INTRODUCTION
Zinc serves as a catalytic or structural cofactor for a large number of proteins. Many of these proteins are secreted from cells and acquire their zinc as they pass through the secretory pathway. These include enzymes such as matrix metalloproteases, alkaline phosphatases, and angiotensin-converting enzymes. In addition, many other zinc-dependent proteins are resident in secretory pathway compartments such as the endoplasmic reticulum and Golgi apparatus. These include protein chaperones and co-chaperones (e.g. calreticulin, calnexin, DnaJ orthologs), ER-associated peptidases (e.g. ERAAP), and glycosylphosphatidylinositol phosphoethanolamine transferases (GPI-PET) involved in GPI anchor synthesis. Evidence that the early secretory pathway requires zinc for function comes from recent studies showing that zinc deficiency causes induction of the Unfolded Protein Response (UPR), an indicator of ER stress [1, 2]. The endoplasmic reticulum has also been proposed to be the source of zinc that acts as an intracellular second messenger of IgE receptor activation in mast cells [3]. Conversely, excess zinc may also be disruptive to ER function. ER zinc overload was proposed to cause the spondylocheiro dysplastic form of Ehlers-Danlos Syndrome (EDS-SCD) resulting from mutations in the SLC39A13/ZIP13 zinc transporter gene [4, 5].
Given these various roles, it is clear that cells must have efficient systems for the transport of zinc into the ER and Golgi under zinc deficiency and regulatory mechanisms to maintain zinc homeostasis within those compartments. Several zinc transporters in the early secretory pathway have been identified in recent years. In vertebrates, the ZnT-5, ZnT-6, and ZnT-7 proteins have been found to contribute to secretory pathway zinc and the metallation of secreted proteins [6-8]. These three proteins are members of the CDF/ZnT/SLC30A family of zinc transporters. While ZnT-7 is active as a homodimer, ZnT-5 and ZnT-6 form a heterodimer complex to be active [9]. In Saccharomyce cerevisiae, the Msc2 and Zrg17 proteins play key roles in maintaining secretory pathway zinc. These proteins are the yeast orthologs of vertebrate ZnT-5 and ZnT-6 and reside in the ER [1, 2]. Previous results suggest that, like ZnT-5 and ZnT-6, Msc2 and Zrg17 are only active as heterodimeric complexes [2, 9]. Specifically, it was shown that Msc2 and Zrg17 physically interact and that both proteins are required for zinc transport function. Moreover, it was shown that Msc2/Zrg17 activity is required only under zinc-limiting conditions and that other transport systems are sufficient to maintain ER zinc levels in zinc-replete cells [1, 2].
Little is known about how secretory pathway zinc transporters are regulated in response to zinc status or other signals. In this report, we address the transcriptional regulation of ZRG17 in response to zinc by the Zap1 transcriptional activator. In yeast, Zap1 is the central player in the response of cells to zinc deficiency [10]. We currently estimate that Zap1 activates the transcription of ~80 genes in zinc-limited cells and expression of several other genes are repressed directly or indirectly by Zap1 [11-15]. Zap1 regulates target gene expression by binding to 11 bp sequences known as Zinc-Responsive Elements (ZREs) in those target gene promoters [16]. The consensus sequence for the ZRE is 5’-ACCTTNAAGGT-3’ and flanking sequences may also contribute to Zap1 ZRE recognition [17]. Regulation of Zap1 activity is controlled by zinc binding directly to the protein to repress activation domain function in zinc-replete cells [18, 19].
Previous analyses of the Zap1 regulon suggested that ZRG17 is a direct target of Zap1 activation [11, 12, 20]. Using whole genome DNA microarrays, we found that ZRG17 expression increased in zinc-limited cells in a Zap1-dependent manner. In this report, we test this hypothesis and confirmed that ZRG17 is indeed a direct target of Zap1 regulation. In addition, we also show the physiological importance of this regulation to ER function.
EXPERIMENTAL
Yeast strains and growth conditions
Media used were YPD, SD, YPGE, and LZM as described previously [21]. LZM contains 1 mM EDTA and 20 mM citrate to both limit and buffer available zinc levels. Yeast strains DY1457 (MATa ade6 can1 his3 leu2 trp1 ura3), DY150 (MATa ade2 can1 his3 leu2 trp1 ura3), and ZHY6 (DY1457 zap1Δ::TRP1) have also been described previously [22]. The chromosomal ZRG17 mutant, zrg17-1m2ZRE, was constructed by first integrating the counterselectable reporter (CORE) cassette [23] containing the Kluveromyces lactis URA3 gene and kanMX4 into the ZRG17 promoter of DY1457. The promoter fragment from pZRG17-m2ZRE-lacZ (see below) was then amplified by PCR and transformed into the CORE cassette-containing strain and selected for loss of the URA3 gene by selection on 5-fluoroorotic acid [24]. Correct mutation of the chromosomal ZRG17 promoter was confirmed by PCR and DNA sequencing. The reconstructed wild-type ZRG17 strain, ZRG17rZRE, was constructed in the same way with a promoter fragment amplified from pZRG17-lacZ containing the wild-type promoter. To generate zrg17Δ mutant in the DY1457 background, the KanMX cassette with 500 bp flanking the ZRG17 open reading frame was amplified by PCR from the CEY9 (DY150 zrg17Δ::KanMX) [2]. The PCR fragment was then transformed into DY1457 strain to generate DY1457 zrg17Δ.
Yeast plasmids
pYef2 (Vec) [25], pAFH35 (Zap1up) [26], YEp353 (Vec) [27], pZRG17-HA [2] and pMCZ-Y [28] (UPRE-lacZ; provided by A. Cooper, Garvan Institute, Sydney) were described previously. Reporter plasmid pZRG17-lacZ was constructed in YEp353 by homologous recombination. PCR products were generated from genomic DNA that contained 1000 bp of ZRG17 promoter sequence (bases −1000 to +1) flanked by homology to the vector. This fragment was gel purified and co-transformed with EcoRI- and BamHI-digested YEp353; transformants were selected for URA3 prototrophy. The mutant alleles of ZRG17 ZRE (pZRG17-m1ZRE-lacZ, pZRG17-m2ZRE-lacZ) were constructed in a similar fashion after generation of the mutant promoter fragments by overlapping PCR. pYef2L(Vec) and pYef2L-Zap1-6x-myc (Zap1-myc) used in chromatin immunoprecipitation were constructed as previously described [18]. All plasmid constructs were confirmed by sequencing.
