Abstract
Introduction
The possible benefit of endoscopic submucosal dissection (ESD) for early neoplasia arising in Barrett’s esophagus remains controversial. We aimed to assess the efficacy and safety of ESD for the treatment of early Barrett’s neoplasia.
Methods
All consecutive patients undergoing ESD for the resection of a visible lesion in a Barrett’s esophagus, either suspicious of submucosal infiltration or exceeding 10 mm in size, between February 2012 and January 2015 were prospectively included. The primary endpoint was the rate of curative resection of carcinoma, defined as histologically complete resection of adenocarcinomas without poor histoprognostic factors.
Results
Thirty-five patients (36 lesions) with a mean age of 66.2 ± 12 years, a mean ASA score of 2.1 ± 0.7, and a mean C4M6 Barrett’s segment were included. The mean procedure time was 191 ± 79 mn, and the mean size of the resected specimen was 51.3 ± 23 mm. En bloc resection rate was 89%. Lesions were 12 ± 15 mm in size, and 81% (29/36) were invasive adenocarcinomas, six of which with submucosal invasion. Although R0 resection of carcinoma was 72.4%, the curative resection rate was 66% (19/29). After a mean follow-up of 12.9 ± 9 months, 16 (45.7%) patients had required additional treatment, among whom nine underwent surgical resection, and seven further endoscopic treatments. Metachronous lesions or recurrence of cancer developed during the follow-up period in 17.2% of the patients. The overall complication rate was 16.7%, including 8.3% perforations, all conservatively managed, and no bleeding. The 30-day mortality was 0%.
Conclusion
In this early experience, ESD yielded a moderate curative resection rate in Barrett’s neoplasia. At present, improvements are needed if ESD is to replace piecemeal endoscopic mucosal resection in the management of Barrett’s neoplasia.
Keywords: Endoscopic submucosal dissection, Barrett’s esophagus, early esophageal adenocarcinoma, high-grade dysplasia
Introduction
Endoscopic submucosal dissection (ESD) has been extensively used in Japan and increasingly in Europe within the last decade for the resection of early esophageal neoplasms.1–3 On the one hand, esophageal surgical resection is associated with 35%–42% morbidity and 2%–3.4% mortality4–6 even in high-volume, specialized centers. On the other hand, endoscopic mucosal resection (EMR), although reported safe and effective for small intramucosal esophageal cancer, either squamous cell carcinoma7,8 or adenocarcinoma,9 does not allow oncologically adequate en bloc resection of tumors larger than 20 mm. Therefore, lesions are resected piecemeal, leaving remnants of neoplastic tissue in situ, and recurrence rates of 9.8% to 14.5% require cautious follow-up and additional endoscopic resections.9,10
In 2010, Takahashi et al. reported a 99.1% curative resection rate for intramucosal esophageal squamous cell carcinoma resected with ESD, as compared to 78.3% obtained with EMR, even though the lesions resected by EMR were almost twice as small as those resected with ESD.10 The superiority of ESD over EMR in terms of en bloc resection, curative resection, and local recurrence rate of esophageal neoplasms was confirmed in three large studies10–12 as it had been demonstrated earlier for gastric neoplasms.13 Furthermore, ESD provides a single specimen, obviously more appropriate for an optimal histological assessment than the multiple tissue chunks retrieved after piecemeal EMR.
If the resection of squamous neoplasms invading the submucosa is questionable because of the high frequency of lymph node involvement,14 ESD could allow for safer resection of early adenocarcinoma (EAc) invading the submucosa, as compared to EMR. Yet, current data on ESD for the treatment of Barrett’s carcinoma are scarce and do not demonstrate a benefit of ESD in this indication,15 possibly because the experience of Western teams in ESD is still limited, and second because the endoscopic delineation of the extent of EAc in Barrett’s esophagus is difficult and results in high rates of positive lateral margins. Therefore, we sought to assess in our experience the effectiveness and safety of ESD for the treatment of visible lesions arising in Barrett’s esophagus suspicious of early adenocarcinoma.
Methods
Patient selection and data collection
This is a retrospective study from a prospectively collected database including 69 esophageal ESDs performed in our department since January 2012. Patients included had histologically confirmed Barrett’s esophagus with intestinal metaplasia and a visible lesion deemed not amenable to en bloc EMR because of elevated type or a size exceeding 10 mm. All consecutive patients with lesions of more than 10 mm and/or suspicion of submucosal ingrowth were treated with ESD. Patients with signs of deep submucosal invasion such as Paris type 0–III type, or evidence of advanced disease on preoperative or computed tomography (CT) scanner were not offered ESD. An oncologic workup including endoscopic ultrasound (EUS) and thoraco-abdomino-pelvic CT scan was performed preoperatively in cases in which preoperative histology mentioned invasive adenocarcinoma and worrisome endoscopic features, such as polypoid (Paris 0–Is) or ulcerated (Paris 0–III) lesion were seen. Demographic data and procedural characteristics such as the date, the size of the resection, the endoscopic device used, the early outcomes and complications, histological results and possible complementary treatments were found in the database. Further data on the endoscopic procedure such as procedure duration, use of esophageal stricture prevention, patients’ comorbid conditions, and most recent follow-up data were found in the patients’ files.
