Abstract
Background
Gastro-esophageal reflux disease (GERD) leads to frequent medical visits, and available therapies fail in up to 40% of patients. Food allergies may be involved in GERD pathogenesis; however, allergens other than food have received little attention. Nickel allergy is common in the general population and some high-nickel foods are associated with GERD. However, the potential relationship between nickel allergy and GERD remains unaddressed.
Aim
This study aimed to evaluate the prevalence of nickel sensitization in patients with and without GERD and to compare clinical and demographic features.
Methods
This prospective, multicenter study included 210 adult GERD patients and 140 patients without GERD who presented at the general practitioner. All GERD patients had undergone treatment with proton pump inhibitors and upper digestive endoscopy within the previous five years. Demographic and clinical data were collected by questionnaire and patients underwent a nickel patch allergy test.
Results
Patients with and without GERD presented similar characteristics, with the exception of nickel sensitization, which was significantly more prevalent among GERD patients than controls (39.5% vs. 16.4%; p = 0.001). Nickel-positive GERD patients were more frequently female (90.4% vs. 65.4%, p = 0.003) and asthmatic (18.1% vs. 4.7%; p = 0.038), compared to nickel-negative GERD patients. At six-month follow-up, most of the patients, with or without nickel sensitization, reported improved symptoms without differences in drug prescription.
Conclusion
Nickel sensitization is particularly prevalent in GERD patients seen in general practice. Whether allergies other than food allergy play a role in GERD remains to be elucidated.
Keywords: Gastro-esophageal reflux disease, allergy, nickel, symptoms, functional gastrointestinal disorders
Introduction
Gastro-esophageal reflux disease (GERD) is one of the most frequently diagnosed digestive diseases in Western countries,1 as well as one of the main complaints requiring medical visits to general practicioners2 and referral centers alike.1,3 Frequent referral is due, at least in part, to the issue that a substantial proportion of affected individuals show an unsatisfactory response to available therapies.4,5 Patients with non-erosive reflux disease (NERD) respond even less favorably to antisecretory therapies than those with endoscopically demonstrated erosive reflux disease (ERD).6,7 Overall, up to 40% of GERD patients do not respond to proton pump inhibitors (PPIs), when prescribed at either standard or double doses.5,8 GERD exhibits multifactorial pathophysiology, including an increased number of transient lower esophageal sphincter relaxations (TLESR), decreased LES tone, delayed gastric emptying, and increased esophageal chemo-mechano-sensitivity.9 Whereas the involvement of food allergies and sensitization of the esophageal mucosa to allergens has been suspected to play a role, the evidence remains inconclusive.10 Allergens other than food have as yet received little attention among potential causative mechanisms.11 Nickel is diffusely present in the ground, water, and air and it is frequently used for industrial purposes.12 Nickel is also present at high concentration in foods including tomatoes, cocoa, peanuts, oats, beans, whole wheat, lentils, hazelnuts, walnuts, peas, and soy.13 Nickel is the primary cause of atopic contact dermatitis (ACD), and it has also been reported to be responsible for a systemic condition named “systemic nickel allergy syndrome” (SNAS), which is characterized by gastrointestinal, respiratory, and neurologic manifestations.14–17 Data on nickel allergy and digestive symptoms, however, are scarce and studies are difficult to compare.11,18 It has been proposed that there may be a relationship between reflux symptoms and a diet rich in nickel,19–25 but no data were available regarding a possible role of nickel allergy in ERD and NERD, nor regarding its potential effect on the response of these conditions to therapy.
The aims of the present study were to evaluate in a general practice setting: 1) the prevalence of nickel sensitization in patients with and without GERD; and 2) the clinical features of GERD patients with and without nickel sensitization and changes over time, as determined on their first evaluation and again at follow-up six months later.
Materials and methods
This prospective, multicenter study included GERD patients aged 18 to 75 years, presenting at their general practitioner (GP) for any reason, not limited to GERD-related complaints. GERD was previously diagnosed in these individuals based on symptoms of heartburn and/or regurgitation requiring a continuous or intermittent pharmacological treatment with PPIs.26,27 GERD patients were eligible for the study if they had undergone upper gastrointestinal endoscopy during a symptomatic flare-up within the previous five years. To limit the already heavy daily workload of the GPs, study participation was limited to the first GERD patient presenting each day of a prefixed period comprising 15 consecutive working days. The control group included participants without present or past symptoms of heartburn and/or regurgitation requiring a continuous or intermittent pharmacological treatment with PPIs (non-GERD patients). The first non-GERD patient seen by the same GP on each one of 10 consecutive working days was assigned to the control group.