RNA and protein analyses
S1 nuclease protection assays were performed with total RNA as previously described [29]. Total RNA was extracted from cells grown to mid-log phase with hot acid phenol. For each reaction, 15 μg of total RNA was hybridized to 32P-end-labeled DNA oligonucleotide probes for ZRG17 (CGGGGGAAATGCCTCTTACCGGTGATCTTGTTCTGG-GAGGAGGCGGCACCAGCTTTGGTGCTGGTACGCGCC) and CMD1 (GGGCAAAGG-CTTCTTTGAATTCAGCAATTTGTTCTTCGGTGGAGCC) before digestion with S1 nuclease and separation on a 10% polyacrylamide, 5 M urea polyacrylamide gel. Band intensities were quantified by phosphorimager analysis (PerkinElmer Life Sciences). Protein extracts were generated by lysis in trichloroacetic acid, and immunoblot analysis was performed as described previously [30]. The primary antibodies used were mouse anti-HA (12CA5, Roche Applied Science) and mouse anti-Pgk1 (Molecular Probes). The secondary antibody used was HRP-conjugated goat anti–mouse IgG (Pierce Chemical Co.). Band intensities were measured using NIH ImageJ.
β-galactosidase assays
Cells were grown to mid-log phase in LZM supplemented with the indicated amount of ZnCl2. β-galactosidase activity was measured in permeabilized cells as described previously [31], and activity was normalized to cell density. For UPRE-lacZ analysis, β-galactosidase assays were performed on protein extracts and specific activity was normalized to protein content [32].
Electrophoretic mobility shift assays
The Zap1 DNA-binding domain (Zap1DBD, residues 687–880) was expressed in Escherichia coli as a fusion to glutathione S-transferase and purified [33]. Electrophoretic mobility shift assays were performed as described previously using purified Zap1DBD [33] and radiolabeled oligonucleotides (Table 1). Fifty pmol of 32P-end-labeled oligonucleotides were purified using G-50 Quick Spin columns (Roche Applied Science). Double-stranded oligonucleotides were prepared by annealing complementary single-stranded oligonucleotides (1 μM) in 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 1 mM EDTA. The annealed mixtures were incubated for 15 min at 85 °C and then 55 °C for 4 h. For electrophoretic mobility shift assays, 15-μl reactions were prepared containing 0.5 pmol of radiolabeled oligonucleotide (20,000 cpm/pmol), 10 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 50 mM KCl, 1 mM dithiothreitol, 0.02 mg/ml poly(dI-dC)•poly(dI-dC), 0.2 mg/ml bovine serum albumin, 0.04% IGEPAL CA-630, 10% glycerol, and the indicated concentrations of purified Zap1DBD. After incubation for 1 h at room temperature, the samples were resolved on 6% polyacrylamide gels. Gels were dried onto blotting paper, and the signals were measured by autoradiography.
Table 1.
Oligonucleotides used for EMSAs
| ZRE | Oligonucleotide |
|---|---|
| TSA1 ZRE1 | 5′-ggccCTGTTCTGGCCCGTCGGGTTTTCTGACAAA-3′ |
| 3′-GACAAGACCGGGCAGCCCAAAAGACTGTTTagct-5′ | |
| ZRG17 ZRE | 5′-ggccACTGAAAATGATGAACCTTGAAGGTATTTTTGTTACT-3′ |
| 3′-TGACTTTTACTACTTGGAACTTCCATAAAAACAATGAagct-5′ | |
| ZRG17 m1ZRE | 5′-ggccACTGAAAATGATGACAAGGTCCTTGATTTTTGTTACT-3′ |
| 3′-TGACTTTTACTACTGTTCCAGGAACTAAAAACAATGAagct-5′ |
ZRG17 m1ZRE was mutated such that each position in the potential ZRE was altered by a transversion mutation. ZREs or regions mutated in each complementary oligonucleotide pair are indicated by the underline. The lower-case letters indicate EagI- and SalI-complementary overhangs included for cloning these fragments into a lacZ reporter plasmid for future studies.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as described [34]. Wild-type cells transformed with either the vector (pYef2L) or a plasmid expressing a myc-tagged Zap1 protein (pYef2L-Zap1-6x-myc) [18] were grown to an OD600 ~ 0.5 and then treated with 1% formaldehyde to cross-link protein-DNA complexes. The cross-linking reaction was quenched by adding 2.5 mL of 2 M glycine. After two 25 mL washes with ice-cold PBS, the cells were lysed with glass beads in buffer containing complete protease inhibitor cocktail (Roche), 1 mM PMSF, and 2 mM benzamidine. Following centrifugation for 10 minutes at 16,000 × g, the supernatants were immunoprecipitated with anti-myc antibody at 4 °C overnight and isolated with Protein A-Sepharose. The cross-links were reversed in TES and co-immunoprecipitation of specific promoter fragments with Zap1-myc was assessed by PCR using primers flanking the ZRG17 ZRE by 100 base pairs. Primers specific to the ZRT1 ZRE and CMD1 were used as positive control and negative controls, respectively. PCR products generated from 10-fold serially diluted input samples were used to confirm the semi-quantitative nature of the analysis.
