Abstract
Echinococcus multilocularis is a zoonotic tapeworm with a sylvatic lifecycle and an expanding range in Europe. Monitoring efforts following its first identification in 2011 in Sweden have focused on the parasite's definitive host, the red fox (Vulpes vulpes). However, identifying rodent intermediate hosts is important to recognize opportunities for parasite transmission. During 2013–2015, livers from a total of 1566 rodents from four regions in Sweden were examined for E. multilocularis metacestode lesions. Species identity of suspect parasite lesions was confirmed by PCR and sequencing. E. multilocularis positive lesions >6 mm in diameter were also examined histologically. One Microtus agrestis out of 187 (0.5%, 95%CI: 0–2.9%), 8/439 (1.8%, 95%CI: 0.8–3.6%) Arvicola amphibius, 0/655 (0%, 95%CI: 0–0.6%) Myodes glareolus, and 0/285 (0%, 95%CI: 0–1.3%) Apodemus spp. contained E. multilocularis metacestode lesions. Presence of protoscoleces was confirmed in the infected M. agrestis and in three of eight infected A. amphibius. Six of the nine positive rodents were captured from the same field. This is the first report of E. multilocularis in intermediate hosts in Sweden. The cluster of positive rodents in one field shows that local parasite prevalence can be high in Sweden despite overall low national prevalence in foxes (<0.1%). The presence of protoscoleces in infected M. agrestis and A. amphibius indicate these species can serve as competent intermediate hosts in Sweden. However, their relative importance for E. multilocularis transmission in the Swedish environment is not yet possible to assess. In contrast, the negative findings in all M. glareolus and Apodemus spp. suggest that these species are of no importance.
Keywords: Echinococcus multilocularis, Arvicola amphibius, Microtus agrestis, Intermediate host, Sweden, Rodent
Graphical abstract
Highlights
-
•
Overall prevalence of Echinococcus multilocularis in rodents is low in Sweden.
-
•
The distribution of infected rodents was focalized.
-
•
Absence of competent intermediate hosts may limit parasite occurrence.
-
•
Arvicola amphibius and Microtus agrestis are confirmed intermediate hosts in Sweden.
1. Introduction
Echinococcus multilocularis is a tapeworm with a sylvatic lifecycle between canids and rodents. In humans, E. multilocularis causes alveolar echinococcosis, which is a highly fatal disease without treatment (Torgerson et al., 2008). The known geographic range of E. multilocularis has been expanding from its high endemic areas in central Europe in recent decades, and it is now considered an emerging disease throughout Europe (Romig et al., 2006, Torgerson et al., 2010). To better understand risk for human exposure, there is an increased need for understanding of the transmission dynamics of this parasite in the wild. This is particularly true in areas, such as Sweden, where the parasite has only recently been detected in red foxes (Vulpes vulpes) (Osterman Lind et al., 2011).
Although host species vary throughout the parasite's range globally, suitable intermediate hosts in Europe are rodent species mainly within the subfamily Arvicolinae (Eckert and Deplazes, 2004). The importance of a particular rodent species as an intermediate host for E. multilocularis transmission is dependent on such physiological and ecological factors as species susceptibility, species abundance, and predator preferences (Giraudoux et al., 2003). In central Europe, the common vole (Microtus arvalis) and the water vole (Arvicola terrestris) are considered the most important intermediate hosts with the bank vole (Myodes glareolus) of lesser importance (Stieger et al., 2002, Romig et al., 2006). In high endemic areas in Switzerland, prevalence in A. terrestris has been reported up to 39% (11/28 voles examined) and 23% (12/52 voles examined) in M. arvalis (Gottstein et al., 2001). Both M. arvalis and A. terrestris are important prey items of the red fox, the most common definitive host for E. multilocularis in central Europe. During cyclic peaks of A. terrestris in France, the red fox may feed almost exclusively on this species (Viel et al., 1999). Although a generalist predator, some studies have also shown that the red fox prefers to feed on Microtus spp. even in areas of low Microtus spp. densities (Guislain et al., 2008, Raoul et al., 2010). In contrast to M. arvalis and A. terrestris, M. glareolus is not heavily preyed upon by foxes (Dell'Arte et al., 2007, Raoul et al., 2010) and prevalence of E. multilocularis in M. glareolus is usually low even in high endemic areas (Stieger et al., 2002, Hanosset et al., 2008).
