Abstract
Crohn’s disease is a chronic inflammatory bowel disease potentially involving any segment of the gastrointestinal tract. Extra-intestinal manifestations may occur in 6%–40% of patients, and disorders of the skin are among the most common. This manuscript will review skin manifestations associated to Crohn’s disease, with a particular focus on lesions associated to anti-tumour necrosis factor therapy.
Keywords: Crohn’s disease, dermatologic manifestation, inflammatory bowel disease, anti-tumour necrosis factor-alpha
Introduction
Crohn’s disease (CD) is a chronic disorder of unknown aetiology characterised by noncaseating granulomatous inflammation of the gastrointestinal tract. Extra-intestinal manifestations may occur in 6%–40% of patients.1–6 Some of these manifestations are related to the activity of CD, whereas others have an independent course.6 Epidemiological and clinical variables that may influence the development of extra-intestinal manifestations have not clearly been defined. In this review, we will analyse skin manifestations which may occur in CD patients. Moreover, mounting evidence suggests that a number of skin lesions in CD patients may be related to the use of tumour necrosis factor alpha (TNF-α) antagonists which are being increasingly used for the management of CD patients. Therefore, we will also focus on the prevalence of skin lesions related to anti-TNF therapy, including skin malignancies, such as malignant melanoma (MM) or non-melanoma skin cancers (NMSCs).
Skin and oral lesions
It is estimated that between 6% and 40% of all patients with CD may suffer from an extra-intestinal manifestation.1–6 A number of these manifestations may occur during treatment and may lead to interruption of therapy in order to assess whether they are drug related or simply linked to the underlying inflammatory bowel disease (IBD).3
Cutaneous manifestations of CD are among the most common extra-intestinal disorders and have been classified into specific manifestations, characterised by granulomatous inflammation, and nonspecific manifestations, where the inflammatory process is not of the granulomatous type.4 Nonspecific manifestations may be further differentiated into subtypes according to pathogenesis:
reactive cutaneous manifestations of CD with immunological mechanisms triggered by common antigens shared by gut bacteria and skin,
cutaneous disorders or dermatosis associated with CD,
secondary cutaneous manifestations due to complications of CD.
Specific cutaneous manifestations or metastatic CD may appear at any site as solitary or multiple nodules, plaques, ulcers or purple perifollicular papules. Histology shows noncaseating granulomas with prevalence of epithelioid cells surrounded by lymphocytes, plasma cells and eosinophils (Table 1). Specific cutaneous alterations can be divided into two clinical forms: genital form, which accounts for 56% of specific cutaneous manifestations of CD and mainly occurs in children, being characterised by oedema, erythema, fissures or ulcers of the labia, scrotum, or penis, and non-genital form, which accounts for 44% specific cutaneous manifestations of CD and most commonly affects the lower extremities (38%), abdomen and trunk (24%), upper extremities (15%), face and lips (11%), and intertriginous areas (8%).5
Table 1.
Specific cutaneous manifestations of Crohn’s disease
| Specific cutaneous manifestations with same histological features as the underlying intestinal disease | |
| Oral ulcerations | Non-caseating granuloma with similar mechanism to underlying bowel pathology. |
| Perianal and peristomal: fissures, fistulas, ulcers | Direct local involvement of skin and mucosa by underlying bowel disease. |
| Metastatic | Specific granulomatous cutaneous lesions with the same histopathology as the intestinal lesions. |
Nonspecific manifestations encompass reactive alterations of the skin such as aphthous stomatitis, occurring in 5%–10% of CD patients, pyostomatitis vegetans, erythema nodosum (EN), and a variety of neutrophilic dermatoses (Table 2). They are often associated with intestinal inflammatory activity, therefore reflecting a common pathogenic mechanism.
Table 2.
