mathiasen LS, Mirza NR, Rodgers RJ (2008). Strain‐ and model‐dependent effects of chlordiazepoxide, L‐838,417 and zolpidem on anxiety‐like behaviours in laboratory mice. Pharmacol Biochem Behav 90: 19–36. [DOI] [PubMed] [Google Scholar]
- Mechan AO, Moran PM, Elliott M, Young AJ, Joseph MH, Green R (2002). A comparison between Dark Agouti and Sprague‐Dawley rats in their behaviour on the elevated plus‐maze, open‐field apparatus and activity meters, and their response to diazepam. Psychopharmacology 159: 188–195. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D (1996). Lateral and medial septal lesions reduce anxiety in the plus‐maze and probe‐burying tests. Physiol Behav 60: 845–853. [DOI] [PubMed] [Google Scholar]
- Michalikova S, van Rensburg R, Chazot PL, Ennaceur A (2010). Anxiety responses in Balb/c, c57 and CD‐1 mice exposed to a novel open space test. Behav Brain Res 207: 402–417. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Wit H (2008). Challenges for translational psychopharmacology research—some basic principles. Psychopharmacology (Berl.) 199: 291–301. [DOI] [PubMed] [Google Scholar]
- Milner LC, Crabbe JC (2008). Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav 7: 496–505. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR (2007). Differential effects of scopolamine and lorazepam on working memory maintenance versus manipulation processes. Cogn Affect Behav Neurosci 7: 120–129. [DOI] [PubMed] [Google Scholar]
- Mostafa RM, Michalikova S, Ennaceur A (2002). A 3D spatial navigation task for assessing memory in rodents. Neurosci Res Commun 31: 19–28. [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN (2007). Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 176:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N, Foster RG, Salmon PA (1999). Thresholds for masking responses to light in three strains of retinally degenerate mice. J Comp Physiol A 184: 423–428. [DOI] [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. (2003). Limbic corticotropin‐releasing hormone receptor 1 mediates anxiety‐related behavior and hormonal adaptation to stress. Nat Neurosci 6: 1100–1107. [DOI] [PubMed] [Google Scholar]
- Nakamura‐Palacios EM, Roelke CE (1997). Effects of acute daily administration of diazepam on spatial learning and working memory. Drug Alcohol Depend 46: 181–190. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB (2002). Recent advances in the neurobiology of depression. Psychopharmacol Bull 36: 6–23. [PubMed] [Google Scholar]
- Nesher E, Peskov V, Rylova A, Raz O, Pinhasov A (2012). Comparative analysis of the behavioral and biomolecular parameters of four mouse strains. J Mol Neurosci 46: 276–284. [DOI] [PubMed] [Google Scholar]
- Nicholson A, Malcolm RD, Russ PL, Cough K, Touma C, Palme R, et al. (2009). The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci 48: 740–753. [PMC free article] [PubMed] [Google Scholar]
- Nowakowska E, Chodera A, Kus K (1996). Anxiolytic and memory improving activity of fluoxetine. Pol J Pharmacol 48: 255–260. [PubMed] [Google Scholar]
- Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, Harvey‐White J, Holmes A (2008). Effects of adolescent fluoxetine treatment on fear‐, anxiety‐ or stress‐related behaviors in C57BL/6J or BALB/c J mice. Psychopharmacology (Berl). 200:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowska E, Kus K, Chodera A, Rybakowski J (2000). Behavioural effects of fluoxetine and tianeptine, two antidepressants with opposite action mechanisms, in rats. Arzneimittelforschung 50: 5–10. [DOI] [PubMed] [Google Scholar]
- Ohl F, Roedel A, Binder E, Holsboer F (2003). Impact of high and low anxiety on cognitive performance in a modified hole board test in C57BL/6 and DBA/2 mice. Eur J Neurosci 17: 128–136. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Reichardt HM, Joëls M, de Kloet ER (2001). Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. PNAS 98: 12790–12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary TP, Gunn RK, Brown RE (2013). What are we measuring when we test strain differences in anxiety in mice? Behav Genet 43: 34–50. [DOI] [PubMed] [Google Scholar]
