Abstract
Background
While central obesity increases gastroesophageal reflux (GER) by mechanically disrupting the anti-reflux barrier, limited data exist on pathways by which central obesity may potentiate esophageal injury by non-mechanical means. Obesity has been associated with an impaired epithelial intestinal barrier.
Objective
We aimed to assess the influence of central obesity and reflux on the squamous esophageal epithelial intercellular space diameter (ICSD).
Methods
The ICSD was measured using electron microscopy in esophageal biopsies from individuals who underwent ambulatory pH monitoring and endoscopy. Anthropometric measurements were obtained on all participants. Participants were classified into four groups: with and without central obesity and reflux.
Results
Sixteen individuals were studied with four in each study group. The mean ICSD was almost three-fold greater (p < 0.001) in the group with central obesity without reflux, compared to controls without central obesity and reflux. It was also comparable to the ICSD in groups with acid reflux only and those with both reflux and central obesity.
Conclusions
There is evidence of esophageal squamous ICSD increase in individuals with central obesity who do not have evidence of acid and nonacid reflux on ambulatory pH monitoring. This may reflect a mechanism by which central obesity potentiates reflux-induced esophageal injury and inflammation.
Keywords: Central obesity, intercellular dilation, electron microscopy, Barrett’s, esophageal reflux
Introduction
The incidence of esophageal adenocarcinoma (EAC) and obesity are increasing rapidly.1 Central obesity is an independent risk factor for Barrett’s esophagus (BE) and EAC.2 Central obesity is associated with mechanical disruption of the gastroesophageal junction (GEJ) and increased gastroesophageal reflux (GER) that causes esophageal injury.3,4 In addition, central obesity (and visceral abdominal fat) has been shown to be a reflux-independent risk factor for esophagitis and BE, suggesting a non-mechanical mechanism of action.3,5 However, the potential mechanisms by which visceral abdominal fat released cytokines and adipokines predispose to esophageal mucosal injury are unknown.
Obesity has been implicated to increase intestinal permeability in animal models.6–9 Epithelial tight junctions that maintain epithelial integrity can be damaged by circulating proinflammatory cytokines released from visceral abdominal fat in centrally obese individuals.10,11 In addition, GER has been shown to lead to esophageal epithelial barrier damage characterized by dilated intercellular spaces (DIS) in the squamous epithelium.12
Impairment of the epithelial barrier in central obesity could facilitate paracellular permeation of noxious compounds in the refluxate, accentuating the injury and inflammation cascade, and promoting esophageal metaplasia and neoplasia. This phenomenon could provide the “second hit” for the development of BE in a background of mildly increased or physiologic levels of GER.13 There are currently limited data on the morphological characterization of the esophageal epithelial barrier in individuals with increased abdominal visceral fat (with and without reflux). In addition it is also unclear if the effects of these two risk factors are additive when present concurrently.
We hypothesized that the esophageal epithelial barrier (as determined by the squamous epithelial intercellular space diameter (ICSD)) is altered in patients with central adiposity without GER. The aim of this study was to: 1) determine the independent effects of central obesity and GER on the intercellular space diameter (ICSD) in the esophageal squamous epithelium of individuals with central obesity with and without physiologic evidence of GER; and 2) determine the effect of these risk factors on the ICSD when present in isolation and in combination.
Methods
This was a prospective cohort study. Sixteen individuals who underwent clinically indicated ambulatory esophageal pH monitoring were recruited to this study. Ambulatory pH monitoring was performed using a 24-hour combined pH impedance catheter assembly (Given,Yoqneam, Israel) with the proximal pH sensor placed at 5 cm from the upper border of the manometrically localized lower esophageal sphincter or a 48-hour wireless pH recording using the radiocapsule (Bravo) catheter-less technique (Given, Yoqneam, Israel). None of these patients had endoscopic evidence of BE (columnar mucosa in the distal esophagus >1 cm in length), a history of prior esophageal/gastric surgery or prior chemotherapy or radiation. Anthropometric measurements (height, weight, waist and hip circumference) were obtained using standard methods by a trained research coordinator. All participants underwent upper endoscopy 24–48 hours after completing the ambulatory pH study. Research biopsies were taken from the esophageal squamous mucosa at 5 cm above the GEJ following patient consent. The tissue was then formalin fixed, paraffin embedded, cut into 1 μ thick sections and stained with toluidine blue to assist in selecting the appropriate area to make the ultrathin sections (Figure 1).