RESULTS
Zinc regulation of ZRG17 mRNA abundance
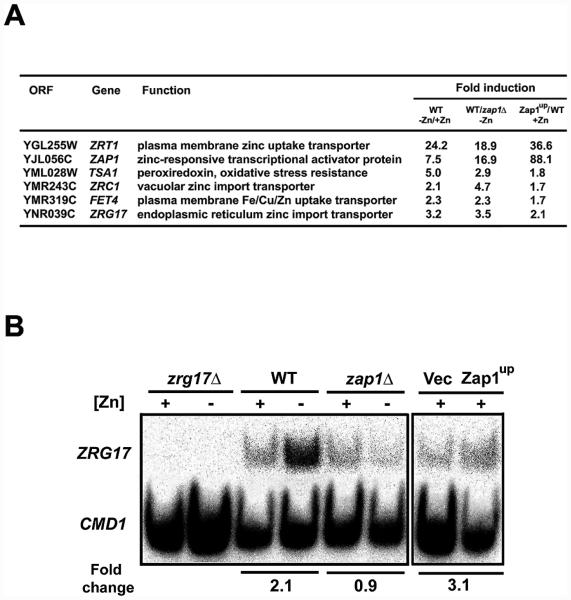
Our previous results suggested that ZRG17 is a direct target of Zap1 regulation [11, 12]. Microarray analyses indicated that ZRG17 mRNA levels were elevated in zinc-limited cells in a Zap1-dependent manner and were also elevated in zinc-replete cells expressing a constitutive allele of Zap1, Zap1up, which contains mutations that disrupt zinc sensing (Fig. 1A). These effects are similar to those observed for several known Zap1 target genes including, ZRT1, ZAP1, TSA1, ZRC1, and FET4. The results for ZRG17 were confirmed when we assayed mRNA levels by S1 nuclease protection assay with cells grown under the same conditions as were used for the microarray experiments (Fig. 1B). ZRG17 mRNA abundance increased ~2-fold in zinc-limited wild-type cells but not zap1Δ mutant cells. In addition, ZRG17 mRNA abundance increased ~3-fold in zinc-replete cells expressing the constitutive Zap1up allele. When a functional hemagglutinin (HA) epitope-tagged ZRG17 allele was expressed from its own promoter, we found that ZRG17 mRNA was induced by relatively severe zinc deficiency (LZM + ≤10 μM ZnCl2) and that the levels of Zrg17-HA protein showed a similar dose response and magnitude of regulation (Fig. 2, data not shown). These results suggest that control of ZRG17 transcription is the principal mechanism regulating Zrg17 protein accumulation in response to zinc. CMD1 mRNA, encoding calmodulin, and the Pgk1 3-phosphoglycerate kinase protein are not zinc regulated and these served as loading controls for the S1 nuclease protection assays and immunoblots, respectively.
Figure 1. S1 nuclease protection assays confirm microarray results.
A) Previous microarray results for ZRG17 and select previously confirmed Zap1 target genes [11, 12]. B) ZRG17 mRNA levels were analyzed by S1 nuclease protection assays. Total RNA was isolated from cells grown under the same conditions as the microarray experiments. zrg17Δ mutant cells, wild-type cells (DY1457), or zap1Δ mutant cells (ZHY6) were grown in low zinc medium (LZM) supplemented with high zinc (+, 1000 μM ZnCl2) or low zinc (−, 1 μM ZnCl2) (left panel). LZM contains 1 mM EDTA and 20 mM citrate to buffer available zinc levels. Also, wild-type (DY1457) cells expressing either the vector (pYef2) or a Zap1up plasmid (pAFH35) were grown in zinc-replete SD medium containing 1 μM ZnCl2 and 2% galactose as the carbon source to drive expression of the constitutively active Zap1up allele from the GAL1 promoter (right panel). CMD1, encoding calmodulin, was used as a loading control. The fold changes shown were quantified from the level of ZRG17 mRNA normalized to CMD1 in each lane. The data shown are representative of two independent experiments.
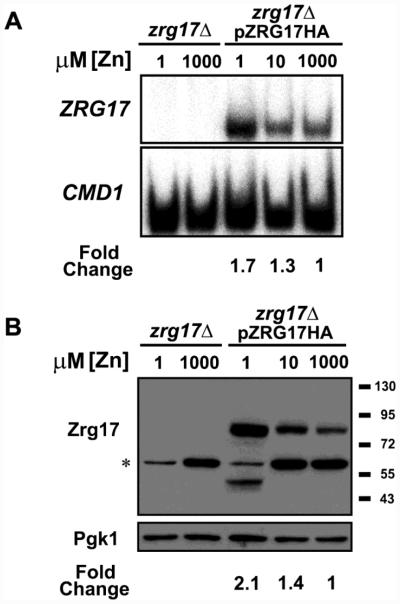
Figure 2. Zrg17 protein levels correlate with ZRG17 mRNA.
zrg17Δ mutant cells or zrg17Δ cells expressing a C-terminal hemaggluttinin (HA) epitope-tagged allele of Zrg17 expressed from its own promoter (pZRG17-HA) were grown in LZM supplemented with the indicated concentration of ZnCl2. A) S1 nuclease protection assays were performed to detect ZRG17 and CMD1 mRNA. The fold changes shown were quantified from the level of ZRG17 mRNA normalized to CMD1. B) Immunoblot analysis of Zrg17-HA protein levels. Lysates were prepared from the same cells used in panel A and subjected to immunoblotting with anti-HA antibody. Pgk1 (phosphoglycerate kinase 1) served as a loading control. The asterisk indicates a non-specific background band. The fold changes shown were quantified from the level of Zrg17 protein normalized to Pgk1. A smaller molecular mass Zrg17-HA band was observed in the LZM + 1 μM ZnCl2-grown pZRG17-HA transformants, perhaps resulting from partial proteolysis, and both full-length and truncated Zrg17 forms were included in the quantification. The data shown are representative of two independent experiments.
A potential Zap1-binding site in the ZRG17 promoter
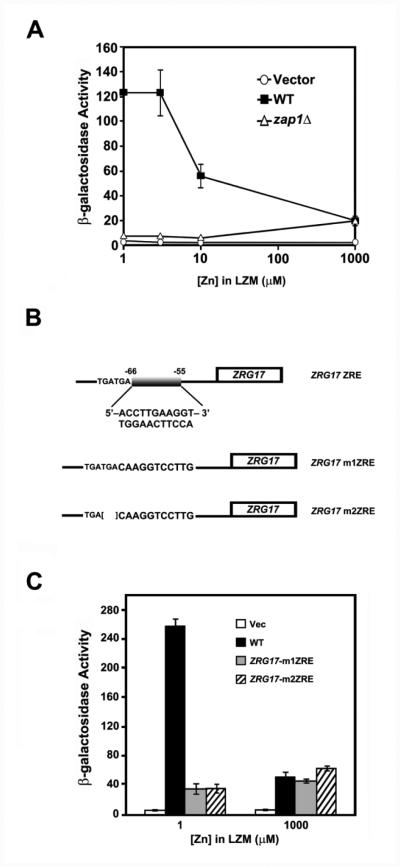
To further examine the mechanism of ZRG17 regulation, we constructed a lacZ reporter gene in which the ZRG17 promoter, extending 1000 bp upstream of the open reading frame, was fused to the E. coli lacZ gene. As shown in Figure 3A, the ZRG17-lacZ reporter was highly regulated by zinc in wild-type cells being induced approximately 6-fold in zinc-limited cells relative to zinc-replete conditions. This induction was not observed in zap1Δ mutant cells. Expression in zinc-replete zap1Δ cells was similar to that observed in wild-type cells but expression decreased in zinc-limited cells to very low levels. This indicated that basal expression of ZRG17 in zinc-replete cells is Zap1-independent and Zap1 function is required for the elevated expression observed in zinc-limited cells.