Endoscopic procedures
A diagnostic staging endoscopy was systematically conducted in our center within one month prior to ESD, using high-definition endoscopes and narrow-band imaging, in order to characterize the extent of Barrett’s esophagus, and determine the endoscopic resectability of the neoplasia. All ESDs were conducted under general anesthesia with endotracheal intubation and CO2. Antiplatelet agents other than aspirin and anticoagulant therapy were discontinued before the procedure. ESDs were performed by three experienced operators (FP, SL, SC), each of whom has previous experience with more than 50 rectal, gastric, and esophageal ESDs in patients. High-definition gastroscopes with narrow-band imaging (GIF-H180J, GIF-2TH180 or GIF-HQ190, Olympus, Japan) were used for ESD. Procedures were carried out with a soft distal attachment cap and waterjet. The choice of ESD knives was left to the operator, among them the 1.5 mm dual knife (Olympus, Japan), the 1.5 or 1 mm flush knife (Fujifilm, Japan), and the triangle tip (TT) knife (Olympus, Japan). After delineation of the lesion with soft coagulation dots positioned 2–3 mm external to the lateral margins of visible lesions, submucosal injection of indigo-carmine-stained lifting solution (a mixture of 5% fructose and 10% glycerol with saline16) was performed; peripheral incision was conducted using the endocut mode and submucosal dissection was then achieved using the swift coagulation mode. Hemostasis of submucosal vessels was achieved either with the ESD knife, coagulation forceps in soft coagulation mode (Coagrasper, Olympus, Japan), or 1% epinephrine lavage. The modus operandi for the most recent cases of esophageal neoplasia generally followed the “tunnel method,” in which we perform distal and proximal marginal incisions before undertaking the submucosal dissection in an antegrade fashion, and finish by cutting the left and right margins.17 Standard ESD procedure with a circumferential incision all around the lesion at the early phase of the procedure was performed in other cases. An example of ESD for the resection of a Barrett’s carcinoma is presented in Figure 1. When circumferential or subcircumferential mucosal resection was achieved, post-endoscopic stricture prevention was attempted by injecting endoscopically 100 mg of triamcinolone into the residual submucosa and muscularis propria, as reported by Hanaoka et al.18
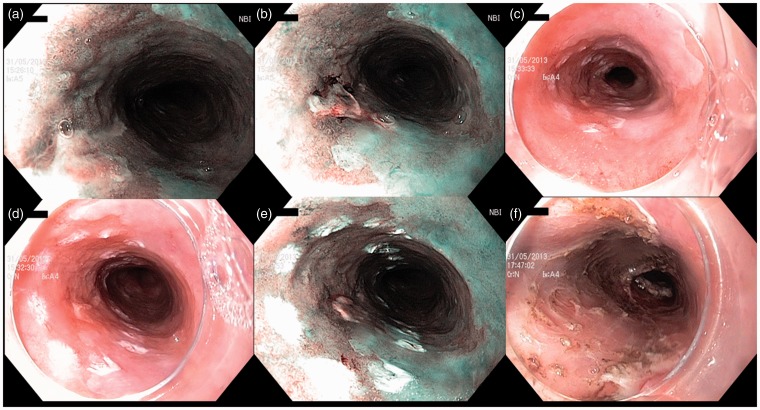
Figure 1.
Endoscopic submucosal dissection of a 15 mm, Paris 0–IIa + IIc, ulcerated intramucosal carcinoma, arising on a C14M15 Barrett’s esophagus. Panels (a) and (b) show the distal and proximal side of the lesion in narrow-band imaging, respectively. Panels (c), (d) and (e) show the lesion delineated with coagulation markings in white light endoscopy ((c) and (d)) and narrow-band imaging (e). The markings are placed at least 5 mm away from the lesion margins. Panel (f) shows the resection site immediately after the hemicircumferential endoscopic submucosal dissection on a 4 cm height. Resection was curative with lateral margins positive for intestinal metaplasia, and radiofrequency ablation of the remaining Barrett’s esophagus was performed.
