Abstract
Background:
Obstructive sleep apnea (OSA) is a common sleep disorder and is characterized by airway collapse at multiple levels of upper airway. The effectiveness of nasal surgery has been discussed in several studies and shows a promising growing interest. In this study, we intended to evaluate the effects of nasal surgery on the upper airway dimensions in patients with OSA using three-dimensional (3D) reconstruction of cone-beam computed tomography (CT).
Methods:
Twelve patients with moderate to severe OSA who underwent nasal surgery were included in this study. All patients were diagnosed with OSA using polysomnography (PSG) in multi sleep health centers associated with Massachusetts General Hospital, Massachusetts Eye and Ear Infirmary and the Partners Health Care from May 31, 2011 to December 14, 2013. The effect of nasal surgery was evaluated by the examination of PSG, subjective complains, and 3D reconstructed CT scan. Cross-sectional area was measured in eleven coronal levels, and nasal cavity volume was evaluated from anterior nasal spine to posterior nasal spine. The thickness of soft tissue in oral pharynx region was also measured.
Results:
Five out of the 12 patients were successfully treated by nasal surgery, with more than 50% drop of apnea–hypopnea index. All the 12 patients showed significant increase of cross-sectional area and volume postoperatively. The thickness of soft tissue in oral pharynx region revealed significant decrease postoperatively, which decreased from 19.14 ± 2.40 cm2 and 6.11 ± 1.76 cm2 to 17.13 ± 1.91 cm2 and 5.22 ± 1.20 cm2.
Conclusions:
Nasal surgery improved OSA severity as measured by PSG, subjective complaints, and 3D reconstructed CT scan. 3D assessment of upper airway can play an important role in the evaluation of treatment outcome.
Keywords: Nasal Surgery, Obstructive Sleep Apnea, Postoperative Outcome, Three-dimensional Computed Tomography Scan
INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic and increasingly common condition affecting adults today. It is characterized by recurrent collapse of the upper airway during sleep and has an impact on a series of biomechanical and physiological changes that occur during the development of upper airway stenosis.[1] The typical symptoms of OSA include snoring, apnea and hypopnea during sleep, and excessive daytime sleepiness.[2] Left untreated, OSA may also have a strong association with increased cardiovascular morbidity and mortality, impairment of cognitive function, motor vehicle collisions, and reduced quality of life (QOL).[3,4,5] Examination by polysomnography (PSG) is used for the diagnosis and evaluation of OSA traditionally. Treatment for OSA consists of continuous positive airway pressure (CPAP), which has become the standard therapy for OSA since first described in 1982 by Bridgman and Dunn. Recently, there is a rapidly growing body of literature studying surgical intervention as the treatment of OSA. The long-term effectiveness of surgical treatment is estimated to range between 50% and 78%, depending on the surgical procedure applied.[6,7] Nasal surgery, in particular, has been appraised in multiple studies. As a solitary intervention, nasal surgery may not be supported as an OSA treatment modality, but this procedure has been shown to be effective at decreasing CPAP pressure settings.[8,9,10] Although it is a fact that nasal cavity obstruction should be the source of upper airway obstruction and have impact on the reconstruction of pharynx and larynx cavity, to date, there have not been any published studies specifically addressing this issue. Nonetheless, nasal surgery, and septoplasty in particular, continues to be widely deployed for this purpose.
To date, there is growing interest in the ways of understanding the narrowing upper airway, including both theories of neuromuscular regulation and theories of fluid structure interaction.[11,12] Evaluation of the airway has become an important aspect in OSA treatment planning. When trying to understand the status of OSA, with or without a diagnostic PSG study, it is important to find out the changes that narrowed nasal cavity causes in the airway dimensions. Imaging methods to view the airway include cephalometric radiographs, cone-beam computed tomography (CBCT), and magnetic resonance imaging (MRI). However, the disadvantage of cephalometry is that it is generally performed when individuals are either sitting or standing, positions that clearly differ from their position during sleep. Therefore, 2D evaluation methods such as cephalometry are considered inappropriate for the evaluation of changes in upper airway form during OSA therapy. Studies have shown that both CBCT and MRI are accurate ways to measure the airway in a 3D patent.[13,14,15,16] Compared with MRI, CBCT offers an easier, faster, and more accurate method to obtain a view of a patient's 3D airway image.