Exclusion criteria for both GERD patients and controls were the following: neoplastic diseases, relevant skin lesions, dermatitis, known autoimmune diseases or previously diagnosed nickel allergy, or current treatment with steroidal or antihistaminergic therapies. None of the included patients was on a nickel exclusion-diet. All study participants gave their informed written consent. The study was performed according to the Helsinki Declaration (Edinburgh revision, 2000).
Nickel sensitization was assessed by patch test, which is considered a simple an accurate method to detect sensitization both in clinical and epidemiological studies.10,14–18,28 and it is the current gold standard for diagnosing nickel allergy.29
Nickel skin patch tests were administered to each patient included in the study, according to a standard procedure: four very small amounts of Vaseline, two of which were enriched with nickel, were applied to the skin and covered with small patches, which remained on the skin for two days before removal.29 A diagnosis of nickel sensitization was made in cases where the skin under both nickel patches was inflamed when the patch was removed and the skin remained inflamed on the following day. The physician administered a standardized questionnaire to collect demographic and clinical data from each patient. Upper digestive complaints were evaluated using a previously validated questionnaire designed to assess symptom severity, based on their impact on patients’ usual activities. 30 Similarly, extra-digestive symptoms were also scored according to their influence on usual activities. GERD patients were classified as either ERD or NERD based on the results of the previous upper gastrointestinal endoscopy, with esophagitis being graded according to the Los Angeles classification.31
All GERD patients were instructed to follow a diet devoid of foods that are traditionally considered to be capable of facilitating gastro-esophageal reflux. Those who tested positive for nickel allergy were also instructed to observe a diet with low nickel content.
Approximately six months later, GERD patients with and without nickel sensitization were contacted again for follow-up that entailed scoring of their GERD symptoms, and evaluation of their compliance and their response to therapy.
Statistical analysis was performed by Chi-squared test and multivariate analysis of variance.32 Two-tailed p < 0.05 was chosen as the significance cut-off value.
Results
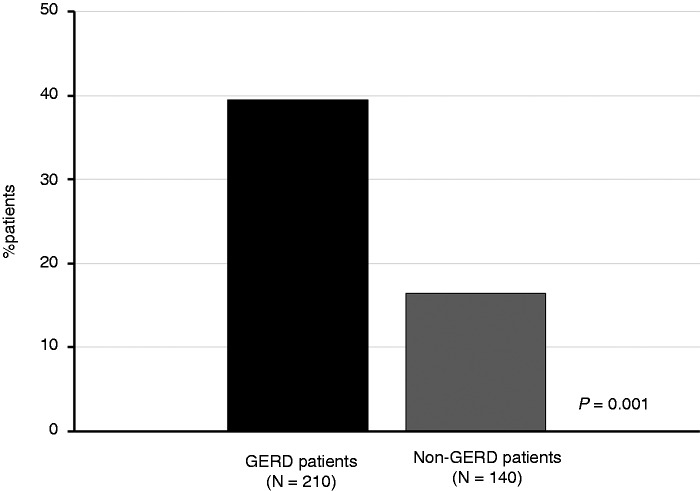
Overall, 210 GERD patients and 140 non-GERD patients were enrolled in the study. The main demographic and clinical features are summarized in Table 1. Both groups included a slightly higher prevalence of females than males, which is consistent with the different referral ratio in general practice,33,34 although the gender difference was not statistically significant. Patients with and without GERD presented similar demographic features, smoking habits, and overlapping pathological conditions, with the exception of nickel sensitization, which was significantly more common among patients with GERD than patients without GERD (39.5% vs. 16.4%, respectively; p = 0.001) (Figure 1).
Table 1.
Characteristics of GERD patients and controls
| GERD patients (N = 210) | Non-GERD patients (N = 140) | Significance | |
|---|---|---|---|
| Females | 158 (75.2%) | 93 (66.4%) | ns |
| Males | 52 (24.8%) | 47 (33.6%) | ns |
| Age (years; mean ± SD) | 52.9 ± 13.6 | 50.1 ± 13.9 | ns |
| BMI (kg/m2; mean ± SD) | 25.4 ± 3.7 | 25.1 ± 3.4 | ns |
| Smokers | 41 (19.5%) | 24 (17.1%) | ns |
| Asthma | 21 (10.0%) | 7 (5.0%) | ns |
| Chronic diarrhea | 7 (3.3%) | 4 (2.9%) | ns |
| Celiac disease | 3 (1.4%) | 2 (1.4%) | ns |
| IBS | 26 (12.4%) | 13 (9.3%) | ns |
| Gastric/duodenal ulcer | 7 (3.3%) | 1 (0.7%) | ns |
GERD: gastro-esophageal reflux disease; BMI: body mass index; IBS: irritable bowel syndrome; ns: not significant, multivariate analysis of variance.