Histological assessment
Resected specimens were pinned on polystyrene boards and fixed in 10% formalin for 24 hours. After fixation, specimens were cut into 2–3 mm slices and embedded in paraffin. Blocks were further sliced at 4 µm and stained with hematoxylin-eosin-saffron. Histological slides were assessed by two pathologists experienced in Barrett’s esophagus pathology (FB and BT). The following data were assessed: en bloc resection, size of the lesion with worst histology, invasion of the lateral margins, grade of dysplasia; in case of invasive carcinomas, the following data were recorded: grade of differentiation, presence of lymphatic or vascular invasion, deepest tumor extension in the esophageal wall, and tumoral invasion of the vertical (deep) margin. In case of submucosal invasion, the extent of the tumor front beyond the muscularis mucosae was measured in µm, as well as the width of that extension. For invasive adenocarcinoma, the margins could be either tumor free (R0) or infiltrated with tumor (R1). Submucosal invasion was assessed according to the Japanese classification of esophageal cancer.19 The processing of the samples and histopathological analysis are presented in Figure 2.
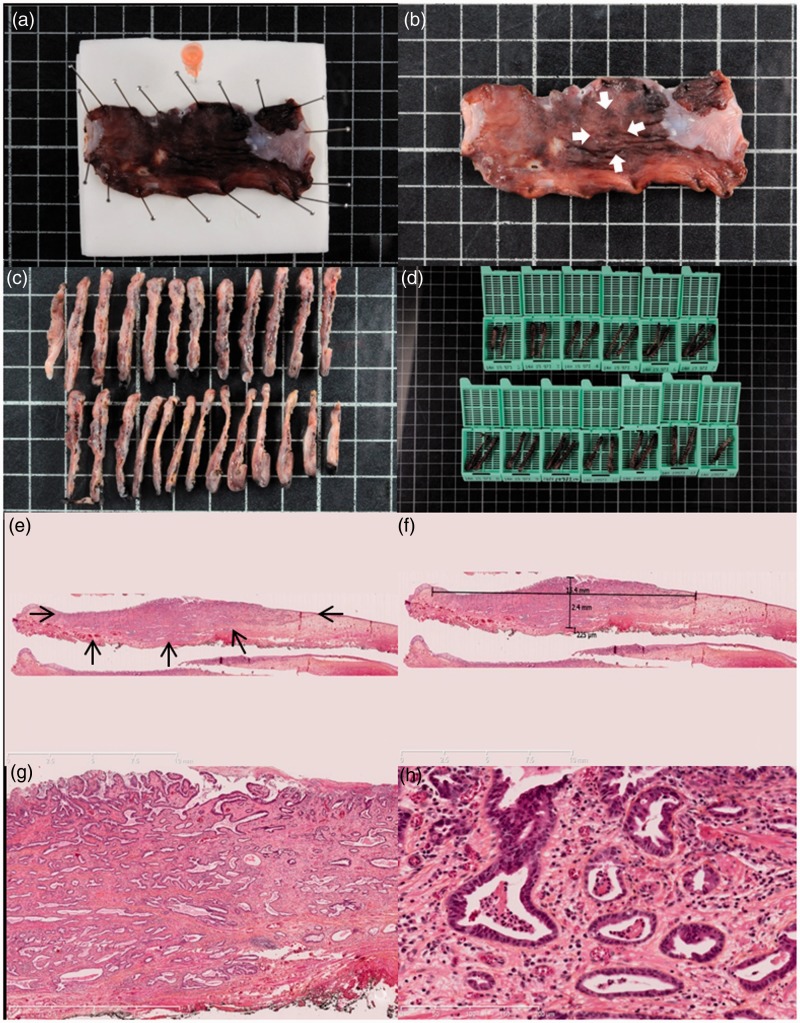
Figure 2.
Histopathological assessment of early Barrett’s carcinoma resected by endoscopic submucosal dissection. (a) Macroscopic view of the resected specimen after 24 hours of formaldehyde fixation, pinned to the polystyrene board with an orange needle to mark the oral side of the resection. (b) Macroscopic view of the resected specimen after fixation. Arrowheads show the 15 mm suspicious nodular lesion. (c) and (d) Processing of the specimen in 2–3 mm sections further included in paraffin blocks. (e) Histological view of the resected specimen showing an invasive adenocarcinoma (arrowheads) with submucosal infiltration and R0 margins, hematoxylin-eosin-saffron, 5×. (f) After digitization of the slide, measurement shows a submucosal invasion of 225 µm, making the lesion a pT1a sm1 lesion, with curative endoscopic treatment. (g) and (h) Histological view of the resected specimen at higher magnifications, Histological view of the resected specimen showing a well-differentiated tubulous adenocarcinoma with submucosal infiltration and R0 margins, and no vascular or lymphatic invasion, hematoxylin-eosin-saffron, 25× and 200×, respectively.
Complications and follow-up
We recorded complications as early (within 48 hours after ESD) or late complications (>48 hours after ESD). Bleeding was defined as a either a two-point hemoglobin drop in 24 hours or hematemesis, melena or hematochezia requiring either blood cell transfusion or control endoscopy. Per-procedural bleeding managed with hemostatic forceps was not recorded as a complication. Esophageal perforation was defined as a visible hole in the esophageal muscle layer and/or postoperative air or liquid collection in the mediastinum or the abdomen. Esophageal strictures were recorded as a narrowing of the esophageal lumen either too small to admit a 10 mm endoscope, or associated with dysphagia.