Figure 3. A candidate ZRE in the ZRG17 promoter is required for its induction.
A) β-galactosidase activity was measured in wild-type cells (DY1457) or zap1Δ mutant cells (ZHY6) bearing the wild-type ZRG17-lacZ reporter and grown in LZM over a range of zinc concentrations. B) The ZRG17 promoter with the wild-type ZRE (ZRG17 ZRE) and two different promoter mutants constructed (m1ZRE, m2ZRE). Both ZRG17 m1ZRE and ZRG17 m2ZRE were mutated such that each of the eleven positions of the ZRE was altered by transversion mutations. ZRG17 m2ZRE has three additional base pairs (TGA) adjacent to the ZRE deleted. C) β-galactosidase activity was measured in wild-type cells (DY1457) bearing the vector (YEp353), the wild-type ZRG17-lacZ reporter (WT), or the mutated reporters (ZRG17-m1ZRE, ZRG17-m2ZRE) and grown in low zinc (LZM + 1 μM ZnCl2) or high zinc (LZM + 1000 μM ZnCl2) conditions. The data shown are the means of three independent cultures for each condition and are representative of two independent experiments. The error bars represent ± 1 S.D.
Motif analysis of the ZRG17 promoter indicated the presence of a candidate ZRE located at position −66 to −55 upstream of the ZRG17 transcription start site (Fig. 3B) [11, 35]. This sequence matched the consensus ZRE sequence compiled from experimentally verified and other candidate Zap1 target genes. To determine the importance of this potential ZRE to ZRG17 regulation, we constructed two different promoter mutant constructs. In mutant m1ZRE, transversion mutations were introduced in all positions of the ZRE. Because adjacent base pairs may also contribute to Zap1 binding [17], we constructed a second promoter mutant, m2ZRE, in which an adjacent 3 bp were also deleted. As shown in Figure 3C, mutation of the ZRG17 ZRE, with or without deletion of the adjacent base pairs, completely eliminated induction of ZRG17 expression in zinc-limited cells. Notably, neither ZRE mutation affected zinc-replete expression confirming that expression under these conditions is Zap1-independent.
Zap1 binds specifically to the ZRG17 ZRE in vitro and in vivo
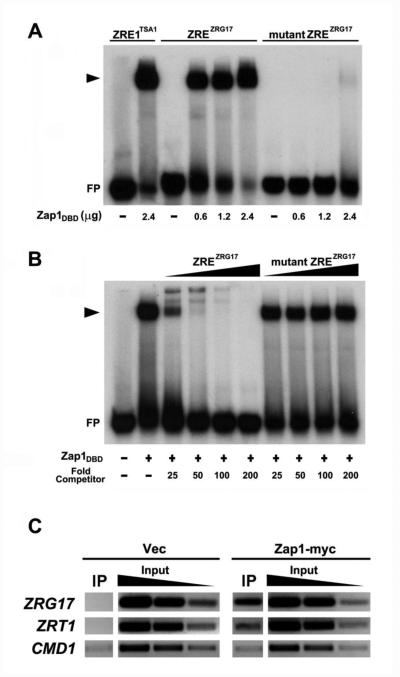
To determine whether Zap1 binds to the ZRG17 ZRE sequence, electrophoretic mobility shift assays were performed using the Zap1 DNA binding domain (Zap1DBD) purified from E. coli. An oligonucleotide containing a known Zap1 binding site from the TSA1 promoter was used as a positive control (Fig. 4A, lanes 1, 2). When increasing amounts of Zap1DBD were incubated with a ZRG17 ZRE oligonucleotide, increased abundance of a Zap1DBD-DNA complex was also observed (Fig. 4A, lanes 3-6). However, no binding was observed when the mutant m1ZRE was used as the probe except when the highest amounts of Zap1DBD protein (2.4 μg) were used in the reaction (Fig. 4A, lanes 7-10). In addition, a binding competition experiment confirmed that the Zap1DBD binds specifically to the ZRG17 ZRE. In this experiment, either the wild-type ZRG17 ZRE or the mutant m1ZRE oligonucleotides were assessed for their ability to compete for binding with the TSA1 ZRE fragment. While the wild-type ZRE was an effective competitor, no decrease in binding was observed with the mutant ZRE (Fig. 4B). These results indicate specific binding of Zap1 to the potential ZRG17 ZRE.
Figure 4. Zap1 binds specifically to the ZRG17 ZRE in vitro and in vivo.
A) Electrophoretic mobility shift assay (EMSA) showing sequence specificity of Zap1 DNA-binding domain (Zap1DBD) binding to the ZRG17 ZRE. Radiolabeled oligonucleotides with the wild-type TSA1 ZRE1 (ZRE1TSA1), wild-type ZRG17 ZRE (ZREZRG17), or the mutated ZRG17 m1ZRE (mutant ZREZRG17) were incubated with purified Zap1DBD for 1 hour prior to native gel electrophoresis. The experiments were performed with the indicated amounts of Zap1DBD. FP denotes the free probe, and the arrowhead indicates the Zap1DBD-ZRE complex. B) EMSA competition assay showing that the ZRG17 ZRE competes with TSA1 ZRE1 for Zap1DBD binding. Radiolabeled wild-type TSA1 ZRE1 (ZRE1TSA1) oligonucleotides were incubated with 0 (−) or 2.4 μg (+) Zap1pDBD and a 25-, 50-, 100-, or 200-fold excess of the nonradiolabeled wild-type ZRG17 ZRE (ZREZRG17) or the mutated ZRG17 m1ZRE (mutant ZREZRG17) oligonucleotides for 1 h at room temperature prior to native gel electrophoresis. C) Chromatin immunoprecipitation verifies Zap1 binding to the ZRG17 promoter in vivo. Wild-type cells transformed with either the vector (pYef2L) or a plasmid expressing a myc-tagged Zap1 protein (Zap1-myc) were grown under low zinc conditions (LZM + 3 μM ZnCl2). Chromatin immunoprecipitation was performed as described in the Experimental section. Enrichment of the ZRG17 ZRE was observed in the immunoprecipitates (IP) from Zap1-myc expressing cells relative to the vector-only cells. The ZRT1 ZRE and CMD1 promoter regions were used as positive and negative controls, respectively. PCR products generated from 10-fold serially diluted input samples were used to confirm the semi-quantitativeness of the analysis. The data shown are representative of two independent experiments.