In this study, we intended to evaluate the effects of nasal surgery on the upper airway dimensions in patients with OSA using 3D reconstruction of CBCT.
METHODS
Subjects
This study was performed with the approval of the Institutional Review Board of Massachusetts Eye and Ear Infirmary. The study subjects included 12 patients (10 males and 2 females) who were diagnosed with OSA using PSG in multi sleep health centers associated with Massachusetts General Hospital, Massachusetts Eye and Ear Infirmary and the Partners Health Care. The diagnosis of OSA was based on recognized criteria, including an apnea–hypopnea index (AHI) of >5/h during sleep and pathological daytime sleepiness. We judged the therapeutic effect of nasal surgery by PSG, which showed an AHI of <10 or a decrease of 50% in AHI of postnasal surgery when compared with AHI changes of prenasal surgery. Mean age, preoperation body mass index (BMI), AHI, and minimum pulse oxygen saturation (minSpO2) of the patients were 46.5 ± 10.77 years, 29.36 ± 3.65 kg/m2, 50.93 ± 30.84/h, and 80.67 ± 8.21%, respectively [Table 1]. The severity of OSA was moderate (AHI: 15–29.9/h during sleep) in two patients and severe (AHI: over 30/h during sleep) in ten patients.
Table 1.
Clinical characteristics of the 12 patients with obstructive sleep apnea
| Patients | n | Age (years) | Preoperation | Postoperation | ||||
|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | AHI (/h) | Minimum SpO2 (%) | BMI (kg/m2) | AHI (/h) | Min SpO2 (%) | |||
| Male | 10 | 45.30 ± 11.48 | 29.52 ± 3.90 | 43.58 ± 17.63 | 82.90 ± 4.43 | 28.43 ± 3.49 | 21.78 ± 8.53 | 85.50 ± 3.31 |
| Female | 2 | 52.50 ± 2.12 | 28.65 ± 2.90 | 87.65 ± 66.54 | 69.50 ± 16.62 | 28.65 ± 2.90 | 42.80 ± 38.47 | 75.75 ± 18.74 |
| Total | 12 | 46.50 ± 10.77 | 29.38 ± 3.65 | 50.93 ± 30.84 | 80.67 ± 8.21 | 28.47 ± 3.28 | 25.28 ± 16.15 | 83.88 ± 7.43 |
BMI: Body mass index; AHI: Apnea–hypopnea index; minSpO2: Minimum pulse oxygen saturation.
Surgery
After preoperation assessment including subjective complains (snoring, sleep apnea, nasal blockage, mouth breathing, and daytime fatigue) and objective assessment (BMI, ENT examination, PSG, and computed tomography [CT] scan), all 12 patients went through bilateral endoscopic total ethmoidectomy, bilateral endoscopic middle meatal antrostomy with removal of maxillary sinus tissue, bilateral submucous resection of the inferior turbinates, and bilateral outfracturing of the inferior turbinates. Following surgery, patients were assessed initially at 3 weeks postoperatively and then instructed to follow-up with their pulmonologist and CPAP vendor to restart nasal CPAP therapy, if necessary.
Computed tomography scan evaluation
In all patients, CT from the head to the neck was performed pre- and post-operation with a slice thickness of 2 mm. Each patient was placed in the supine position, and the head was fixed such that the Frankfurt plane was perpendicular to the floor. CT was performed without swallowing or respiratory movements during inspiration at rest, and it was consecutively performed pre- and post-operation in each patient. The definition of nasal cavity is described by four boundaries [Figure 1]. Anterior boundary: line connecting the anterior nasal spine (ANS) and the apex of the nasal bone; posterior boundary: line extending from sella (S) to posterior nasal spine (PNS); superior boundary: line connecting basion or the highest point on the nasal bone 1 mm inferior to the edge of the field of view and S; and inferior boundary: line extending from ANS to PNS. After defining the boundaries, cross-sectional area of the nasal cavity was divided into eleven levels every 4 mm from anterior boundary to posterior boundary pre- and post-operatively. We also measured the thickness of basion posterior airway wall and the most anterior point on the anterior arch of the atlas vertebrae (AA)-posterior airway wall. The thickness of the airway wall is defined as soft tissue measurements. From the obtained CT images, 3D image reconstruction of nasal cavity form was examined, nasal cavity volume and surface area were measured preoperatively, and the volume of the nasal cavity was measured using software OsiriX Lite (http://www.osirix-viewer.com) pre- and post-operatively. The volume rendering technique was utilized for the measurement of volume of the nasal cavity.