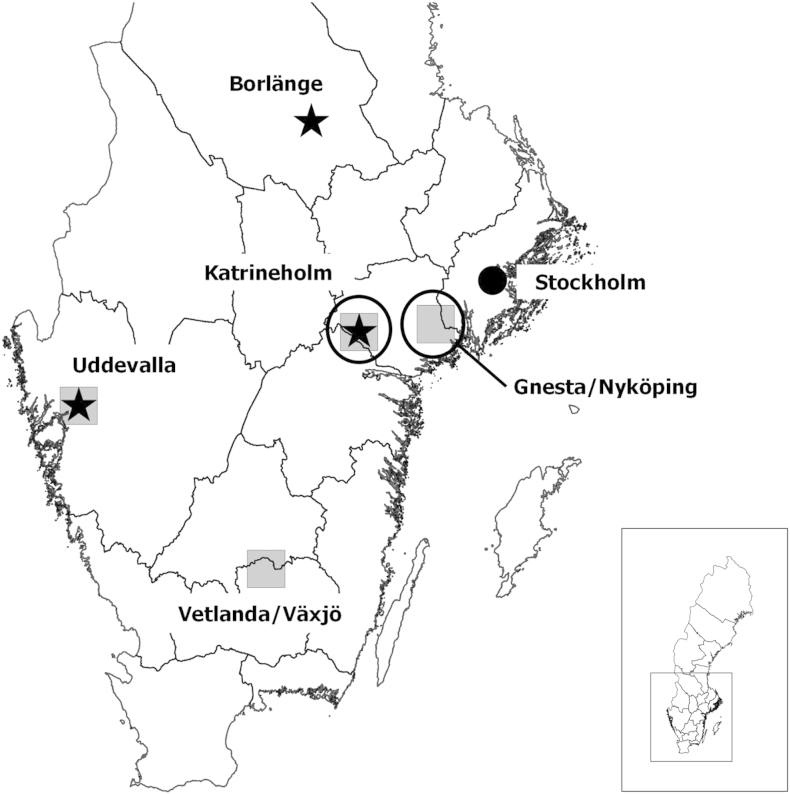
E. multilocularis was first identified in Sweden in a red fox shot December 2010 in the municipality of Uddevalla (Osterman Lind et al., 2011) (Fig. 1). Increased nationwide monitoring during 2011 identified three infected areas in Sweden, and prevalence in foxes on a country level was estimated to be approximately 0.1% (Wahlstrom et al., 2012) (Fig. 1). This low prevalence has remained and is intriguing as in large parts of Sweden conditions for the existence of the parasite are considered to be favorable. These conditions include presence of the red fox and a cool, moist environment ideal for survival of tapeworm eggs (Veit et al., 1995, Eckert and Deplazes, 2004). Nevertheless, the absence of Europe's most important intermediate hosts could be a limiting factor for the presence of the parasite in the Swedish environment. The common vole (M. arvalis) does not exist in Sweden (Wilson and Reeder, 2005). Furthermore, A. terrestris has recently been recognized as two species, Arvicola scherman and Arvicola amphibius (Wilson and Reeder, 2005). Of the two, only A. amphibius exists in Sweden (Wilson and Reeder, 2005). In the absence of M. arvalis and A. scherman, the intermediate hosts most likely to maintain the E. multilocularis lifecycle in Sweden are A. amphibius, Microtus agrestis and M. glareolus (Wahlstrom et al., 2012).
Fig. 1.
Study areas and positive findings of E. multilocularis in southern Sweden at the beginning of the study, 2013. Boxes show study areas and stars indicate where positive foxes/fox fecal samples had been found. Circles encompass the study areas where rodents positive for E. multilocularis were captured. The lines are county boundaries. (CRS: WGS 84, QGIS 2.12.3).
Thus far, there have been no reports of E. multilocularis in intermediate hosts in Sweden. Monitoring of rodents around Uddevalla where the parasite was first detected red foxes, included examination of 236 rodents (mostly A. amphibius) with no positive findings (Wahlstrom et al., 2012). The purpose of this paper is to describe the first findings of E. multilocularis in rodents in Sweden and to discuss their importance for transmission of the parasite in the Swedish ecosystem.
2. Methods
2.1. Field methods
During 2013–2015, rodents were collected from four regions in Sweden (Fig. 1). Two regions within the municipalities of Uddevalla and Katrineholm were sites where E. multilocularis had been identified through national surveillance in foxes in 2011 (Wahlstrom et al., 2012). Two additional regions within the municipalities of Vetlanda/Växjö and Gnesta/Nyköping were part of a National Environmental and Wildlife Monitoring and Assessment program (FoMA, http://www.slu.se/en/environment) where seasonal rodent trapping had been occurring since 2012 and where the E. multilocularis status in foxes was unknown.
Trapping occurred during 2013–2015 over a period of 4–6 weeks in both the spring (April–June) and autumn (September–October) seasons. Due to logistical constraint and to focus trapping in an area with high numbers of positives, only the FoMA sites (Vetlanda/Växjö and Gnesta/Nyköping) were trapped spring 2015. Although every effort was made to use the same trap sites each season, environmental changes (e.g. plowing or forestry practices) sometimes necessitated dropping or moving a site for trapping. Fieldwork was performed with ethical permits from the Swedish Environmental Protection Agency (NV-02939-11) and the Swedish Board of Agriculture (A-135-12).
2.1.1. Snap trapping
Small rodent trapping was performed within an approximately 20 × 20 km square region. Snap trapping design was based on the small quadrat method described in Myllymäki et al. (1971). Briefly, a snap trap site consisted of two to four 15 × 15 m small quadrats placed at least 50 m apart from each other. At the corner of each small quadrat a marking stick was placed and three snap traps (Etutuote Ky, Vaasa, Finland) were laid in the best microhabitat as possible within an approximately 1 m2 area. Traps were baited with hempseed sandwiched between two pieces of beeswax.
The study regions of Uddevalla and Katrineholm were divided into 10–11 4 × 4 km areas that encompassed both forest and field habitats. These areas were designed to simulate a fox territorial home range (von Schantz, 1981) with the idea that rodents captured within these areas would be a reflection of prey availability for one fox. Each 4 × 4 km area contained one to two snap trap sites. In contrast, snap trap sites in the Växjö and Gnesta/Nyköping study regions were set at points previously designated by the wildlife monitoring (FoMA) design. In all study regions, every attempt was made to set snap trap sites along ecotone borders with the purpose to maximize catches of the bank vole (M. glareolus), the field vole (M. agrestis), the wood mouse (Apodemus sylvaticus) and the yellow-necked mouse (Apodemus flavicollis) (Mouse species are hereafter referred to as Apodemus spp.). Although Apodemus spp. were collected both years, only those from 2014 were examined due to the low likelihood for Apodemus spp. to contain E. multilocularis lesions (Stieger et al., 2002) and logistical constraint. All snap traps were kept open for two nights and checked daily. To increase catches, in autumn 2013, some snap trap sites were kept open up to seven nights.