Figure 1.
Prevalence of positive nickel patch test in gastro-esophageal reflux disease (GERD) patients (black column) and patients without GERD (gray column).
Table 2 shows the demographic and clinical features of the GERD patients with and without nickel sensitization. Nickel-sensitive GERD patients were more frequently female than non-allergic GERD patients (90.4% vs. 65.4%, respectively; p = 0.003) and were more frequently asthmatic (18.1% vs. 4.7%; p = 0.038). Moreover, nickel-sensitive GERD patients more frequently complained of postprandial itching sufficiently severe to influence their usual activities (7.2% vs. 0.0%; p = 0.019). There was no significant difference in the quantity of PPI tablets prescribed in the previous 12 months, nor in the use of alginate, between GERD patients with or without nickel sensitization.
Table 2.
Characteristics of GERD patients with or without nickel sensitization
| GERD patients with positive nickel patch test (N = 83) | GERD patients with negative nickel patch test (N = 127) | Significance | |
|---|---|---|---|
| Males | 8 (9.6%) | 44 (34.6%) | p = 0.003 |
| Females | 75 (90.4%) | 83 (65.4%) | |
| Age (years; mean ± SD) | 51.4 ± 12.9 | 53.9 ± 14.0 | ns |
| BMI (kg/m2; mean ± SD) | 25.0 ± 4.1 | 25.6 ± 3.4 | ns |
| Smokers | 17 (20.4%) | 24 (18.9%) | ns |
| Asthma | 15 (18.1%) | 6 (4.7%) | p = 0.038 |
| Urticaria | 5 (6.0%) | 2 (1.6%) | ns |
| Chronic diarrhea | 5 (6.0%) | 2 (1.6%) | ns |
| Celiac disease | 2 (2.4%) | 1 (0.8%) | ns |
| IBS | 7 (8.4%) | 19 (15.0%) | ns |
| Gastric/duodenal ulcer | 1 (1.2%) | 6 (4.7%) | ns |
| NERD | 63 (75.9%) | 75 (59.0%) | ns |
| Hiatal hernia | 51/76 (67.1%) | 71/126 (56.3%) | ns |
| Use of alginate | 27/77 (35.1%) | 34/127 (27.7%) | ns |
| PPI tablets/year (mean ± SD) | 231.4 ± 78.4 | 235.2 ± 83.0 | ns |
| Acid regurgitationa | 24 (28.9%) | 44 (34.7%) | ns |
| Heartburna | 32 (38.6%) | 48 (37.8%) | ns |
| Epigastric pain or burninga | 30 (36.1%) | 43 (33.9%) | ns |
| Epigastric bloatinga | 32 (38.6%) | 30 (23.6%) | ns |
| Postprandial discomforta | 25 (30.2%) | 15 (11.8%) | ns |
| Nauseaa | 7 (8.4%) | 3 (2.4%) | ns |
| Postprandial itchinga | 6 (7.2%) | 0 (0.0%) | p = 0.019 |
Severe enough to influence usual activities.
BMI: body mass index; NERD: non-erosive reflux disease; PPI tablets: number of proton pump inhibitor tablets prescribed in one year (mean ± SD); ns: not significant, multivariate analysis of variance.
All GERD patients with or without nickel sensitization were contacted for follow-up six months after the patch test. Clinical data and data regarding the number of PPI tablets prescribed were collected from 74 GERD patients with nickel sensitization (89.2%) and from 95 GERD patients without nickel sensitization (74.8%). Upon follow-up, a total of 13.5% of patients with and 16.1% of the patients without nickel sensitization reported their symptoms to be the same as before the patch test, 62.1% and 64.3 reported moderately improved symptoms, and 24.3% and 19.6% reported markedly improved symptoms, respectively. Analysis of PPI use revealed no differences between the mean number of tablets prescribed in the six months before and after the patch test in GERD patients with nickel sensitization (113.9 ± 48.3 vs. 108.2 ± 52.5; mean ± SD) and in GERD patients without nickel sensitization (119.1 ± 50.5 vs. 122.3 ± 53.1).