Figure 1.
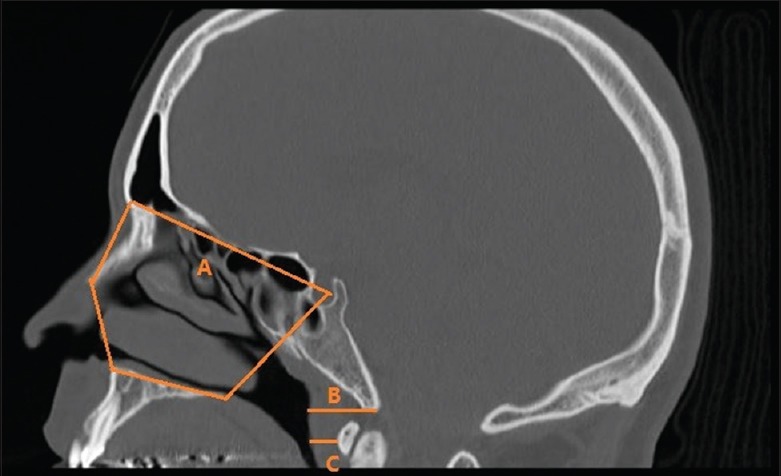
Nasal cavity boundaries (A) Basion posterior airway wall (B) AA-posterior airway wall (C).
Statistical analysis
Student's t-test was used to evaluate changes in nasal cavity's cross-sectional area, volume, as well as soft tissue thickness pre- and post-operatively. One-way analysis of variance (ANOVA) was used to evaluate the difference of nasal cavity's cross–sectional area and level of obstruction among patients with different surgical outcomes. All statistical analyses were conducted using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics
A total of 12 patients received systematical assessment before and after nasal surgery. Septoplasty and turbinoplasty were performed on all patients as primary treatment. There were no complications reported. After 3 months, there was improvement in subjective symptoms, and intranasal endoscopy revealed that the nasal airway had widened and nasal obstruction had significantly improved in all patients.
Analysis of sleep parameters after nasal surgery
The result of postoperation PSG is shown in Table 1. The mean AHI for 12 patients after the surgery was 25.28 ± 16.15 events/h, which had a statistically significant reduction compared with preoperative one (t = 4.610, P < 0.05). The mean SpO2 (%) postoperatively was 83.88 ± 7.43%, also increased significantly after surgical correction (t = −3.412, P < 0.05).
Radiological evaluation
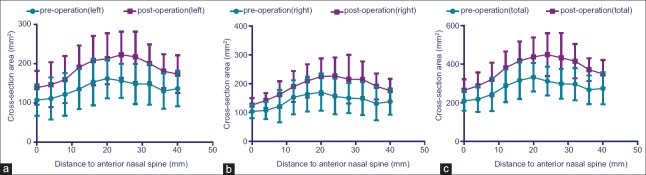
The mean nasal cavity cross-sectional area at each level [Figure 2a and 2b], as calculated from CT images, revealed a significant enlargement after the surgery, either left side, right side, or bilateral [Figure 3a–3c]. The mean area enlargement ratio of left side was 136.96 ± 42.33 at level 1, 148.42 ± 75.56 at level 2, 144.93 ± 67.45 at level 3, 154.24 ± 61.67 at level 4, 152.76 ± 78.26 at level 5, 146.16 ± 82.69 at level 6, 154.53 ± 72.96 at level 7, 175.22 ± 126.25 at level 8, 157.49 ± 95.30 at level 9, 158.56 ± 120.27 at level 10, and 150.71 ± 116.85 at level 11. The mean area enlargement ratio of right side was 126.30 ± 33.29 at level 1, 139.21 ± 35.77 at level 2, 128.76 ± 25.88 at level 3, 129.61 ± 21.99 at level 4, 132.50 ± 19.28 at level 5, 137.05 ± 23.17 at level 6, 152.64 ± 34.78 at level 7, 146.67 ± 40.01 at level 8, 151.71 ± 34.09 at level 9, 137.81 ± 28.04 at level 10, and 134.36 ± 29.29 at level 11. The mean area enlargement of bilateral nasal cavity was 127.31 ± 19.26 at level 1, 135.31 ± 23.91 at level 2, 130.99 ± 28.49 at level 3, 135.55 ± 18.50 at level 4, 135.35 ± 30.46 at level 5, 134.67 ± 30.46 at level 6, 147.38 ± 41.86 at level 7, 153.45 ± 68.98 at level 8, 148.74 ± 51.28 at level 9, 140.62 ± 46.89 at level 10, and 135.48 ± 47.23 at level 11.