Non-specific cutaneous manifestations of Crohn’s disease (CD)
| Reactive cutaneous manifestations with immunological mechanisms related to CD |
| Aphthous stomatitis |
| Erythema nodosum |
| Pyoderma gangrenosum |
| Sweet’s syndrome |
| Bowel-associated dermatosis-arthritis syndrome (BADAS) |
| Pyostomatitis vegetans |
| Leucocytoclastic vasculitis |
| Autoimmune cutaneous disorders associated with CD |
| Psoriasis |
| Secondary amyloidosis |
| Vitiligo |
| Acquired epidermolysis bullosa |
| Alopecia areata |
| Systemic lupus erythematosus |
| Secondary cutaneous manifestations/complications |
| Acrodermatitis enteropathica (zinc deficiency) |
| Purpura, angular cheilitis, hair and nail abnormalities |
EN is the most common cutaneous manifestation of CD, affecting about 4%–15% of patients. Typical EN presents as painful, tender, warm nodules (1–5 cm in diameter), raised bluish-red subcutaneous lesions or plaques predominantly located on the anterior surface of the lower extremities. The clinical presentation is often associated with systemic symptoms, such as fever, chills or arthralgias.6 The differential diagnosis may include metastatic skin cancer and histology may help ascertain the nature of the lesions. EN severity may parallel CD activity (90%) or may be independent of the underlying CD (10%).
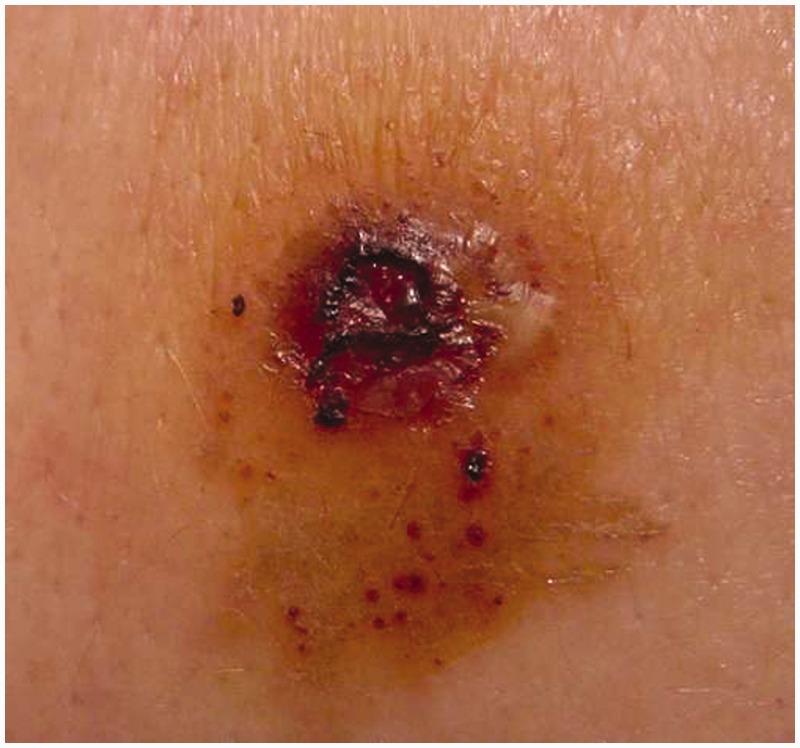
Pyoderma gangrenosum (PG) and Sweet’s syndrome (SS) are two neutrophilic dermatoses, frequently associated with CD. The hallmark of these diseases is the accumulation of neutrophils in the skin and, rarely, in the internal organs. PG is a very debilitating chronic ulcerative skin disorder. The ulcers have sharply circumscribed and demarcated borders with a necrotic yellowish base (Figure 1). The lesions can be single or multiple, unilateral or bilateral, and may extend from several centimetres to the surface of an entire limb. PG often occurs on the extensor surface of the legs, but may appear anywhere.5 SS is characterised by abrupt onset with fever, peripheral neutrophilia and leucocytosis. Cutaneous lesions consist of painful, tender, erythematous or purple papules or nodules that may coalesce into plaques, distributed in an asymmetrical fashion over the upper extremities, face and neck.6
Figure 1.
Pyoderma gangrenosum of the extensor aspect of the leg in a Crohn's disease patient.
