Abstract
Background:
The aim of this study was to assess the efficacy and safety of vinorelbine and cisplatin (NP chemotherapy) alone or in combination with Aidi injection for the treatment of advanced nonsmall cell lung cancer (NSCLC).
Methods:
Pertinent publications were identified in PubMed, EMBASE, Cochrane Library, CNKI, CQVIP, and Wanfang databases, up to December 8, 2015. After quality assessment of all included randomized controlled trials evaluating Aidi injection combined with NP chemotherapy for the treatment of advanced NSCLC, a meta-analysis was performed by Review Manager 5.2 and STATA 12.0 for statistical analyses.
Results:
Twelve studies including 509 and 503 cases in the experimental and control groups, respectively, were finally analyzed. The meta-analysis revealed that when cisplatin dose ranging from 20 to 40 mg/m2, combination of Aidi injection and NP chemotherapy was statistically different compared with NP chemotherapy alone in enhancing efficiency (relative risk [RR] = 1.24, 95% confidence interval [CI] [1.05–1.47], P = 0.010) and reducing the incidence of Grade II or above nausea and vomiting (RR = 0.49, 95% CI [0.30–0.80], P = 0.005). Meanwhile, with cisplatin ranging from 80 to 120 mg/m2, no significant differences in efficiency (RR = 1.11, 95% CI [0.87–1.42], P = 0.390) and Grade II or above nausea and vomiting (RR = 0.88, 95% CI [0.71–1.10], P = 0.260) were obtained. In addition, Aidi injection combined with NP chemotherapy was superior to NP chemotherapy alone in improving the quality of life, alleviating Grade II or above leukopenia and thrombocytopenia.
Conclusions:
Aidi injection combined with NP chemotherapy can enhance efficiency, improve the quality of life, and decrease adverse effects in patients with advanced NSCLC.
Keywords: Aidi Injection, Meta-analysis, Nonsmall Cell Lung Cancer, Randomized Controlled Trials
INTRODUCTION
Lung cancer is one of the most common malignancies in China, with the incidence increasing year by year. Nonsmall cell lung cancer (NSCLC) accounts for 80% of all lung cancer cases, and nearly two-thirds of NSCLC patients are diagnosed at an advanced stage, with no opportunity of radical surgery.[1] Chemotherapy is a major treatment option for advanced NSCLC. Aidi injection, a traditional Chinese medicine, is an extraction obtained from cantharidin, ginseng, astragalus, and acanthopanax, with effects such as heat-clearing, detoxification, and swelling reduction.[2] Its main components include cantharidin, ginsenoside, astrogaloside, and acanthopanax senticosus polysaccharide. Aidi injection has various pharmacological effects, including tumor angiogenesis inhibition, induction of apoptosis in tumor cells, enhancement of immunity, and relief of chemotherapy-related side effects.[3,4] In recent years, Aidi injection has been widely used in the treatment of lung cancer,[5] primary liver cancer,[6] colorectal cancer,[7] gastric carcinoma,[8] and malignant lymphoma.[9] In NSCLC specifically, an increasing number of clinical trials evaluating Aidi injection combined with platinum-containing chemotherapy have been reported. These studies all showed that Aidi injection significantly enhances the clinical efficacy of chemotherapy, decreases the incidence of adverse side effects, and improves immunity.[10,11] Therefore, this study selected Aidi injection combination with vinorelbine and cisplatin (NP chemotherapy) as research object, evaluating its efficacy and safety in the treatment of advanced NSCLC based on Cochrane systematic evaluation.
METHODS
Literature and search strategy
All randomized controlled trials (RCTs) involving Aidi injection combined with vinorelbine and cisplatin (NP chemotherapy) for patients with advanced NSCLC were meta-analyzed. PubMed, EMBASE, Cochrane Library, CNKI, CQVIP and Wanfang databases were used to source all relevant articles published by December 8, 2015. Search terms included “Aidi injection,” “cisplatin,” “vinorelbine,” “NSCLC,” and “RCT.”
Inclusion and exclusion criteria
Studies were considered for inclusion if they met the following criteria: (1) published RCTs comparing NP chemotherapy versus NP chemotherapy plus Aidi injection for the treatment of NSCLC; (2) Study subjects (a) were patients with stages III and IV NSCLC diagnosed pathologically and (or) cytologically, (b) had Karnofsky status scale ≥50 and (or) time of survival ≥3 months; (3) had no other anti-cancer treatment before the study regimen within 1 month; and (4) had no chemotherapy contraindication before treatment, no significant abnormalities in liver, kidney, and heart functions. Studies were excluded according to the following criteria: (1) non-RCTs; (2) animal experiments, reviews, and other irrelevant studies; (3) studies without relevant indicators of endpoints; (4) including subjects with severe internal medicine diseases and severe infection; and (5) including subjects with other malignancies.