- Packard MG (2009). Anxiety, cognition, and habit: a multiple memory systems perspective. Brain Res 1293: 121–128. [DOI] [PubMed] [Google Scholar]
- Palm S, Hävermark Å, Meyerson BJ, Nylander I, Roman E (2011). When is a Wistar a Wistar? Behavioral profiling of outbred Wistar rats from five different suppliers using the MCSF test. Appl Anim Behav Sci 135: 128–137. [Google Scholar]
- Parra A, Rama E, Vinader‐Caerols C, Monleón S (2013). Inhibitory avoidance in CD1 mice: sex matters, as does the supplier. Behav Process 100: 36–39. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Yu AJ (2012). Emotion and decision‐making: affect‐driven belief systems in anxiety and depression. Trends Cogn Sci 16: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M (1985). Validation of open:closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Brown RE (2002). Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes Brain Behav 1: 96–110. [DOI] [PubMed] [Google Scholar]
- Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk B, Krieter P, et al. (2006). The anxioselective agent 7‐(2‐chloropyridin‐4‐yl)pyrazolo‐[1,5‐a]‐pyrimidin‐3‐yl](pyridin‐2‐yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA‐gated currents at alpha1 subunit‐containing GABAA receptors. J Pharmacol Exp Ther 319: 1244–1252. [DOI] [PubMed] [Google Scholar]
- Post AM, Weyers P, Holzer P, Painsipp E, Pauli P, Wultsch T, Reif A, Lesch KP( 2011). Gene‐environment interaction influences anxiety‐like behavior in ethologically based mouse models. Behav Brain Res. 218:99–105. [DOI] [PubMed] [Google Scholar]
- Rachman S (1989). The return of fear: review and prospect. Clin Psychol Rev 9: 147–168. [Google Scholar]
- Ravenelle R, Santolucito HB, Byrnes EM, Byrnes JJ, Donaldson ST (2014). Housing environment modulates physiological and behavioral responses to anxiogenic stimuli in trait anxiety male rats. Neuroscience 270: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus GZ, Valvassori SS, Machado RA, Martins MR, Gavioli EC, Quevedo J (2008). Acute treatment with low doses of memantine does not impair aversive, non‐associative and recognition memory in rats. Naunyn Schmiedebergs Arch Pharmacol 376: 295–300. [DOI] [PubMed] [Google Scholar]
- Robert G, Drapier D, Bentué‐Ferrer D, Renault A, Reymann JM (2011). Acute and chronic anxiogenic‐like response to fluoxetine in rats in the elevated plus‐maze: modulation by stressful handling. Behav Brain Res 220: 344–348. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ (1997). Animal models of ‘anxiety’: where next? Behav Pharmacol 8: 477–496. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC (1993). Influence of social isolation, gender, strain and prior novelty on plus‐maze behaviour in mice. Physiol Behav 54: 729–736. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A (1997). Anxiety, defence and the elevated plus‐maze. Neurosci Biobehav Rev 21: 801–810. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Shepherd JK (1993). Influence of prior maze experience on behaviour and response to diazepam in the elevated plus‐maze and light/dark tests of anxiety in mice. Psychopharmacology (Berl.) 113: 237–242. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC, Aboualfa K, Stephenson LH (1995). Ethopharmacological analysis of the effects of putative ‘anxiogenic’ agents in the mouse elevated plus‐maze. Pharmacol Biochem Behav 52: 805–813. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cutler MG, Jackson JE (1997). Behavioural effects in mice of subchronic buspirone, ondansetron and tianeptine. II. The elevated plus‐maze. Pharmacol Biochem Behav 56: 295–303. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Davies B, Shore R (2002a). Absence of anxiolytic response to chlordiazepoxide in two common background strains exposed to the elevated plus‐maze: importance and implications of behavioural baseline. Genes Brain Behav 1: 242–251. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A (2002b). Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration‐based tests of anxiety‐related behaviour. Physiol Behav 77: 301–310. [DOI] [PubMed] [Google Scholar]