Pyostomatitis vegetans is a rare cutaneous manifestation of IBD characterised by yellowish flat ulcerations on the oral and gingival mucosa, typically in the ‘snail tracks’ shape.5
Malnutrition and/or malabsorption may be the cause of secondary cutaneous manifestations of CD such as purpura, angular cheilitis, hair and nail abnormalities. The most frequent is acrodermatitis enteropathica-like syndrome, due to zinc deficiency, that presents with eroded, crusted, sharply bordered erythematous patches, surrounded by vesicles or pustules, located at the extremities and peri-orificially.7
Other skin alterations such as alopecia areata, psoriasis, vitiligo, or systemic lupus erythematosus (SLE) can be associated with CD but they are independent of the underlying disease and may simply reflect an increased susceptibility to autoimmunity.3
The pathogenic mechanism underlying the development of cutaneous manifestations in CD patients is still unknown. Recent evidence from mouse models of intestinal inflammation suggests that T helper (Th)17-dependent interleukin (IL)-23 production is one of the major players in the pathogenesis of CD.7 Because Th17 cells are involved in the pathogenesis of many autoimmune skin diseases, this might represent the mechanistic link between CD and cutaneous manifestations.5 Moreover, neutrophil dysfunction, abnormal T-cell response, over-expression of pro-inflammatory cytokines such as IL-8, IL-16, IL-17, IL-23 and TNF-α, have been proposed as the possible pathogenic mechanism underlying neutrophilic dermatoses.6
Skin lesions induced by anti-TNF therapy
An emerging problem in the course of CD is represented by the occurrence of skin lesions during anti-TNF therapy. Skin adverse events in patients treated with anti-TNF agents are classified as: a) local or systemic manifestations related to treatment such as diffused skin rash following drug administration or skin reaction at the injection site; b) skin infection; c) malignancy; d) autoimmune-related skin disease.8
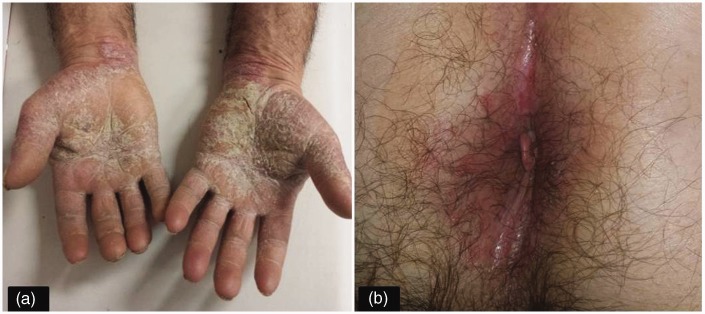
Anti-TNF agents, which are approved for the treatment of moderate to severe psoriasis, may paradoxically cause psoriasiform skin lesions in patients with IBD.9 An incidence of psoriasiform lesions of 1.6%–8.8%, which is consistent with data derived from anti-TNF-treated patients with rheumatoid arthritis.7,10,11 has been reported. Psoriasiform lesions during anti-TNF therapy occur more frequently in patients with CD than in those with UC (i.e. 90%–96% vs 4%–10%, respectively).7,12 The main risk factors for developing psoriasiform skin lesions are: smoking (past or active), increased body mass index (BMI), female sex, familial history of inflammatory skin disease and short disease duration.7 The most frequent localisation of psoriasiform lesions is hand palm or feet plant, scalp and flexures (Figure 2). The pathogenic mechanism of anti-TNF agents-related psoriasiform skin lesions is still an object of debate. Tillack et al. reported that psoriasiform lesions induced by anti-TNF therapy were characterised by infiltrates of IL-17/IL-22-expressing Th17 cells and interferon (IFN)-γ-expressing Th1 cells, the severity of lesions being correlated with the density of Th17 cell infiltrates. This suggests that Th1 and Th17 cells may play a pathogenic role in development of these lesions and indicates that anti-TNF-induced psoriasiform lesions and non-anti-TNF-related psoriasis may have a common pathogenic mechanism. In fact, in both conditions, an increased expression of the Th17-derived cytokines IL-17 and IL-22 has been demonstrated.7,13,14 Also, a role for over-production of IFN-α by plasmacytoid dendritic cells (pDCs) has been suggested.7,15 In fact, TNFα inhibits pDC maturation from haematopoietic progenitor cells and consequently inhibits IFN-α production.7,16 Therefore, anti-TNF treatment may result in unlimited IFNα production by pDCs, which might in turn favour the formation of psoriasiform lesions. In agreement with this hypothesis, Tillack et al. found increased IFNα protein expression in skin biopsy of patients with anti-TNF-associated psoriasiform lesions.7 Other studies suggested that altered lymphocyte migration caused by anti-TNF therapy may also contribute to skin lesions through the expression of CXCR3 ligands which, by interacting with their receptor, regulate leucocyte trafficking.7,17,18 Finally, Scaldaferri et al. hypothesised that anti-TNF agents may induce a ‘patchy cutaneous’ immune suppression leading to the development of psoriasis-like lesions.19
Figure 2.
