Abstract
Aim
The aim of this article is to clarify the epidemiologic, clinical, endoscopic, biological and genetic characteristics of type 1 serrated polyposis patients.
Patients and methods
Consecutive patients responding to the WHO definition of type 1 serrated polyposis in one reference center for polyposis patients accepted genetic counseling. Detailed data on previous endoscopies, histology, and life habits were recorded, after informed consent, germline analysis of MUTYH, APC, and PTEN germline mutations. Molecular biology was tested on available fixed tissue from different lesion types.
Results
We included 29 patients (mean age 53.5 years, 21 women (72.4%)), four with a personal history of colorectal cancer (CRC), with a mean of 11.6 SSAs, with associated hyperplastic polyps in 93.1% and adenomas in 82.8%. SSAs showed no dysplasia in 46.9% of lesions (three of 29 patients), LGD in 51.9% (22/29 patients), and HGD in 1.2% (four of 29 patients). Dysplasia was more frequent in proximal SSAs and in women. Colectomy 15 patients (51.7%), upper digestive neoplasms: eight patients (27.5%); smokers: 24 patients (82.8%); family history of CRC: 16 patients (55.2%). Biology: MSI-H phenotype in one SSA, V600E BRAF mutation in 95% of SSAs; MGMT hypermethylation in three of 17 SSAs. No germline mutation was detected in MYH, APC or PTEN genes.
Conclusion
Type 1 serrated polyposis corresponds to a majority of women, with a high prevalence of smokers, a high prevalence of dysplastic serrated adenomas, particularly in females, without identified germline mutation in targeted predisposing genes.
Keywords: Polyposis, sessile serrated adenomas, genetics, molecular biology, endoscopy, epidemiology
Introduction
Serrated polyposis1 responds to well-defined international criteria established in 20002 and revised in 2010.1 This entity is characterized by multiple and/or large serrated lesions throughout the colon. The current World Health Organization (WHO) definition corresponds either to: i) at least five serrated polyps proximal to the sigmoid colon with two or more of these being greater than 10 mm; ii) at least 20 serrated polyps of any size distributed throughout the colon; or iii) any number of serrated polyps proximal to the sigmoid colon in an individual who has a first-degree relative with serrated polyposis. Prevalence of serrated polyposis has been estimated at one in 3000.3 Sessile serrated adenomas (SSAs), also called sessile serrated polyps, are part of the histological definition of serrated polyposis.4,5 Serrated polyposis is thus a phenotypically heterogeneous condition, with no identified genetic background. Very few data are available regarding specifically patients with a majority of SSAs, which is a relatively frequent situation in clinical practice.
Some authors suggested two distinct subtypes of serrated polyposes, although some overlap may be present.1,2,6 Type 1 serrated polyposis is characterized by multiple (five or more), large, proximally located SSAs (possibly coexisting with traditional serrated adenomas and conventional adenomas); this situation is associated with a significant risk of colorectal cancer (CRC).5 Type 1 serrated polyposis is associated with a high frequency of BRAF mutations and CpG island promoter hypermethylation (CpG island methylation phenotype, CIMP) in serrated lesions. Type 2 serrated polyposis is a more heterogeneous condition characterized by numerous (≥20) small hyperplastic polyps (HPs) distributed throughout the colon; lesions in type 2 serrated polyposis generally have KRAS mutations. The risks of cancer and the molecular pathways of carcinogenesis may differ: Cancers with a high degree of microsatellite instability (MSI-High, MSI-H) are more closely linked to the type 1 entity, which seems to bear a higher risk of CRC as compared to type 2.7
The purpose of this study was to clarify the epidemiologic, endoscopic, clinical, biological and genetic characteristics of consecutive type 1 serrated polyposis patients.
Patients and methods
Participants and endoscopy
This was a prospective study including patients from different families corresponding to the WHO definition of type 1 serrated polyposis: at least five endoscopically characteristic non-flat or slightly elevated colorectal lesions (respectively, 0–IIb, 0–IIa, 0–Is in Paris classification); at least two being greater than 10 mm in maximal diameter; histology of the largest lesion compatible with SSA. Patients were included in a prospective national database (clinical trial number: NCT01987518). All patients gave informed consent for the study and genetic counselling.