- Roesler R, Vianna M Sant'Anna MK, Kuyven CR, Kruel AV, Quevedo J, Ferreira MB (1998). Intrahippocampal infusion of the NMDA receptor antagonist AP5 impairs retention of an inhibitory avoidance task: protection from impairment by pretraining or preexposure to the task apparatus. Neurobiol Learn Mem 69: 87–91. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, et al. (1999). Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res 105: 207–217. [DOI] [PubMed] [Google Scholar]
- Rogóz Z, Skuza G (2011). Anxiolytic‐like effects of olanzapine, risperidone and fluoxetine in the elevated plus‐maze test in rats. Pharmacol Rep 63: 1547–1552. [DOI] [PubMed] [Google Scholar]
- Rosa VP, Vandresen N, Calixto AV, Kovaleski DF, Faria MS (2000). Temporal analysis of the rat's behavior in the plus‐maze: effect of midazolam. Pharmacol Biochem Behav 67: 177–182. [DOI] [PubMed] [Google Scholar]
- Rosat R, Da‐Silva RC, Zanatta MS, Medina JH, Izquierdo I (1992). Memory consolidation of a habituation task: role of N‐methyl‐D‐aspartate, cholinergic muscarinic and GABA‐A receptors in different brain regions. Braz J Med Biol Res 25: 267–273. [PubMed] [Google Scholar]
- Salomons AR, Arndt SS, Ohl F (2012). Impact of anxiety profiles on cognitive performance in BALB/c and 129P2 mice. Cogn Affect Behav Neurosci 12: 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams‐Dodd F (2013). Is poor research the cause of the declining productivity of the pharmaceutical industry? An industry in need of a paradigm shift. Drug Discov Today 18: 211–217. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Joly D (1991). The effects of NMDA antagonists on punished exploration in mice. Behav Pharmacol 2: 57–63. [PubMed] [Google Scholar]
- Saucier D, Cain DP (1995). Spatial learning without NMDA receptor‐dependent long‐term potentiation. Nature 378: 186–189. [DOI] [PubMed] [Google Scholar]
- Saucier D, Hargreaves EL, Boon F, Vanderwolf CH, Cain DP (1996). Detailed behavioral analysis of water maze acquisition under systemic NMDA or muscarinic antagonism: nonspatial pretraining eliminates spatial learning deficits. Behav Neurosci 110: 103–116. [PubMed] [Google Scholar]
- Schmitt U, Hiemke C (1998). Strain differences in open‐field and elevated plus‐maze behavior of rats without and with pretest handling. Pharmacol Biochem Behav 59: 807–811. [DOI] [PubMed] [Google Scholar]
- Scott D, Taylor JR (2014). Chronic nicotine attenuates phencyclidine‐induced impulsivity in a mouse serial reaction time task. Behav Brain Res. 259:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, O'Connor C (1992). N‐methyl‐D‐aspartate receptor antagonist MK‐801 and spatial memory representation: working memory is impaired in an unfamiliar environment but not in a familiar environment. Behav Neurosci 106: 604–612. [DOI] [PubMed] [Google Scholar]
- Shekhar A, McCann UD, Meaney MJ, Blanchard DC, Davis M, Frey KA, et al. (2001). Summary of a National Institute of Mental Health workshop: developing animal models of anxiety disorders. Psychopharmacology 157: 327–339. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT (1994). Behavioural and pharmacological characterisation of the elevated “zero‐maze” as an animal model of anxiety. Psychopharmacology 116: 56–64. [DOI] [PubMed] [Google Scholar]
- Sherwin CM, Glen EF (2003). Cage colour preferences and effects of home cage colour on anxiety in laboratory mice. Anim Behav 66: 1085–1092. [Google Scholar]
