Abstract
When adding peroxide (H2O2), β subunits of hemoglobin (Hb) bear the burden of oxidative changes due in part to the direct oxidation of its Cys93. The presence of unpaired α subunits within red cells and/or co-inheritance of another β subunit mutant, HbE (β26 Glu→Lys) have been implicated in the pathogenesis and severity of β thalassemia. We have found that although both HbA and HbE autoxidize at initially comparable rates, HbE loses heme at a rate almost 2 fold higher than HbA due to unfolding of the protein. Using mass spectrometry and the spin trap, DMPO, we were able to quantify irreversible oxidization of βCys93 to reflect oxidative instability of β subunits. In the presence of free α subunits and H2O2, both HbA and HbE showed βCys93 oxidation which increased with higher H2O2 concentrations. In the presence of Alpha-hemoglobin stabilizing protein (AHSP), which stabilizes the α-subunit in a redox inactive hexacoordinate conformation (thus unable to undergo the redox ferric/ferryl transition), Cys93 oxidation was substantially reduced in both proteins. These experiments establish two important features that may have relevance to the mechanistic understanding of these two inherited hemoglobinopathies, i.e. HbE/β thalassemia: First, a persistent ferryl/ferryl radical in HbE is more damaging to its own β subunit (i.e., βCys93) than HbA. Secondly, in the presence of excess free α-subunit and under the same oxidative conditions, these events are substantially increased for HbE compared to HbA, and may therefore create an oxidative milieu affecting the already unstable HbE.
Keywords: Hemoglobin E, Thalassemia, Oxidation, Alpha-hemoglobin stabilizing protein
Graphical abstract

Highlights
-
•
A compromised redox ferric/ferryl cycle promotes oxidative instability in hemoglobin E (HbE).
-
•
The presence of unmatched alpha subunits aggravates oxidative instability of HbE.
-
•
Alpha-hemoglobin stabilizing protein (AHSP) reverses alpha subunit destabilizing effects on HbE.
1. Introduction
The hemoglobin (Hb) tetramer consists of two pairs of α-globin and β-globin subunits with one heme in each globin subunit. The redox active heme/iron, forming the Hb oxygen (O2) binding site, undergoes spontaneous and chemically induced oxidation reactions. These events are well controlled under the reductive environment of normal red blood cells (RBCs). However, oxidation reactions and the turnover of oxidation intermediates of cell-free Hb in a number of chemically/genetically modified Hbs have been the subject of intense investigations in recent years as these oxidative pathways have been shown to contribute to the pathophysiology associated with the use of Hb as oxygen therapeutics and in some hemoglobinopathies [1], [2], [3].
Oxidants such as hydrogen peroxide (H2O2) drive a catalytic pseudoperoxidase cycle that includes the formation of a transient oxyferryl Hb, when the reaction starts with ferrous Hb. The ferryl species autoreduces to ferric iron (Fe3+) and in the presence of additional H2O2, ferryl iron (Fe4+) is regenerated back in a classic cycle reported for both Hb and myoglobin (See Eqs (1), (3)) [4]. When H2O2 reacts with the ferric form of Hb, a protein radical is formed, but unlike true peroxidases this “unharnessed” radical is escaped through βCys93 [5]. Both the ferryl heme and its associated protein cation radical induce oxidative reactions affecting the protein itself and other biological molecules due to their high midpoint redox potentials, (E°1/2~1.0 V) [3]. These internal reactions result in the modification of heme, its subsequent attachment to nearby amino acids, and the irreversible oxidation of amino acids in oxidation “hotspot”, particularly the β Cys93 side-chain leading to a collapse of β subunits [5]. Experimental evidence from animal studies supports the notion that these oxidative activities of Hb occur in vivo with some potentially serious consequences [6], [7].
| HbFe2+O2+H2O2→HbFe4+=O+H2O+O2 | (1) |
| (2) |
| HbFe3++H2O2→·HbFe4+=O+H2O | (3) |
Hb synthesis in erythroid progenitor cells is well controlled in order to minimize the accumulation of free α-or β-Hb subunits, which are cytotoxic. β-thalassemia is an autosomal recessive inherited disease causing anemia of variable degrees of severity in Southeast Asia and the Mediterranean regions where in some cases malaria is or has been endemic [8]. The molecular defects are due to point mutations or small deletions within the chromosome 11 β-globin gene (or immediate flanking regions of the Hb β gene) leading to reduction or absence of β-globin chain synthesis. In the latter scenario, unmatched α-Hb (because of its oxidative instability) is particularly damaging to itself and other cellular proteins, lipids, and nucleic acids [9], [10]. This results in a short half-life for circulating RBCs and also impairs the viability of erythroid precursors in hematopoietic tissues, causing ineffective erythropoiesis [11].
Hemoglobin E (HbE; 26Glu→Lys), is a common human Hb variant, synthesized at a slightly reduced rate. Although HbE was first identified in the 1950s, there are still uncertainties regarding its pathophysiology [12]. When it is inherited together with a β-thalassemia allele, the resulting condition, HbE/β-thalassemia, is often severe requiring a transfusion. HbE oxidative instability may contribute to the severity and variability in HbE/β-thalassemia [13]. The oxidative stress resulting from free α-chains within the HbE/β-thalassemic RBC has been implicated in events that result in HbE degradation and membrane damage [9], [14], [15]. In a study involving 240 HbE/β thalassemia patients, those inheriting an α deletion and/or point mutation were found to have mild symptoms, while patients with α triplication had severe phenotypes requiring frequent transfusion. These observations seem to suggest that the α globin levels are an important modifier of HbE/β thalassemia [16]. This is further supported by a mouse knock out study comparing HbE knock out (KO) mice with HbE mice; the KO mice lacked an abundance of α subunits that would approximate a mouse thalassemia thereby supporting a model where HbE instability is exacerbated by free α chains [17].
Overall HbE is structurally similar to HbA; however crystal structural studies showed that, while intersubunit contacts remain the same as those of HbA, the E26 K substitution results in the loss of interactions required for optimal stabilized tertiary structure, particularly at higher temperatures [18]. Recent structural and functional comparisons between HbE and HbA (using high resolution x-ray crystallography, spectroscopy, and solution phase circular dichroism) revealed substantial differences in nitrite reductase activity and l-Cys-mediated reduction of metHb both in the T and R states of HbE and HbA. These findings are consistent with the HbE mutation causing an increase in the redox potential of both the T and R states for this Hb [19].
α-Hemoglobin stabilizing protein (AHSP) is an erythroid scavenger protein (primarily expressed during erythropoiesis) that rapidly and reversibly binds to monomeric forms of the α subunit. AHSP binds in a 1:1 stoichiometry and has been shown to modulate heme iron oxidation and subunit folding [20], [21]. The affinity of AHSP is dependent on the oxidation state; the rate of ferric α dissociation from AHSP is dramatically slower than that for the ferrous α subunit [21]. Recent mechanistic investigations of isolated α and β subunits with H2O2 revealed that met-β subunits are oxidized more prominently to form the ferryl heme species and protein-based radicals than met-α subunits. Furthermore it was shown that AHSP binding renders met-α Hb nearly inert to oxidative degradation by H2O2, with no ferryl heme species or protein-based radicals detected by either optical absorbance or EPR. The AHSP binding dramatically lowers the redox potential of α subunit to a much more negative value, and thermodynamically favors the ferric over ferrous iron [21].
In this investigation, both HbE and HbA were contrasted in their autooxidation reactions, and heme loss kinetics. Although both HbE and HbA autooxidized initially at the same rate, HbE loses heme more rapidly due to unfolding of the protein as the reaction proceed to longer time periods. We also sought to determine the precise role of free α subunits in destabilizing HbE and HbA in solution under oxidative conditions. This however is experimentally challenging as α subunits, will exist at any given time as α dimers or complexed with β subunits to form tetramers of either HbE or HbA. We developed a mass spectrometry method that specifically quantifies the levels of irreversible oxidation of hotspot residues including βCys93 in the presence of H2O2 in a reaction mixture containing either HbE or HbA. Addition of free α subunits to HbA or HbE in the presence of H2O2 resulted in a dose dependent increase in the relative level of βCys93 oxidation in both proteins. We also show that AHSP when complexed with α subunits resulted in a complete reversal of oxidative damage mediated by ferryl Hb.
2. Experimental procedure
2.1. Protein purification and handling
Fresh blood for HbA preparation was obtained by patient consent from the Division of Transfusion Medicine, National Institutes of Health. Human HbA was purified using established methods [22]. Specifically, HbA was purified using an XK50/100 column containing Superdex 200 medium on an AKTA FPLC system to remove erythrocyte catalase [23]. Catalase activity assays were performed on Hb samples to confirm the complete removal of catalase [24]. Human HbE was purified from red blood cells obtained from transgenic mice expressing human HbE as described earlier [25]. Human HbE purified from transgenic mouse RBC contains solely HbE, lacking contamination from HbA2 as occurs in human red blood cells. Since HbA2 and HbE have a very similar isoelectric point, separation is not feasible, hence the advantage of human HbE expressed in the transgenic full KO mouse. The criteria of purity of both proteins were verified by isoelectric focusing and HPLC. The molar extinction coefficients used to calculate Hb concentrations in heme equivalents in potassium phosphate buffer were 15.15 mM−1 cm−1 at 576 nm for HbO2; 14.95 mM−1 cm−1 at 568.5 nm for HbCO in heme equivalents; 4.4 mM−1 cm−1 at 630 nm for aquomet Hb in heme equivalents; and 43.6 M−1 cm−1 at 240 nm for H2O2; 24 mM−1 cm−1 at 620 nm for sulfheme Hb in heme equivalents [26], [27], [28]. Both α and β subunits were isolated using established methods [29]. AHSP used in this study was a gift from Dr. Mitchell Weiss (Department of Hematology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA). In the case of HbA and α/β subunits, previously determined extinction coefficients were used to determine protein concentrations in heme equivalents. The AHSP extinction coefficient utilized was 11,460 M−1 cm−1 at 280 nm [29].