Discussion
Here we present the first study showing a higher frequency of nickel sensitization in GERD patients than in non-GERD controls. The main limitations of the study are that no attempt was made to blind the result of the patch test, nor to control patient compliance with the dietary recommendations; consequently, no definitive conclusion can be drawn regarding a causative link between nickel sensitization and the pathological esophageal condition. Furthermore, skin sensitization to nickel does not by itself demonstrate true allergy, since a clinical correlate to positive diagnostic tests is required to formulate this diagnosis and GERD cannot be considered at present a clinical correlate of food or non-food allergies. Although nickel allergy is one of the most frequently observed allergies in the general population, its actual prevalence has not been established. Studies investigating the prevalence of nickel allergy in the general population of different countries have reported a wide range of results, from 4% to 20%, but they agree in consistently confirming a higher prevalence in females.35–42 Chemical intolerance occurs in one out of five primary care patients, yet is rarely diagnosed by busy practicing physicians, as the clinical relevance is traditionally considered to be substantially limited to dermatologic conditions, while potential systemic manifestations are hardly considered.43
The control sample used in this study consisted of a non-GERD patient population of individuals who presented at the GP for any non-selected reason, excluding serious autoimmune diseases. The demographic characteristics of the control population, including the preponderance of the female gender, reflects the features of outpatients typically seen by Italian GPs.44 The prevalence of positive nickel patch tests in this control sample is similar to that reported in the literature for tests in comparable populations.35–42 Thus, the higher prevalence of nickel sensitization observed in GERD patients appears to be suggestive of a specific feature of this disease; however, whether and to what extent it may play a pathogenic role in GERD remains to be elucidated.
No previous studies have specifically evaluated the involvement of nickel allergy in the pathogenesis of GERD. GERD patients are generally given lifestyle recommendations, including avoidance of foods that may decrease LES tone and transient relaxations, such as tomato and chocolate.45,46 Notably, these foods also happen to be rich in nickel.47 The role of allergens other than food in the determinism of GERD deserves to be addressed in specific studies.11 It has been hypothesized that a relationship may exist between the presence of nickel in the gastrointestinal tract and the general perception of digestive symptoms.10 A recent study of more than 20,000 patients seen in general practice also demonstrated a substantial overlap between atopy and functional digestive disorders (irritable bowel syndrome, functional dyspepsia, and constipation) that was only partially explained by a common connection to mood disorders.48
No differences were observed in the frequency of heartburn and acid regurgitation among GERD patients with and without nickel sensitization, or in the frequency of gastric or duodenal ulcer, hiatal hernia, chronic diarrhea, irritable bowel syndrome, or celiac disease. The group of nickel-sensitive GERD patients that we evaluated was characterized by a greater prevalence of females and asthma than we observed in non-GERD controls and in non-sensitive GERD patients. The increased prevalence of asthma is not unexpected, as it represents one of the many possible manifestations of an allergy disorder, as well as one of the possible extra-esophageal manifestations of GERD.26 Previous reports had already found asthma to be more frequent in patients with functional digestive diseases than in controls.48 Although asthma may be secondary to GERD, the observation that non-sensitive GERD patients presented a prevalence of overlapping asthma identical to that of controls and three to four times smaller than that of sensitive GERD patients argues in favor of a possible causative role of allergy in the determinism of GERD, rather than the other way around. Indeed the nickel allergy presents some potential mechanisms that could contribute to develop a pro-inflammatory condition in the mucosa of the alimentary canal that causes either directly GERD-like symptoms, or increased susceptibility to acid injury at