- Silva RC, Brandão ML (2000). Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus‐maze: an ethological analysis. Pharmacol Biochem Behav 65: 209–216. [DOI] [PubMed] [Google Scholar]
- Silva MT, Alves CR, Santarem EM (1999). Anxiogenic‐like effect of acute and chronic fluoxetine on rats tested on the elevated plus‐maze. Braz J Med Biol Res 32: 333–339. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN (2010). Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Nadal R, Pallares M, Ferre N (1997). Acute effects of ketamine in the holeboard, the elevated‐plus maze, and the social interaction test in Wistar rats. Depress Anxiety 5: 29–33. [PubMed] [Google Scholar]
- Smith KS, Engin E, Meloni EG, Rudolph U (2012). Benzodiazepine‐induced anxiolysis and reduction of conditioned fear are mediated by distinct GABAA receptor subtypes in mice. Neuropharmacology 63: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati J (2011). Dorsal hippocampal N‐methyl‐D‐aspartate glutamatergic and d‐opioidergic systems modulate anxiety behaviors in rats in a noninteractive manner. Kaohsiung J Med Sci 27: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati J, Salari A‐A (2011). Involvement of dorsal hippocampal NMDA‐glutamatergic system in anxiety‐related behaviors of rats. Neurochem J 5: 194–199. [Google Scholar]
- Soto PL, Ator NA, Rallapalli SK, Biawat P, Clayton T, Cook JM, et al. (2013). Allosteric modulation of GABA(A) receptor subtypes: effects on visual recognition and visuospatial working memory in rhesus monkeys. Neuropsychopharmacology 38: 2315–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Almeida OF, Wotjak CT (2006). A hitchhiker's guide to behavioral analysis in laboratory rodents. Genes Brain Behav 5(Suppl 2): 5–24. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Owa T, Nishino T, Kamei C (2010). Assessing anxiolytic‐like effects of selective serotonin reuptake inhibitors and serotonin‐noradrenaline reuptake inhibitors using the elevated plus maze in mice. Methods Find Exp Clin Pharmacol 32: 113–121. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Gould TJ, Druhan JP, Bucan M (2000). Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome 11: 555–564. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA (1966). Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73: 16–43. [DOI] [PubMed] [Google Scholar]
- Thompson T, Grabowski‐Boase L, Tarantino LM (2015). Prototypical anxiolytics do not reduce anxiety‐like behavior in the open field in C57BL/6J mice. Pharmacol Biochem Behav 133: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake WD, Birch D (1967). Complexity, novelty, and food deprivation as determinants of speed of shift of behavior. J Comp Physiol Psychol 63: 545–548. [DOI] [PubMed] [Google Scholar]
- Tiplady B, Bowness E, Stien L, Drummond G (2005). Selective effects of clonidine and temazepam on attention and memory. J Psychopharmacol 19: 259–265. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J, Royan C (1993). Anxiogenic stimuli in the elevated plus‐maze. Pharmacol Biochem Behav 44: 463–469. [DOI] [PubMed] [Google Scholar]
- Treit D, Engin E, McEown K (2010). Animal models of anxiety and anxiolytic drug action. Curr Top Behav Neurosci 2: 121–160. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P (1993). Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl.) 111: 323–331. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Steckler T (2001). Behavioural phenotyping of mouse mutants. Behav Brain Res. 125: 3–12 [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Steckler T (2000). Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res 115: 95–106. [DOI] [PubMed] [Google Scholar]
- Venâncio C, Magalhães A, Antunes L, Summavielle T (2011). Impaired spatial memory after ketamine administration in chronic low doses. Curr Neuropharmacol 9: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleye M, Dumas S, Heulard I, Krafft N, Gillardin JM (2011). Differential effects of etifoxine on anxiety‐like behaviour and convulsions in BALB/cByJ and C57BL/6J mice: any relation to overexpression of central GABA A receptor beta2 subunits? Eur Neuropsychopharmacol 21: 457–470. [DOI] [PubMed] [Google Scholar]