After ESD, patients were looked after in the department for at least two nights and received high-dose proton pump inhibitors, pain medication, and oral feeding was resumed progressively within 24 hours of the procedure. All cases of invasive adenocarcinoma were discussed in a multidisciplinary meeting. All patients had a one-month follow-up visit after ESD to check for symptoms and plan further management. Control endoscopy was conducted at three months and biopsies of the scar, as well as the remaining Barrett’s mucosa or the neosquamocolumnar junction, were taken. If radiofrequency ablation for Barrett’s esophagus was needed and accepted by the patient, radiofrequency ablation eradication protocol was started within six months of ESD. Otherwise, control endoscopies were performed every six months. When necessary, esophageal dilation using Savary-Gilliard bougies was conducted every two weeks until resolution of symptoms.
Study endpoints and definitions
The main study endpoint was the curative resection of carcinoma, defined as R0 resection of a well- or moderately differentiated invasive adenocarcinoma without lymphatic or vascular invasion. In cases with submucosal tumor invasion, curative resection was considered only when submucosal tumor invasion was less than 500 µm (T1Bsm1).
Secondary endpoints were as follows:
‐ Rate of ESD success: ESD was considered as failed when the procedure had to be stopped before complete dissection of the specimen was achieved with the ESD knife.
‐ Rate of en bloc resection, defined by the resection of the whole neoplastic lesion in a single piece.
‐ Rate of R0 resection rate of carcinoma, defined as en bloc resection of an invasive carcinoma with vertical and lateral tumor-free margins and a minimum of 1 mm safety margins.
‐ Rate of curative resection of high-grade dysplasia (HGD) or carcinoma, defined as a histologically complete resection with margins free of carcinoma or HGD.
‐ Rate of curative resection of neoplasia, defined as a histologically complete resection with margins free of carcinoma or any dysplastic tissue.
‐ Rates of complete remission of carcinoma, neoplasia (HGD or carcinoma), dysplasia and intestinal metaplasia at the most recent follow-up.
‐ Early and late complication rates.
‐ 30-day mortality after ESD.
Statistical analysis and ethical aspects
Statistical analyses were performed using GraphPad Software (GraphPad Software Inc, San Diego, CA). Results are expressed as mean (±SD) in case of a normal distribution of variables and median (interquartile range (IQR) 25%–75%) for variables with a skewed distribution, or absolute numbers and percentages. Patient written informed consent was obtained before each endoscopic procedure. The study received approval from our local institutional review board.
Results
Baseline patient characteristics
Thirty-five patients, with a mean age of 66.2 ± 12, had 36 lesions resected between February 2012 and January 2015. Main patient characteristics are presented in Table 1. A total of 11.6% had a history of chronic alcohol abuse and 45.7% a history of tobacco smoking. There were 68.6% of patients who had a major comorbid condition, mainly ischemic cardiomyopathy; 34.3% of patients were using aspirin or clopidogrel, and 5.7% were on anticoagulant therapy. Patients reported heartburn or regurgitations in 45.7% of cases, although they received proton pump inhibitor therapy in 88.6% of cases. The mean number of years since the diagnosis of Barrett’s esophagus was 3.6 ± 5, and 65.7% of patients had been diagnosed during the previous year. A hiatal hernia was present in 45.7%, and the mean (±SD) Barrett’s esophagus segment was C4 ± 3M6 ± 4 within a range of C0M1 to C14M15. Lesions were located in the right anterior, right posterior, left posterior, and right anterior quadrant of the esophagus in 43.8%, 34.4%, 9.4%, and 12.5% of cases, respectively.
Table 1.
Baseline patient characteristics
| n = 35 | |
|---|---|
| Age (mean ± SD), years | 66.2 ± 12 |
| Gender, male, n (%) | 29 (82.9) |
| ASA score (mean ± SD) | 2.1 ± 0.7 |
| Comorbid conditions, n (%) | |
| Cardiomyopathy | 9 (25.7) |
| COPD | 5 (14.3) |
| Liver cirrhosis | 3 (8.6) |
| Body mass index (mean ± SD), kg/m2 | 26.7 ± 5 |
| Anticoagulant or antiplatelet therapy, n (%) | 13 (37.1) |
| Previous therapeutic intervention on the esophagus, n (%) | |
| Endoscopic resection | 10 (28.6) |
| Argon plasma coagulation | 1 (2.9) |
| Radiofrequency ablation | 2 (5.7) |
| Radiotherapy | 1 (2.9) |
| Esophagectomy | 1 (2.9) |
| Barrett’s length (median, IQR), cm | |
| Circumferential extent (C) | 2.5 (1–5.5) |
| Maximal extent (M) | 5 (3–8) |
| Preoperative histology, n (%) | |
| Low grade dysplasia | 1 (2.9) |
| High grade dysplasia | 14 (38.9) |
| Adenocarcinoma | 21 (58.3) |
ASA: American Society of Anesthesiologists; COPD: chronic obstructive pulmonary disease; IQR: interquartile range.