the gastro-esophageal junction, or increased esophageal mucosal permeability and increasing antigenic exposure. On the other hand, nickel-sensitive and non-sensitive patients presented similar prevalence of erosive and non-erosive GERD and were prescribed a similar number of PPI tablets and alginates over time. Moreover, GERD patients showed no difference in clinical data nor in the use of PPIs after the diagnosis of nickel allergy and recommendation of appropriate diet regimen. These observations seem to argue against a role of nickel allergy in the determinism of GERD, but ad hoc designed studies are needed to evaluate the role of allergies other than food allergies in GERD pathology in different sub-groups of GERD patients, including the patients who do not respond to therapy and those with early relapse, who are often seen in referral centers.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag H, Hill C, Jones R. Systematic review: The epidemiology of gastro-oesophageal reflux disease in primary care, using the UK General Practice Research Database. Aliment Pharmacol Ther 2009; 29: 470–480. [DOI] [PubMed] [Google Scholar]
- 3.Cantù P, Savojardo D, Carmagnola S, et al. Impact of referral for gastro-oesophageal reflux disease on the workload of an academic gastroenterology unit. Dig Liver Dis 2005; 37: 735–740. [DOI] [PubMed] [Google Scholar]
- 4.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 2012; 61: 1340–1354. [DOI] [PubMed] [Google Scholar]
- 5.Fass R, Gasiorowska A. Refractory GERD: What is it? Curr Gastroenterol Rep 2008; 10: 252–257. [DOI] [PubMed] [Google Scholar]
- 6.Lee ES, Kim N, Lee SH, et al. Comparison of risk factors and clinical responses to proton pump inhibitors in patients with erosive oesophagitis and non-erosive reflux disease. Aliment Pharmacol Ther 2009; 30: 154–164. [DOI] [PubMed] [Google Scholar]
- 7.Bytzer P, van Zanten SV, Mattsson H, et al. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis—a post hoc analysis of 5796 patients. Aliment Pharmacol Ther 2012; 36: 635–643. [DOI] [PubMed] [Google Scholar]
- 8.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009; 58: 295–309. [DOI] [PubMed] [Google Scholar]
- 9.Boeckxstaens GE, Rohof WO. Pathophysiology of gastroesophageal reflux disease. Gastroenterol Clin North Am 2014; 43: 15–25. [DOI] [PubMed] [Google Scholar]
- 10.Falagiani P, Di Gioacchino M, Ricciardi L, et al. Systemic nickel allergy syndrome (SNAS): A review. Rev Port Imunoalergologia 2008; 16: 135–147. [Google Scholar]
- 11.Walker MM, Powell N, Talley NJ. Atopy and the gastrointestinal tract—a review of a common association in unexplained gastrointestinal disease. Expert Rev Gastroenterol Hepatol 2014; 8: 289–299. [DOI] [PubMed] [Google Scholar]
- 12.McIlveen WD, Negusanti JJ. Nickel in the terrestrial environment. Sci Total Environ 1994; 148: 109–138. [DOI] [PubMed] [Google Scholar]
- 13.Sharma AD. Relationship between nickel allergy and diet. Indian J Dermatol Venereol Leprol 2007; 73: 307–312. [DOI] [PubMed] [Google Scholar]
- 14.Erdmann SM, Werfel T. Hematogenous contact eczema induced by foods [article in German]. Hautarzt 2006; 57: 116–120. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Morillas L, Reaño Martos M, Rodríguez Mosquera M, et al. Baboon syndrome [article in Spanish]. Allergol Immunopathol (Madr) 2004; 32: 43–45. [DOI] [PubMed] [Google Scholar]
- 16.Kolodziej T, Szepietowski JC, Sikora J, et al. The baboon syndrome due to nickel. Acta Dermatovenerol Croat 2003; 11: 29–31. [PubMed] [Google Scholar]
- 17.Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol 2007; 56: 88–90. [DOI] [PubMed] [Google Scholar]
- 18.Turi MC, Di Claudio F, Schiavone C, et al. Systemic nickel allergy syndrome: An update. It J Allergy Clin Immunol 2008; 18: 98–102. [Google Scholar]
- 19.Veien NK, Hattel T, Justesen O, et al. Dietary treatment of nickel dermatitis. Acta Derm Venereol 1985; 65: 138–142. [PubMed] [Google Scholar]
- 20.Veien NK, Menné T. Nickel contact allergy and a nickel-restricted diet. Semin Dermatol 1990; 9: 197–205. [PubMed] [Google Scholar]
- 21.Veien NK, Hattel T, Justesen O, et al. Dietary restrictions in the treatment of adult patients with eczema. Contact Dermatitis 1987; 17: 223–228. [DOI] [PubMed] [Google Scholar]
- 22.Schiavino D, Nucera E, Alonzi C, et al. A clinical trial of oral hyposensitization in systemic allergy to nickel. Int J Immunopathol Pharmacol 2006; 19: 593–600. [DOI] [PubMed] [Google Scholar]