Palmar (a) and perianal (b) psoriasis in a Crohn's disease patient on anti-TNF therapy.
There are no clear guidelines for management of anti-TNF-induced psoriasiform lesions. Paradoxical psoriasis can be treated with topical corticosteroids, vitamin D analogues, phototherapy, methotrexate or cyclosporine, but it is occasionally refractory to conventional treatments.20,21 In the case of localised lesions, topical corticosteroids or vitamin D analogues are indicated. In the case of diffuse lesions, there is an indication for systemic treatment with steroids or methotrexate or cyclosporine. Discontinuation of anti-TNF therapy may be required in about 30% of patients with severe lesions or in those who do not respond to conventional therapy leading to an improvement of skin lesions in 24% to 88%. After the resolution of skin lesions, the reintroduction of anti-TNF therapy should be considered. If anti-TNF therapy does no longer represent an option, alternative therapies for IBD have to be considered. Some studies reported efficacy of ustekinumab, an anti-IL-12/IL-23 p40 antibody, in patients non-responsive to local therapy after stopping anti-TNF therapy.7 Whether switching from one anti-TNF to another may have a positive impact on skin lesions is not completely clear. Tillack et al.7 in a large prospective study found that in 21/434 (4.8%) patients on anti-TNF therapy who developed psoriasiform lesions the switching strategy resulted in no significant improvement. Afzali et al.12 in a retrospective study including 1004 IBD patients on anti-TNF therapy found that 27 patients (i.e. 2.7%) developed psoriasiform skin lesions. In particular, skin lesions developed in eight of 620 (i.e. 1.3%) patients on infliximab, in 10/243 (i.e 4.1%) on adalimumab and in nine of 141 (i.e. 6.4%) on certolizumab. Of these 27 patients, six were managed by switching to another anti-TNF agent and four of these six patients showed a significant improvement in skin lesions. Therefore, therapeutic approach for paradoxical psoriasis must be discussed properly with patients because it may have a negative impact on the underlying intestinal disease and must be based on psoriasis severity, response to the standard therapy, and possibility of using alternative therapies for IBD.7,22
Anti-TNF therapy is associated with the development of skin lesions other than psoriasiform lesions with an incidence of approximately 18%. The most common non-psoriasiform skin disorders associated with anti-TNF therapy are infusion reactions to infliximab with skin erythema (5.8%), followed by viral skin infections (2.5%), eczematiform skin lesions (2.1%), xerosis cutis (2.1%) and bacterial skin infections (1.4%).7 SLE is an uncommon phenomenon with most cases reported in rheumatological series with an incidence of about 1%. Ninety-four per cent of the cases responded to interruption of therapy.23,24
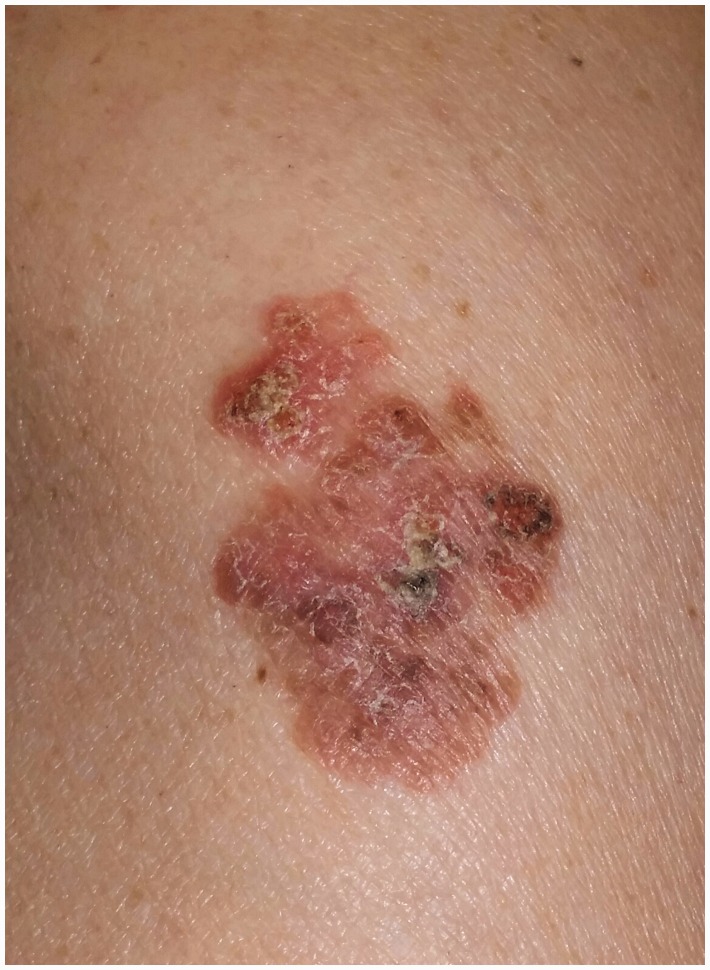
Cutaneous malignancies have also been reported in patients treated with anti-TNF agents or thiopurines. Askling et al., in a meta-analysis including 74 randomised controlled trials using anti-TNF therapy with over 22,000 patients reported a relative risk of NMSC (Figure 3) associated with all anti-TNF monotherapies equal to 2.02 (95% confidence interval (CI) 1.11–3.95).25 Data from the Therapy, Resource, Evaluation, and Assessment Tool (TREAT) registry including 6273 patients, 3420 treated with anti-TNF agents and 2853 