All patients had at least one colonoscopy. Most patients had optimized colonoscopy with chromoscopy using total dye spraying of the proximal colon to the splenic flexure and targeted dye spraying of the distal colon with indigo carmine solution (0.4% solution). Information regarding the number, size (using the open biopsy forceps technique), distribution (lesions from the cecum to the splenic flexure were regarded as proximal, and lesions from the descending colon to the rectum were regarded as distal), gross morphology, and histology of polyps were derived from the colonoscopy and histopathology reports. Polyps were classified as SSAs with or without cytological dysplasia, traditional serrated adenomas (TSAs), HPs, and tubular (TAs), tubulovillous (TVAs), or villous (VAs) adenomas. The total number of each polyp type was estimated during colonoscopy or from the surgical specimen if a colectomy was performed. Predominant proximal-disease was defined by a number of SSAs greater in the proximal than in the distal colon. When possible, presence or absence of fundic gland polyps and of duodenal neoplasia was stated during esophagogastroduodenoscopy with a lateral or axial viewing endoscope.
Personal and family history, toxic habits and genetics
All patients had genetic counselling, with informed consent for germline mutation analysis in the MUTYH, APC and PTEN genes. The following data were recorded: personal and family history of CRC, colorectal polyps, and polyposis, digestive diseases, and other cancers. Details of CRC were reported. Gender, age, and age at first diagnosis of SSA were documented. A genealogic tree was drawn. Patients were asked about tobacco use, alcohol use, and use of nonsteroidal anti-inflammatory drugs (NSAIDs) or aspirin. When possible, patients were analyzed for MUTYH (polymerase chain reaction (PCR) and targeted sequencing on exons 1 to 16), APC (PCR and sequencing on exons 1 to 15), and PTEN (Enhanced Mismatch Mutation Analysis and sequencing on exons 1 to 9) germline mutations using two blood specimen collections on ethylenediaminetetraacetic acid (EDTA).
Molecular biology
MSI and CIMP
MSI analysis was performed on tumor DNA with the commercial kit, MSI Analysis System, Promega, USA, which studied a panel of five microsatellite loci: bat25, bat26, NR21, NR24 and mono27. The amplified PCR products were separated by capillary electrophoresis using the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems) and data were analyzed with Genemapper Analysis software, version 4 (Applied Biosystems). Instability at two or more mononucleotide loci was interpreted as MSI-H, instability at one mononucleotide locus as MSI-Low (MSI-L), and no instability at any of the loci tested as microsatellite stability (MSS). CIMP status was tested used a predefined protocol.
Immunohistochemistry
Immunohistochemistry was performed with antibodies for MSH2 (DBS Clinisciences clone 25D12), MSH6 (BD Biosciences clone 44), MLH1 (BD Pharmingen, clone G168-15) and PMS2 (BD Pharmingen clone A16-4) on deparaffined tissue sections. Labeling was detected with the Dako Envision Plus detection kit (DAKO, USA). Normal epithelial, stroma or lymphocytes served as positive internal control in every tissue section.
KRAS and BRAF mutation analysis
KRAS exon 2 and BRAF exon 15 were amplified by PCR. The presence of somatic mutations at codons 12 and 13 of the KRAS gene and the V600E mutation of the BRAF gene were searched by simple nucleotide primer extension using the ABI PRISM SNaPshot multiplex kit (Applied Biosystems).
DNA extraction and methylation-specific PCR (MSP)
DNA from tumor tissues was extracted from fresh-frozen specimens using a commercial kit (Master Pure DNA and RNA purification kit-Epicentre Biotechnologies, Madison, WI, USA) according to the manufacturer's instructions. CIMP status was determined by analysis of methylation of the promoters p16, MLH1, Mint1, Mint2 and Mint31 genes by pyrosequencing. CIMP-high status was defined as positive when three or more promoters were methylated. DNA methylation of the promoters p16, MLH1, Mint1, Mint2, Mint31, and MGMT genes was determined by MSP after chemical modification of genomic DNA with sodium bisulfite as described by Herman et al. DNA modification was performed using the Methylamp DNA modification kit (Epigentek, USA) according to the manufacturer's instructions.8,25 The specific primers for the methylated and the unmethylated MSP were selected according to published sequences or designed with the website www.urogene.org/methprimer/. PCRs were performed in a total volume of 25 µl using 0.5 U of Taq Hot Star (Qiagen) and 1 µl of bisulfite-treated DNA. DNA isolated from normal peripheral lymphocytes served as negative methylation control. This same DNA treated in vitro with SssI methyl transferase served as the positive methylation control. MSP products were analyzed by electrophoresis on 2% agarose.