A thoraco-abdomino-pelvic CT scan had been performed prior to ESD in 12 patients; it was unremarkable in nine patients, showed a thickening of the lower esophagus in two patients, regional lymph nodes in one patient, and a concomitant primary lung tumor in one patient. A pre-ESD EUS had been performed in 20 patients; it showed regional lymph nodes in two patients; in one case, the lymph node did not show suspicious features and was not punctured, and in the other case, an EUS-guided fine-needle aspiration was negative.
Procedural characteristics
Thirty-six ESDs were performed, with a 91.7% (33/36) success rate. In two cases, ESD was stopped because of a difficult dissection through submucosal fibrosis, and in one case because of severe hypotension complicating the anesthesia: In all three cases, the endoscopic resection was finally achieved with a snare. Resection specimens (assessed endoscopically) had a mean size of 50.6 ± 22 mm and ranged from 10 to 100 mm; they exceeded the three-quarters of the esophageal circumference for 27.8% (10/36) of the lesions, and were circumferential in 16.7% (6/36) of the cases. The tunnel procedure was performed in 13 (36.1%) cases. Coagrasper hemostatic forceps were used in 81% of the cases, and hemoclips in 11.1%. In three cases, superficial tears in the circular muscle layer, without endoscopic or clinical evidence of perforation, were observed and closed by hemoclips during the procedure. These were not regarded as complications, nor was intraprocedural bleeding of submucosal vessels requiring hemostasis with hemostatic forceps. En bloc resection of the lesion was achieved in 88.9% (32/36) of cases. Triamcinolone acetonide was injected into the residual submucosa for post-ESD stricture prevention in three patients with almost circular mucosal defects deemed at high risk of stricture. Main procedural characteristics are presented in Table 2.
Table 2.
Endoscopic findings and procedural characteristics of the 36 endoscopic submucosal dissections (ESDs) for Barrett’s early neoplasia
| Procedure duration (mean ± SD), mn | 191 ± 79 |
| Size of the endoscopic resection (mean ± SD), mm | 50.6 ± 22 |
| Circumferential extent of the lesion (median, IQR), % of the circumference | 50 (50–77.8) |
| Paris classification of resected lesions, n (%) | n = 22 patients |
| 0–Is | 3 (13.6) |
| 0–IIa | 8 (36.4) |
| 0–IIb | 5 (22.7) |
| 0–IIc | 3 (13.6) |
| 0–IIb + IIc or 0–IIa + IIc | 3 (13.6) |
| ESD knife used, n (%) | n = 33 patients |
| Flush knife 1.5 mm | 18 (54.5) |
| Dual knife 1.5 mm | 10 (30.3) |
| TT knife | 3 (9.1) |
| Flush knife 1 mm | 2 (6.1) |
| Success rate, n (%) | 33 (91.7) |
| En bloc resection, n (%) | 32 (88.9) |
IQR: interquartile range; TT: triangle tip.
Complications
Early procedure-related complications were recorded in four patients (11.1%): Perforation occurred three times and complications of the anesthesia (respiratory distress and vasoplegia) in two cases. One patient had a perforation and respiratory distress in the immediate postoperative course. All three perforations were acute perforations diagnosed during the index endoscopy. They were managed conservatively with endoclips, nil per os, intravenous proton pump inhibitors, broad-spectrum antibiotics, and 24 - to 48-hour surveillance in the intensive care unit. None of the patients developed mediastinitis. Patients were authorized to resume oral intake after a barium swallow had showed no leakage two to three days after the procedure. They stayed in the hospital for a total of six, eight and 10 days. No surgical intervention was required for the management of the complicated cases.
Late complications were recorded in two patients (5.6%) and were esophageal strictures, successfully managed by endoscopic dilation. Of the three patients with triamcinolone local injections, one had an uneventful follow-up, one had to stay overnight in the intensive care unit for early esophageal perforation, and the last eventually developed an esophageal stricture.
When comparing the 16 ESDs performed in our early experience (2012–2013) and the 20 ESDs performed in our late experience (2014), the rate of early complications dropped from 18.8% to 5% (p = 0.3), whereas the rate of late complications remained stable moving from 6.3% to 5% (p = 1). Subgroup analysis of the complications according to the type of ESD knife used showed an early complication rate of 5.9%, 10%, and 33.3% (p = 0.12) and a late complication rate of 0%, 10%, and 33.3% for the 1.5 mm flush knife, 1.5 mm dual knife, and TT knife groups, respectively.