2.2. Spectrophotometry
The comparative oxidative stability of HbA and HbE were examined using an Agilent 8453 UV–visible light optical absorbance spectrophotometer (Agilent, Santa Clara, California, US). All spectrophotometric studies listed below were performed with 65 µM Hb (heme equivalents) in 20 mM potassium phosphate buffer, pH 7.4 at 37 °C. In the first set of experiments, spectral scans in the range of 450–700 were recorded every 2 min for 24 h to monitor the impact of autooxidation (with and without catalase) on HbA and HbE denaturation and precipitation. Specifically, any light scattering associated with precipitation (and changes in turbidity) was identified by increases in absorbance at 700 nm; importantly oxyHb, metHb and hemichrome have little absorbance at this wavelength.
A second set of experiments required multi-wavelength analysis to calculate accurate autooxidation rate constants due to complications associated with HbE precipitation. Specifically, data representing the absorbance at 540 nm, 560 nm, 576 nm, 630 nm and 700 nm were recorded every 5 min for 24 h. The concentration and percentage of oxyHb (ferrous) were calculated using multicomponent analysis as previously described [30]. Data for the autooxidation experiments were normalized to total absorbance signal changes, considering the 8th hour as maximum amount of MetHb formation. Estimates of the autoxidation rate constants were obtained by fitting the initial phase (8 h) of the normalized absorbance decreases at 576 nm to a single-exponential expressions: y=ymax * e−kt+y0, where y is the observed absorbance reading as a function of time. The time courses were fitted using the Excel Microsoft Solver program. In a third set of experiments, H2O2 mediated ferryl Hb formation for HbA and HbE was monitored upon the addition of 30 equivalents of H2O2 (30% (w/w)) After a reaction time of 2 min, catalase (200 units/mL) was added (for 1 min) to remove excess H2O2 followed by ferryl detection using 2 mM sodium sulfide (Na2S was added to transform ferryl Hb to sulfHb). Visible light optical absorbance spectra were recorded between 500 and 700 nm. The concentration of sulfHb was calculated using the extinction coefficient of sulfheme listed above.
2.3. Kinetics of ferryl hemoglobin decay
MetHb (ferric) was freshly prepared prior to the start of each experiment. From a 1.5 mM stock solutions of HbO2 to which slight molar excesses of K3[Fe(CN)6] was added to generate metHb. Removal of K3[Fe(CN)6] was accomplished using a column containing Sephadex G-25 media (Sigma-Aldrich, St. Louis, Missouri, US). Ferryl HbA and HbE were generated by the addition of 10, 30, or 50 heme equivalents of H2O2 to metHb (65 μM) in 50 mM phosphate buffer pH 7.4 to ensure maximum ferryl production in the Hb solutions. After 2 min of reaction time, excess catalase (250 units) was added to stop the reaction and to prevent heme bleaching. Decay of the resulting ferryl species back to metHb was followed at ambient temperature. Spectra were recorded between 500 and 700 nm for 1 h, scanning every 30 s. The optical changes were monitored and the time courses for reduction of ferryl Hb were plotted as absorbance (541 nm or 544 nm) and (630 nm) versus time (seconds) and fitted to a single exponential function using Microsoft Excel.
2.4. RPHPLC globin chain analyses after peroxide treatment
RP-HPLC was performed with a Zorbax 300 SB C3 column (4.6×250 mm) using Waters HPLC system consisting of a Waters 626 pumps, 2487 dual-wavelength detector and a 600 s controller installed with Empower 2 (Waters Corp, Milford, MA). 20 μg of Hb in 25 µL of water was loaded on the C3 column equilibrated with 35% acetonitrile containing 0.1% TFA. Globin chains were eluted with a gradient of 35–50% ACN within 100 min at a flow rate of 1 mL/min. The eluent was monitored at 280 nm for globin chains and at 405 nm for the heme components. For the H2O2 oxidation experiments, 250 µM of Hbs (in 1 mL of 50 mM phosphate buffer, pH7.4) were oxidized with escalating does of H2O2 (0.25, 0.55, 0.75, 1.25, 2.5, 5.0, and 10.0 mM) at 25 °C. Catalase (70 µL) in PBS (37,840 U/mL, MP Biomedical) was added into the 1 mL of reaction solution at 1 h to terminate the oxidation reactions.
2.5. Kinetics of heme loss from hemoglobins
To assess the rate of heme transfer, we measured the absorbance changes when a heme acceptor, a double-mutant (H64Y/V86 F) apomyoglobin (ApoMb), binds the heme released from HbA and HbE to yield holomyoglobin, a green adduct [31]. In these experiments, spectra between 350 and 800 nm were recorded every 2 min for 16 h at 37 °C using 200 mM potassium phosphate buffer, 600 mM sucrose, at pH 7.0. Final concentration of Hb in heme equivalents was 2 μM and the final concentration of H64Y/V86F ApoMb was 20 μM in a 1 mL total reaction volume. Data collection started immediately after mixing the two solutions.
2.6. Mass spectrometric analysis of hemoglobin subunits oxidation reactions with and without AHSP
MS experiments were performed with 178 μM (heme) HbA and HbE and were carried out after incubation overnight in 20 mM phosphate buffer, pH 7.4 at ambient temperature. The following 10 experimental conditions were designed to study the effect of excess α subunits (associated with β thalassemia) and increasing H2O2 concentration on HbA and HbE oxidation in the presence and absence of AHSP: (experiments 1) (control) ferrous HbE and HbA were incubated in air equilibrated phosphate buffer; (experiments 2) ferrous HbA and HbE were incubated with 2.0 M excess of H2O2 per heme; (experiments 3–4) ferrous HbA and HbE were incubated with equimolar ferric alpha Hb subunits and 2.0 and 5.0 M excess of H2O2 per heme; (experiments 5–6) ferrous HbA and HbE were incubated with equimolar ferric alpha Hb subunits, equimolar AHSP and 2.0 and 5.0 M excess of H2O2 per heme; (experiments 7–8) ferrous HbA and HbE were incubated with equimolar ferrous alpha Hb subunits and 2.0 and 5.0 M excess of H2O2 per heme; (experiments 9–10) ferrous HbA and HbE were incubated with equimolar ferrous alpha Hb subunits, equimolar AHSP and 2.0 and 5.0 M excess of H2O2 per heme.
2.7. Spin trapping reaction conditions
HbA or HbE (65 µM in heme), in 100 mM ammonium bicarbonate, pH 8.0 were subjected to both a 5 fold and 10 M excess of H2O2 over heme in the presence of DMPO (5,5-Dimethyl-1-Pyrroline-N-Oxide) at 37 °C for 2 h. The reactions were stopped by freezing at −80 °C. All samples were prepared for LC-MS/MS analysis as described below.
2.8. LC-MS/MS analysis
All Hb samples were tryptically digested, desalted and analyzed by mass spectrometry using our previously described method. [32] Briefly, tryptic peptides were analyzed by reverse phase liquid chromatography mass spectrometry (RP LC/MS/MS) using an Easy nLC II Proxeon nanoflow HPLC system coupled online to a Q-Exactive Orbitrap mass spectrometer (Thermo Scientific). Data were acquired using a top 10 method (for 60 min) dynamically choosing the most abundant precursors (scanned at 400–2000 m/z) from the survey scans for HCD fragmentation.
Data were searched against the Swiss-Prot Human database (release 2014_03; contains 542,782 sequence entries) supplemented with HbE and porcine trypsin using the Mascot (version 2.4) search engine (Matrix Sciences, London, UK) as described previously [32] with the following amendments to Mascot searches: variable modifications including cysteine trioxidation (+48 Da), cysteine dioxidation (+32 Da), tryptophan oxidation (+16), tyrosine oxidation (+16 Da), and methionine oxidation (+16) were included for identifying “hotspot” oxidation and DMPO labeled cysteine and tyrosine (+111 Da) were included as variable modifications for all DMPO treated samples. Mascot output files were analyzed using the software Scaffold 4.2.0 (Proteome Software Inc.). Hb peptide identifications were accepted if they could be established at greater than 99.9% probability and contained at least 2 identified peptides. Peptide probabilities were assigned by the Protein Prophet algorithm [33].