2.1.2. Topcat trapping
Water vole (A. amphibius) fields were defined as fields that contained water vole signs (i.e. tunnels and tumuli). If possible, at least one water vole field was located near the snap trap sites described in Section 2.1.1. A. amphibius in all study areas were captured using topcat traps (Andermatt Biocontrol AG, Grossdietwil, Switzerland). These traps may also capture burrowing M. agrestis. Topcat traps were kept open a minimum of 2 h but not more than 30 h.
2.2. Laboratory methods
2.2.1. Dissection methods and parasite lesion identification
Rodent specimens were frozen in the field at −20 °C. At dissection information collected included species identification, weight, morphometric measurements, and breeding status. Rodents missing a liver (due to scavenging in the field) were excluded from the analysis. Upon dissection, each rodent liver was examined macroscopically for suspect parasite lesions. In most cases, the lesion was taken in its entirety. However, if parasite lesions >6 mm in diameter were present, only small pieces (weighing up to 10 mg) of the lesions were collected. If the liver contained more than three parasite lesions of similar morphology, only three were excised. From all collected lesions, DNA was extracted using the QIAamp® DNA mini kit (Qiagen, Sollentuna, Sweden). Parasite species were then identified using PCR with primers specific for E. multilocularis, Echinococcus granulosus, and Taenia spp. targeting the mitochondrial gene of 12S rRNA (Stieger et al., 2002) or the NADH dehydrogenase subunit 1 (Trachsel et al., 2007). All negative results from the multiplex PCR (Trachsel et al., 2007) were tested using a PCR specific for E. multilocularis (Stieger et al., 2002). Observed bands from either PCR method were purified using the Illustra ExoProStar 1-step kit (VWR International, Stockholm, Sweden) or, in cases where two bands were present, with the QIAquick® Gel Extraction Kit (Qiagen, Sollentuna, Sweden) and sent for sequencing (Macrogen, Amsterdam, The Netherlands). Sequence results were analyzed using the software CLC Main Workbench (CLC Bio, v5.6.1) and submitted for nucleotide BLAST search through the NCBI database. Sequences with ≥95% quality cover and identity were considered to be positive for E. multilocularis. Percentages of rodents positive for E. multilocularis are presented with 95% confidence intervals (95% CI) based on binomial exact calculations performed in R (i386, v 3.2.2).
2.2.2. Protoscolex identification and preparation of histologic sections
Protoscoleces were identified in parasite lesions >6 mm in diameter by examining fluid from freshly cut parasite vesicles by light microscopy, by histologic examination of tissue sections, or by a combination of both methods. For histology, pieces of thawed E. multilocularis parasite lesions >6 mm in diameter were fixed in 10% formalin, routinely processed and embedded in paraffin. Four μm thick sections were cut and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS).
3. Results
3.1. Field and laboratory results
Over the five trapping seasons in 2013–2015, 1702 rodents were collected. Of these, 1566 were examined for E. multilocularis (Table 1). One of 187 (0.5%, 95%CI 0–2.9%) M. agrestis and 8/439 (1.8%, 95% CI 0.8–3.6%) A. amphibius were positive for E. multilocularis (Table 1, Table 2). All 655 M. glareolus (0%, 95%CI 0–0.6%) and 285 Apodemus spp. (0%, 95%CI 0–1.3%) were negative (Table 1, Table 2).
Table 1.
Results of 1566 rodents captured in four different regions in Sweden during 2013–2015 and examined for Echinococcus multilocularis. Number of examined rodents (n), number of positive rodents (N), percent positive (%), and 95% confidence interval (95 CI) are given for each region and for each species.
| Uddevalla |
Katrineholm |
Gnesta/Nyköping |
Vetlanda/Växjö |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % (95 CI) | n | N | % (95 CI) | n | N | % (95 CI) | n | N | % (95 CI) | |
| Arvicola amphibius | 147 | 0 | 0 (≤2.5) | 159 | 3 | 1.9 (0.4–5.4) | 131 | 5 | 3.8 (1.3–8.7) | 2 | 0 | 0 (≤84.2) |
| Microtus agrestis | 60 | 0 | 0 (≤6.0) | 73 | 0 | 0 (≤5.0) | 44 | 1 | 2.3 (0.1–12.0) | 10 | 0 | 0 (≤30.8) |
| Myodes glareolus | 205 | 0 | 0 (≤1.8) | 166 | 0 | 0 (≤2.2) | 124 | 0 | 0 (≤2.9) | 160 | 0 | 0 (≤2.3) |
| Apodemus spp. | 78 | 0 | 0 (≤4.6) | 84 | 0 | 0 (≤4.3) | 36 | 0 | 0 (≤9.7) | 87 | 0 | 0 (≤4.2) |
| TOTAL | 490 | 0 | 482 | 3 | 335 | 6 | 259 | 0 | ||||
Table 2.