Histological outcomes
The resected specimens contained invasive adenocarcinoma in 80.5% (29/36) of cases, limited to the mucosa (pT1a m) or the superficial third of the submucosa (pT1b sm1) in 82.8% of the cases. On pathological examination, the mean size of the lesion was 12 ± 15 mm, and the lesions ranged from 2 to 90 mm. The R0 resection rate of carcinomas, as defined by the absence of carcinoma in the lateral or vertical margins of the resection with 1 mm safety margins for the lateral margins, was 72.4% (21/29). Vertical margins and lateral margins contained adenocarcinoma in 20.7% (6/29) and 17.2% (5/29) of cases, respectively. Lateral resection margins contained HGD or low-grade dysplasia (LGD) in 2.9% and 17.2% of cases, respectively. Poor histoprognostic factors such as low differentiation of lymphovascular invasion were observed in 13.8% of the cases. As a result, rates of curative resection of carcinoma, HGD or carcinoma, and neoplasia were 65.5%, 51.4%, and 44.5%. Details about the histological outcomes of the ESD are given in Table 3.
Table 3.
Outcomes of the 36 endoscopic submucosal dissections (ESDs) for Barrett’s early neoplasia
| Hospital stay (mean ± SD), days | 2.9 ± 2 |
| Early complications, n (%) | 4 (11.1) |
| Bleeding | 0 (0) |
| Perforation | 3 (8.3) |
| Other (anesthesia-related) | 2 (5.6) |
| Late complications, n (%) | |
| Esophageal stricture | 2 (5.6) |
| 30-Day mortality, n (%) | 0 (0) |
| Size of neoplasia on pathological examination (mean ± SD), mm | 12 ± 15 |
| Histological analysis, n (%) | |
| Low-grade dysplasia | 1 (2.8) |
| High-grade dysplasia | 6 (16.7) |
| Adenocarcinoma | 29 (80.5) |
| Infiltration deptha, n (%) | |
| Mucosa | 23 (79.3) |
| SM1 | 1 (3.4) |
| SM > 1 | 5 (17.2) |
| Other histoprognostic factorsa, n (%) | |
| Well differentiated | 26 (89.7) |
| Moderately differentiated | 2 (6.9) |
| Poorly differentiated | 1 (3.4) |
| Lymphovascular invasion | 3 (10.3) |
| Histological quality of the endoscopic resection, n (%) | |
| R0 resection of carcinoma | 21/29 (72.4) |
| Curative resection of carcinomaa | 19/29 (65.5) |
| Curative resection of high-grade dysplasia | 18/35 (51.4) |
| Curative resection of all neoplasia | 16/36 (44.5) |
For the 29 adenocarcinomas.
Late outcomes
No 30-day mortality was observed. After a mean follow-up of 12.9 ± 9 months, 48.6% (16/35) patients had required additional treatment, among whom nine patients (25.7%) with deep submucosal adenocarcinoma or R1 endoscopic resection underwent surgical esophagogastric resection. Six patients were operated on for positive vertical margins (associated in two cases with deep submucosal tumor infiltration, in one case with deep submucosal tumor infiltration and lymphovascular invasion), one for deep submucosal tumor infiltration associated with poor tumoral differentiation, one for deep submucosal tumor infiltration, and one for positive lateral margins associated with lymphovascular invasion. Among these nine patients operated on, three had no residual tumor (intestinal metaplasia, LGD and HGD), three had residual—or most probably synchronous—intramucosal adenocarcinoma, and three had either N1, T2 or T3 tumors.
Metachronous lesions (3/29) or recurrence of cancer (2/29) developed during the follow-up period in five patients (17.2%), all successfully treated by further endoscopic resection, among which one second ESD. Four patients received ablative therapy, with argon plasma coagulation in one case and radiofrequency ablation in three cases. Among the 26 patients who were not operated on at the end of follow-up, complete remission of carcinoma, HGD, dysplasia and intestinal metaplasia were observed in 100% (26/26), 73.1% (19/26), 57.7% (15/26) and 38.5% (10/26) of cases, respectively. Buried, non-dysplastic Barrett’s glands were seen on follow-up biopsies in three cases, two of which were treated for an adenocarcinoma and one for HGD; one of them had been treated with EMR before ESD.
Discussion
In this work, we sought to determine the effectiveness and safety of ESD for the treatment of large visible lesions arising in Barrett’s esophagus. The R0 resection rate of EAc was 72.4% and the curative resection rate of EAc and HGD/EAc were 65.5% and 51.4%, respectively. ESD appeared feasible, with an 88.9% en bloc resection rate, and resulted in 11.1% early complications and no 30-day mortality. The recurrence rate of EAc was 6.9%. Nine patients ultimately underwent esophagectomy, suggesting that ESD for large Barrett’s neoplasia spared surgical esophageal resection in almost three-quarters of our study patients.