2.9. Quantitative mass spectrometric analysis
Peptides listed in Table 2 from LC-MS/MS data were analyzed to quantify changes in HbE and HbA under the oxidative conditions for experiments 1–10 (listed above). Each peptide was further validated by retention time reproducibility. All quantitative experiments were performed in triplicate and standard deviations were obtained by averaging relative abundance data from three different experiments. Briefly, extracted ion chromatograms (XICs) were generated from the most abundant monoisotopic peak of each isotopic profile (representing charged states of each peptide). To construct XICs, Xcalibur (version 2.2) software was used with a designated mass tolerance of 0.01 Da, and mass precision set to three decimals. For relative quantification, the ratio of each isoform was calculated based on the sum of the XIC peak area from all forms (including all charge states and versions that result from different cleavage sites), which was normalized to 100%.
Table 2.
All Hotspot peptides including charge state and cleavage variants derived from HbA and HbE.
| Peptides | Modified residue | (+) Charge State | m/z |
|---|---|---|---|
| 83GTFATLSELHCDK96 | βCys93 | 2 | 735.33 |
| 83GTFATLSELHCDKLHVDPENFR104 | βCys93 | 3 | 860.06 |
| 4 | 645.31 | ||
| 31LLVVYPWTQR40 | βTrp37 | 2 | 645.86 |
| 9SAVTALWGK17 | βTrp15 | 2 | 476.76 |
| 105LLGNVLVCVLAHHFGK121 | βCys112 | 3 | 589.99 |
| 2 | 884.48 | ||
| 32MFLSFPTTK40 | αMet32 | 2 | 544.27 |
| 2 | 1087.54 | ||
| 41FFSFGDLSTPDAVMGNPK59 | βMet55 | 3 | 692.32 |
| 2 | 1037.97 | ||
| 17VGAHAGEYGAEALER31 | αTyr24 | 3 | 525.58 |
| 41TYFPHFDLSHGSAQVK56 | αTyr42 | 3 | 626.97 |
| 4 | 470.48 | ||
| 62VADALTNAVAHVDDMPNALSALSDLHAHK90 | αMet76 | 2 | 628.92 |
| 3 | 524.26 | ||
| 100LLSHCLLVTLAAHLPAEFTPAVHASLDK127 | αCys104 | 4 | 754.66 |
3. Results
3.1. Autooxidation kinetics of HbA and HbE
We monitored spectral scans in the range of 450–700 nm every 2 min for 24 h. Panels A-C in Fig. 1 represent hourly time dependent spectra (up to 16 h) that were taken at 37 °C during the autooxidation of 65 µM HbA and HbE (in heme equivalents). Since HbO2, metHb and hemichromes have little absorbance at 700 nm any increases in absorbance at 700 nm is purely due to light scattering caused by sample precipitation [34]. The spectra in Panel A (representing HbA) show an increase in absorbance at 630 nm but little if any increase at 700 nm. The increase at 630 nm (λmax for ferric Hb) is associated with oxidation of the ferrous (Fe2+) to the ferric (Fe3+) state. Conversely, the spectra in Panel B (representing HbE) clearly show an increase in absorbance at 630 nm and 700 nm indicating both the formation of ferric HbE and protein denaturation/precipitation. Notably, the HbA spectra (Panel A) maintained clear isosbestic points (unlike those shown for HbE), reflecting the specific transition of ferrous to ferric heme. In the case of HbE (after 6–8 h) there was a clear deviation in spectra from the isosbestic points reflecting the onsets of protein unfolding/denaturation. The addition of catalase to HbE eliminates the observed denaturation (see Fig. 1, Panel C) verifying that precipitation seen in Panel B is directly linked to the autooxidative production of H2O2.
Fig. 1.
Autooxidation kinetics of HbA and HbE. Spectral changes were measured during the autooxidation of 65 μM (heme) HbA and HbE in 20 mM phosphate buffer, pH7.4 at 37 °C, and monitored in an Agilent 8453 spectrophotometer. Panels A (HbA)-B HbE): represent time dependent spectra measured every hour up to 16 h during autooxidation. Panel C represents the same conditions for HbE in the presence of 200 units catalase.
Due to complications associated with HbE precipitation, multi-wavelength analysis was employed to subtract out the elevated absorbance at 700 nm due to unfolding and precipitation in order to obtain accurate autooxidation rate constants (see Experimental Procedures). Estimates of the autooxidation rate constants were obtained by fitting the initial linear portion of the time courses (first 8 h) to a single exponential expression. The autooxidation rate constants listed in Table 1 show nearly identical kauto values of 0.051 h−1±0.0021 and 0.054 h−1±0.0067 for HbA and HbE respectively. The HbA rate constant reported in this work is similar to previously reported rates by our group under similar conditions [32]. Autooxidation rates for both Hbs were substantially reduced by nearly identical levels with the use of 200 units of catalase in each sample. The identical kauto rate constants taken together with the above spectral scan data (represented in Fig. 1 Panels A-C) indicate that the autooxidation kinetics for both are similar. However, the accumulation of oxidants (H2O2) during spontaneous oxidation is not the same among the two proteins in the presence of identical levels of catalase (Table 1). This suggests that the structural impact of autooxidation is more profound for HbE, in keeping with previous observations on oxidatively less stable Hbs [35], [36].
Table 1.
Observed rate constants for the initial phase of Hb autooxidation in phosphate buffer (n=3).
| Sample | kauto(h−1) | kauto(h−1)+Catalase |
|---|---|---|
| HbA | 0.054±0.0024 | 0.007±0.003 |
| HbE | 0.051±0.056 | 0.089±0.0016 |
3.2. Peroxide-mediated ferryl formation
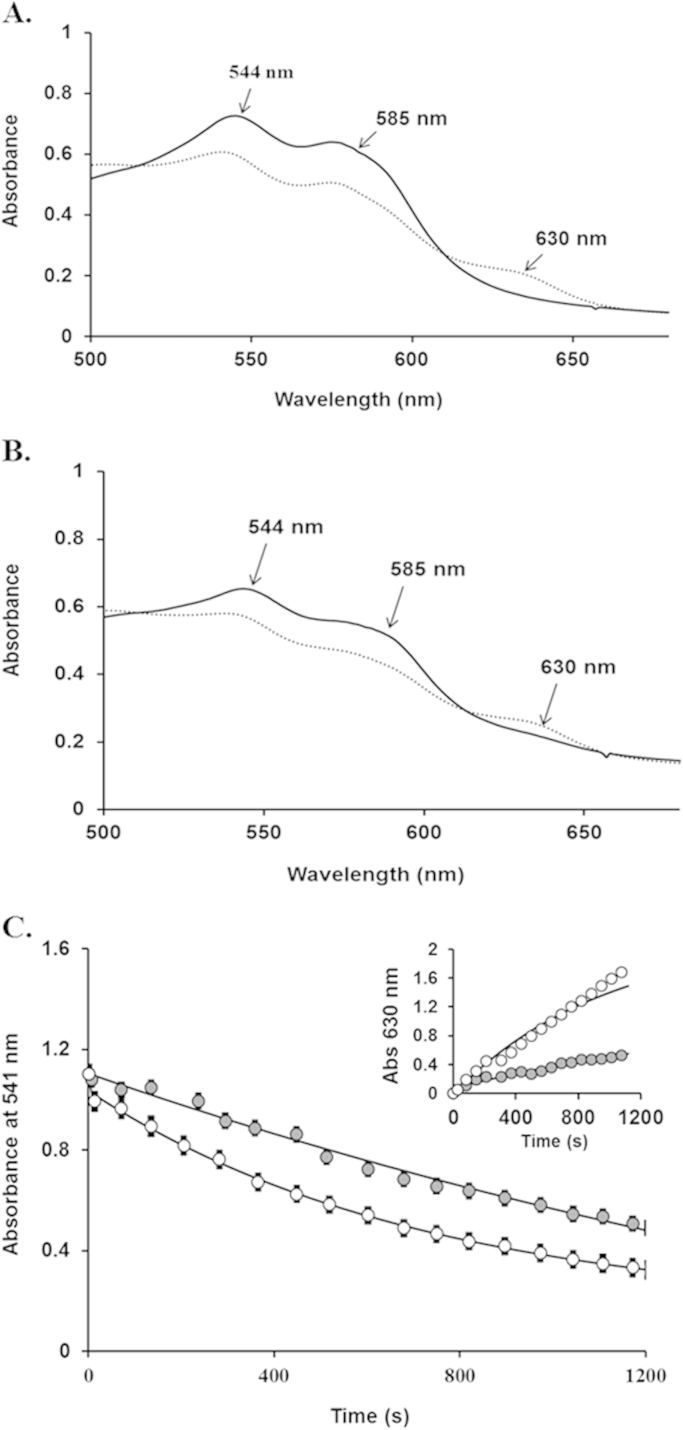
The pseudoperoxidase reactivity of both Hbs with H2O2 was examined using UV/Vis absorbance spectroscopy, with the formation of the oxo-ferryl heme species monitored by absorbance changes in the visible spectrum (see Eqs. (1), (3)). Fig. 2 shows typical spectral transitions for ferrous HbA and the HbE mutant after a 2 min incubation with 30 fold excess H2O2; the ferryl species is characterized by major peaks at 544 and 585 nm and a flattened region between 600 and 650 nm (Panels A and B) (equation 1). To confirm the spectral identity of the ferryl species, Na2S was added to the reaction mixture and sulfheme formation was followed by the appearance of an absorbance band at 620 nm. Catalase was added prior to the addition of sulfide to remove any excess H2O2 so that any sulfur addition to the heme group was due only to reaction with ferryl heme complexes. In these experiments, the levels of ferryl Hb in HbA and HbE were estimated to be 20.2 and 20.4 μM, respectively, based on the reported extinction coefficients for sulfheme [28]. These spectral changes, like the autooxidation kinetics reveal nearly identical levels of ferryl HbA and HbE after incubation with H2O2.