Figure 2.
(a) Nasal cavity cross-sectional area at level 1–11 preoperatively in patient no. 2. He is a 37-year-old male (BMI = 33.3 kg/m2, severe right-sided septal deviation with bilaterally enlarged inferior turbinate, AHI = 78.5/h, minimum SaO2 = 87%) and did not adherent to CPAP treatment. (b) Nasal cavity's cross-sectional area at level 1–11 postoperatively in patient no. 2 (postoperative BMI = 29.6 kg/m2, AHI = 21.8/h, minimum SaO2 = 85%). BMI: Body mass index; AHI: Apnea–hypopnea index; CPAP: Continuous positive airway pressure.
Figure 3.
(a) Changes in mean cross-sectional area at level 1–11 of left-sided nasal cavity, pre- and post-operation. Significant increase was found (P < 0.05) when comparing the pre- and post-operative value. (b) Changes in mean cross-sectional area at level 1–11 of right-sided nasal cavity, pre- and post-operation. Significant increase was found (P < 0.05) when comparing the pre- and post-operative value. (c) Changes in mean cross-sectional area at level 1–11 of bilateral nasal cavity, pre- and post-operation. Significant increase was found (P < 0.05) when comparing the pre- and post-operative value.
We also measured the nasal cavity volume both pre- and post-operatively after using OsiriX Lite for 3D image reconstruction. The mean volume of nasal cavity preoperatively was 5.99 ± 1.81 cm3 (left side), 5.83 ± 2.07 cm3 (right side), and 11.82 ± 2.60 cm3 (bilateral). The mean volume of nasal cavity postoperatively was 7.97 ± 1.90 cm3 (left side), 8.02 ± 1.44 cm3 (right side), and 15.99 ± 2.75 cm3 (bilateral), all of which were significantly enlarged compared with preoperative measurement.
No significant difference of cross-sectional area and volume was found between patients who were successfully treated with nasal surgery and patients who did not achieve the goal of successful management.
The measurement of thickness of basion posterior airway wall and AA (most anterior point on the anterior arch of the atlas vertebrae)-posterior airway wall revealed a significant decline from the preoperative status to the postoperative status. The mean preoperative thickness was 19.14 ± 2.40 cm2 and 6.11 ± 1.76 cm2 and the mean postoperative thickness was 17.13 ± 1.91 cm2 and 5.22 ± 1.20 cm2.