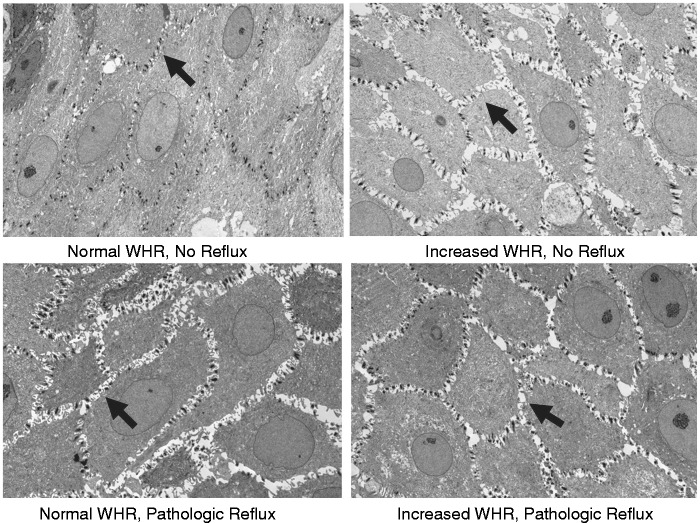
Figure 1.
Representative photomicrographs of intercellular spaces in the squamous esophageal epithelium of individuals with and without reflux and central obesity (measured using waist-to-hip ratio (WHR) as defined in the manuscript (4000×).
Sections 60 nm thick were then prepared from the squamous mucosal biopsies. Using a JEOL ExII Transmission Electron Microscope, four images at 4000× power were obtained randomly at the prickle layers in the Optical Microscopy Core Laboratory (Figure 1). Then, using a computerized image analyzer (Digimizer, version 4.2.2.0, MedCalc Software), 10 transects were randomly drawn across perpendicular cell membranes with each transect no closer than 1 µm apart. The intercellular space was calculated at these transecting lines using standard and commonly accepted techniques by an observer who was blinded to the clinical features.12,14 The measurements were obtained at the prickle layer of each patient to maintain consistency between patients, given the known inter-layer variability that has been reported.15 The mean ICS measurement for each patient was calculated from the four sets of 10 measurements taken in the photomicrographs.
All pH/impedance studies were read by a single investigator (PGI) who was blinded to other clinical information. Excessive total distal esophageal acid exposure was defined as greater than 4.2% of the time with a pH less than 4 in the distal esophagus over 24 hours and a composite DeMeester score of >14.7. For those who underwent impedance pH testing, we defined an abnormal number of total reflux episodes to be >48 in those on proton pump inhibitors (PPIs) and >73 in those off PPIs.16 The number of total, acid and nonacid reflux episodes were also separately assessed in those undergoing pH-impedance testing on PPIs (11 out of 16 participants) and were also used for classifying individuals into the study groups defined below.
Patients were divided into four groups: Group 1: Normal body mass index (BMI) (19–25 kg/m2) and waist-to-hip ratio (WHR) (women < 0.85 and men < 0.90), physiologic number of acid and nonacid reflux events (if on PPI); Group 2: Increased BMI (>30 kg/m2) and WHR (women > 0.85 and men > 0.90) and physiologic number of acid and nonacid reflux events (if on PPI); Group 3: Normal BMI and WHR with pathologic GER (greater than 4.2% time with pH less than 4 or elevated total number of reflux events (if on PPI); Group 4: Increased BMI and WHR with pathologic GER (greater than 4.2% time with pH less than 4 or elevated total number of reflux events (if on PPI). The WHR cutoff used for defining central obesity was as per World Health Organization standards.17
Statistical analysis was conducted using JMP® 10.0.0 statistical software (SAS Institute). Data were summarized as the mean (standard deviation) or median (interquartile range) as per data distribution. Comparison between groups was performed using the analysis of variance (ANOVA) (for continuous variables) or the chi square test (for dichotomous variables).
Results
Demographic and clinical features of the 16 patients who underwent ambulatory pH monitoring, endoscopy with research biopsy and anthropometric measurements and were divided into the four study groups described above are shown in Table 1. Age, smoking, alcohol consumption, and medication use were not significantly different among groups (p > 0.05). The ratio of men to women was higher in Group 1. Indications for endoscopy, as documented in a clinic visit with a gastroenterologist, included esophageal (heartburn, regurgitation) and extra-esophageal (cough, hoarseness) reflux symptoms (10, 63%), dysphagia (two, 12.5%), nausea/vomiting (one, 6%), dyspepsia (one, 6%), non-cardiac chest pain (one, 6%), and asthma (one, 6%).