Fig. 2.
Spectral analysis of hydrogen peroxide oxidation reaction with ferrous HbA and HbE and the formation of their respective sulfhemoglobin. Absorbance spectra were recorded before and after the addition of 2 mM Na2S to 65 µM HbA (Panel A) and HbE (Panel B) in solutions containing 30 equivalent of H2O2. The spectra (shown in both panels) with λmax at 541 nm and 576 nm are representative for ferrous heme (Fe2+) before the addition of H2O2. The spectra shown in both panels characterized by peaks at 544 and 585 nm corresponds to the transient ferryl Hb spectrum (characterized by peaks at 544 and 585 nm) immediately after the addition of H2O2. To further verify the ferryl intermediate Na2S was added to transform ferryl Hb to form sulfHb which exhibits a characteristic strong peak at 620 nm.
3.3. Hydrogen peroxide induced oxidative changes in hemoglobins
One of the properties of the Hb ferric/ferryl redox cycle is subunit oxidative changes that can be detected by HPLC. The H2O2 specific impact on HbA and HbE were evaluated using an RP-HPLC method such that the absorbance at 280 and 405 nm was recorded to monitor protein and heme components of Hb, respectively. As shown in Fig. 3A, the α-globin chains, β-globin chains, and heme moieties of untreated HbA were separated into three distinct peaks. Under our chromatographic conditions, the HbA β and α chains eluted at 42.5 and 46.5 min, respectively, and the HbE β and α chains eluted at 38 and 46.5 min, respectively. There was a corresponding decrease in peak height of α and β subunits with increasing H2O2 (Figs. 3A and B). Based on peak integration and the retention time of each subunit we were able to plot retention fraction of each subunit as function of heme: H2O2 ratios as shown in Fig. 3C. As can be seen, the β subunit retention fractions are much lower than α subunit fractions (in both proteins), indicating that the β subunit is more prone to oxidative damage. As shown in Fig. 3 (A and B), HbE is more susceptible to oxidative changes than HbA upon treatment with higher concentrations of H2O2. These results are also consistent with our previous observation which confirms that H2O2 induces extensive decomposition of β-globin chains of Hb [5].
Fig. 3.
RP-HPLC analyses of oxidative changes in hemoglobin A and hemoglobin E and the retention fractions of their subunits as a function of peroxide concentration. Panel A and B represent RP-HPLC chromatograms of HbA and HbE treated with different H2O2 concentrations. RP-HPLC was performed using a Zorbax 300 SB C3 column (4.6×250 mm). Hbs (250 µM in heme) were incubated in 20 mM phosphate buffer pH 7.4, 25 °C in the presence or absence of 0.25, 0.5, 0.75, 1.25, 2.5, 5.0, and 10.0 mM H2O2. 70 µL of catalase (0.57 mg/mL) was added to 1 mL of reaction solution to terminate the oxidation reactions. Oxidized Hb samples (40 µL) were loaded into the autosampler of Waters HPLC. Panel C, represents a plot of the reaction fraction of Hb solutions (%) versus the ratios of Hb: H2O2 used. The open circle and square represent the α and βA chains of HbA. The closed circle and square represent the α and βE chains of HbE. The values in (C) were derived from the areas of β and α chains for each Hb from panel A and B. The area of each peak was integrated with Origin 6.0 software. The retention fraction of each peak was calculated by dividing the area of the subunit peak subjected to oxidation with the area of the subunit peak without oxidation.
3.4. Autoreduction rates of ferryl HbA and HbE and decay to methemoglobin
To explore possible differences in ferryl Hb autoreduction rates, we followed the autoreduction (ferryl decay) of ferryl HbA and HbE to their respective ferric states at varying H2O2 concentrations. Ferryl decay was followed at room temperature by monitoring changes in absorbance at 541 nm and 544 nm as a function of time and observed to be identical. Any observable rate variance between HbA and HbE, as monitored through the decrease of ferryl and increase in ferric heme, reflected differences in autoreduction rate constants. While ferryl decay differences were undetectable at a majority of the H2O2 concentrations studied, we were able to see a clear difference in the ferryl decay time courses for ferryl HbA and HbE after treatment with 50 M excess H2O2 (Fig. 4). Indeed, the decay of the ferryl species is clearly slower for HbE than HbA indicating that the former has a lower autoreduction rate constant. The time courses for ferryl HbE decay were fit to a single exponential expression with a rate constant equal to 1.37±0.08 h−1, compared to 5.05±0.17 h−1, for HbA; the ferryl decay rate of HbA was 3.4 times slower than the calculated rate for HbE solutions (Fig. 4C). The inset to Panel 4 C further substantiates the ferryl decay results; the transition back to metHb (monitored at 630 nm) is much slower for HbE. Although extremely high H2O2 levels were required to detect differences, these results suggest that ferryl HbE likely persist longer and as a result is oxidatively less stable and more damaging to it and other biological molecules.
Fig. 4.
Oxidative stability of the ferryl intermediate in HbA and HbE and autoreduction to the ferric forms. Samples of 65 μM metHbA or metHbE were initially incubated with 3.25 mM H2O2 for 2 min 20 mM phosphate buffer, pH 7.4 at 25 °C. After the addition of catalase (250 units) to stop the reaction, absorbance spectra were recorded at intervals of 30 sec for 1 h within 350 nm and 700 nm. Spectral Panels A-B: time dependent spectra representing the first and final (56 min) recorded spectra during the ferryl decay of HbA (A) and HbE (B). Panel C: Ferryl decay was evaluated by plotting changes in absorbance at 541 nm as a function of time. The resulting ferryl decay time courses for metHbA and metHbE were fit to a single exponential expression (open and closed circles represent HbA and HbE respectively). Inset to Panel C: MetHb formation time courses were evaluated by plotting changes in absorbance at 630 nm (open and closed circles represent HbA and HbE respectively).
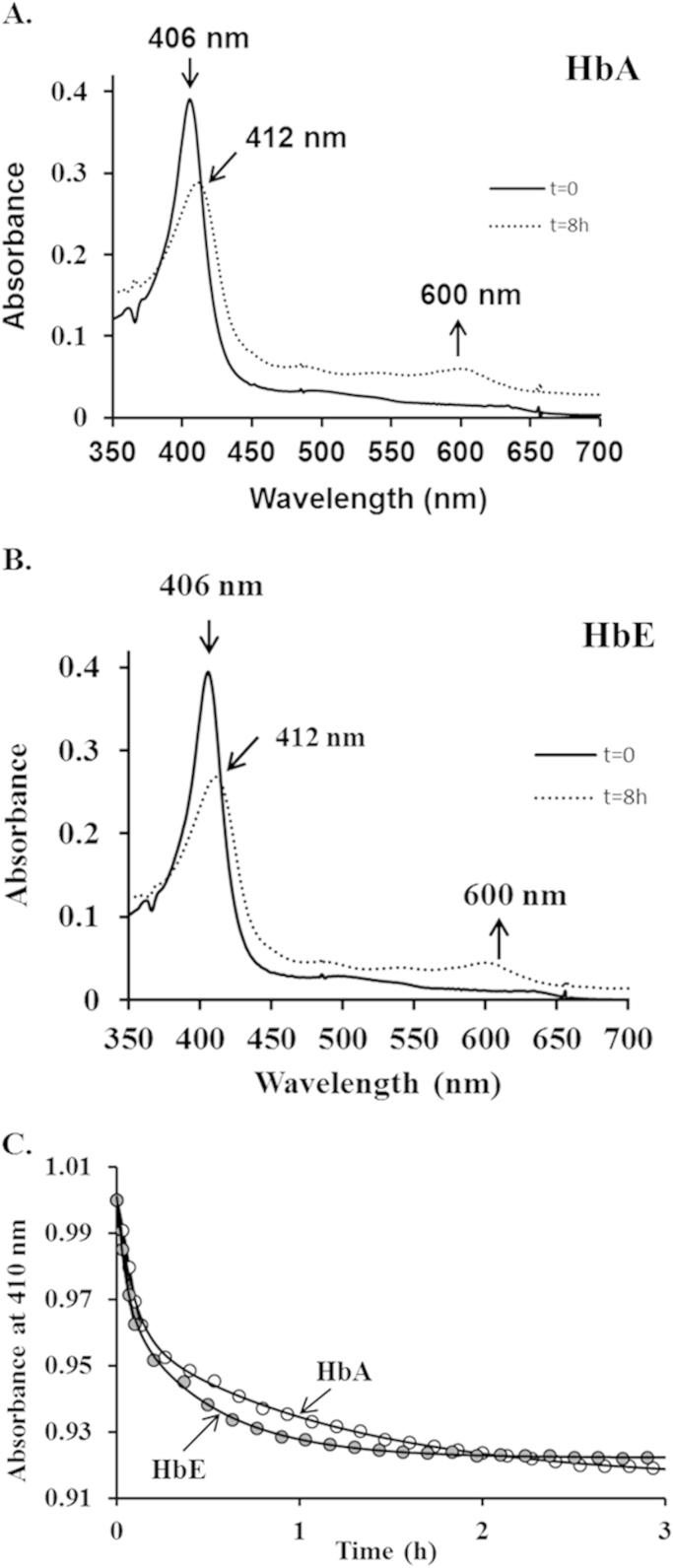
3.5. Kinetics of heme loss from HbA and HbE
In order to further probe the apparent difference in structural stability, we performed experiments to determine the rates of heme loss for both HbA and HbE. Typical spectral changes occurring during heme exchange between metHbA and H64Y/V86 F, the receptor apomyoglobin, are shown in Fig. 5, Panel A and B. The heme transfer is confirmed by the red shift in the Soret peak and the appearance of a new peak at 600 nm. Time courses for heme loss measuring the decrease in A410 nm as heme is transferred to the H64Y/V68 F apoMb reagent are shown in Fig. 5 Panel C. As shown in earlier reports, the time courses in this study were biphasic with fast components represent heme loss from β-subunits of both proteins [37]. Unlike HbA, an increase in absorbance at extended time reflects aggregation of the HbE apoglobin. Estimates of averaged absorbance changes at the Soret peak at 410 nm were normalized and the initial phases of absorbance were fit to double exponential decay expressions where k1 and k2 are the fast and slow first-order observed rate constants. Rate constants equal to, k1=11.75 h−1 and k2=0.86 h−1 were derived for metHbA and were similar to the reported rates for normal HbA [23]. Rate constants for the heme transfer from HbE were k1=19.07 h−1and k2=2.12 h−1 respectively. These results confirm that HbE loses its heme about 1.5–2.0-fold faster than HbA. Taken together with the autooxidation, slower decay of the ferryl heme data described above suggest that oxidative changes within HbE leads to rapid denaturation and subsequent heme loss.