To test whether Zap1 binds to the ZRG17 ZRE in vivo, we performed a chromatin immunoprecipitation experiment. Wild-type cells transformed with either the vector or a plasmid expressing a myc-tagged Zap1 protein were grown under low zinc conditions and then treated with 1% formaldehyde to cross-link protein-DNA complexes. Chromatin was then isolated, sheared by sonication, and Zap1 was immunoprecipitated with anti-myc antibody. The cross-links were reversed and co-immunoprecipitation of specific promoter fragments with Zap1-myc was assessed by PCR using primers flanking the ZRG17 ZRE. Enrichment of the ZRG17 ZRE was observed in the immunoprecipitates from Zap1-myc expressing cells relative to those from the vector control cells (Fig. 4C). Enrichment by immunoprecipitation was also observed for the ZRT1 promoter, a known Zap1 target gene, but no enrichment was detected for the non-zinc responsive CMD1 promoter, which served as a negative control. These results indicate that Zap1 binds specifically to the ZRG17 promoter in vivo as well as in vitro.
Biological importance of ZRG17 transcriptional control
Having established that ZRG17 is indeed a direct Zap1 target gene, we next addressed the importance of Zap1 regulation to the function of the Zrg17 protein. As shown in Figures 1 and 3, our results indicated that Zap1 regulation was required for maximal expression of ZRG17 in zinc-limited cells but was not needed for basal expression in zinc-replete cells. Therefore, to assess the importance of Zap1 regulation to Zrg17 function, we replaced the ZRE in the chromosomal ZRG17 gene with the nonfunctional m2ZRE mutant sequence to generate the zrg17-1m2ZRE allele. The rest of the ZRG17 promoter and the complete open reading frame are unaltered in the zrg17-1m2ZRE allele, and thus only its regulation by Zap1 was affected. As a control, we simultaneously generated a strain identical to zrg17-1m2ZRE in which we restored the wild-type sequence and this strain was designated ZRG17rZRE (“rZRE” for reconstructed ZRE, see Experimental Procedures). S1 nuclease protection assays indicated that the chromosomal m2ZRE mutation disrupted induction of chromosomal ZRG17 under low zinc conditions but did not greatly affect expression in zinc-replete cells (Fig. 5A). Expression of ZRG17 in the ZRG17rZRE strain was similar to wild type. These data confirmed the results obtained with the ZRG17-lacZ reporters indicating that Zap1 contributes to ZRG17 expression in zinc-limited cells but not in replete cells.
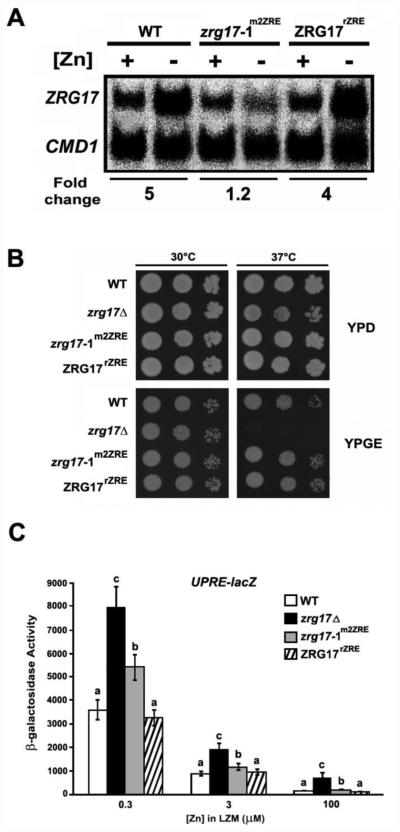
Figure 5. Zap1 regulation of ZRG17 expression is important to ER function.
A) ZRG17 mRNA levels were analyzed by S1 nuclease protection assay of RNA isolated from wild-type (DY1457), a chromosomal ZRG17 ZRE mutant (zrg17-1m2ZRE), and reconstructed wild-type ZRE (ZRG17rZRE) cells grown in LZM supplemented with 1000 μM ZnCl2 (+) or 1 μM ZnCl2 (−). CMD1 was used as a normalization control. The data shown are representative of two independent experiments. B) Wild-type (DY1457), zrg17Δ mutant (DY1457 zrg17Δ), chromosomal ZRG17 ZRE mutant (zrg17-1m2ZRE), and reconstructed wild-type ZRE (ZRG17rZRE) cells were grown in SD liquid medium overnight. Cultures were then diluted in fresh medium, and 5-μl volumes containing 104, 103, and 102 cells (from left to right) were plated onto YPD (YP medium + 2% glucose) and YPGE (YP medium + glycerol and ethanol) plates. The plates were incubated at 30 or 37°C and photographed after 3 days. C) UPRE-lacZ activity (pMCZ-Y) was assayed in the same cells as in panel B grown in LZM supplemented with 0.3 μM ZnCl2, 3 μM ZnCl2, and 100 μM ZnCl2. The data shown are the means of three independent cultures for each condition and are representative of two independent experiments. The error bars represent ± 1 S.D. Letters denote statistically significant differences of UPRE-lacZ activity between strains and growth conditions (P < 0.05).