- 23.Veien NK, Hattel T, Justesen O, et al. Oral challenge with nickel and cobalt in patients with positive patch tests to nickel and/or cobalt. Acta Derm Venereol 1987; 67: 321–325. [PubMed] [Google Scholar]
- 24.Veien NK, Hattel T, Laurberg G. Low nickel diet: An open, prospective trial. J Am Acad Dermatol 1993; 29: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 25.Antico A, Soana R. Chronic allergic-like dermatopathies in nickel-sensitive patients. Results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc 1999; 20: 235–242. [DOI] [PubMed] [Google Scholar]
- 26.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am J Gastroenterol 2006; 101: 1900–1920; quiz 1943. [DOI] [PubMed] [Google Scholar]
- 27.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108: 308–328. [DOI] [PubMed] [Google Scholar]
- 28.Mathias CGT, Maibach HI. When to read a patch test? Int J Derm 1979; 18: 127–128. [DOI] [PubMed] [Google Scholar]
- 29.Josefson A, Färm G, Meding B. Validity of self-reported nickel allergy. Contact Dermatitis 2010; 62: 289–293. [DOI] [PubMed] [Google Scholar]
- 30.Stanghellini V, Tosetti C, Paternicò A, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology 1996; 110: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 31.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of esophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinfurt KP. Multivariate analysis of variance. In: Grimm LH, Yarnold PR. (eds). Reading and understanding multivariate statistics 1995; Vol. ix, Washington, DC, US: American Psychological Association, pp. 245–276. [Google Scholar]
- 33.Pinkhasov RM, Wong J, Kashanian J, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract 2010; 64: 475–487. [DOI] [PubMed] [Google Scholar]
- 34.Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–02. Vital Health Stat 13 2006; 159: 1–66. [PubMed] [Google Scholar]
- 35.Peltonen L. Nickel sensitivity in the general population. Contact Dermatitis 1979; 5: 27–32. [DOI] [PubMed] [Google Scholar]
- 36.Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis 2007; 57: 287–299. [DOI] [PubMed] [Google Scholar]
- 37.Schäfer T, Böhler E, Ruhdorfer S, et al. Epidemiology of contact allergy in adults. Allergy 2001; 56: 1192–1196. [DOI] [PubMed] [Google Scholar]
- 38.Dotterud LK, Smith-Sivertsen T. Allergic contact sensitization in the general adult population: A population-based study from Northern Norway. Contact Dermatitis 2007; 56: 10–15. [DOI] [PubMed] [Google Scholar]
- 39.Dotterud LK. The prevalence of allergic contact sensitization in a general population in Tromsø, Norway. Int J Circumpolar Health 2007; 66: 328–334. [DOI] [PubMed] [Google Scholar]
- 40.Landeck L, Schalock PC, Baden LA, et al. Patch-testing with the standard series at the Massachusetts General Hospital, 1998 to 2006. Dermatitis 2009; 20: 89–94. [PubMed] [Google Scholar]
- 41.Nielsen NH, Menné T. Allergic contact sensitization in an unselected Danish population. The Glostrup Allergy Study, Denmark. Acta Derm Venereol 1992; 72: 456–460. [PubMed] [Google Scholar]
- 42.The ESSCA Writing Group. The European Surveillance System of Contact Allergies (ESSCA): Results of patch testing the standard series, 2004. J Eur Acad Dermatol Venereol 2008; 22: 174–181. [DOI] [PubMed] [Google Scholar]
- 43.Katerndahl DA, Bell IR, Palmer RF, et al. Chemical intolerance in primary care settings: Prevalence, comorbidity, and outcomes. Ann Fam Med 2012; 10: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gini R, Francesconi P, Mazzaglia G, et al. Chronic disease prevalence from Italian administrative databases in the VALORE project: A validation through comparison of population estimates with general practice databases and national survey. BMC Public Health 2013; 13: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dibley LB, Norton C, Jones R. Don’t eat tomatoes: Patient’s self-reported experiences of causes of symptoms in gastro-oesophageal reflux disease. Fam Pract 2010; 27: 410–417. [DOI] [PubMed] [Google Scholar]
- 46.Murphy DW, Castell DO. Chocolate and heartburn: Evidence of increased esophageal acid exposure after chocolate ingestion. Am J Gastroenterol 1988; 83: 633–636. [PubMed] [Google Scholar]
- 47.Flyvholm MA, Nielsen GD, Andersen A. Nickel content of food and estimation of dietary intake. Z Lebensm Unters Forsch 1984; 179: 427–431. [DOI] [PubMed] [Google Scholar]
- 48.Jones MP, Walker MM, Ford AC, et al. The overlap of atopy and functional gastrointestinal disorders among 23,471 patients in primary care. Aliment Pharmacol Ther 2014; 40: 382–391. [DOI] [PubMed] [Google Scholar]