Table 1.
Demographic and clinical features of patients in the study as divided into four predetermined study groups
| Group 1 (N = 4) | Group 2 (N = 4) | Group 3 (N = 4) | Group 4 (N = 4) | p value | |
|---|---|---|---|---|---|
| Mean age (SD), years | 47.3 (23.5) | 49.8 (19) | 36.8 (9.8) | 35.8 (28.1) | 0.72 |
| Male gender (%) | 50 | 25 | 25 | 0 | 0.89 |
| Mean BMI (SD) | 22.5 (1.2) | 35.4 (2.9) | 23.1 (1.8) | 37.5 (3.5) | <0.0001 |
| Mean WHR (SD) | 0.79 (0.03) | 0.82 (0.06) | 0.97 (0.04) | 0.96 (0.09) | 0.002 |
| Mean % time pH <4 in distal esophagus (SD) | 1.4 (1.9) | 1.1 (1.1) | 5.8 (2.1) | 10.3 (6.9) | 0.015 |
| Mean number of total reflux episodes (N, SD)a | 39 (4, 26.3) | 42.3 (4, 13.7) | 60.5 (4, 32.4) | 105 (4, 25.6) | 0.012 |
| Mean number of nonacid reflux episodes (N, SD) | 11 (2, 11.3) | 31.3 (4, 5.3) | 28 (4, 9.3) | 91 (1, –) | 0.001 |
| Symptomatic reflux as indication of EGD (%) | 75 | 25 | 75 | 100 | 0.137 |
| Smoking (%) | 0 | 0 | 25 | 0 | 0.362 |
| Alcohol consumption (%) | 50 | 50 | 50 | 75 | 0.859 |
| PPI or H2 antagonist use (%) | 50 | 50 | 75 | 100 | 0.362 |
| NSAID or ASA use (%) | 25 | 50 | 0 | 50 | 0.362 |
Includes patients with 48-hour Bravo study or 24-hour impedance study.
BMI: body mass index; WHR: waist-to-hip ratio; EGD: esophagogastroduodenoscopy; PPI: proton pump inhibitor; NSAID: nonsteroidal anti-inflammatory drug; ASA: acetylsalicylic acid.
Eleven (69%) patients were on PPIs at the time of ambulatory pH testing, of whom one patient was on a histamine receptor antagonist as well at the time of pH testing. Five of 16 participants underwent ambulatory pH monitoring using the Bravo pH monitoring system (performed off medication) with the remaining undergoing combined pH impedance testing (performed on medication). Of the two patients in Group 1 who were on a PPI, the one who completed the Bravo study had an esophageal pH that was <4 for 0.8% of the recording time and the other patient who underwent 24-hour impedance had only three episodes of acid reflux with the pH being <4 for 0.4% of the time. This is far below the 95th percentile of the number of GER episodes in those on PPI in previous studies.18
As seen in Table 1, the mean percentage time pH was less than 4 in the distal esophagus was substantially lower and in the physiologic range in Groups 1 (no central obesity and no reflux) and 2 (centrally obese and no reflux) and elevated in Groups 3 (no central obesity, with reflux) and 4 (centrally obese with reflux). The mean number of nonacid reflux events in participants in Groups 1, 2 and 3 who underwent pH impedance testing (two individuals in Group 1: 11 events, four patients in Group 2: 31 events and four patients in Group 3: four events) was also in the physiologic range;18 with only one patient in Group 4 (centrally obese, with reflux) demonstrating an elevated number (91) of nonacid reflux episodes. This suggests that participants in Groups 1, 2 and 3 did not have evidence of excessive nonacid reflux as well.
At endoscopy, the esophageal mucosa was normal in 13 (81%) patients. Two patients (13%) had esophagitis, one each with Los Angeles classification-A (Group 4) and Los Angeles classification-B (both in Group 3), with increased GER on pH monitoring. One patient (6%) with treated eosinophilic esophagitis had esophageal furrowing and a normal esophageal pH study. Biopsies revealed histologically quiescent disease.