Individual description of rodents confirmed Echinococcus multilocularis positive in Sweden from 2013 to 2015.
| Functional group | Breeding statusa | Sexb | Seasonc | Region | Protoscoleces identified | |
|---|---|---|---|---|---|---|
| Microtus agrestis | ||||||
| Adult | B | F | S 2014 | Gnesta/Nyköping | Yes | |
| Arvicola amphibius | ||||||
| Adult | B | F | S 2013 | Gnesta/Nyköping | No | |
| Subadult | NB | M | A 2013 | Katrineholm | No | |
| Adult | B | F | S 2014 | Gnesta/Nyköping | Yes | |
| Adult | B | F | S 2014 | Gnesta/Nyköping | No | |
| Adult | NDd | F | S 2015 | Gnesta/Nyköping | Yes | |
| Adult | B | M | S 2015 | Gnesta/Nyköping | Yes | |
| Subadult | NDd | M | A 2013 | Katrineholm | No | |
| Subadult | NB | F | A 2013 | Katrineholm | No | |
(B) breeding, (NB) non-breeding (ND) not determined.
(F) female, (M) male.
(S) spring, (A) autumn.
Rodent breeding status was not clearly categorizable.
Most (6/9) positive voles (five A. amphibius, one M. agrestis) were captured in the Gnesta/Nyköping region during the spring seasons of 2013–2015 (Table 2). These six positive voles were caught within the same water vole field. During 2013–2014 the proportion of voles positive for E. multilocularis in this field was 3/10 (30%, 95%CI 6.7–65.2%) for A. amphibius and 1/17 (5.9%, 0.1–28.7%) for M. agrestis. In 2015, the proportion of A. amphibius positive for E. multilocularis collected in this field was 2/43 (4.7%, 0.6–15.8%) and for M. agrestis 0/1 (0%, 95%CI 0–97.5%).
3.2. Macroscopic and histologic parasite description of metacestode lesions
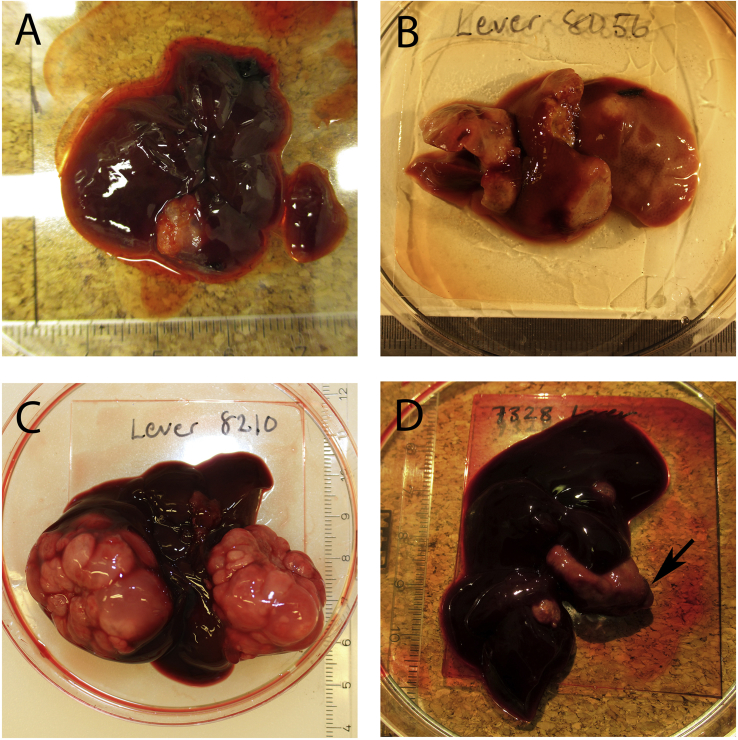
Three of the nine positive rodent livers (three A. amphibius) contained metacestode lesions of ≤3 mm in diameter. The six remaining positive rodents contained livers with one or multiple metacestode lesions that in some cases invaded half of the normal liver tissue (Fig. 2). Four of these six rodent livers (one M. agrestis, three A. amphibius) contained protoscoleces. The protoscoleces were present in soft, fluid filled vesicles (Fig. 2, A and C). Of the remaining two positive rodent livers, one A. amphibius liver contained multiple hard metacestode lesions with no fluid and no protoscoleces (Fig. 2, B). One A. amphibius liver contained two metacestode lesions ≤6 mm in diameter, and one metacestode lesion >6 mm in diameter with no fluid and with no protoscoleces (Fig. 2, D).
Fig. 2.
Macroscopic photos of rodent livers containing Echinococcus multilocularis metacestode lesions. The ruler in each picture is in millimeters. (A) Liver from Microtus agrestis with one lesion that contained protoscoleces. (B) Liver from Arvicola amphibius with multiple lesions that did not contain protoscoleces. (C) Liver from Arvicola amphibius with multiple lesions that did contain protoscoleces. (D) Liver from Arvicola amphibius. Arrow points to the only lesion examined for protoscoleces, which were absent.