Statistical analysis
For statistical analysis, we used SPSS software, version 12.0 (SPSS Inc, Chicago, IL, USA) in a Windows XP (Microsoft, Seattle, WA, USA) environment. Variables were compared using the Mann-Whitney-Wilcoxon test and χ2 test, and considered as significant at a p value < 0.05.
Results
Endoscopy
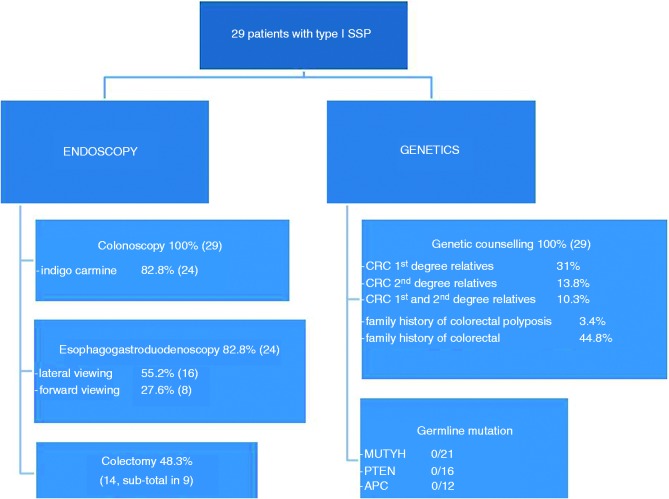
We included prospectively 29 patients from different families. There were more women (21, 72.4%) than men (eight, 27.6%)). The mean age at time of diagnosis of the first SSA was 53.5 years (range 21.2–76.3, median 52.9). All patients had at least one complete colonoscopy, 24 (82.8%) with indigo-carmine dye chromoscopy. Twenty-four patients (82.8%) had esophagogastroduodenoscopy: 16 of them (55.2%) with a lateral-viewing endoscope and eight patients (27.6%) with a forward-viewing endoscope. Five patients (17.2%) had no upper digestive endoscopy (Table 1). The mean follow-up interval between the first and last endoscopy for the patient cohort was 51.3 months (median 33 months, range 1–204 months).
Table 1.
Demographic and endoscopy data
| Patients | 29 |
|---|---|
| Female gender (%) | 21 (72.4) |
| Age (years) | |
| Mean | 53.5 |
| Median | 52.9 |
| Range | 21.2–76.3 |
| Number of lower digestive system endoscopies | |
| Total | 123 |
| Mean (range) | 4.2 (1–13) |
| Patients undergoing chromoendoscopy (%) | 24 (82.8) |
| Esophagogastroduodenoscopy (%) | |
| Total | 24 (82.8) |
| Duodenoscope | 16 (55.1) |
| Gastroscope | 8 (27.5) |
Colonic phenotype
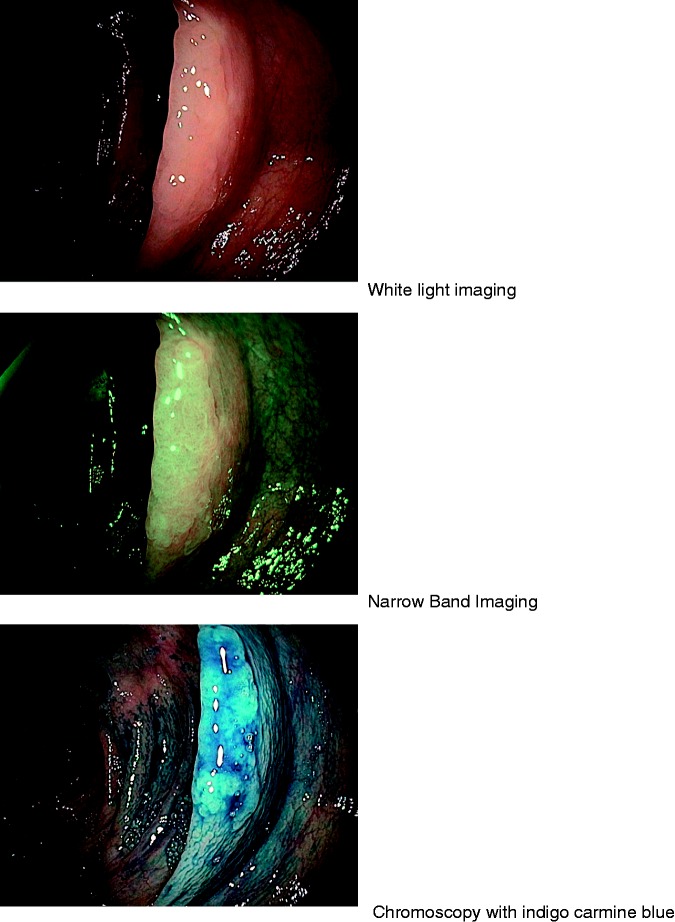
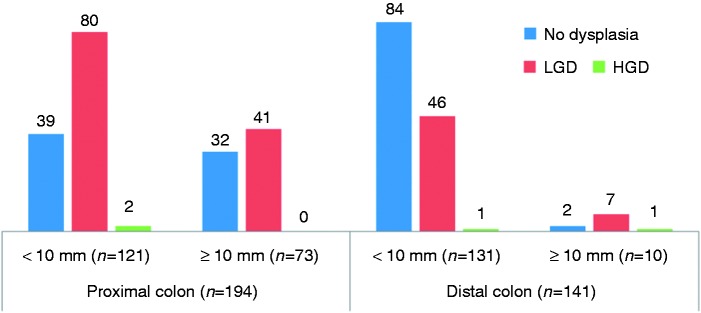
In 29 patients, 335 flat serrated-like lesions (SSL, Figure 1) were recorded, 194 proximal (57.9%) and 141 distal (42.1%). Patients had a mean number of 11.6 SSL (range 1–42). Males had a higher mean number of SSL (15.7 for male vs 10.0 for female) but without reaching statistical significance (p = 0.405). There were more frequently large SSL (≥10 mm) in the proximal colon (37.6%) than in the distal colon (7.1%) (Table 2). From large SSL, 88% (73/83) were located in the proximal colon (Figure 2). Nineteen patients (65.5%) had a predominant proximal disease (women: 71%; men: 50%, p = 0.47), and six patients (20.7%) a predominantly distal involvement. A colectomy was scheduled in 15 patients (51.7%, subtotal in nine) because the endoscopic treatment could not control the polyposis and/or because of cancer (see Table 2), at a mean age of 59.5 years. HPs were detected in 27/29 patients (93.1%), and conventional adenomas in 24 (82.8%) (Table 3).