non-anti-TNF-treated, with a mean follow-up/patient of 5.2 years, show that there is no statistically significant difference in the incidence of NMSC between patients treated with anti-TNF and those treated differently (0.16 vs 0.18 events per 100 patient-years, respectively).26 Biancone et al., showed that patients treated with anti-TNF agents have an increased risk of lymphoma and NMSC.27 However, it has been questioned whether IBD by itself may carry an increased risk of NMSC. In this respect, Long et al. demonstrated that IBD patients have an increased risk of NMSC independently of anti-TNF therapy compared to non-IBD controls with an incidence rate ratio (IRR) equal to 1.64 (95% CI 1.51–1.78). Moreover, persistent use (>365 days) of both thiopurine (6 MP/azathioprine) and anti-TNF is associated to an increased risk of developing NMSC with an adjusted odds ratio (OR) equal to 2.18 (95% CI 1.07–4.46).28 More recently, Long et al.29 in a retrospective case-control study of administrative data including108,579 patients with IBD (50,920 CD, 56,390 ulcerative colitis and 1269 IBD of undefined type) demonstrated that IBD was associated with an increased incidence of MM (IRR, 1.29; 95% CI, 1.09–1.53). Risk was greatest among individuals with CD (IRR, 1.45; 95% CI, 1.13–1.85; adjusted hazard ratio (HR), 1.28; 95% CI, 1.00–1.64). The incidence of NMSC also increased among patients with IBD (IRR, 1.46; 95% CI, 1.40–1.53) and was greatest among those with CD (IRR, 1.64; 95% CI, 1.54–1.74). In this study, therapy with anti-TNF appeared to increase the risk of MM (OR 1.88; 95% CI, 1.08–3.29). Patients who had been treated with thiopurines had an increased risk of NMSC (OR, 1.85; 95% CI, 1.66–2.05).25 Therefore, based on this study, use of biologics increases the risk of MM whereas the use of thiopurines increases the risk of NMSC. However, the absolute risk of developing MM or NMSC in IBD patients is as low as 57/100,000 person-years and 912/100,000 person-years, respectively. Therefore, taking into account the benefits of anti-TNF agents or thiopurines, the mildly increased relative risk of developing skin malignancies should not deter the use of these efficacious therapeutical agents. Avoidance of the sun, use of sun-protective agents or clothing may represent a valuable option for primary prevention of both MM and NMSC.
Figure 3.
Bowen's disease (i.e. squamous cell carcinoma in situ) on the extensor aspect of the arm in a Crohn's disease patient on anti-TNF therapy.
While thiopurine-associated increase in the risk of NMSC may be linked to the activation of thiopurine into a mutagenic DNA-reactive moiety after exposure to ultraviolet light,30 the mechanism whereby anti-TNF agents may increase the risk of skin malignancies is not fully understood. However, an alteration in immune surveillance due to anti-TNF may contribute.24,25 TNF-α has a complex effect on carcinogenesis and, depending on doses, promotes cell proliferation and thus tumour growth or induces apoptosis and inhibits angiogenesis, thus suppressing the development of a number of cancers.31–33 No causal association between anti-TNF agents and cancer has been demonstrated so far and caution is required when the available data are interpreted, because the follow-up of IBD patients treated with anti-TNF drugs is quite short. Multicentre studies in large populations with long-term follow-up are needed to address the issue of anti-TNF therapy and cancer development.