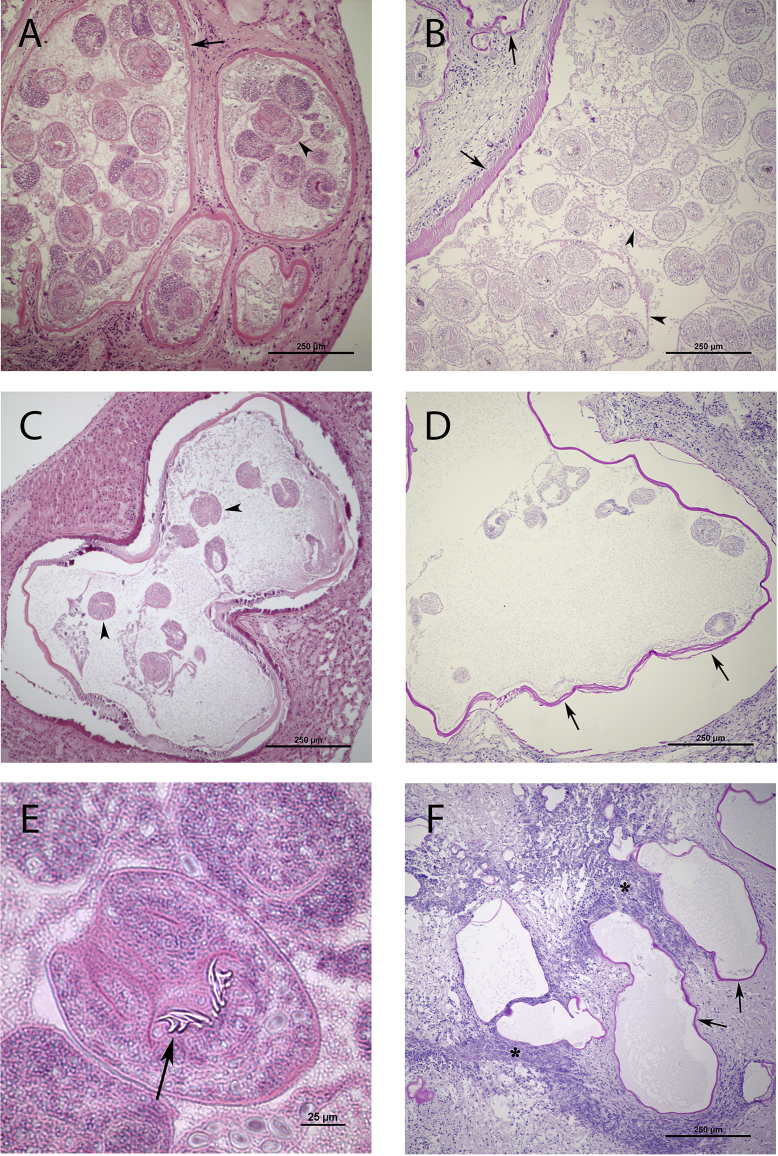
In liver sections from four rodents (one M. agrestis, three A. amphibius) large cyst-like vesicles outlined by an eosinophilic homogenous to laminated PAS-positive wall were present (Fig. 3, A–D). Multifocal remnants of a germinative epithelium were detected along vesicle walls in some sections. Basophilic ovals, consistent with calcareous corpuscles, and varying numbers of protoscoleces were detected within vesicular lumina in sections from all four animals. Protoscoleces were sometimes grouped in brood capsules with individual protoscoleces measuring approximately 80–180 μm in cross section (Fig. 3, B). Rostrellar hooks were often identified (Fig. 3, E). Lesions were surrounded by a fibrous capsule and varying amount of inflammatory cells, including granulocytes, lymphocytes, plasma cells, macrophages and multinucleated giant cells.
Fig. 3.
Photomicrographs of liver sections. Sections in A, C, and E are stained with hematoxylin and eosin (H&E) and sections in B, D, and F are stained with periodic acid-Schiff (PAS). (A) Section from Arvicola amphibius with multiple fluid filled parasite vesicles outlined by an eosinophilic wall (black arrow) containing large numbers of protoscoleces (arrow head). (B) Section from Arvicola amphibius with fluid filled parasite vesicles outlined by PAS-positive laminar layer (arrows). Protoscoleces are organized in places into brood capsules (lining wall indicated by arrow heads). (C) Fluid-filled parasite vesicle with protoscoleces (arrow heads) in a Microtus agrestis. (D) Fluid filled parasite vesicle outlined by a PAS-positive laminar layer (arrows) in a Microtus agrestis. Protoscoleces are seen within the vesicle. (E) Protoscolex with rostrellar hooks (arrow). (F) Numerous empty variably sized parasite vesicles outlined by a PAS-positive laminar layer (arrows), multifocally surrounded by dense inflammatory infiltrates (asteriks). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Smaller vesicular lesions with empty lumina were seen in sections from the remaining two water voles and in some sections from the voles with protoscolex-containing vesicles. These smaller lesions were typically surrounded by a severe granulocytic inflammatory reaction, fibrosis and areas of necrosis (Fig. 3, F).
4. Discussion
These findings are the first records of E. multilocularis in rodent intermediate hosts in Sweden. Importantly, nearly all (6/9) positive rodents were captured in one field from an area previously unknown to have the parasite. The morphologic and histologic observations described here are consistent with descriptions of E. multilocularis lesions in rodent species from previous studies (Houin et al., 1982, Barabási et al., 2011, Liccioli et al., 2013). The identification of protoscoleces in M. agrestis (1/1) and A. amphibius (3/8) show that these rodent species are competent intermediate hosts for E. multilocularis in Sweden.