Figure 1.
Flowchart of sessile serrated polyposis patient management. 1 SSP: type 1 serrated polyposis; CRC: colorectal cancer.
Table 2.
Colectomy data
| Colectomy | |
| Patients | 51.7% (15/29) |
| Right | 4 |
| Right then left* | 1 |
| Partial | 1 |
| Subtotal | 9 |
| Because of cancer | 1 |
| Because of benign polyposis | 13 |
| Because of cancer then benign polyposisa | 1 |
| Age at colectomy (years) | |
| Mean | 59.5 |
| Range | 47.1–76.7 |
| Time from diagnosis to colectomy because of polyposis (years) | |
| Mean | 1.2 |
| Median | 0.7 |
| Range | 0–6.1 |
This was the same patient.
Figure 2.
Typical aspect of sessile serrated lesion located in the right colon with different colorations (narrow band imaging (NBI), chromoscopy using indigo-carmin dye).
Table 3.
Histology and topography of lesions
| % patients (no.) | Mean-median no. (range) | Proximal colon | Proximal ≥10 mm | Distal ≥10 mm | No dysplasia (%) (no.) | LGD (%) (no.) | HGD (%) (no.) | |
|---|---|---|---|---|---|---|---|---|
| SSAs (n = 335) | 100% (29) | 11.6–9 (1-42) | 57.9% | 37.6% | 7.1% | 46.9 (157) | 51.9 (174) | 1.2 (4) |
| HPs (n = 404) | 93.1% (27) | 15–14 (1-30) | 50.7% | 13.7% | 4% | X | X | X |
| Conventional adenomas (n = 164) | 82.8% (24) | 6.8–3.5 (1–33) | 53.7% | 15.9% | 19.7% | X | 87.2 (143) | 12.8 (21) |
SSAs: sessile serrated adenomas; HPs: hyperplastic polyps; LGD: low-grade dysplasia; HGD: high-grade dysplasia.
As regards the degree of dysplasia (Figures 3 and 4), there was no dysplasia in three patients (10.3%) patients, low-grade dysplasia (LGD) in 75.9% (22/29) of patients, and high-grade dysplasia (HGD) in 13.8% (four of 29 patients). Dysplasia was more frequent in proximal (63.4%) versus distal (39.0%) SSAs (p < 0.001, Figure 4). Dysplasia was detected in at least one SSA in 64.1% of women versus 34.9% of men (p < 0.001). Proximal SSA showed dysplasia in 75.6% of females as compared to 38.1% of males (p < 0.001) (Table 4).