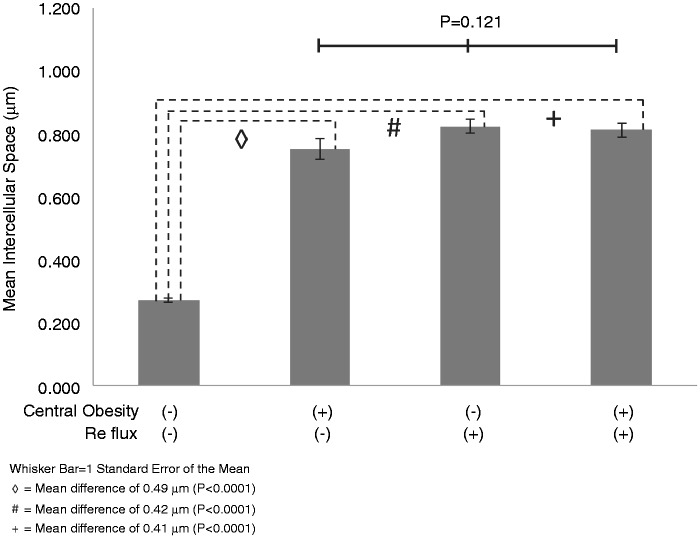
As seen in Figures 1 and 2, patients in Group 1 (i.e. controls with no reflux and no central obesity) had the smallest ICSD, with a mean (SD) ICSD of 0.275 µm (0.1). The ICSD was more than twofold greater in Groups 2, 3 and 4 than Group 1; each of these differences was statistically significant (p < 0.0001). The mean (SD) ICSD for these groups were 0.753 µm (0.4), 0.826 µm (0.3) and 0.814 µm (0.3), respectively. However, differences between the ICSD of Groups 2, 3 and 4 were not significant (p = 0.12). Increased esophageal ICSD was not associated with age, tobacco use, alcohol use, PPI or H2 receptor antagonist (H2RA) use (Table 2). The mean ICSD was higher in females than males. There was no statistical difference in ICSD between those on an acid-suppressing medication versus those who were not.
Figure 2.
Comparison of intercellular space diameter measurements from the esophageal squamous epithelium of groups with and without central obesity and reflux.
Table 2.
Influence of clinical variables on the intercellular space diameter in the esophageal squamous epithelium on transmission electronic microscopy in the four study groups
| Men | Women | p value | |
|---|---|---|---|
| Sex | 0.4 (4, 0.23) | 0.76 (12, 0.25) | 0.03 |
| Yes | No | ||
| Tobacco use | 0.99 (1, –) | 0.65 (15, 0.28) | 0.25 |
| Alcohol use | 0.64 (9, 0.31) | 0.69 (7, 0.28) | 0.727 |
| Aspirin use | 0.61 (4, 0.36) | 0.69 (12, 0.28) | 0.69 |
| NSAID use | 0.64 (3, 0.33) | 0.67 (13, 0.29) | 0.87 |
| PPI or H2 antagonist use | 0.69 (11, 0.28) | 0.62 (5, 0.34) | 0.685 |
Key: Mean in (N, SD)
NSAID: nonsteroidal anti-inflammatory drug; PPI: proton pump inhibitor.
Discussion
Dilation of intercellular spaces in the esophageal squamous epithelium has been shown to be an early pathologic event in GER disease (GERD). In this small but prospective study with four carefully characterized patient groups with and without central obesity and GER (both documented using validated objective criteria), we have demonstrated that this pathologic change occurs not only in response to abnormal esophageal acid exposure (Groups 3 and 4) but also in patients with central obesity without evidence of acid or nonacid reflux on ambulatory pH monitoring (Group 2). Specifically, our data show that among patients with central obesity with or without GER (Groups 2, 3), the ICSD of the squamous esophageal epithelium was significantly greater than in controls without central obesity or reflux (Group 1). Furthermore, the ICSD in patients with central obesity with or without GERD was comparable to that of patients with GER alone in this and previous studies of patients with GERD.12 Our results were not affected by relevant clinical variables.