Summary and conclusion
Cutaneous lesions are the most common extra-intestinal manifestations associated with CD, with almost one-third of CD patients developing skin lesions during the course of their disease. The most frequent skin lesions are represented by EN and PG.34 Neither of these two skin diseases is found exclusively in CD, and the finding of one or the other lesion is not specific for either major form of IBD.34 The clinical course of these cutaneous manifestations often parallels the course of the underlying intestinal disease and generally improves with therapy.
An emerging problem is represented by the occurrence of skin lesions during anti-TNF therapy, psoriasiform skin lesions being the most common anti-TNF-related skin disease. These lesions generally respond to conventional therapy, but, in a number of cases, discontinuation of biologic therapy is needed and this may affect the course of the underlying intestinal disease. Switching from one anti-TNF to another is not always effective, but could be worth trying.
One major concern associated with the increased use of anti-TNF agents is the occurrence of MM.35 However, a causal relationship between ant-TNF therapy and development and progression or reactivation of cutaneous malignancy has not yet been fully ascertained. Further long-term controlled clinical trials and registries are required to investigate this potentially serious association.36,37
In conclusion, this review strengthens once again the concept that CD needs a multidisciplinary approach and that, in particular, a careful medical history and assessment for dermatologic conditions should be carried out before starting any biologic therapy.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Kethu SR. Extraintestinal manifestations of inflammatory bowel diseases. J Clin Gastroenterol 2006; 40: 467–475. [DOI] [PubMed] [Google Scholar]
- 2.Pellicer Z, Santiago JM, Rodriguez A, et al. Management of cutaneous disorders related to inflammatory bowel disease. Ann Gastroenterol 2012; 25: 21–26. [PMC free article] [PubMed] [Google Scholar]
- 3.Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol 2005; 11: 7227–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruocco E, Cuomo A, Salerno R, et al. Crohn’s disease and its mucocutaneous involvement. Skinmed 2007; 6: 179–185. [DOI] [PubMed] [Google Scholar]
- 5.Huang BL, Chandra S, Shih DQ. Skin manifestations of inflammatory bowel disease. Front Physiol 2012; 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohn’s Colitis 2010; 4: 63–101. [DOI] [PubMed] [Google Scholar]
- 7.Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut 2014; 63: 567–577. [DOI] [PubMed] [Google Scholar]
- 8.Hernández MV, Sanmartí R, Cañete JD, et al. Cutaneous adverse events during treatment of chronic inflammatory rheumatic conditions with tumor necrosis factor antagonists: Study using the Spanish registry of adverse events of biological therapies in rheumatic diseases. Arthritis Care Res 2013; 65: 2024–2031. [DOI] [PubMed] [Google Scholar]
- 9.Cullen G, Kroshinsky D, Cheifetz AS, et al. Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: A new series and a review of 120 cases from the literature. Aliment Pharmacol Ther 2011; 34: 1318–1327. [DOI] [PubMed] [Google Scholar]
- 10.Harrison MJ, Dixon WG, Watson KD, et al. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2009; 68: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres J, Buche S, Delaporte E, et al. Skin side effects of inflammatory bowel disease therapy. Inflamm Bowel Dis 2013; 19: 1086–1098. [DOI] [PubMed] [Google Scholar]
- 12.Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: A single academic center case series. J Crohns Colitis 2014; 8: 480–488. [DOI] [PubMed] [Google Scholar]
- 13.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur J Immunol 2006; 36: 1309–1323. [DOI] [PubMed] [Google Scholar]
- 14.Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol 1998; 111: 645–649. [DOI] [PubMed] [Google Scholar]
- 15.Wollenberg A, Wagner M, Günther S, et al. Plasmacytoid dendritic cells: A new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol 2002; 119: 1096–1102. [DOI] [PubMed] [Google Scholar]
- 16.de Gannes GC, Ghoreishi M, Pope J, et al. Psoriasis and pustular dermatitis triggered by TNF-{alpha} inhibitors in patients with rheumatologic conditions. Arch Dermatol 2007; 143: 223–231. [DOI] [PubMed] [Google Scholar]