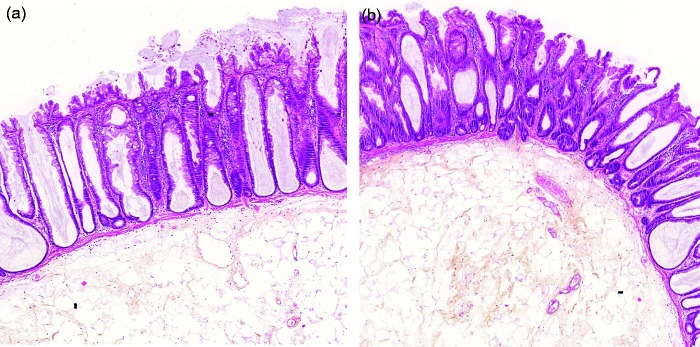
Figure 3.
Typical histological images of sessile serrated adenoma without dysplasia (a) and with low-grade dysplasia (b).
Figure 4.
Rate of dysplasia in sessile serrated adenomas (SSAs) according to the topography and size of the lesion. Dysplasia was more frequent in SSA located in the proximal colon (123/194, 63.4%) as compared to SSAs located in the distal colon (55/141, 39.0%).
Table 4.
Dysplasia (low and high grade) in SSA according to patient's sex and location of neoplasia
| Women | Men | p | |
|---|---|---|---|
| % of proximal SSAs with dysplasia (No.) | 75.6 (99/131) | 38.1 (24/63) | p < 0.001 |
| % of distal SSAs with dysplasia (No.) | 44.9 (35/78) | 31.7 (20/63) | p = 0.112 |
| % of total SSAs with dysplasia (No.) | 64.1 (134/209) | 34.9 (44/126) | p < 0.001 |
SSAs: sessile serrated adenomas.
Upper digestive phenotype
Eight patients (27.6%) had upper digestive lesions. Four patients (13.8%) had fundic gland polyps, and three (10.3%) had duodenal neoplasms: two (6.9%) duodenal adenomas (one TA in LGD in the first duodenum, one TVA in HGD in the third duodenum) and one (3.45%) ampullary adenoma (TA in LGD). One patient had a macroscopically characteristic duodenal adenoma, but no biopsy was possible because of peristalsis.
Personal history and toxic habits
Four patients (13.8%) had a personal history of CRC. Three of them (10.3%) had a previous CRC at 40, 48 and 64 years (two in the rectum, one in the right colon). The last patient had one proximal intramucosal carcinoma identified at diagnosis of serrated polyposis. Three patients (10.3%) had asymptomatic carcinoid tumors (one ileal, one appendicular and one rectal) detected at surgery or after endoscopic mucosal resection. Five patients (17.2%) had a history of extra-digestive cancer (three uterine cervical neoplasms, one Hodgkin disease, and one meningioma).
There were 24 current or previous smokers (82.8%) with a mean consumption of 13.2 pack-years (range 1–42), including 54.2% current smokers. Seventeen patients (58.6%) reported occasional, and six (20.7%) moderate regular alcohol consumption. Chronic use (≥1 per month) of NSAIDs or aspirin was reported in six patients (20.7%).
Family history and genetics
All patients had genetic counseling. Family history of CRC at first and/or second degrees was noted in 16 patients (55.2%): first-degree relatives in nine patients (31%), second-degree relatives in four patients (13.8%), first- and second-degree relatives in three patients (10.3%). Only one patient (3.4%) had a family history of colorectal polyposis. Family history of colorectal “polyps” was noted for 13 patients (44.8%). We looked for germline mutations in the MUTYH, APC and PTEN genes in a panel of patients with an available blood sample. We detected no MUTYH mutation in 21 patients, no PTEN germline mutation in 16 cases, and no APC mutation in 12 cases tested.