This study supports two important concepts in GERD. The first is that dilation of intercellular spaces in esophageal epithelium is an early event in and sensitive marker for GERD as has been shown in previous studies.8,9 Moreover, reflux did not augment the effect of obesity on ICSD dilation, suggesting a threshold effect attributable to obesity. From a mechanistic view, these early changes makes intuitive sense as the dilation of these spaces allows diffusion of HCl-rich fluid into the esophageal epithelium thereby facilitating caustic injury and potential cytokine release.19 The pathogenesis of this process is unclear but, conceivably, disruption of the structure or function of tight junction proteins may, at least partly, mediate these effects.20 The mechanism by which acid induces these changes is unclear and warrants further study. In addition, the role of nonacid reflux on intercellular space dilation is unclear. Of the 11 patients in our study who underwent impedance testing, the mean number of nonacid reflux episodes was 33, which is well below the 73 episodes that is thought to be pathologic.16 The low rate of nonacid reflux in our study patients likely mitigates the potential confounding nature of nonacid reflux on ICSD dilation.
The second important concept is that central obesity is associated with a morphologically impaired esophageal epithelial barrier in the absence of pathologic levels of acid exposure. Since central obesity may predispose to GER by mechanically disrupting the GEJ reflux barrier and increasing intra-abdominal pressure, it also makes intuitive sense that obesity leads to dilation of intercellular spaces by promoting GER. Consistent epidemiologic evidence, however, suggests a reflux-independent mechanism by which central obesity may increase the risk of esophageal inflammation and neoplasia.21 Indeed, with stratification of groups by acid reflux and central obesity status that enabled an accurate and objective assessment of the effects of central obesity independent of acid reflux, we demonstrated that ICSD occurs in centrally obese patients in the absence of documented GER. Thus, disruption of the esophageal epithelial barrier may be an underlying mechanism by which increased visceral abdominal fat increases the susceptibility of the esophageal epithelium to reflux-mediated injury and inflammation that potentiates the development of metaplasia and neoplasia.3
The cause of ICSD without pathologic reflux is unclear but it is postulated that the disruption of tight junctions may occur through pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin 6 (IL6), which are elevated in obese patients. It has also been demonstrated that TNFα decreases barrier function in esophageal epithelium.22 Indeed, these cytokines have been shown to be involved in remodeling tight junctions by altering epithelial barrier proteins such as claudins and occludins.23 Notably, recent data from our group in patients with eosinophilic esophagitis, a strongly cytokine-mediated disease, also demonstrate that pathologic dilation of intercellular spaces correlates inversely to tight junction protein expression.24
This study has some potential limitations. We recognize the small sample size of this study and conducted this exploratory study to obtain pilot data, but were struck by the statistically significant differences between Group 3 (centrally obese with evidence of excessive acid or nonacid reflux) and the control groups (Group 1 and 3). These data should be confirmed in larger studies. While most participants were on PPIs, the lack of not only excessive acid reflux but also total and nonacid reflux events (particularly in those on PPIs in Groups 1 and 2) argues against misclassification bias. Moreover, the mean ICSD values in Groups 1 (normal controls) and 3 (excessive acid and nonacid reflux) are comparable to those described in the literature as well. It is conceivable that the increased ICSD in centrally obese individuals without pathological GER (i.e. Group 2) reflects the effects of ongoing but undetected GER, i.e. the pH study was falsely negative. However, this seems unlikely given the extremely low mean acid exposure duration in this group, which was lower than in Group 1, and normal findings on endoscopy. Furthermore, extra-esophageal symptoms of GERD were the predominant indication for pH testing in Group 2 (with central obesity, without reflux). We cannot exclude the possibility that the increased ICSD in Group 2 is attributable to weakly or nonacidic and/or bile reflux, which has been reported in animals.25 The physiologic number of nonacid reflux episodes in patients in Group 2, however, makes this relatively unlikely. Lastly, there are various methods for assessing epithelial ICSD that include routine histologic analysis assessing spongiosis.24 We chose electron microscopy as this appears to be the most objective and precise method. We also ensured that all our photomicrographs included the basal membrane for orientation, which is critical for obtaining standardized measurements.15
In summary, central obesity with or without reflux may be associated with DIS, reflecting morphological impairment of the esophageal epithelial barrier. These observations should be confirmed in larger studies. This early change, which does not appear to be additive in the presence of concomitant reflux, may occur independently of either central obesity or gastroesophageal reflux. Further studies are necessary to clarify if the pathogenesis of DIS involves cytokine-mediated or independent mechanisms.
Acknowledgements
We appreciate review of the manuscript by Dr Marcelo Vela.
Author contributions are as follows:
Christopher H Blevins was responsible for the study concept, study design, data acquisition, data analysis, drafting the manuscript and statistical analysis.
Anamay N Sharma was responsible for technical and material support.