- 17.Seneschal J, Milpied B, Vergier B, et al. Cytokine imbalance with increased production of interferon-alpha in psoriasiform eruptions associated with antitumour necrosis factor-alpha treatments. Br J Dermatol 2009; 161: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 18.Schroepf S, Kappler R, Brand S, et al. Strong overexpression of CXCR3 axis components in childhood inflammatory bowel disease. Inflamm Bowel Dis 2010; 16: 1882–1890. [DOI] [PubMed] [Google Scholar]
- 19.Scaldaferri F, Petito V, Papa A, et al. Anti-TNF-α-induced psoriasiform lesions in IBD: An abnormal immune activation or a ‘patchy cutaneous’ immune suppression? Gut 2014; 63: 699–670. [DOI] [PubMed] [Google Scholar]
- 20.Fiorino G, Danese S, Pariente B, et al. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-α agents. Autoimmun Rev 2014; 13: 15–19. [DOI] [PubMed] [Google Scholar]
- 21.Denadai R, Teixeira FV, Steinwurz F, et al. Induction or exacerbation of psoriatic lesions during anti-TNF-α therapy for inflammatory bowel disease: A systematic literature review based on 222 cases. J Crohns Colitis 2013; 7: 517–524. [DOI] [PubMed] [Google Scholar]
- 22.Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel disease with infliximab: Should biological therapy be suspended? Arq Gastroenterol 2012; 49: 172–176. [DOI] [PubMed] [Google Scholar]
- 23.Verma HD, Scherl EJ, Jacob VE, et al. Anti-nuclear antibody positivity and the use of certolizumab in inflammatory bowel disease patients who have had arthralgias or lupus-like reactions from infliximab or adalimumab. J Dig Dis 2011; 12: 379–383. [DOI] [PubMed] [Google Scholar]
- 24.Moran GW, Lim AW, Bailey JL, et al. Review article: Dermatological complications of immunosuppressive and anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2013; 38: 1002–1024. [DOI] [PubMed] [Google Scholar]
- 25.Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: Meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf 2011; 20: 119–130. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: More than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol 2012; 107: 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biancone L, Calabrese E, Petruzziello C, et al. Treatment with biologic therapies and the risk of cancer in patients with IBD. Nat Clin Pract Gastroenterol Hepatol 2007; 4: 78–91. [DOI] [PubMed] [Google Scholar]
- 28.Long MD, Herfarth HH, Pipkin C, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2010; 8: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012; 143: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011; 141: 1621–1628. [DOI] [PubMed] [Google Scholar]
- 31.Le Blay P, Mouterde G, Barnetche T, et al. Risk of malignancy including non-melanoma skin cancers with anti-tumor necrosis factor therapy in patients with rheumatoid arthritis: Meta-analysis of registries and systematic review of long-term extension studies. Clin Exp Rheumatol 2012; 30: 756–764. [PubMed] [Google Scholar]
- 32.Johansson M, Tan T, de Visser KE, et al. Immune cells as anti-cancer therapeutic targets and tools. J Cell Biochem 2007; 101: 918–926. [DOI] [PubMed] [Google Scholar]
- 33.Sgagias MK, Kasid A, Danforth DN., Jr Interleukin-1 alpha and tumor necrosis factor-alpha (TNF alpha) inhibit growth and induce TNF messenger RNA in MCF-7 human breast cancer cells. Mol Endocrinol 1991; 5: 1740–1747. [DOI] [PubMed] [Google Scholar]
- 34.Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4: 142–148. [DOI] [PubMed] [Google Scholar]
- 35.Magro F, Peyrin-Biroulet L, Sokol H, et al. Extra-intestinal malignancies in inflammatory bowel disease: Results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J Crohns Colitis 2014; 8: 31–44. [DOI] [PubMed] [Google Scholar]
- 36.Kouklakis G, Efremidou EI, Pitiakoudis M, et al. Development of primary malignant melanoma during treatment with a TNF-α antagonist for severe Crohn’s disease: A case report and review of the hypothetical association between TNF-α blockers and cancer. Drug Des Devel Ther 2013; 7: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fryrear RS, 2nd, Wiggins AK, Sangueza O, et al. Rapid onset of cutaneous squamous cell carcinoma of the penis in a patient with psoriasis on etanercept therapy. J Am Acad Dermatol 2004; 51: 1026–1026. [DOI] [PubMed] [Google Scholar]