Molecular biology of polypoid lesions
MSI was tested in 46 lesions from 23 patients. Of 23 SSAs, one (proximal colon, HGD, <10 mm) showed an MSI-H phenotype with MLH1 and PMS2 protein loss of expression and hMLH1 gene promoter methylation. Of 12 SSAs with LGD tested, none had an MSI-H phenotype. Eight HPs, 13 adenomas, and two adenocarcinomas of distal location were tested: All were of MSS phenotype with normal immunochemistry. We looked for BRAF and KRAS mutations in 41 lesions from 19 patients: Nineteen of 20 SSAs (95%) showed a V600E BRAF mutation. One of 20 SSAs (5%) had both G12D and G12V KRAS mutations. BRAF V600E mutation was also identified in one of 11 conventional adenomas and five of 10 HPs. A G12A KRAS mutation was present in one LGD tubular adenoma out of 11 tested (Table 5). MGMT hypermethylation was tested in 27 lesions and was present in three of 17 SSAs, one of four adenomas and one of six HPs. The CIMP status was tested in 24 lesions: Five of 16 SSAs (31.2%) were CIMP-high.
Table 5.
RER, immunochemistry, BRAF and KRAS mutations on resected lesions
| MSI | MMR protein loss of expression | BRAF V600-E mutation | KRAS mutation | |
|---|---|---|---|---|
| SSAs | 4.6% (1/22)a | 4.3% (1/23)a | 95% (19/20) | 5% (1/20)b |
| HPs | 0/8 | 0/7 | 50% (5/10)c | 0/10 |
| Conventional adenomas | 0/13 | 0/12 | 9.1% (1/11)d | 9.1% (1/11)e |
| Adenocarcinomas | 0/1 | 0/2 | Xf | Xf |
One proximal SSA in HGD < 10 mm with loss of expression of MLH1 and PMS2 (normal for MSH2 and MSH6) and methylation of promoter of the hMLH1 gene.
G12D and G12V mutations.
Four distal lesions.
One proximal TA in LGD.
G12A mutation in TVA in LGD.
Technical failure.
RER: replication error; MSI: microsatellite instability; MMR: mismatch repair; SSAs: sessile serrated adenomas; HPs: hyperplastic polyps; HGD: high-grade dysplasia; LGD: low-grade dysplasia; TA: tubular adenoma; TVA: tubulovillous adenoma.
Discussion
Despite sessile serrated polyps becoming a subject of interest in recent years, knowledge about the specific situation of type 1 serrated polyposis is limited, as most studies present incomplete data focusing on one aspect of serrated polyposis (genotype, phenotype, family history, etc.). Our study, in contrast, includes complete evaluation of clinical history, endoscopic phenotype, molecular biology, and germline genetic data of the disease.
A large population-based screening study of 40,674 asymptomatic patients aged 55–64 years prospectively estimated the prevalence of serrated polyposis, but not of type I sessile serrated polyposis, at one in 3000.3 Patients in our study had a mean age at time of diagnosis of the first SSA of 53.5 years (median 52.9 years), comparable to previous serrated polyposis series.3,5,9 We observed a high female (f)/male (m) sex ratio (f/m 3:1) and a proximal predominance of SSAs. In contrast, most studies show serrated polyposis with neither female predominance3,9,10 nor predominantly proximal location.9 Serrated polyposis presents many hallmarks of a genetic predisposition (early age of onset of CRC, multiple polyps and cancers, increased CRC risk both in patients and relatives, and restricted ethnicity11); to date no responsible gene, but recently germline mutations in senescence genes, is considered as responsible for the disease.12 A small number of serrated polyposis patients have been shown to harbor bi-allelic germline mutations of MUTYH and germline PTEN mutations.10,13,14 In our series, none of 21 patients tested showed an MUTYH mutation, nor PTEN (zero of 16) nor APC (zero of 12) mutations. In our cohort, family history of CRC was frequent (55.2% of patients) and familial history of polyposis was very infrequent (one patient, 3.4%). The high rate of CRC in patients' relatives is compatible with a genetic predisposition, but we had only one family with serrated polyposis affecting two relatives in two generations. However, one limitation of our study is that no systematic endoscopy was proposed to patients' first-degree relatives. In the literature, a family history of CRCs in serrated polyposis patients has been reported in up to 59% of cases, and presence of one first-degree relative with polyposis in 17%.15 The risk of CRC in first-degree relatives of serrated polyposis patients has been estimated at fivefold greater than that in the general population.4,11
Four patients (13.8%) in our study had a personal history of CRC (three previous (10.3%) and one synchronous (3.4%)). In the literature this prevalence varies between 7% and 59%.3,9,10,15 In the three largest series published to date,11 25% to 38% of serrated polyposis patients presented with at least one CRC, and multiple CRCs were common.16 This relatively low rate of CRC in our study could be explained by the fact that partial or subtotal colectomy was performed in 51.7% of our patients. In our experience, despite the fact that a majority of our patients had at least partial colectomy, there is a significant place for endoscopic treatment in patients with sessile serrated polyposis. Optimized diagnostic endoscopy, including high-definition endoscopes and indigo-carmin dye, allows a precise identification of these difficult lesions and drives the possibility of efficient resection. Of course only the prospective follow-up of patients will prove the efficacy of this approach. The decision of surgery or no surgery thus relies on the initial endoscopist's evaluation, using optimized endoscopy, of the polyposis burden.