Although prevalence in M. glareolus has been reported as high as 10.3% (6/58) (Reperant et al., 2009), prevalence in central Europe has generally been low with reports by Hanosset et al. (2008) of 4.3% (1/23) and by Stieger et al. (2002) of 2.4% (2/83). Experimentally, M. glareolus has also shown a limited capacity for infection with <5% animals developing cysts two months after inoculation (Woolsey, 2015). Reports of infected wild Apodemus spp. are very few and are often limited to single reports, such as the infection of one Apodemus agrarius in Romania (Barabási et al., 2011). Even in a high endemic area of Switzerland, the prevalence of E. multilocularis in Apodemus spp. was 0% (0/154) (Stieger et al., 2002). In a low endemic area, such as Sweden, the probability to find infected M. glareolus and Apodemus spp. would likely be low. Therefore, the negative findings in all of the M. glareolus and Apodemus spp. in this study were not unexpected.
The significance of M. glareolus and Apodemus spp. to the transmission of E. multilocularis depends not only on susceptibility to infection but also rates of fox predation. According to a fox fecal analysis performed in central Sweden, fox predation on M. glareolus was almost half that of M. agrestis (Lindström, 1982). In addition, the presence of Apodemus spp. in a fox fecal analysis in southern Sweden was negligible (Erlinge et al., 1983). This, together with our negative findings in these species, suggest that M. glareolus and Apodemus spp. are of no importance for E. multilocularis transmission in the Swedish environment.
The majority of our positive findings were identified in the water vole, A. amphibius. Due to reclassification of species within the Arvicola genus, many studies on E. multilocularis report all examined water voles as A. terrestris. However, A. terrestris has been separated into two species, A. scherman and A. amphibius (Wilson and Reeder, 2005). A. scherman is mainly a fossorial vole with a range limited to central Europe (Wilson and Reeder, 2005, Piras et al., 2012). Investigations in France suggest that population peaks of the fossorial form (A. scherman) induce prey specialization by foxes and thereby increase the prevalence of the parasite in the fox (Viel et al., 1999, Giraudoux et al., 2003). In contrast, A. amphibius is a semi-aquatic vole with a much wider distribution which also includes Sweden (Wilson and Reeder, 2005, Piras et al., 2012). It is unclear if the same prey specialization occurs with A. amphibius, a vole of differing ecology (Piras et al., 2012).
Although prevalence in Arvicola spp. has been reported as high as 41–79% in localized areas of Europe (Burlet et al., 2011), the potential infectivity of these water voles may be much lower. In a study by Hofer et al. (2000) only 2/19 Arvicola spp. positive for E. multilocularis had protoscoleces (i.e. were infectious to the definitive host). Similar figures were reported by Reperant et al. (2009) where 2/31 positive Arvicola spp. contained protoscoleces, and Stieger et al. (2002) where 26/81 positive Arvicola spp. contained protoscoleces. Although the age of the rodent and size of the metacestode lesions may explain some of this variation, it is likely that many of these rodents contained non-infectious metacestode lesions. Burlet et al. (2011) demonstrated that Arvicola spp. in Switzerland were unlikely to have E. multilocularis lesions containing mature protoscoleces until the rodents were at least three months of age. Furthermore, only metacestode lesions >3 mm in diameter contained protoscoleces (Burlet et al., 2011). In our study, 3/8 positive A. amphibius contained infectious metacestode lesions. Of the five rodents with non-infectious metacestode lesions, two met the age and lesion size limitations outlined by Burlet et al. (2011). It is possible that sampling method may have affected outcome in one of these two rodents, as two lesions ≤6 mm in diameter were taken for PCR (Fig. 2, D). Our findings, particularly with respect to those in Switzerland, suggest a lesser importance for A. amphibius in the transmission of E. multilocularis in Sweden. To better address this question, there is a need for a laboratory study to investigate the growth and development of E. multilocularis metacestode lesions in Arvicola spp. In addition, the extent to which Swedish foxes prey on A. amphibius needs to be determined.
While few studies of rodent intermediate hosts differentiate between Microtus spp., in those that do, M. arvalis seems to be more common. For instance, Hanosset et al. (2008) reports a capture of 914 M. arvalis and only 39 M. agrestis. These results may reflect interspecific competition in areas where both species coexist, and where it is known that M. arvalis outcompete M. agrestis for grassland/open field habitats (Myllymäki, 1977). These habitats can be associated with high levels of fox fecal egg contamination if infected foxes are attracted to prey on abundant grassland rodents (Viel et al., 1999, Giraudoux et al., 2003). Because M. arvalis does not exist in Sweden, M agrestis inhabits the open fields and grassland habitat in southern Sweden where E. multilocularis is found (Hansson, 1971, Wahlstrom et al., 2012). Our findings show that M. agrestis can contribute to parasite transmission. It is possible that M. agrestis could fulfill the role that M. arvalis has as a major intermediate host in central Europe. However, we can make no conclusion about this with only one positive finding.
Recent laboratory studies (Woolsey et al., 2015a, Woolsey et al., 2015b) have demonstrated susceptibility of both M. agrestis and M. arvalis to E. multilocularis. Following infection with parasite eggs, metacestode development differed with M. arvalis creating larger and more numerous metacestode lesions than M. agrestis at the same age time point (Woolsey et al., 2015b). Although protoscolex production was not examined in M. agrestis, Woolsey et al. (2015b) speculates that the smaller metacestode lesion size (as compared to M. arvalis) would reduce the potential for protoscolex production and limit the transmission potential of M. agrestis. Such a limitation could be of massive importance in environments such as in Sweden where M. arvalis is absent. Indeed, at one of the southernmost border of the E. multilocularis range in the Ticino canton of Switzerland, Guerra et al. (2014) observed an overlap between the presence of M. arvalis and E. multilocularis. These authors noted that the occurrence of E. multilocularis infected foxes was limited to the range of M. arvalis—despite the presence of other Microtus spp. (albeit not M. agrestis).