- Violle N, Balandras F, Le Roux Y, Desor D, Schroeder H (2009). Variations in illumination, closed wall transparency and/or extramaze space influence both baseline anxiety and response to diazepam in the rat elevated plus‐maze. Behav Brain Res 203: 35–42. [DOI] [PubMed] [Google Scholar]
- Võikar V, Kõks S, Vasar E, Rauvala H (2001). Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav 72: 271–281. [DOI] [PubMed] [Google Scholar]
- Võikar V, Vasar E, Rauvala H (2004). Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav 3: 27–38. [DOI] [PubMed] [Google Scholar]
- Võikar V, Polus A, Vasar E, Rauvala H (2005). Long‐term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav 4: 240–252. [DOI] [PubMed] [Google Scholar]
- Wahlsten D (2001). Standardizing tests of mouse behavior: reasons, recommendations, and reality. Physiol Behav 73: 695–704. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL ii, Burkhart‐Kasch S, Dorow J, et al. (2003). Different data from different labs: lessons from studies of gene‐environment interaction. J Neurobiol 54: 283–311. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Wadsworth G, Fletcher A, Dourish CT (1998). Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neurosci Biobehav Rev 23: 265–271. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Auer RN (1989). Immediate and long‐lasting effects of MK‐801 on motor activity spatial navigation in a swimming pool and EEG in the rat. Psychopharmacology (Berl.) 98: 500–507. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gharbawie OA, Clark BJ, Lehmann H (2006). The exploratory behavior of rats in an open environment optimizes security. Behav Brain Res 171: 230–239. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Szewczyk B, Palucha A, Branski P, Smialowska M (2003). Involvement of CRF but not NPY in the anxiety regulation via NMDA receptors. Pol J Pharmacol 55: 1119–1124. [PubMed] [Google Scholar]
- Wiley JL, Cristello AF, Balster RL (1995). Effects of site‐selective NMDA receptor antagonists in an elevated plus‐maze model of anxiety in mice. Eur J Pharmacol 294: 101–107. [DOI] [PubMed] [Google Scholar]
- Wilking JA, Hesterberg KG, Nguyen VH, Cyboron AP, Hua AY, Stitzel JA (2012). Comparison of nicotine oral consumption and baseline anxiety measures in adolescent and adult C57BL/6J and C3H/Ibg mice. Behav Brain Res 233: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10‐year review and evaluation. Psychopharmacology 134: 319–329. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD (2004). Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav 78: 445–458. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ (2012). Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev 36: 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer DP, Litvin O, Morf S, Nitsch RM, Lipp HP, Würbel H (2004). Laboratory animal welfare: cage enrichment and mouse behaviour. Nature 432: 821–822. [DOI] [PubMed] [Google Scholar]
- Würbel H (2002). Behavioral phenotyping enhanced–beyond (environmental) standardization. Genes Brain Behav 1: 3–8. [DOI] [PubMed] [Google Scholar]
- Yagi K, Onaka T, Yoshida A (1998). Role of NMDA receptors in the emotional memory associated with neuroendocrine responses to conditioned fear stimuli in the rat. Neurosci Res 30: 279–286. [DOI] [PubMed] [Google Scholar]
- Yaski O, Eilam D (2007). The impact of landmark properties in shaping exploration and navigation. Anim Cogn 10: 415–428. [DOI] [PubMed] [Google Scholar]
- Yilmazer‐Hanke DM, Roskoden T, Zilles K, Schwegler H (2003). Anxiety‐related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behav Brain Res 145: 145–159. [DOI] [PubMed] [Google Scholar]