DISCUSSION
It is known that the development of OSA has a strong correlation with anatomy and structure of upper airway. The main pathologic condition in OSA is airway collapse and many anatomical factors may contribute to this airway collapse. But to date, the relationship between the structural abnormity of upper airway and pathogenesis of OSA still remains unclear. The epidemiology of OSA shows that half of the patients suffer from the symptoms of nasal blockage and/or obstruction. Abnormalities of the nose, such as septal deviation, nasal polyps, intranasal benign tumors, inferior turbinate hypertrophy, rhinitis, and even malignancies, may cause or aggravate the symptoms of OSA due to severe nasal obstruction and elevated nasal airway resistance.[17] Therefore, nasal surgery with the goals of altering structural abnormalities and improving nasal patent, such as septoplasty, submucous resection, and outfracturing of the inferior turbinates and functional endoscopic sinus surgery may play a positive role in the treatment and management of OSA. There is controversy over whether nasal surgery is effective in managing OSA and whether nasal surgery should still be considered a treatment alternation for OSA. Some evidence suggests that nasal surgery may significantly improve sleep quality, symptoms related to sleep apnea, and furthermore has a positive impact on sleep parameters and sleep structure.[18]
In our study, all 12 patients reported significant relief of symptoms such as nasal obstruction, snoring, and apnea. In addition, we observed significant improvement of sleep parameters, such as AHI and SpO2, and 5 out of 12 patients were considered as successfully managed with a >50% drop of AHI. The measurement of cross-sectional area and volume of nasal cavity shows significant enlargement postoperatively. The structure of nasal cavity after the surgery suggests that by performing nasal surgeries including bilateral endoscopic total ethmoidectomy, bilateral endoscopic middle meatal antrostomy with the removal of maxillary sinus tissue, bilateral submucous resection of the inferior turbinates, and bilateral outfracturing of the inferior turbinates, we can achieve the goals of correction of abnormal structure, enlargement of ventilation cavity, and symmetry of bilateral nasal cavity. Moreover, due to the fact that the upper airway possesses the potential of self-flexibility, thickness of soft tissue in larynx region also appeared to be significantly decreased postoperatively, though the surgery was only operated in the nasal cavity.
For patients with appropriate anatomy, nasal surgery treatment with its associated complete adherence rate may still define a successful outcome even without formal AHI cure. In addition, surgical treatment may also lead to greater sustained overall improvement in patient-specific OSA outcomes. The cure rate in our study sample is 41.67% (5/12). The subgroup of patients we studied in this project was considered anatomically optimal for nasal surgery in terms of having severe septal deviation, obstructed nasal cavity, a favorable oropharynx relationship, and relatively low BMI. For patients who did not achieve the goal of successfully treated with nasal surgery alone, they still benefit from improvement in nasal airflow and are able to decrease the pressure settings of the CPAP machine. Friedman et al. and Sériès et al. both reported decreased CPAP pressures setting for nasal CPAP following nasal surgery.[8,9]
Another interesting result in our study was the decrease of the parameter of soft tissue thickness in oral pharyngeal region. The thickness of basion posterior airway wall and AA (most anterior point on the anterior arch of the atlas vertebrae) - posterior airway wall both revealed a significant decline post operatively. Studies using nasal pharyngoscopy, CT and MRI, or pharyngeal pressure monitoring have shown that one or more sites within the oral pharyngeal region are usually where closure occurs in most subjects with OSA, and this region is also smaller in OSA patients versus controls even during wakefulness.[19,20] Enlargement of soft tissue structures both within and surrounding the airway contributes significantly to pharyngeal airway narrowing and closure in most cases of OSA.[21] However, evidence suggests that experimental reduction of nasal patency and flow has a significant effect on breathing during sleep and oral pharynx region patency. Basner et al. suggested that the activity of the dilator muscles of the upper airway can be modulated by receptors in the nasal mucosa, sensitive to airflow or pressure.[22] In addition, when nasal resistance exceeds a certain level, an air bypass occurs and leads to mouth breathing, resulting in a decrease in the retroglossal dimension, due to the subsequent retraction of the tongue, narrowing of the pharyngeal lumen, increased oscillation and vibration of the soft palate, and redundant tissue of the pharynx.[23] This shift from nasal to oral breathing is physiologically disadvantageous to the individual, leading to an unstable breathing pattern.[24] Relief of nasal obstruction may have positive impact on nasal breathing pattern during sleep time and reduce the risks of soft tissue collapse, which can lead to snoring and other OSA symptoms.
Traditionally, the management of OSA is evaluated with objective medical outcomes such as PSG result. However, subjective assessment such as Epworth Sleepiness Scale (ESS) and Sleep Apnea Quality of Life Index (SAQLI) scores also play important roles in the evaluation of preoperative status and postoperative outcomes. OSA is a disease where medical outcomes (e.g., AHI) frequently do not correlate well with QOL outcomes. In recent years, radiological assessment, especially 3D reconstruction technique has become a novel and well-interested technique for the evaluation of medical outcomes. The advantage of 3D evaluation of the upper airway, especially nasal cavity, during OSA therapy is the accurate visual confirmation of morphological changes in each region of the upper airway and it might promote the patient's motivation for the treatment of OSA. 3D evaluation has been used in other therapies such as the determination of mandibular position during OSA because of its association with treatment effects.[25] 3D evaluation of nasal cavity volume using methods such as CT during OSA therapy may lead to improvement in treatment results. In addition, if 3D reconstructed CT and other radiological assessment can be used to predict results before OSA therapy is initiated, it may be useful for effective evaluation of OSA treatment.