Fig. 5.
Time courses for heme dissociation from ferric HbA and HbE. Samples of 2 μM metHb were mixed with 20 μM H64Y/V86 F apomyoglobin in 200 mM potassium phosphate buffer at pH 7, 37 °C, containing 600 mM sucrose and the transfer of heme to the apoMb reagent was followed at 410 nm. Panel A represents exemplary absorbance spectrum for holoMb (H64Y/V86 F) with HbA. Panel B represents exemplary absorbance spectrum for holoMb (H64Y/V86 F) with HbE. Panel C represents absorbance changes at 410 nm plotted as a function of time for 3 h for HbA (open circles) and HbE (closed circles).
3.6. Quantitative mass spectrometry analysis of amino acids oxidation in hemoglobins
To investigate the impact free α subunits have on HbA and HbE oxidative stability, we utilized a quantitative mass spectrometric strategy aimed at exploring the consequential post-translational modification of "oxidation hotspots" for both Hbs under increasing H2O2 conditions. Specifically, HbA and HbE were incubated with either ferric or ferrous free α subunits with increasing H2O2 concentrations. To further substantiate the effect free α subunits have on H2O2 induced oxidation, we also included these experimental conditions in the presence of alpha hemoglobin stabilizing protein (AHSP) as described below.
LC/MS/MS analysis was utilized to target all “hotspot” peptide charge states reproducibly identified by Mascot database searches (see Table 2). Amino acids residues identified by LC-MS/MS analysis in this study correlated well with previously published data [5], [38]. As observed in our previous studies, the most prevalent oxidative changes between HbA and HbE were found to be restricted to peptides containing βCys93 (an important endpoint for free radical induced protein oxidation with Hb[5]). The difference in oxidation between other α and β residues listed in Table 2 (regardless of H2O2 amount) was negligible. To quantify changes, extracted ion chromatograms (XICs) were generated from the most abundant monoisotopic peak of each peptide isotopic profile and the resulting ratio differences were compared. For example, the most abundant monoisotopic peak (860.074 m/z) represented in Fig. 6A for the βCys93 containing peptide, GTFATLSELHCDKLHVDPENFR, was used to construct the resulting XIC. Because βCys93 residue exists in either the oxidized or unoxidized form the percentage of both isoforms were calculated based on the sum of the XIC peak area from all charged isoforms of βCys93 peptides. To confirm that the oxidized Cys93 moiety did not impact trypsin digestion and that trioxidation was the prevalent modification at this cite we compared the averaged total ion current ratio of an internal peptide (β subunit tryptic peptide VNVDEVGGEALGR, does not get oxidized) and the C93 containing peptide (oxidized and unoxidized); the ratio changed very little for both Hbs regardless of the H202 condition (data not shown).
Fig. 6.
Isotopic profile and extracted ion chromatogram (XIC) of βCys93 tryptic peptide. XICs were generated from the most abundant monoisotopic peak of each isotopic profile. To construct XICs, Xcalibur (version 2.2) software was used with a designated mass tolerance of 0.01 Da, and mass precision set to three decimals. For relative quantification, the ratio of each isoform was calculated based on the sum of the XIC peak area from all forms (including all charge states and versions that result from different cleavage sites), which was normalized to 100%. Top Panel A represents the isotopic profile of the triply charged βC93 tryptic peptide GTFATLSELHCDKLHVDPENFR and the representative XIC of βC93 tryptic peptide (residues 83–104). Lower Panel B represent the isotopic profile of the DMPO labeled +4 charge βC93 tryptic peptide GTFATLSELHCDKLHVDPENFR and the representative XIC.
As an initial step, we performed control quantitative proteomic experiments with HbA and HbE in 2.0 M excess H2O2; the results from these studies show that both Hbs were oxidized at similar levels (see Table 3). These data like the autooxidation kinetic results in this report clearly indicate that H2O2 induced oxidation is similar between HbA and HbE. The next series of MS experiments were aimed at probing the effect free α subunits (in the presence of H2O2) have on oxidative stability (Table 4, Table 5). For example, the relative abundance difference associated with a 5.0 M excess H2O2 (with ferric free α subunits) resulted in a 3–4 fold higher level of Cys93 oxidation for both Hbs. This trend was also observed for experimental conditions involving ferrous free α subunits and HbE. These data are the first quantitative results reported that directly link the impact of free α subunits to oxidative effects on Hb in solutions as reflected by the substantial increases in HbA and HbE βCys93 oxidation in the presence of H2O2.
Table 3.
Quantitative mass spectrometry data representing control oxidative reactions of ferric HbA and HbE with H2O2.
| Reaction conditions | HbA Cys93 Oxidation (%) | HbE Cys93 Oxidation (%) |
|---|---|---|
| No H2O2 | 0.6±0.05 | 0.2±0.05 |
| 2XH2O2 | 3.0±0.2 | 4.0±0.1 |
| 5XH2O2 | 8.6±0.12 | 8.1±0.03 |
Table 4.
These quantitative MS data represent oxidative reactions of HbA and HbE with ferrous α subunits and H2O2. Reactions were also performed in the presence of AHSP.
| Reaction conditions | HbA Cys93 Oxidation (%) | HbE Cys93 Oxidation (%) |
|---|---|---|
| 2XH2O2 | 1.2±0.09 | 8.4±0.02 |
| 5XH2O2 | 3.5±0.04 | 12.3±0.4 |
| 2XH2O2+AHSP | 1.3±0.07 | 3.1±0.01 |
| 5XH2O2+AHSP | 0.5±0.1 | 2.6±0.01 |
Table 5.
These quantitative MS data represent oxidative reactions of HbA and HbE with ferric α subunits and H2O2. Reactions were also performed in the presence of AHSP.
| Reaction conditions | HbA Cys93 Oxidation (%) | HbE Cys93 Oxidation (%) |
|---|---|---|
| 2XH2O2 | 1.7±0.23 | 7.5±0.13 |
| 5XH2O2 | 9.3±0.24 | 14.4±3.2 |
| 2XH2O2+AHSP | 0.4±0.03 | Below Detection |
| 5XH2O2+AHSP | 2.7±0.06 | 3.2±0.7 |
To further explore and confirm the oxidative impact of α subunits on Hb oxidation, we added alpha stabilizing protein (AHSP) to the reaction mixture. AHSP preferentially bind ferric α subunits (binds ferrous α subunits less tightly) to form a non-reactive hexacoordinate ferric form which inhibits ferryl heme formation (see Fig. 7), redox chemistry, catalysis, and heme loss [29]. Fig. 7 shows deconvoluted spectra of a typical α subunit ferryl heme after treatment with H2O2. The resulting spectrum is characterized by absorbance peaks at 544 and 588 nm (confirmed by derivtization with Na2S). When H2O2 is added to the reaction mixture of ferric α subunits complexed with AHSP, the resulting spectrum resembles a hexacoordinate hemichrome with prominent absorbance peaks at 535 and 565 nm confirming the lack of any formed ferryl heme [29]. The structural comparisons among the two heme pockets in Panels B and C show that AHSP induces alternative bis-His hexacoordination which restricts access of H2O2 to the heme in addition to inhibiting oxidation of Fe3+to Fe4+. Complexation of AHSP with α subunits therefore should reduce the oxidative impact which would be reflected by a decrease in hotspot oxidation. As shown in Table 4, Table 5 the presence of AHSP substantially reduced βCys93 oxidation for both proteins. The level of βCys93 oxidation for ferric α subunit experiments was reduced to nearly undetectable levels with 2.0 M excess H2O2 (peptide precursor ion current was near baseline levels preventing the Xcalibur software from generating accurate extracted ion chromatogram) and decreased by more than 4 fold with 5.0 M excess H2O2. The decrease in βCys93 oxidation was not as profound when AHSP was incubated with Hbs containing ferrous α subunits which is likely associated with the weaker affinity AHSP has for the ferrous form. Additionally, in the presence and absence of AHSP, H2O2 exposure resulted in completely oxidizing ferrous α subunits to the ferric state. These experiments unambiguously confirm the role that excess free α subunits have on oxidative stability of both HbA and HbE.