Martinez et al. reported that individuals with more than 20 pack-years had almost twice the risk of having hyperplastic polyps,17 and a seven- to 10-fold risk of serrated adenomas.18 On the contrary, Huang et al. found no association between smoking and advanced serrated polyps.19 Current smoking was associated with a significantly higher polyp number in patients with serrated polyposis,16 and the prevalence of current smokers was higher among serrated polyposis patients than in the general population.11,20 Our study confirms a high rate of tobacco users in type 1 serrated polyposis patients with 82.8% of smokers (a rate of 65.6% is usual in the literature): i.e. 44.8% of current smokers at time of diagnosis of the first SSA and 37.9% of previous smokers, compared to 29.9% and 27.3% in the French population.
The predominant molecular abnormality in colorectal lesions of serrated polyposis patients is the BRAF mutation, whereas few cases have predominant KRAS mutations.7,11,21,22 In our study, 95% of SSAs showed the V600E mutation in the BRAF gene. Concerning HPs, the BRAF V600E mutation was found in 50% of 10 HPs tested, and there were no KRAS mutations (zero of eight). Studies on HPs relate 43% of BRAF mutation in HPs from patients with multiple/large HPs and/or serrated polyposis.21 As clearly evidenced in previous studies, we observed that BRAF and KRAS mutations were exclusive in SSAs. Only one of 22 SSAs tested showed an MSI-H phenotype with a loss of expression of MLH1 and PMS2 proteins, and with methylated hMLH1 promoter. This contrasts with studies from Jass et al.,23 in which approximately one-third of CRCs associated with serrated polyposis showed MSI-H.
Interestingly, women presented more dysplastic SSAs (LGD and HGD) than men, a feature already reported in non-syndromic patients;24 this gender difference was more important in proximal SSAs (dysplasia in 38.1% of men's SSAs versus 75.6% in women) but also concerned distal SSAs (dysplasia in 31.7% of men's SSAs versus 44.9% in women). When stratifying patients with SSAs based on the most advanced polyp present, we identified no dysplasia in 10.3% of our patients, LGD in 75.9%, and HGD in 13.8%. This high proportion of dysplastic patients contrasts with a study by Lash et al.24 concerning a large cohort of patients with sporadic SSAs (respectively, 84.9%, 12%, 2.1% and 1%). Conventional adenomas were detected in 24 patients (82.8%) with a mean number of 6.8 (median 3.5, range 1–33). In the literature, the rate of serrated polyposis patients also bearing “neoplastic polyps” (TAs, TVAs, VAs, SSAs, and/or TSAs) varies highly from 7% to 92%.3,9–11,15
This study shows some limitations. Not all patients in this series had optimized endoscopy of the colon and duodenum, although this was a strong recommendation to our gastroenterological correspondents. Second, the evaluation of lesion size was retrospective. In order to reduce subjective interpretation of endoscopic reports, we chose a simple threshold of 10 mm to describe the size of lesions contrarily to previous studies using a distinction between very small (<5 mm), small or intermediate (6–9 mm), or large (>10 mm) lesions. Third, no systematic expert review of polyp histology was performed. However, we think that the high level of expertise of the pathologists at E. Herriot Hospital, a reference center for polyposis and therapeutic endoscopy, allows a rather accurate identification of sessile serrated lesions. Fourth, germline mutation analysis is incomplete in our study, but we considered, based on the available literature and our data, that the probability of positive dominant transmission through an APC or a PTEN mutation was very low, so we tested only a limited number of cases, all negative. Regarding MUTYH mutation, more compatible with the clinical situation and previous data, we limited this analysis to the 21 first patients, all negative. This shows that these three genes are at least poorly involved in the genetic background of type 1 serrated polyposis.
In conclusion, we report here the first complete characterization of type 1 serrated polyposis patients corresponding to a majority of women bearing a majority of proximal SSAs, coexisting with HPs and conventional adenomas. The frequent family history of CRC suggests a familial predisposition, although we did not detect any germline mutation of targeted predisposing genes.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Snover DC, Ahnen DJ, Burt RW. et al. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH. et al. (eds) WHO classification of tumours of the digestive system, Lyon, France: IARC, 2010, pp. 160–165.