Michele L Johnson was responsible for administrative, technical and material support.
Deborah Geno was responsible for administrative, technical and material support.
Milli Gupta was responsible for acquisition of data and study concept.
David A Katzka was responsible for critical review of manuscript for important intellectual content.
Adil E Bharucha was responsible for critical review of manuscript for important intellectual content.
Prasad G Iyer was responsible for the study concept, study design, critical revision of the manuscript for important intellectual content, obtained funding support and provided study supervision.
Funding
This work was supported by the Mayo Foundation and Mayo Clinic Center for Clinical and Translational Sciences, and CTSA grant number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Conflict of interest
None declared.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005; 97: 142–146. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q, Zhuang H, Liu Y. The association between obesity factor and esophageal cancer. J Gastrointest Oncol 2012; 3: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 1399–1412.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB, Ergun GA, Pandolfino J, et al. Obesity increases oesophageal acid exposure. Gut 2007; 56: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 2006; 74: 443–477. [DOI] [PubMed] [Google Scholar]
- 6.Al-Sadi R, Ye DM, Dokladny K, et al. Mechanism of IL-1 beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 2008; 180: 5653–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Sadi R, Ye DM, Ma TY. Molecular mechanism of IL-1 beta-induced increase in CACO-2 intestinal epithelial tight junction permeability. Gastroenterology 2008; 134: A35-A–A35-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2007; 292: G518–G525. [DOI] [PubMed] [Google Scholar]
- 9.Farhadi A, Gundlapalli S, Shaikh M, et al. Susceptibility to gut leakiness: A possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int 2008; 28: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He F, Peng J, Deng XL, et al. Mechanisms of tumor necrosis factor-alpha-induced leaks in intestine epithelial barrier. Cytokine 2012; 59: 264–272. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Weber CR, Raleigh DR, et al. Tight junction pore and leak pathways: A dynamic duo. Annu Rev Physiol 2011; 73: 283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobey NA, Carson JL, Alkiek RA, et al. Dilated intercellular spaces: A morphological feature of acid reflux—damaged human esophageal epithelium. Gastroenterology 1996; 111: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen AR. Visceral adipose tissue attacks beyond the liver: Esophagogastric junction as a new target. Gastroenterology 2010; 139: 1823–1826. [DOI] [PubMed] [Google Scholar]
- 14.Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11: 190–194. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Chun HJ, Jang JS, et al. Is intercellular space different among layers in normal esophageal mucosa? An electron microscopic study. Dig Dis Sci 2011; 56: 3492–3497. [DOI] [PubMed] [Google Scholar]
- 16.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: A multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol 2004; 99: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Waist circumference and waist-hip ratio. Report of a WHO expert consultation, Geneva, 8–11 December 2008.
- 18.Zerbib F, Roman S, Bruley Des Varannes S, et al. Normal values of pharyngeal and esophageal 24-hour pH impedance in individuals on and off therapy and interobserver reproducibility. Clin Gastroenterol Hepatol 2013; 11: 366–372. [DOI] [PubMed] [Google Scholar]
- 19.Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 2009; 137: 1776–1784. [DOI] [PubMed] [Google Scholar]
- 20.Naydenov NG, Baranwal S, Khan S, et al. Novel mechanism of cytokine-induced disruption of epithelial barriers: Janus kinase and protein kinase D-dependent downregulation of junction protein expression. Tissue Barriers 2013; 1: e25231–e25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Zhang Q, Wang M, et al. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol 2008; 126: 67–80. [DOI] [PubMed] [Google Scholar]
- 22.Shan J, Oshima T, Farre R, et al. IL-4 induces columnar-like differentiation of esophageal squamous epithelium through JAK/PI3K pathway: Possible role in pathogenesis of Barrett’s esophagus. Am J Physiol Gastrointest Liver Physiol 2014; 306: G641–G649. [DOI] [PubMed] [Google Scholar]
- 23.Capaldo CT, Farkas AE, Hilgarth RS, et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell 2014; 25: 2710–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzka DA, Tadi R, Smyrk TC, et al. Effects of topical steroids on tight junction proteins and spongiosis in esophageal epithelia of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014; 12: 1824–18249.e1. [DOI] [PubMed] [Google Scholar]
- 25.Farre R, van Malenstein H, De Vos R, et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut 2008; 57: 1366–1374. [DOI] [PubMed] [Google Scholar]