Fig. 7.
Spectra and Model structures representing ferrous α subunits treated with 10 fold excess of H2O2 and ferric α subunits treated with 10 fold excess of H2O2 and incubated with AHSP. Panel A: Spectra for 65 µM ferric alpha subunits incubated with and without AHSP treated with a 10 fold excess of H2O2 for 2 min followed by catalase to remove excess peroxide. Spectrum with λmax at 544 nm and 585 nm represent ferryl heme (Fe+4) after the addition of 10 fold excess of H2O2. Spectrum with λmax at 535 nm and 565 nm represent represents the α spectrum with AHSP present. Panel B. Heme is shown using a red stick structure, distal and proximal histidines are also shown using orange stick structures. AHSP and α-subunits are shown with cyan and gray90 cartoon structures respectively. Panel C. Heme, distal and proximal histidines are shown using stick structures with red and orange color respectively. α and β subunit structures are shown as cartoons with yellow and gray respectively. Models were generated using the PyMOL Molecular Graphics System and images in both panels were generated from Protein Data Bank code 1NQP and 1LA6. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Because β Cys93 has been described as a free radical endpoint of ferryl ion and globin centered radical oxidation we performed DMPO labeling experiments to comparatively quantify the relative abundance of ferryl radicals in HbA and HbE. The exposure of Hbs to H2O2 has previously been shown to initially produce a porphyrin cation radical (and ferryl ion) that oxidizes cysteinyl and tyrosyl amino acids to form globin centered radicals [39]. The spin trap label DMPO reacts with these modified amino acids to form a nitroxide radical that is further oxidized to the corresponding globin radical derived nitrone adduct by the ferryl moiety. These DMPO derived adducts are stable under fragmentation conditions used in LC-MS/MS analysis [39], [40]. We therefore utilized DMPO to characterize the Hb radicals in HbA and HbE in the presence of H2O2 (5 fold excess relative to heme). Analyses of LC/MS/MS and full MS data identified DMPO labeled Cys112, Tyr42α and Cys93 peptides (most prominently labeled amino acid) with the addition of H2O2 (see Fig. 6B). DMPO Cys93 labeling in the absence of H2O2 was at negligible levels (~1% for both HbA and HbE). As shown in Table 6, the degree of DMPO labeling was similar for HbA and HbE.
Table 6.
These quantitative proteomic data represent DMPO labeled Cys93.
| Reaction conditions | DMPO Labeled Cys93 (%)HbA | DMPO Labeled Cys93 (%)HbE |
|---|---|---|
| DMPO (No H2O2) | 1.1±0.07 | 2.4±0.21 |
| DMPO (H2O2) 5:1 (Heme) | 6.3±0.56 | 7.8±0.5 |
4. Discussion
There are well over 1000 naturally occurring human Hb variants that result mostly from single point mutations in the globin protein. Less commonly found variants are known to be associated with multiple amino acid substitutions, deletions, and altered post-translational processes. In general, these mutations alter Hb structure and biochemical properties with physiological effects ranging from insignificant to severe [41]. While several decades of research have primarily focused on identifying Hb variants that have altered oxygen binding affinities and associated clinical outcome, [41] very little has been done to explore the impact these amino acid substitutions have on the variant Hb iron oxidation state. These Hb mutants which are subjected to evolutionary pressures are viewed as “experiments of nature” and can provide unique model systems to address the question as to why some Hb variants are more oxidatively stable while others develop into a full circulatory disorder [41].
An example of an oxidatively stable Hb mutant is Hb Providence [42], [43]. RBCs from patients with Hb Providence contains two β-subunit variants with single amino acid mutations at βLys82→Asp (βK82D) and at βLys82→Asn (βK82 N) positions; both of these bind oxygen at lower affinity than wild type HbA. However, these variants (when isolated from native HbA) were found to resist H2O2 induced oxidation by internalizing radicals through the ferric/ferryl pseudoperoxidase cycle [42]. Conversely, single amino acid variant Hbs linked to severe pathology, including sickle cell Hb (HbS) (β6 Glu→Val) and HbE (β26 Glu→Lys), are less oxidatively stable than HbA [41], [9], [44], [34]. In the case of HbS, we recently found that the ferryl form of HbS persists longer in solutions than its HbA counterpart and as a consequence induced self-inflicted modifications within the protein, and subsequent heme loss. We have recently shown that this oxidative instability and heme loss induces profound physiological changes (when incubated in a medium of lung epithelial cells) including mitochondrial dysfunction and heme oxygenase (HO-1) translocation to its cytosol (unpublished experiments).
Studies have indicated that HbE is oxidatively unstable. First, drug (menadione)-induced oxidation studies of HbE (and a number of Hb variants) indicated that it oxidizes slightly faster than HbA, and produces larger quantities of low-spin ferric Hb (hemichromes) [45]. Secondly, HbE loses heme to lipid monolayers as well as phospholipid bilayers more readily than other variant Hbs [46], [47]. This unusual response to oxidation has an impact on HbE structural stability and has been suggested to be an important factor influencing the clinical course in HbE/β thalassemia patients particularly during febrile episodes [9]. The combination of HbE with excess unpaired α subunits (such as the case in HbE/β thalassemia) syndrome has been suggested as the cause of the severe/mild thalassemia phenotype [17], [19]. α-Hb is structurally unstable, with a tendency to denature upon oxidation, filling the cytoplasm and cell membrane with precipitated α-globin polypeptides, free heme, porphyrins, and iron, which further propagate ROS production [48].
In this investigation, we sought to identify the molecular basis of oxidative instability of HbE and to understand how this instability is influenced by the presence of unmatched α subunits. We have over the years developed (through in vitro and in vivo studies) a working model that describes oxidative pathways of cell free Hb in relation to its use as an oxygen therapeutic [49], [50], [7], [51]. These oxidative reactions of Hb (described below) can be sustained by oxidants such as H2O2 generated internally (autooxidation) or exogenously (chemical oxidation) when H2O2 is originated from cellular sources during oxidative stress [2], [1].
We first examined the process of spontaneous oxidation (autooxidation) of both Hbs at physiological conditions; our data show that HbE autoxidized at similar initial rates to its HbA counterpart. Importantly, during the later stages of the autooxidation process, HbE underwent extensive unfolding and precipitation. This unfolding and precipitation was completely reversed with the addition of catalase (H2O2 scavenger) suggesting that H2O2 plays a key role in driving these events. This difference in response to oxidation supports previous crystal structure data indicating that HbE is structurally less stable than HbA as the (β26 Glu→Lys) mutation in HbE was found to disrupt salt bridges and packing interactions necessary for optimal tertiary structure [18], [9].
Next, we followed the pseudoperoxidase activities of both Hbs after their reaction with bolus amounts of H2O2. Both ferrous forms of HbA and HbE consumed H2O2 at approximately the same rates, and their respective solutions were populated with almost the same levels of ferryl species. This is further supported by the spin trap DMPO labeling data indicated similar ferryl levels between HbA and HbE. However, when freshly prepared ferryl HbA and HbE heme were allowed to autoreduce to their respective ferric forms, ferryl HbE was slower in its rates of autoreduction in the absence of outside reductant. This is significant because a longer lasting ferryl heme and protein radical can self-inflict oxidative changes within the Hb protein and other biological molecules. Our data further substantiates this point because we also observed a significant increase in HbE heme loss rate (relative to HbA).
We employed both HPLC and mass spectrometric methods to analyze these oxidative pathways in the two Hbs with a focus on the β subunit, since it has been shown by our group and others to bear the burden of these oxidative side reactions [5], [32], [38], [52]. Specifically, βCys93 has emerged as a reliable index among a group of amino acids of the hotspot region in Hb for Hb radicals and their migration to this residue. The quantitative mass spectrometry results from this study clearly correlate with previous reports indicating the most prevalent oxidative “hotspot” changes between HbA and HbE to be restricted to peptides containing βCys93 (an important endpoint for free radical induced protein oxidation with Hb). The MS data from this report also quantitatively indicate for the first time that free α subunits (in the presence of H2O2) act as oxidants and in the presence of H2O2 substantially increase βCys93 oxidation of both proteins. Intriguingly, the addition of AHSP reverses the oxidative impact of free α subunits on both proteins. Since AHSP is known to bind α subunits in an inactive hexacoordinate configuration that prevents ferryl accumulation, these mass spectrometry results unambiguously confirm the destabilizing role of unpaired α subunits and shed light on the mechanism behind the pathology in patients who inherit HbE/β thalassemia.