Although ideally, patients who meet the criteria of appropriated anatomy could be successfully treated via nasal surgery treatment that is manifestly not the case in reality. Verse et al. suggested that nasal surgery alone or combined with uvulopalatopharyngoplasty may only have limited effect on the management of severity of OSA or on improvement of sleep quality compared to CPAP treatment.[26] Another study revealed that OSA is not relieved by nasal surgery despite improvement in nasal resistance, and nasal surgery also does not result in a significant reduction in sleep parameters.[27,28] To be clear, we do not advocate that surgery is the best or only solution to OSA. However, we do suggest patients who are anatomically optimal candidates for nasal surgery go through detailed preoperative assessments, such as PSG, upper airway radiological evaluation, BMI measurement, and thorough ENT examination. Subjective assessments such as ESS and SAQLI scores also play important roles in the evaluation of preoperative status and postoperative outcomes. In the modern era of evidence-based medicine and increasingly limited economic resources, more and more reimbursement decisions use subjective outcomes such as QOL to judge therapy effectiveness and appropriateness. OSA is a disease where medical outcomes (e.g., AHI) frequently do not correlate well with QOL outcomes. In our study, all the 12 patients reported significant improvement in subjective symptoms. Right now, the subjective markers for OSA are either nonspecific (ESS) or treatment-related (SAQLI). It would be useful if an OSA-specific QOL marker could be developed and tested.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. doi:10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. doi:10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. doi:10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–6. doi: 10.1164/rccm.200605-629PP. doi:10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 6.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. doi:10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 7.Bridgman SA, Dunn KM. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2000;2:CD001004. doi: 10.1002/14651858.CD001004. doi:10.1002/14651858.CD001004. [DOI] [PubMed] [Google Scholar]
- 8.Friedman M, Tanyeri H, Lim JW, Landsberg R, Vaidyanathan K, Caldarelli D. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122:71–4. doi: 10.1016/S0194-5998(00)70147-1. doi:10.1016/S0194-5998(00)70147-1. [DOI] [PubMed] [Google Scholar]
- 9.Sériès F, St Pierre S, Carrier G. Effects of surgical correction of nasal obstruction in the treatment of obstructive sleep apnea. Am Rev Respir Dis. 1992;146(5 Pt 1):1261–5. doi: 10.1164/ajrccm/146.5_Pt_1.1261. [DOI] [PubMed] [Google Scholar]
- 10.Friedman M, Soans R, Joseph N, Kakodkar S, Friedman J. The effect of multilevel upper airway surgery on continuous positive airway pressure therapy in obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2009;119:193–6. doi: 10.1002/lary.20021. doi:10.1002/lary.20021. [DOI] [PubMed] [Google Scholar]
- 11.Longobardo GS, Evangelisti CJ, Cherniack NS. Analysis of the interplay between neurochemical control of respiration and upper airway mechanics producing upper airway obstruction during sleep in humans. Exp Physiol. 2008;93:271–87. doi: 10.1113/expphysiol.2007.039917. [DOI] [PubMed] [Google Scholar]
- 12.Van Hirtum A, Pelorson X, Lagrée PY. In vitro validation of some flow assumptions for the prediction of the pressure distribution during obstructive sleep apnoea. Med Biol Eng Comput. 2005;43:162–71. doi: 10.1007/BF02345139. [DOI] [PubMed] [Google Scholar]
- 13.Tai K, Park JH, Hayashi K, Yanagi Y, Asaumi JI, Iida S, et al. Preliminary study evaluating the accuracy of MRI images on CBCT images in the field of orthodontics. J Clin Pediatr Dent. 2011;36:211–8. doi: 10.17796/jcpd.36.2.r7853hp574045414. doi:10.17796/jcpd.36.2.r7853hp574045414. [DOI] [PubMed] [Google Scholar]