- 2.Burt RW and Jass JR. Hyperplastic polyposis. In: Hamilton SR and Aaltonen LA (eds) Pathology and Genetics of Tumours of the Digestive System, ARC Press, Lyon, 2000, pp. 20–65.
- 3.Lockett MJ, Atkin WS. Hyperplastic polyposis: Prevalence and cancer risk (abstract). Gut 2004, pp. 48. [Google Scholar]
- 4.Boparai KS, Mathus-Vliegen EM, Koornstra JJ, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: A multicentre cohort study. Gut 2010; 59: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 5.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology 1996; 110: 748–755. [DOI] [PubMed] [Google Scholar]
- 6.Burt RW, Samowits WS. Serrated adenomatous polyposis: A new syndrome? Gastroenterology 1996; 110: 950–952. [DOI] [PubMed] [Google Scholar]
- 7.Jass JR. Gastrointestinal polyposes: Clinical, pathological and molecular features. Gastroenterol Clin North Am 2007; 36: 927–946, viii. [DOI] [PubMed] [Google Scholar]
- 8.Park SJ, Rashid A, Lee JH, et al. Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol 2003; 162: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrández A, Samowitz W, DiSario JA, et al. Phenotypic characteristics and risk of cancer development in hyperplastic polyposis: Case series and literature review. Am J Gastroenterol 2004; 99: 2012–2018. [DOI] [PubMed] [Google Scholar]
- 10.Lage P, Cravo M, Sousa R, et al. Management of Portuguese patients with hyperplastic polyposis and screening of at-risk first-degree relatives: A contribution for future guidelines based on a clinical study. Am J Gastroenterol 2004; 99: 1779–1784. [DOI] [PubMed] [Google Scholar]
- 11.Rosty C, Parry S, Young JP. Serrated polyposis: An enigmatic model of colorectal cancer predisposition. Patholog Res Int 2011; 2011: 157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gala MK, Mizukami Y, Le LP, et al. Germline mutations in oncogene-induced senescence pathways are associated with multiple sessile serrated adenomas. Gastroenterology 2014; 146: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boparai KS, Dekker E, Van Eeden S, et al. Hyperplastic polyps and sessile serrated adenomas as a phenotypic expression of MYH-associated polyposis. Gastroenterology 2008; 135: 2014–2018. [DOI] [PubMed] [Google Scholar]
- 14.Heald B, Mester J, Rybicki L, et al. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterology 2010; 139: 1927–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: A prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131: 1400–1407. [DOI] [PubMed]
- 16.Buchanan DD, Sweet K, Drini M, et al. Phenotypic diversity in patients with multiple serrated polyps: A genetics clinic study. Int J Colorectal Dis 2010; 25: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez ME, McPherson RS, Levin B, et al. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology 1997; 113: 423–429. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JC, Rangasamy P, Rustagi T, et al. Risk factors for sessile serrated adenomas. J Clin Gastroenterol 2011; 45: 694–699. [DOI] [PubMed] [Google Scholar]
- 19.Huang CS, Farraye FA, Shepherd C, et al. T2038. A case-control study of the risk factors for advanced serrated polyps. Gastroenterology 2008; 134(4 Suppl 1): A-606. [Google Scholar]
- 20.Walker RG, Landmann JK, Hewett DG, et al. Hyperplastic polyposis syndrome is associated with cigarette smoking, which may be a modifiable risk factor. Am J Gastroenterol 2010; 105: 1642–1647. [DOI] [PubMed] [Google Scholar]
- 21.Beach R, Chan AO, Wu TT, et al. BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol 2005; 166: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvajal-Carmona LG, Howarth KM, Lockett M, et al. Molecular classification and genetic pathways in hyperplastic polyposis syndrome. J Pathol 2007; 212: 378–385. [DOI] [PubMed] [Google Scholar]
- 23.Jass JR. Colorectal polyposes: From phenotype to diagnosis. Pathol Res Pract 2008; 204: 431–447. [DOI] [PubMed] [Google Scholar]
- 24.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: Prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 2010; 63: 681–686. [DOI] [PubMed] [Google Scholar]
- 25.Herman JG, Graff JR, Myöhänen S, et al., Methylation-specific PCR : a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996; 93: 9821–9826. [DOI] [PMC free article] [PubMed]