We have recently reported differences in oxidative pathways among α, β, and γ subunits of native and some human Hb variants [32]. In the presence of H2O2, a β subunit mutant Bristol-Alesha (β67(E11)Val→Met), unlike its α subunit analogue, Hb Evans (α62(E11)Val→Met) produced larger ferryl and protein radical signals; the proximity of this substituted methionine to the reactive ferryl heme resulted in post-translational modification (PTM) of the Met 67 to Asp [32]. The absence of Asp conversion in α subunits is attributed to the presence of an α specific Tyr42 that acts as a redox cofactor providing an electron transfer route involved in ferryl heme iron reduction [53]. β subunits do not possess this redox cofactor and are therefore unable to effectively reduce the reactive ferryl responsible for the above PTM; this is supported by the crystal structure (in the same study) revealing a long-lived ferryl (Fe4+=O) complex in the β subunit but not in the α subunit mutant. Separately, we found (in another study) that fetal Hb (γ2/α2) exhibits an enhanced pseudoperoxidase activity and accumulates less damaging ferryl iron. This finding may explain the evolutionary advantage in possessing high levels of oxidatively stable fetal Hb (~10–20%) within RBCs on the course of both sickle cell disease and the thalassemias [52]. The inability of HbE (another β subunit variant Gluβ26 Lys) to effectively reduce its highly damaging ferryl heme under oxidative stress is likely a contributing factor for the damaging role of HbE in the erythrocyte proteome of patients suffering from HbE/β-thalassemia.
In summary, we have shown for the first time that unpaired α globin (if not associated with β subunits in a tetrameric form) is oxidatively unstable and more damaging to HbA and HbE tetramers as a whole. The work here also indicates that α subunit oxidative side reactions have a more profound impact on the β subunit; specifically irreversible oxidation of βCys93 in the presence of H2O2 ultimately leads to β subunit unfolding and heme loss. We have also shown that AHSP is an effective inhibitor of both ferrous and ferric forms of α subunits. AHSP has been investigated in recent years in relationship to its role in modifying the severity of β-thalassemia in mice models [54]. This effect however, is less established in humans [55], although some investigations found that AHSP expression was significantly correlated to excess α subunits expression in individuals with HbE/β-thalassemia [56]. Since the amount of free α globin chain appears to be an important factor contributing to differences in hematologic and clinical severity with β thalassemia. It remains possible that AHSP or its mimetic could potentially offer some therapeutic opportunities in treatment of the complications of β thalassemia and other related conditions. Alternatively down regulation of α-globin expression was recently suggested as a therapeutically feasible approach using RNA interference, epigenetic drug targeting, or genome editing [57].
Funding
This work was supported by National Institutes of Health (NIH/NHLBI) grant P01-HL110900 (MBS, TK, FM, FBW, AIA and JMF), and grants from the U.S. Food and Drug Administration (MODSCI) ((MBS, TK, FM, FBW, AIA) and Einstein Global Health Microgrant and NIH R21 HL106421 (REH and JMF).
Acknowledgements
We thank Dr. John Olson of Rice University for providing recombinant myoglobin (H64Y/V86F) and for his valuable comments and suggestions.
Footnotes
Hotspot in this report refers to amino acids located close to the alpha/beta interface of hemoglobin subunits that are consistently oxidized by hydrogen peroxide.
References
- 1.Alayash A.I. Blood substitutes: why haven’t we been more successful? Trends Biotechnol. 2014;32:177–185. doi: 10.1016/j.tibtech.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alayash A.I. Oxygen therapeutics: can we tame haemoglobin? Nat. Rev. Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 3.Reeder B.J. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid. Redox Signal. 2010;13:1087–1123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- 4.Alayash A.I. Redox biology of blood. Antioxid. Redox Signal. 2004;6:941–943. doi: 10.1089/ars.2004.6.941. [DOI] [PubMed] [Google Scholar]
- 5.Jia Y., Buehler P.W., Boykins R.A., Venable R.M., Alayash A.I. Structural basis of peroxide-mediated changes in human hemoglobin: a novel oxidative pathway. J. Biol. Chem. 2007;282:4894–4907. doi: 10.1074/jbc.M609955200. [DOI] [PubMed] [Google Scholar]
- 6.Buehler P.W., Abraham B., Vallelian F., Linnemayr C., Pereira C.P., Cipollo J.F., Jia Y., Mikolajczyk M., Boretti F.S., Schoedon G., Alayash A.I., Schaer D.J. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113:2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 7.Belcher J.D., Chen C., Nguyen J., Milbauer L., Abdulla F., Alayash A.I., Smith A., Nath K.A., Hebbel R.P., Vercellotti G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams T.N., Maitland K., Bennett S., Ganczakowski M., Peto T.E., Newbold C.I., Bowden D.K., Weatherall D.J., Clegg J.B. High incidence of malaria in alpha-thalassaemic children. Nature. 1996;383:522–525. doi: 10.1038/383522a0. [DOI] [PubMed] [Google Scholar]
- 9.Rees D.C., Clegg J.B., Weatherall D.J. Is hemoglobin instability important in the interaction between hemoglobin E and beta thalassemia? Blood. 1998;92:2141–2146. [PubMed] [Google Scholar]
- 10.Rees D.C., Williams T.N., Maitland K., Clegg J.B., Weatherall D.J. Alpha thalassaemia is associated with increased soluble transferrin receptor levels. Br. J. Haematol. 1998;103:365–369. doi: 10.1046/j.1365-2141.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 11.Sankaran V.G., Weiss M.J. Anemia: progress in molecular mechanisms and therapies. Nat. Med. 2015;21:221–230. doi: 10.1038/nm.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itano H.A., Bergren W.R., Sturgeon P. The abnormal human hemoglobins. Medicine. 1956;35:121–159. doi: 10.1097/00005792-195605000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Frischer H., Bowman J. Hemoglobin E, an oxidatively unstable mutation. J. Lab. Clin. Med. 1975;85:531–539. [PubMed] [Google Scholar]
- 14.Scott M.D., van den Berg J.J., Repka T., Rouyer-Fessard P., Hebbel R.P., Beuzard Y., Lubin B.H. Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J. Clin. Investig. 1993;91:1706–1712. doi: 10.1172/JCI116380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott M.D. H2O2 injury in beta thalassemic erythrocytes: protective role of catalase and the prooxidant effects of GSH. Free Radic. Biol. Med. 2006;40:1264–1272. doi: 10.1016/j.freeradbiomed.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V., Kumar B., Kumar G., Saxena R. Alpha globin gene numbers: an important modifier of HbE/beta thalassemia. Hematology. 2009;14:297–300. doi: 10.1179/102453309X446126. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q., Fabry M.E., Rybicki A.C., Suzuka S.M., Balazs T.C., Etzion Z., de Jong K., Akoto E.K., Canterino J.E., Kaul D.K., Kuypers F.A., Lefer D., Bouhassira E.E., Hirsch R.E. A transgenic mouse model expressing exclusively human hemoglobin E: indications of a mild oxidative stress. Blood Cells Mol. Dis. 2012;48:91–101. doi: 10.1016/j.bcmd.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen U., Dasgupta J., Choudhury D., Datta P., Chakrabarti A., Chakrabarty S.B., Chakrabarty A., Dattagupta J.K. Crystal structures of HbA2 and HbE and modeling of hemoglobin delta 4: interpretation of the thermal stability and the antisickling effect of HbA2 and identification of the ferrocyanide binding site in Hb. Biochemistry. 2004;43:12477–12488. doi: 10.1021/bi048903i. [DOI] [PubMed] [Google Scholar]
- 19.Roche C.J., Malashkevich V., Balazs T.C., Dantsker D., Chen Q., Moreira J., Almo S.C., Friedman J.M., Hirsch R.E. Structural and functional studies indicating altered redox properties of hemoglobin E: implications for production of bioactive nitric oxide. J. Biol. Chem. 2011;286:23452–23466. doi: 10.1074/jbc.M110.183186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng L., Zhou S., Gu L., Gell D.A., Mackay J.P., Weiss M.J., Gow A.J., Shi Y. Structure of oxidized alpha-haemoglobin bound to AHSP reveals a protective mechanism for haem. Nature. 2005;435:697–701. doi: 10.1038/nature03609. [DOI] [PubMed] [Google Scholar]
- 21.Mollan T.L., Alayash A.I. Redox reactions of hemoglobin: mechanisms of toxicity and control. Antioxid. Redox Signal. 2013;18:2251–2253. doi: 10.1089/ars.2013.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiedermann B.L., Olson J.S. Acceleration of tetramer formation by the binding of inositol hexaphosphate to hemoglobin dimers. J. Biol. Chem. 1975;250:5273–5275. [PubMed] [Google Scholar]