The presence of 6/9 positive rodents in the same field further demonstrates the heterogeneous spatial distribution of E. multilocularis (Hansen et al., 2004) and the potential for prevalence in rodent populations in small areas to be quite high (Gottstein et al., 2001, Burlet et al., 2011). The local prevalence of 30% (95%CI 6.7–65.2%) found in A. amphibius reflects an area with an aggregation of E. multilocularis eggs from fox feces during 2013–2014 that may also reflect a particular risk for human exposure (Miller et al., unpublished). Although the sample size for 2013–2014 was low (n = 10), repeated sampling in 2015 (n = 43, A. amphibius) demonstrated the continued presence of E. multilocularis in this field (4.7%, 95%CI 0.6–15.8%). While the fox prevalence in Sweden is presumed to be very low (≤1%) on a nationwide level, these findings indicate that local contamination of E. multilocularis eggs in the Swedish environment will vary.
5. Conclusions
Our findings of infected A. amphibius and M. agrestis with protoscoleces show that these rodents can act as suitable intermediate hosts for E. multilocularis in Sweden. The identification of these intermediate hosts increases the knowledge about the lifecycle of the parasite in Sweden and thereby the possibilities in the future to predict areas where a higher prevalence of E. multilocularis could be expected.
Acknowledgments
This work is funded through an EU Formas grant (EMIDA-ERA NET) for a project entitled “Echinococcus Multilocularis in ROdents (EMIRO)” (221-2011-2212). The samples from Vetlanda/Växjö and Gnesta/Nyköping were mainly collected within the National Environmental and Wildlife Monitoring and Assessment program (FoMA, http://www.slu.se/en/environment). The authors thank Miloš Anděra for providing the rodent pictures used in the graphical abstract. We also thank the local landowners that put their land at our disposal and the students that helped complete fieldwork.
References
- Barabási S.S., Marosfői L., Barabási Z.S., Cozma V. Natural alveolar echinococcosis with Echinococcus multilocularis in wild rodents. Sci. Parasitol. 2011;12:11–21. [Google Scholar]
- Burlet P., Deplazes P., Hegglin D. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris. Parasit. Vectors. 2011;4:6. doi: 10.1186/1756-3305-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Arte G.L., Laaksonen T., Norrdahl K., Korpimäki E. Variation in the diet composition of a generalist predator, the red fox, in relation to season and density of main prey. Acta Oecol. 2007;31:276–281. [Google Scholar]
- Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge S., Göransson G., Hansson L., Högstedt G., Liberg O., Nilsson I.N., Nilsson T., von Schantz T., Sylvén M. Predation as a regulating factor on small rodent populations in southern Sweden. Oikos. 1983;40:36–52. [Google Scholar]
- Giraudoux P., Craig P.S., Delattre P., Bao G., Bartholomot B., Harraga S., Quéré J.-P., Raoul F., Wang Y., Shi D., Vuitton D.-A. Interactions between landscape changes and host communities can regulate Echinococcus multilocularis transmission. Parasitology. 2003;127:S121–S131. [PubMed] [Google Scholar]
- Gottstein B., Saucy F., Deplazes P., Reichen J., Demierre G., Busato A., Zuercher C., Pugin P. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg. Infect. Dis. 2001;7:408–412. doi: 10.3201/eid0703.010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra D., Hegglin D., Bacciarini L., Schnyder M., Deplazes P. Stability of the southern European border of Echinococcus multilocularis in the Alps: evidence that Microtus arvalis is a limiting factor. Parasitology. 2014;141:1593–1602. doi: 10.1017/S0031182014000730. [DOI] [PubMed] [Google Scholar]
- Guislain M.-H., Raoul F., Giraudoux P., Terrier M.-E., Froment G., Ferté H., Poulle M.-L. Ecological and biological factors involved in the transmission of Echinococcus multilocularis in the French Ardennes. J. Helminthol. 2008;82:143–151. doi: 10.1017/S0022149X08912384. [DOI] [PubMed] [Google Scholar]
- Hanosset R., Saegerman C., Adant S., Massart L., Losson B. Echinococcus multilocularis in Belgium: prevalence in red foxes (Vulpes vulpes) and in different species of potential intermediate hosts. Vet. Parasitol. 2008;151:212–217. doi: 10.1016/j.vetpar.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Hansen F., Jeltsch F., Tackmann K., Staubach C., Thulke H.H. Processes leading to a spatial aggregation of Echinococcus multilocularis in its natural intermediate host Microtus arvalis. Int. J. Parasitol. 2004;34:37–44. doi: 10.1016/j.ijpara.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hansson L. Habitat, food and population dynamics of the field vole Microtus agrestis (L.) in south Sweden. Viltrevy. 1971;8:267–378. [Google Scholar]
- Hofer S., Gloor S., Müller U., Mathis A., Hegglin D., Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zürich. Switz. Parasitol. 2000;120:135–142. doi: 10.1017/s0031182099005351. [DOI] [PubMed] [Google Scholar]
- Houin R., Deniau M., Liance M., Puel F. Arvicola terrestris an intermediate host of Echinococcus multilocularis in France: epidemiological consequences. Int. J. Parasitol. 1982;12:593–600. doi: 10.1016/0020-7519(82)90058-3. [DOI] [PubMed] [Google Scholar]