Five studies have specifically studied the outcomes of ESD for Barrett’s neoplasia,1,20–24 most of which took place in Europe. These studies included a total of 262 patients and reported en bloc resection rates from 90 to 100%, R0 resection rates from 64% to 85%, curative resections rates of EAc of 48% to 96.4%, and curative resection rates of HGD/EAc of 38.5% to 64%. Complications included 0 to 6% bleeding, 0 to 10% perforations, and 0 to 60% strictures. Recurrences rates of EAc ranged from 0 to 5.6%. Results can, however, be difficult to compare among studies especially because the outcome parameters are not standardized yet: “Curative resection of EAc” is for instance not always reported, or the “R0 resection” follows several definitions; R0 resection might refer to margins free of cancer or of any kind of dysplastic Barrett’s.20,23,24 Since ESD is primarily aiming at treating cancer, we chose, along with other authors,1,21 to define R0 resection as cancer-free margins. Our figures, as well as those of other teams, are disappointing, especially when compared to the outcomes of ESD in squamous cell carcinoma or adenocarcinomas of the gastric cardia, where R0 resection rates reach 89%–91.9%, in Japan but also in Europe.1,2,8,25 However, ESD is designed for the resection of a visible lesion. Therefore, it is expected to yield optimal results for a unique tumor surrounded by normal mucosa in the upper or mid-esophagus such as a squamous cell neoplasm. In Barrett’s esophagus, dysplasia is often multifocal, extends circumferentially around the esophagogastric junction, and is not always detectable by the eye and state-of-the-art endoscopic techniques: it is then little wonder that ESD margins frequently cut through dysplastic Barrett’s, and that additional treatments of residual Barrett’s esophagus, such as radiofrequency ablation, are often required.
Our data reflect the early experience of a Western center, as demonstrated by the extensive procedure durations and the relatively high complication rate. The R0 resection rate can be explained by the difficult delineation of Barrett’s neoplasia, which might extend laterally further than a non-magnifying endoscope can show, and even under the squamous epithelium as was demonstrated using optical coherence tomography.26,27 Therefore, many authors suggest extending the delineation margins 5 to 10 mm away from the visible lesion.20,24,27 Our choice of only 2–3 mm lateral safety margins was made by analogy with ESD procedures in other organs. It might have accounted for the 17.2% cancer-infiltrated lateral margins we found, even though the mean resection was 51.3 mm for a mean lesion size (on histopathological examination) of 12 mm. This might also suggest that not only lateral margins, but also training for adequate tumor delineation, is critical for ESD to be curative in Barrett’s neoplasia. We resected three protruded Paris 0–Is lesions by ESD in our study: noticeably, two had deep submucosal invasion and were operated on, confirming the high proportion of submucosal invasion for esophageal and gastric 0–Is lesions.28 Preoperative esophageal EUS or CT did not change patient management, as was shown by others.29 The low rates of complete remission of intestinal metaplasia and dysplasia are explained by the absence of eradication of Barrett’s esophagus without HGD after endoscopic resection ESD in our center, and the relatively short follow-up. Breaking down the cases in early and late experience showed that the rate of early complications was divided by three between the 2012–2013 period and the year 2014. Even if this difference was not statistically significant, it should reflect the learning curve in esophageal ESD. The choice of the ESD knife drifted in time toward the use of the 1.5 mm flush knife, and therefore associations between the ESD knife type and complication rates are not interpretable.
The current strategy for the management of Barrett’s early neoplasia relying on the resection of a visible lesion using EMR, by definition suspicious of EAc, followed by the eradication of all remaining Barrett’s mucosa with radiofrequency ablation,30 could theoretically be improved with ESD. Indeed, the R0 resection rate of EAc by EMR, even in expert hands, is as low as 33%,31 and could account for recurrent lesions. However, this strategy, despite frequent R1 or Rx resection, has proven safe, with low complication rates and in particular virtually no esophageal perforations, and effective, with complete remission of neoplasia after four years of follow-up in 96% of cases.30 By comparison, ESD for Barrett’s neoplasia did not reach high R0 resection rates, and was marked by a relatively high number of complications in our study. Furthermore, the flat learning curve, the longer duration of the procedures, and the need for general anesthesia—usually with endotracheal intubation—with ESD plead against this technique as long as clinical or oncological benefit is not clearly established. There is currently no established role for a routine use of ESD in the care of early Barrett’s cancer. However, ESD might be preferable for the resection of lesions larger than 15 mm, pretreated and/or poorly lifting, too bulky to consider resection with a cap-based technique, or suspicious for submucosal invasion, such as Paris 0–IIc lesions. Finally, prospective randomized trials are needed to compare EMR and ESD, starting with those specific indications.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Probst A, Aust D, Märkl B, et al. Early esophageal cancer in Europe: Endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015; 47: 113–121. [DOI] [PubMed] [Google Scholar]