- 23.Mollan T.L., Jia Y., Banerjee S., Wu G., Kreulen R.T., Tsai A.L., Olson J.S., Crumbliss A.L., Alayash A.I. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free Radic. Biol. Med. 2014;69:265–277. doi: 10.1016/j.freeradbiomed.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzym. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q., Bouhassira E.E., Besse A., Suzuka S.M., Fabry M.E., Nagel R.L., Hirsch R.E. Generation of transgenic mice expressing human hemoglobin E. Blood Cells Mol. Dis. 2004;33:303–307. doi: 10.1016/j.bcmd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee R., Alpert Y., Leterrier F., Williams R.J. Visible absorption and electron spin resonance spectra of the isolated chains of human hemoglobin. Discussion of chain-mediated heme-heme interaction. Biochemistry. 1969;8:2862–2867. doi: 10.1021/bi00835a025. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Z.Y., Woollard A.C., Wolff S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- 28.Berzofsky J.A., Peisach J., Blumberg W.E. Sulfheme proteins. I. Optical and magnetic properties of sulfmyoglobin and its derivatives. J. Biol. Chem. 1971;246:3367–3377. [PubMed] [Google Scholar]
- 29.Mollan T.L., Banerjee S., Wu G., Parker Siburt C.J., Tsai A.L., Olson J.S., Weiss M.J., Crumbliss A.L., Alayash A.I. Alpha-hemoglobin stabilizing protein (AHSP) markedly decreases the redox potential and reactivity of alpha-subunits of human HbA with hydrogen peroxide. J. Biol. Chem. 2013;288:4288–4298. doi: 10.1074/jbc.M112.412064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benesch R.E., Benesch R., Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal. Biochem. 1973;55:245–248. doi: 10.1016/0003-2697(73)90309-6. [DOI] [PubMed] [Google Scholar]
- 31.Hargrove M.S., Singleton E.W., Quillin M.L., Ortiz L.A., Phillips G.N., Jr., Olson J.S., Mathews A.J. His64(E7)–>Tyr apomyoglobin as a reagent for measuring rates of hemin dissociation. J. Biol. Chem. 1994;269:4207–4214. doi: 10.2210/pdb1mgn/pdb. [DOI] [PubMed] [Google Scholar]
- 32.Strader M.B., Hicks W.A., Kassa T., Singleton E., Soman J., Olson J.S., Weiss M.J., Mollan T.L., Wilson M.T., Alayash A.I. Post-translational transformation of methionine to aspartate is catalyzed by heme iron and driven by peroxide: a novel subunit-specific mechanism in hemoglobin. J. Biol. Chem. 2014;289:22342–22357. doi: 10.1074/jbc.M114.568980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller A., Nesvizhskii A.I., Kolker E., Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald V.W., Charache S. Drug-induced oxidation and precipitation of hemoglobins A, S and C. Biochim. Biophys. Acta. 1982;701:39–44. doi: 10.1016/0167-4838(82)90309-0. [DOI] [PubMed] [Google Scholar]
- 35.Jia Y., Alayash A.I. Effects of cross-linking and zero-link polymerization on oxygen transport and redox chemistry of bovine hemoglobin. Biochim. Biophys. Acta. 2009;1794:1234–1242. doi: 10.1016/j.bbapap.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Vandegriff K.D., Malavalli A., Minn C., Jiang E., Lohman J., Young M.A., Samaja M., Winslow R.M. Oxidation and haem loss kinetics of poly(ethylene glycol)-conjugated haemoglobin (MP4): dissociation between in vitro and in vivo oxidation rates. Biochem. J. 2006;399:463–471. doi: 10.1042/BJ20060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hargrove M.S., Krzywda S., Wilkinson A.J., Dou Y., Ikeda-Saito M., Olson J.S. Stability of myoglobin: a model for the folding of heme proteins. Biochemistry. 1994;33:11767–11775. doi: 10.1021/bi00205a012. [DOI] [PubMed] [Google Scholar]
- 38.Pimenova T., Pereira C.P., Gehrig P., Buehler P.W., Schaer D.J., Zenobi R. Quantitative mass spectrometry defines an oxidative hotspot in hemoglobin that is specifically protected by haptoglobin. J. Proteome Res. 2010;9:4061–4070. doi: 10.1021/pr100252e. [DOI] [PubMed] [Google Scholar]
- 39.Deterding L.J., Ramirez D.C., Dubin J.R., Mason R.P., Tomer K.B. Identification of free radicals on hemoglobin from its self-peroxidation using mass spectrometry and immuno-spin trapping: observation of a histidinyl radical. J. Biol. Chem. 2004;279:11600–11607. doi: 10.1074/jbc.M310704200. [DOI] [PubMed] [Google Scholar]
- 40.Gunther M.R., Tschirret-Guth R.A., Witkowska H.E., Fann Y.C., Barr D.P., Ortiz De Montellano P.R., Mason R.P. Site-specific spin trapping of tyrosine radicals in the oxidation of metmyoglobin by hydrogen peroxide. Biochem. J. 1998;330:1293–1299. doi: 10.1042/bj3301293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thom C.S., Dickson C.F., Gell D.A., Weiss M.J. Hemoglobin variants: Biochemical properties and Clinical Correlates. Cold Spring Harb. Perspect. Med. 2013;3:a011858. doi: 10.1101/cshperspect.a011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham B., Hicks W., Jia Y., Baek J.H., Miller J.L., Alayash A.I. Isolated Hb providence beta82Asn and beta82Asp fractions are more stable than native HbA(0) under oxidative stress conditions. Biochemistry. 2011;50:9752–9766. doi: 10.1021/bi200876e. [DOI] [PubMed] [Google Scholar]
- 43.Graves P.E., Henderson D.P., Horstman M.J., Solomon B.J., Olson J.S. Enhancing stability and expression of recombinant human hemoglobin in E. coli: progress in the development of a recombinant HBOC source. Biochim. Biophys. Acta. 2008;1784:1471–1479. doi: 10.1016/j.bbapap.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Hebbel R.P., Morgan W.T., Eaton J.W., Hedlund B.E. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc. Natl. Acad. Sci. USA. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macdonald V.W., Charache S. Differences in the reaction sequences associated with drug-induced oxidation of hemoglobins E, S, A, and F. J. Lab. Clin. Med. 1983;102:762–772. [PubMed] [Google Scholar]
- 46.Banerjee M., Pramanik M., Bhattacharya D., Lahiry M., Chakrabarti A. Faster heme loss from hemoglobin E than HbS, in acidic pH: effect of aminophospholipids. J. Biosci. 2011;36:809–816. doi: 10.1007/s12038-011-9163-5. [DOI] [PubMed] [Google Scholar]
- 47.Chiu D.T., van den Berg J., Kuypers F.A., Hung I.J., Wei J.S., Liu T.Z. Correlation of membrane lipid peroxidation with oxidation of hemoglobin variants: possibly related to the rates of hemin release. Free Radic. Biol. Med. 1996;21:89–95. doi: 10.1016/0891-5849(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 48.Kong Y., Zhou S., Kihm A.J., Katein A.M., Yu X., Gell D.A., Mackay J.P., Adachi K., Foster-Brown L., Louden C.S., Gow A.J., Weiss M.J. Loss of alpha-hemoglobin-stabilizing protein impairs erythropoiesis and exacerbates beta-thalassemia. J. Clin. Investig. 2004;114:1457–1466. doi: 10.1172/JCI21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alayash A.I. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat. Biotechnol. 1999;17:545–549. doi: 10.1038/9849. [DOI] [PubMed] [Google Scholar]
- 50.Alayash A.I., Patel R.P., Cashon R.E. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid. Redox Signal. 2001;3:313–327. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- 51.Schaer D.J., Buehler P.W., Alayash A.I., Belcher J.D., Vercellotti G.M. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratanasopa K., Strader M.B., Alayash A.I., Bulow L. Dissection of the radical reactions linked to fetal hemoglobin reveals enhanced pseudoperoxidase activity. Front. Physiol. 2015;6:39. doi: 10.3389/fphys.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeder B.J., Grey M., Silaghi-Dumitrescu R.L., Svistunenko D.A., Bulow L., Cooper C.E., Wilson M.T. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J. Biol. Chem. 2008;283:30780–30787. doi: 10.1074/jbc.M804709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasimuzzaman M., Khandros E., Wang X., Kong Y., Zhao H., Weiss D., Rivella S., Weiss M.J., Persons D.A. Analysis of alpha hemoglobin stabilizing protein overexpression in murine beta-thalassemia. Am. J. Hematol. 2010;85:820–822. doi: 10.1002/ajh.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viprakasit V., Tanphaichitr V.S., Chinchang W., Sangkla P., Weiss M.J., Higgs D.R. Evaluation of alpha hemoglobin stabilizing protein (AHSP) as a genetic modifier in patients with beta thalassemia. Blood. 2004;103:3296–3299. doi: 10.1182/blood-2003-11-3957. [DOI] [PubMed] [Google Scholar]
- 56.Lim W.F., Muniandi L., George E., Sathar J., Teh L.K., Gan G.G., Lai M.I. Alpha-haemoglobin stabilising protein expression is influenced by mean cell haemoglobin and HbF levels in HbE/beta-thalassaemia individuals. Blood Cells Mol. Dis. 2012;48:17–21. doi: 10.1016/j.bcmd.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Mettananda S., Gibbons R.J., Higgs D.R. Alpha-globin as a molecular target in treatment of beta-thalassemia. Blood. 2015;125:3694–3701. doi: 10.1182/blood-2015-03-633594. [DOI] [PMC free article] [PubMed] [Google Scholar]