- Liccioli S., Duignan P.J., Lejeune M., Deunk J., Majid S., Massolo A. A new intermediate host for Echinococcus multilocularis: the southern red-backed vole (Myodes gapperi) in urban landscape in Calgary, Canada. Parasitol. Int. 2013;62:355–357. doi: 10.1016/j.parint.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Lindström E. Department of Zoology, University of Stockholm; Stockholm, Sweden: 1982. Population Ecology of the Red Fox (Vulpes Vuples L.) in Relation to Food Supply; p. 154. PhD Thesis. [Google Scholar]
- Myllymäki A. Interactions between the field vole Microtus agrestis and its microtine competitors in Central-Scandinavian populations. Oikos. 1977;29:570–580. [Google Scholar]
- Myllymäki A., Paasikallio A., Pankakoski E., Kanervo V. Removal experiments on small quadrats as a means of rapid assessment of the abundance of small mammals. Ann. Zool. Fenn. 1971;8:177–185. [Google Scholar]
- Osterman Lind, E., Juremalm, M., Christensson, D., Widgren, S., Hallgren, G., Ågren, E.O., Uhlhorn, H., Lindberg, A., Cedersmyg, M., Wahlström, H., First detection of Echinococcus multilocularis in Sweden, February to March 2011. Euro Surveill. 16 pii19836, available online. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19836 [PubMed]
- Piras P., Marcolini F., Claude J., Ventura J., Kotsakis T., Cubo J. Ecological and functional correlates of molar shape variation in European populations of Arvicola (Arvicolinae, Rodentia) Zool. Anz. 2012;251:335–343. [Google Scholar]
- Raoul F., Deplazes P., Rieffel D., Lambert J.-C., Giraudoux P. Predator dietary response to prey density variation and consequences for cestode transmission. Oecologia. 2010;164:129–139. doi: 10.1007/s00442-010-1647-8. [DOI] [PubMed] [Google Scholar]
- Reperant L.A., Hegglin D., Tanner I., Fischer C., Deplazes P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology. 2009;136:329–337. doi: 10.1017/S0031182008005428. [DOI] [PubMed] [Google Scholar]
- Romig T., Dinkel A., Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol. Int. 2006;55:S187–S191. doi: 10.1016/j.parint.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Stieger C., Hegglin D., Schwarzenbach G., Mathis A., Deplazes P. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology. 2002;124:631–640. doi: 10.1017/s0031182002001749. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Keller K., Magnotta M., Ragland N. The global burden of alveolar echinococcosis. PLoS Negl. Trop. Dis. 2010;4:e722. doi: 10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson P.R., Schweiger A., Deplazes P., Pohar M., Reichen J., Ammann R.W., Tarr P.E., Halkik N., Müllhaupt B. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008;49:72–77. doi: 10.1016/j.jhep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- von Schantz T. Female cooperation, male competition, and dispersal in the red fox Vulpes vulpes. Oikos. 1981;37:63–68. [Google Scholar]
- Veit P., Bilger B., Schad V., Schäfer J., Frank W., Lucius R. Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology. 1995;110(Pt 1):79–86. doi: 10.1017/s0031182000081075. [DOI] [PubMed] [Google Scholar]
- Viel J.-F., Giraudoux P., Abrial V., Bresson-Hadni S. Water vole (Arvicola terrestris scherman) density as risk factor for human alveolar echinococcosis. Am. J. Trop. Med. Hyg. 1999;61:559–565. doi: 10.4269/ajtmh.1999.61.559. [DOI] [PubMed] [Google Scholar]
- Wahlstrom H., Lindberg A., Lindh J., Wallensten A., Lindqvist R., Plym-Forshell L., Osterman Lind E., Agren E.O., Widgren S., Carlsson U., Christensson D., Cedersmyg M., Lindström E., Olsson G.E., Hörnfeldt B., Barragan A., Davelid C., Hjertqvist M., Elvander M. Investigations and actions taken during 2011 due to the first finding of Echinococcus multilocularis in Sweden. Eurosurveillance. 2012;17 doi: 10.2807/ese.17.28.20215-en. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20215 pii=20215, available online. [DOI] [PubMed] [Google Scholar]
- Wilson D.E., Reeder D.M., editors. Mammal Species of the World: a Taxonomic and Geographic Reference. third ed. Johns Hopkins University Press; Baltimore, Maryland: 2005. p. 2142.http://vertebrates.si.edu/msw/mswCFApp/msw/index.cfm Online database from Smithsonian National Museum of Natural History. Accessed Jan. 26, 2016. [Google Scholar]
- Woolsey I.D. Faculty of Science, University of Copenhagen; Copenhagen, Denmark: 2015. Experimental Echinococcus multilocularis Infection in Intermedate Hosts; p. 118. PhD Thesis. [Google Scholar]
- Woolsey I.D., Bune N.E.T., Jensen P.M., Deplazes P., Kapel C.M.O. Echinococcus multilocularis infection in the field vole (Microtus agrestis): an ecological model for studies on transmission dynamics. Parasitol. Res. 2015;114:1703–1709. doi: 10.1007/s00436-015-4355-9. [DOI] [PubMed] [Google Scholar]
- Woolsey I.D., Jensen P.M., Deplazes P., Kapel C.M.O. Establishment and development of Echinococcus multilocularis metacestodes in the common vole (Microtus arvalis) after oral inoculation with parasite eggs. Parasitol. Int. 2015;64:571–575. doi: 10.1016/j.parint.2015.08.006. [DOI] [PubMed] [Google Scholar]