- 14.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. doi:10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 15.Ghoneima A, Kula K. Accuracy and reliability of cone-beam computed tomography for airway volume analysis. Eur J Orthod. 2013;35:256–61. doi: 10.1093/ejo/cjr099. doi:10.1093/ejo/cjr099. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki T, Takemoto Y, Inada E, Sato H, Saitoh I, Kakuno E, et al. Three-dimensional cone-beam computed tomography analysis of enlargement of the pharyngeal airway by the Herbst appliance. Am J Orthod Dentofacial Orthop. 2014;146:776–85. doi: 10.1016/j.ajodo.2014.08.017. doi:10.1016/j.ajodo.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Kohler M, Bloch KE, Stradling JR. The role of the nose in the pathogenesis of obstructive sleep apnoea and snoring. Eur Respir J. 2007;30:1208–15. doi: 10.1183/09031936.00032007. doi:10.1183/09031936.00032007. [DOI] [PubMed] [Google Scholar]
- 18.McLean HA, Urton AM, Driver HS, Tan AK, Day AG, Munt PW, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J. 2005;25:521–7. doi: 10.1183/09031936.05.00045004. doi:10.1183/09031936.05.00045004. [DOI] [PubMed] [Google Scholar]
- 19.Horner RL, Sanford LD, Pack AI, Morrison AR. Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 1997;778:127–34. doi: 10.1016/s0006-8993(97)01045-7. doi:10.1016/S0006-8993(97)01045-7. [DOI] [PubMed] [Google Scholar]
- 20.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385–400. doi: 10.1164/ajrccm/148.5.1385. doi:10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- 21.Posnick JC, Choi E, Adachie A, Troost T. Correction of symptomatic chronic nasal airway obstruction in conjunction with bimaxillary orthognathic surgery: Does it complicate recovery and is it effective? J Oral Maxillofac Surg. 2015 doi: 10.1016/j.joms.2015.10.021. pii: S0278-239101421-4. doi:10.1016/j.joms.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Basner RC, Simon PM, Schwartzstein RM, Weinberger SE, Weiss JW. Breathing route influences upper airway muscle activity in awake normal adults. J Appl Physiol. 1989;66:1766–71. doi: 10.1152/jappl.1989.66.4.1766. [DOI] [PubMed] [Google Scholar]
- 23.Verse T, Pirsig W. Impact of impaired nasal breathing on sleep-disordered breathing. Sleep Breath. 2003;7:63–76. doi: 10.1007/s11325-003-0063-2. doi:10.1007/s11325-003-0063-2. [DOI] [PubMed] [Google Scholar]
- 24.Georgalas C. The role of the nose in snoring and obstructive sleep apnoea: An update. Eur Arch Otorhinolaryngol. 2011;268:1365–73. doi: 10.1007/s00405-010-1469-7. doi:10.1007/s00405-010-1469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dieltjens M, Vanderveken OM, Hamans E, Verbraecken JA, Wouters K, Willemen M, et al. Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: A prospective clinical study. Sleep Breath. 2013;17:565–72. doi: 10.1007/s11325-012-0721-3. doi:10.1007/s11325-012-0721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verse T, Maurer JT, Pirsig W. Effect of nasal surgery on sleep-related breathing disorders. Laryngoscope. 2002;112:64–8. doi: 10.1097/00005537-200201000-00012. doi:10.1097/00005537-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Kim ST, Choi JH, Jeon HG, Cha HE, Kim DY, Chung YS. Polysomnographic effects of nasal surgery for snoring and obstructive sleep apnea. Acta Otolaryngol. 2004;124:297–300. doi: 10.1080/00016480410016252. doi:10.1080/00016480410016252. [DOI] [PubMed] [Google Scholar]
- 28.Virkkula P, Bachour A, Hytönen M, Salmi T, Malmberg H, Hurmerinta K, et al. Snoring is not relieved by nasal surgery despite improvement in nasal resistance. Chest. 2006;129:81–7. doi: 10.1378/chest.129.1.81. doi:10.1378/chest.129.1.81. [DOI] [PubMed] [Google Scholar]