- 2.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: Results from a prospective Western series. Gastrointest Endosc 2010; 71: 715–721. [DOI] [PubMed] [Google Scholar]
- 3.Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: A multicenter retrospective cohort study. Endoscopy 2015; 47: 775–783. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004; 198: 205–211. [DOI] [PubMed] [Google Scholar]
- 5.Peyre CG, DeMeester SR, Rizzetto C, et al. Vagal-sparing esophagectomy: The ideal operation for intramucosal adenocarcinoma and barrett with high-grade dysplasia. Ann Surg 2007; 246: 665–671. discussion 671–674. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014; 260: 259–266. [DOI] [PubMed] [Google Scholar]
- 7.Endo M. Endoscopic resection as local treatment of mucosal cancer of the esophagus. Endoscopy 1993; 25: 672–674. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: A meta-analysis. Dig Dis Sci 2014; 59: 1862–1869. [DOI] [PubMed] [Google Scholar]
- 9.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146: 652–660.e1. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010; 72: 255–264, 264.e1–e2. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008; 68: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 12.Urabe Y, Hiyama T, Tanaka S, et al. Advantages of endoscopic submucosal dissection versus endoscopic oblique aspiration mucosectomy for superficial esophageal tumors. J Gastroenterol Hepatol 2011; 26: 275–280. [DOI] [PubMed] [Google Scholar]
- 13.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006; 64: 877–883. [DOI] [PubMed] [Google Scholar]
- 14.Katada C, Muto M, Momma K, et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae—a multicenter retrospective cohort study. Endoscopy 2007; 39: 779–783. [DOI] [PubMed] [Google Scholar]
- 15.Park CH, Kim EH, Kim HY, et al. Clinical outcomes of endoscopic submucosal dissection for early stage esophagogastric junction cancer: A systematic review and meta-analysis. Dig Liver Dis 2015; 47: 37–44. [DOI] [PubMed] [Google Scholar]
- 16.Tall ML, Salmon D, Diouf E, et al. Aseptic process validation and stability study of an injectable preparation of fructose (5%)-glycerol (10%) as part of a hospital clinical research program on endoscopic curative treatment for early epithelial neoplastic lesions of the gastrointestinal tract [article in French]. Ann Pharm Fr 2015; 73: 139–149. [DOI] [PubMed] [Google Scholar]
- 17.Pioche M, Mais L, Guillaud O, et al. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy 2013; 45: 1032–1034. [DOI] [PubMed] [Google Scholar]
- 18.Hanaoka N, Ishihara R, Takeuchi Y, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: A controlled prospective study. Endoscopy 2012; 44: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 19.Shimoda T. Japanese classification of esophageal cancer, the 10th edition—Pathological part [article in Japanese]. Nihon Rinsho 2011; 69(Suppl 6): 109–120. [PubMed] [Google Scholar]
- 20.Chevaux JB, Piessevaux H, Jouret-Mourin A, et al. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett’s neoplasia. Endoscopy 2015; 47: 103–112. [DOI] [PubMed] [Google Scholar]
- 21.Höbel S, Dautel P, Baumbach R, et al. Single center experience of endoscopic submucosal dissection (ESD) in early Barrett’s adenocarcinoma. Surg Endosc 2015; 29: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 22.Hoteya S, Matsui A, Iizuka T, et al. Comparison of the clinicopathological characteristics and results of endoscopic submucosal dissection for esophagogastric junction and non-junctional cancers. Digestion 2013; 87: 29–33. [DOI] [PubMed] [Google Scholar]
- 23.Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80: 239–245. [DOI] [PubMed] [Google Scholar]
- 24.Neuhaus H, Terheggen G, Rutz EM, et al. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy 2012; 44: 1105–1113. [DOI] [PubMed] [Google Scholar]
- 25.Kakushima N, Yahagi N, Fujishiro M, et al. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy 2006; 38: 170–174. [DOI] [PubMed] [Google Scholar]
- 26.Anders M, Lucks Y, El-Masry MA, et al. Subsquamous extension of intestinal metaplasia is detected in 98% of cases of neoplastic Barrett’s esophagus. Clin Gastroenterol Hepatol 2014; 12: 405–410. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara R, Yamamoto S, Hanaoka N, et al. Endoscopic submucosal dissection for superficial Barrett's esophageal cancer in the Japanese state and perspective. Ann Transl Med 2014; 2: 24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005; 37: 570–578. [DOI] [PubMed] [Google Scholar]
- 29.Pouw RE, Heldoorn N, Alvarez Herrero L, et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc 2011; 73: 662–668. [DOI] [PubMed] [Google Scholar]
- 30.Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: Results of an European multicentre study (EURO-II). Gut. Epub ahead of print 2 March 2015. DOI: 10.1136/gutjnl-2015-309298. [DOI] [PubMed] [Google Scholar]
- 31.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007; 65: 3–10. [DOI] [PubMed] [Google Scholar]