Direct assays of Zrg17/Msc2 complex activity, e.g. 65Zn transport assays, have not yet been developed. However, we could assess function of this complex using phenotypic assays that serve as indirect indicators of transporter function. For example, zrg17Δ mutants are unable to grow at 37 °C on rich YP medium containing glycerol and ethanol as carbon sources [2] (Fig. 5B). No such defect is seen at 30 °C or when high levels of zinc are supplemented in the medium. As shown in Figure 5B, neither the zrg17-1m2ZRE mutant nor the ZRG17rZRE strain showed any growth defect on glycerol/ethanol-containing plates at 37 °C. YP medium is relatively zinc-replete so these data are consistent with the normal levels of Zap1-independent basal expression of ZRG17 observed in cells with adequate zinc supplies.
To assess the impact of these promoter mutations in zinc-limited cells, we used a UPRE-lacZ reporter that responds to ER dysfunction. The Unfolded Protein Response (UPR) is triggered by misfolded proteins in the ER and up-regulates expression of protein chaperones and degradation systems to refold or degrade the aberrant proteins. This control occurs at the transcriptional level and is mediated by regulatory elements (UPREs) in UPR-regulated promoters. We had shown previously that expression of a UPRE-lacZ reporter increased in zinc-limited wild-type cells and that this induction was exacerbated in zrg17Δ mutants [2] (Fig. 5C). UPRE-lacZ expression in the ZRG17rZRE strain was identical to wild type, which was consistent with normal ZRG17 expression in that strain. In the zrg17-1m2ZRE mutant, UPRE-lacZ expression was induced to a higher degree than in wild-type cells albeit not to as high a level as in the zrg17Δ mutant. These results indicate that while basal Zap1-independent expression of ZRG17 does contribute to the function of the Zrg17/Msc2 complex in zinc-limited cells, the additional level of expression provided by Zap1 induction is required for full activity under those conditions.
DISCUSSION
Previous studies have estimated that Zap1 induces the expression of about 80 genes in zinc-limited yeast cells [11, 12]. These include genes involved in adaptation to zinc-limiting conditions and genes involved in maintaining zinc homeostasis within the cell and within intracellular compartments [10]. Among Zap1 targets are several genes encoding zinc transporters that play key roles in zinc homeostasis. For example, ZRT1, ZRT2, and FET4 encode the high affinity and low affinity zinc transporters required for efficient zinc uptake across the plasma membrane [36-38]. ZRT3 encodes a vacuolar zinc transporter responsible for mobilization of zinc stores from the vacuole for use by other compartments of the cell [39]. Conversely, ZRC1 encodes a transporter that moves zinc into the vacuole. Zrc1 activity is important for zinc storage and its induction in zinc-limited cells helps protect those cells from the high level of zinc that can be taken in when they are re-supplied with zinc [40].
To this list of Zap1-regulated genes, we can now add ZRG17 as an important target for maintaining zinc homeostasis. Zap1 induces ZRG17 expression by as much as five fold (Fig. 5). Zinc transport into the ER of zinc-limited cells is mediated largely by the Msc2/Zrg17 complex [1, 2]. The vacuolar zinc transporters Zrc1 and Cot1 also contribute, perhaps while they pass through the early secretory pathway on their way to their final vacuolar localization. Despite the contributions of the vacuolar transporters, Msc2 and Zrg17 appear to play the major role in maintaining ER function. Based on our genetic studies, both Msc2 and Zrg17 are required for transporter activity and biochemical analyses indicated that these proteins form heteromeric complexes. Results from studies of other members of the CDF/ZnT/SLC30A family [9, 41], suggest that Msc2 and Zrg17 form heterodimers and formation of this complex is required for transporter function.
Some CDF/ZnT/SLC30A transporters are active as homodimers. For these proteins, both subunits likely contribute to zinc transport. In the case of the heteromeric complexes in this family, the role of the individual subunits is much less clear. One tool for assessing the zinc transport role of the subunits in these complexes is the crystal structure model of the E. coli YiiP zinc efflux transporter [41, 42]. This structural model has indicated that a zinc-binding site using ligand residues from transmembrane domains II and V is the likely site of transient zinc occupancy during transport. For the ZnT-5/ZntT-6 heterodimer, this binding site is conserved in ZnT-5 but is not present in ZnT-6. This observation suggested that the ZnT-6 subunit is not involved in transport and may play a structural role in the transporter complex [9, 43]. Similarly, the zinc-binding site is conserved in Msc2 but not in Zrg17. Given that Zrg17 expression is regulated by zinc and the possibility that Zrg17 is not acting directly as a transporter, we hypothesize that Zrg17 serves as a regulatory subunit of the complex.
Regardless of its role, Zrg17 appears to be the rate-limiting subunit for transporter function in zinc-limited cells. This was suggested by our observation that disrupting Zap1-mediated induction of ZRG17, but not its basal expression, by mutating the ZRE in its promoter prevented full function in zinc-limited cells when assayed using the UPRE-lacZ ER stress reporter. Levels of Zrg17 protein correlated well with Zap1-mediated mRNA regulation indicating that transcriptional control is the primary mechanism regulating Zrg17 accumulation in response to zinc. However, we note that Zrg17 is phosphorylated in vivo suggesting that post-translational modifications may also contribute to its regulation [44].
As we learn more about how zinc transporters of the early secretory pathway are regulated, we can begin to compare the regulation of the yeast systems with those in higher eukaryotes, specifically heterodimeric ZnT-5/ZnT-6 and homodimeric ZnT-7. In investigations of transcript levels, it was noted that both ZnT-5 and ZnT-7 mRNA levels are increased in zinc-limited cells analogously to the regulation we have observed in yeast [45]. While the mechanism of ZnT-7 regulation has not been explored further, it appears that ZnT-5 is regulated both transcriptionally and at the level of mRNA stability [46]. ZnT-5 promoter activity is increased in zinc-limited cells while mRNA stability decreases in low zinc. Given that the net effect is an increase in mRNA in low zinc, the transcriptional control appears to outweigh the mRNA stability effects. The zinc sensors and regulatory factors responsible for these changes are currently unknown. It has also been found that ZnT-5 is regulated by the Unfolded Protein Response pathway mediated by XBP1 [7]. Given that zinc is required for ER function and the UPR is induced by zinc deficiency [1, 2], it is logical that cells would induce zinc transport activity in the early pathway to fully meet the requirements of those compartments. Microarray studies of yeast suggested that ZRG17 was regulated by the yeast ortholog of XBP1, the Hac1 transcription factor [47]. However, when ZRG17 expression in response to UPR inducers such as tunicamycin and DTT was tested with S1 nuclease protection assays, no such regulation was observed (C. Wu and D. Eide, unpublished data). These results indicate that ZRG17 is likely not a target of the Unfolded Protein Response.
Finally, we are also considering the regulation of Msc2, the other subunit of the yeast complex. MSC2 mRNA was not found to be zinc regulated in our previous microarray experiments [11, 12] and direct analysis by S1 nuclease protection assays confirmed that MSC2 mRNA levels do not change in response to zinc status (C. Wu and D. Eide, unpublished data). We are currently investigating whether Msc2 accumulation or activity are regulated at post-transcriptional levels. These studies will ultimately lead us to an integrated understanding of the regulation of zinc homeostasis in the early secretory pathway of eukaryotic cells.
SYNOPSIS.
The Msc2 and Zrg17 proteins of Saccharomyces cerevisiae are members of the cation diffusion facilitator family of zinc transporters. These proteins form heteromeric complexes that transport zinc into the endoplasmic reticulum. Previous studies suggested that the ZRG17 gene is regulated in response to zinc status by the Zap1 transcription factor. Zap1 activates expression of many genes in zinc-deficient cells. Here, we assessed whether ZRG17 is a direct Zap1 target gene. We showed that ZRG17 mRNA levels were elevated in zinc-limited cells in a Zap1-dependent manner and were also elevated in zinc-replete cells expressing a constitutively active allele of Zap1. Furthermore, Zrg17 protein levels correlated closely with mRNA levels. A candidate Zap1 binding site (ZRE) in the ZRG17 promoter was required for this induction. Using electrophoretic mobility shift assays and chromatin immunoprecipitation, we demonstrated that Zap1 binds specifically to the ZRG17 ZRE both in vitro and in vivo. By using a chromosomal ZRG17 mutant with a nonfunctional ZRE, we found that Zap1 induction of ZRG17 is required for ER function as indicated by elevated ER stress under zinc-limited conditions. Together, these results establish that ZRG17 is a direct Zap1 target gene and its regulation has biological importance in maintaining ER function.
Acknowledgements
This work was supported by grants RO1-GM056285 and T32-DK007665 from the National Institutes of Health and USDA Hatch grant WIS01323.
Abbreviations
- LZM
low zinc medium
- UPR
Unfolded Protein Response
- ZRE
zinc responsive element
REFERENCES
- 1.Ellis CD, Wang F, MacDiarmid CW, Clark S, Lyons T, Eide DJ. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J Cell Biol. 2004;166:325–335. doi: 10.1083/jcb.200401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis CD, Macdiarmid CW, Eide DJ. Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J Biol Chem. 2005;280:28811–28818. doi: 10.1074/jbc.M505500200. [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giunta C, Elcioglu NH, Albrecht B, Eich G, Chambaz C, Janecke AR, Yeowell H, Weis M, Eyre DR, Kraenzlin M, Steinmann B. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome--an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am J Hum Genet. 2008;82:1290–1305. doi: 10.1016/j.ajhg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, Idaira Y, Asada Y, Kitamura H, Yamasaki S, Hojyo S, Nakayama M, Ohara O, Koseki H, Dos Santos HG, Bonafe L, Ha-Vinh R, Zankl A, Unger S, Kraenzlin ME, Beckmann JS, Saito I, Rivolta C, Ikegawa S, Superti-Furga A, Hirano T. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-β signaling pathways. PLoS ONE. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki T, Ishihara K, Migaki H, Nagao M, Yamaguchi-Iwai Y, Kambe T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem. 2005;280:30956–30962. doi: 10.1074/jbc.M506902200. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara K, Yamazaki T, Ishida Y, Suzuki T, Oda K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J Biol Chem. 2006;281:17743–17750. doi: 10.1074/jbc.M602470200. [DOI] [PubMed] [Google Scholar]
- 8.Kirschke CP, Huang L. ZnT7, A novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J. Biol. Chem. 2003;278:4096– 4102. doi: 10.1074/jbc.M207644200. [DOI] [PubMed] [Google Scholar]
- 9.Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, Yamaguchi-Iwai Y, Nagao M, Kambe T. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J Biol Chem. 2009;284:30798–30806. doi: 10.1074/jbc.M109.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eide DJ. Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J Biol Chem. 2009;284:18565–18569. doi: 10.1074/jbc.R900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons TJ, Gasch AP, Gaither LA, Botstein D, Brown PO, Eide DJ. Genome-wide characterization of the Zap1p zinc-responsive regulon in yeast. Proc. Natl. Acad. Sci. USA. 2000;97:7957–7962. doi: 10.1073/pnas.97.14.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu CY, Bird AJ, Chung LM, Newton MA, Winge DR, Eide DJ. Differential control of Zap1-regulated genes in response to zinc deficiency in Saccharomyces cerevisiae. BMC Genomics. 2008;9:370. doi: 10.1186/1471-2164-9-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird AJ, Blankman E, Stillman DJ, Eide DJ, Winge DR. The Zap1 transcriptional activator also acts as a repressor by binding downstream of the TATA box in ZRT2. EMBO J. 2004;23:1123–1132. doi: 10.1038/sj.emboj.7600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird AJ, Gordon M, Eide DJ, Winge DR. Repression of ADH1 and ADH3 during zinc deficiency by Zap1-induced intergenic RNA transcripts. Embo J. 2006;25:5726–5734. doi: 10.1038/sj.emboj.7601453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu CY, Roje S, Sandoval FJ, Bird AJ, Winge DR, Eide DJ. Repression of sulfate assimilation is an adaptive response of yeast to the oxidative stress of zinc deficiency. J Biol Chem. 2009;284:27544–27556. doi: 10.1074/jbc.M109.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H, Butler E, Rodgers J, Spizzo T, Duesterhoeft S, Eide D. Regulation of zinc homeostasis in yeast by binding of the Zap1 transcriptional activator to zinc-responsive promoter elements. J. Biol. Chem. 1998;273:28713–28720. doi: 10.1074/jbc.273.44.28713. [DOI] [PubMed] [Google Scholar]
- 17.Zhu C, Byers KJ, McCord RP, Shi Z, Berger MF, Newburger DE, Saulrieta K, Smith Z, Shah MV, Radhakrishnan M, Philippakis AA, Hu Y, De Masi F, Pacek M, Rolfs A, Murthy T, Labaer J, Bulyk ML. High-resolution DNA-binding specificity analysis of yeast transcription factors. Genome Res. 2009;19:556–566. doi: 10.1101/gr.090233.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird AJ, Zhao H, Luo H, Jensen LT, Srinivasan C, Evans-Galea M, Winge DR, Eide DJ. A dual role for zinc fingers in both DNA binding and zinc sensing by the Zap1 transcriptional activator. EMBO J. 2000;19:3704–3713. doi: 10.1093/emboj/19.14.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Feng LS, Matskevich V, Venkataraman K, Parasuram P, Laity JH. Solution structure of a Zap1 zinc-responsive domain provides insights into metalloregulatory transcriptional repression in Saccharomyces cerevisiae. J Mol Biol. 2006;357:1167–1183. doi: 10.1016/j.jmb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Yuan DS. Zinc-regulated genes in Saccharomyces cerevisiae revealed by transposon tagging. Genetics. 2000;156:45–58. doi: 10.1093/genetics/156.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gitan RS, Luo H, Rodgers J, Broderius M, Eide D. Zinc-induced inactivation of the yeast Zrt1 zinc transporter occurs through endocytosis and vacuolar degradation. J. Biol. Chem. 1998;273:28617–28624. doi: 10.1074/jbc.273.44.28617. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Eide DJ. Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:5044–5052. doi: 10.1128/mcb.17.9.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storici F, Lewis LK, Resnick MA. In vivo site-directed mutagenesis using oligonucleotides. Nat Biotechnol. 2001;19:773–776. doi: 10.1038/90837. [DOI] [PubMed] [Google Scholar]
- 24.Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Meth. Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- 25.Cullin C, Minvielle-Sebastia L. Multipurpose vectors designed for the fast generation of N- or C-terminal epitope-tagged proteins. Yeast. 1994;10:105–112. doi: 10.1002/yea.320100110. [DOI] [PubMed] [Google Scholar]
- 26.Herbig A, Bird AJ, Swierczek S, McCall K, Mooney M, Wu CY, Winge DR, Eide DJ. Zap1 activation domain 1 and its role in controlling gene expression in response to cellular zinc status. Mol Microbiol. 2005;57:834–846. doi: 10.1111/j.1365-2958.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- 27.Myers AM, Tzagoloff A, Kinney DM, Lusty CJ. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene. 1986;45:299–310. doi: 10.1016/0378-1119(86)90028-4. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara T, Yanagi H, Yura T, Mori K. Endoplasmic reticulum stress-induced mRNA splicing permits synthesis of transcription factor Hac1p/Ern4p that activates the unfolded protein response. Mol Biol Cell. 1997;8:1845–1862. doi: 10.1091/mbc.8.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dohrmann PR, Butler G, Tamai K, Dorland S, Greene JR, Thiele DJ, Stillman DJ. Parallel pathways of gene regulation: homologous regulators SWI5 and ACE2 differentially control transcription of HO and chitinase. Genes Dev. 1992;6:93–104. doi: 10.1101/gad.6.1.93. [DOI] [PubMed] [Google Scholar]
- 30.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor Press; Cold Spring Harbor: 1988. [Google Scholar]
- 31.Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- 32.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley & Sons; New York, NY: 1995. [Google Scholar]
- 33.Bird A, Evans-Galea MV, Blankman E, Zhao H, Luo H, Winge DR, Eide DJ. Mapping the DNA binding domain of the Zap1 zinc-responsive transcriptional activator. J Biol Chem. 2000;275:16160–16166.. doi: 10.1074/jbc.M000664200. [DOI] [PubMed] [Google Scholar]
- 34.Kanin EI, Kipp RT, Kung C, Slattery M, Viale A, Hahn S, Shokat KM, Ansari AZ. Chemical inhibition of the TFIIH-associated kinase Cdk7/Kin28 does not impair global mRNA synthesis. Proc Natl Acad Sci U S A. 2007;104:5812–5817. doi: 10.1073/pnas.0611505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Dietrich FS. Mapping of transcription start sites in Saccharomyces cerevisiae using 5' SAGE. Nucleic Acids Res. 2005;33:2838–2851. doi: 10.1093/nar/gki583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci U S A. 1996;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H, Eide D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem. 1996;271:23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]
- 38.Waters BM, Eide DJ. Combinatorial control of yeast FET4 gene expression in response to iron, zinc, and oxygen. J. Biol. Chem. 2002;277:33749–33757. doi: 10.1074/jbc.M206214200. [DOI] [PubMed] [Google Scholar]
- 39.MacDiarmid CW, Gaither LA, Eide D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacDiarmid CW, Milanick MA, Eide DJ. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J Biol Chem. 2003;278:15065–15072. doi: 10.1074/jbc.M300568200. [DOI] [PubMed] [Google Scholar]
- 41.Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- 42.Lu M, Chai J, Fu D. Structural basis for autoregulation of the zinc transporter YiiP. Nat Struct Mol Biol. 2009;16:1063–1067. doi: 10.1038/nsmb.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem. 2009;284:17677–17686. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodenmiller B, Campbell D, Gerrits B, Lam H, Jovanovic M, Picotti P, Schlapbach R, Aebersold R. PhosphoPep--a database of protein phosphorylation sites in model organisms. Nat Biotechnol. 2008;26:1339–1340. doi: 10.1038/nbt1208-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devergnas S, Chimienti F, Naud N, Pennequin A, Coquerel Y, Chantegrel J, Favier A, Seve M. Differential regulation of zinc efflux transporters ZnT-1, ZnT-5 and ZnT-7 gene expression by zinc levels: a real-time RT-PCR study. Biochem Pharmacol. 2004;68:699–709. doi: 10.1016/j.bcp.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Jackson KA, Helston RM, McKay JA, O'Neill E D, Mathers JC, Ford D. Splice Variants of the Human Zinc Transporter ZnT5 (SLC30A5) Are Differentially Localized and Regulated by Zinc through Transcription and mRNA Stability. J Biol Chem. 2007;282:10423–10431. doi: 10.1074/jbc.M610535200. [DOI] [PubMed] [Google Scholar]
- 47.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]