Endpoint indicators
The outcomes investigated included efficiency, quality of life, and adverse effects. According to the World Health Organization Recommendations for Grading of Acute and Subacute Toxicity, toxicity was graded from 0 to IV in severity. The meta-analysis only evaluated the incidence of Grade II or above nausea and vomiting, leukopenia, thrombocytopenia, and hemoglobin decrease.
Data extraction
The included articles were critically appraised by two reviewers, who independently extracted and collected data using a standardized data-extraction protocol. Disagreements were resolved by discussion or expert opinion. For each study, extracted data included title, name of the first author, year of publication, participant characteristics (age, gender), study characteristics (sample size, drug dose in each group, and treatment duration), study outcomes or endpoints, and adverse effects.
Statistical analysis
The meta-analysis was performed using the Review Manager 5.2 software (Cochrane Collaboration, Copenhagen, Denmark) and STATA 12.0 software package (STATA Corporation, College Station, TX, USA). The pooled relative risk (RR) was examined with 95% confidence intervals (CIs). χ2 and I2 tests were used to assess statistical heterogeneity between studies. We used a fixed-effects model in case of no statistical heterogeneity (P > 0.100, I2< 50%); a random-effect model was applied otherwise (P ≤ 0.100, I2≥ 50%). Sensitivity analyses were undertaken by sequentially omitting one single study to estimate the summary effect. Publication bias was assessed by the Begg's funnel plot and Egger's regression test. A value of P < 0.05 was considered statistically significant for all outcomes.
RESULTS
Search results
We identified 693 potentially relevant trials from our initial electronic search, including 21, 40, 1, 203, 212, and 216 from PubMed, EMBASE, Cochrane Library, CNKI, CQVIP, and Wanfang databases, respectively. A total of 643 trials were excluded after screening the titles and abstracts, and 38 trials were excluded after full-text review. Eventually, 12 RCTs[12,13,14,15,16,17,18,19,20,21,22,23] were selected for the meta-analysis. The flowchart presenting the selection process is shown in Figure 1.
Figure 1.
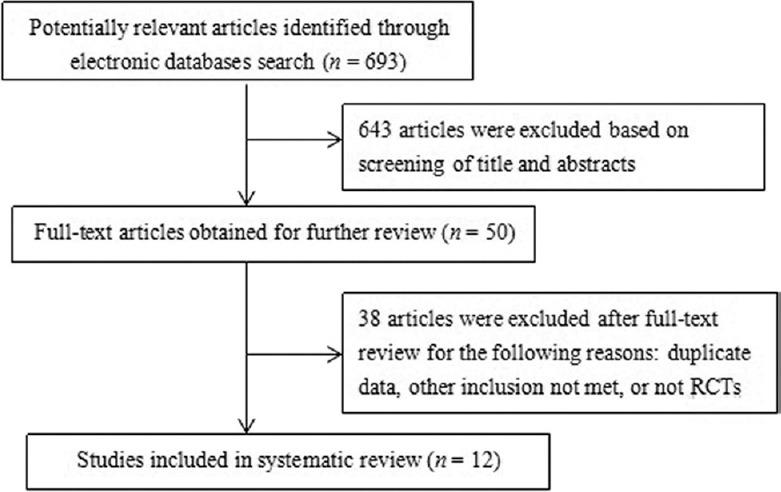
Flow diagram of the literature search for studies on NP chemotherapy versus NP chemotherapy plus Aidi injection for advanced nonsmall cell lung cancer. RCTs: Randomized controlled trials.
Study characteristics and quality assessment
A total of 12 RCTs[12,13,14,15,16,17,18,19,20,21,22,23] met the inclusion criteria, and included 1012 advanced NSCLC patients, with 509 and 503 in the experimental and control groups, respectively; males out-numbered the females. Patients’ ages ranged from 33 to 79 years. There were four pathological types of NSCLC in these studies,[14,15,18,19,22,23] including squamous-cell carcinoma, adenocarcinoma, adeno-squamous carcinoma, and large cell carcinoma. In addition, two studies[14,22] evaluated liver and kidney dysfunction; two studies[12,17] assessed phlebitis; five trials[12,13,15,17,23] evaluated immunological parameters; and one study[23] mentioned survival rate. The main characteristics of the 12 studies were summarized in Table 1. Quality assessment of each study was carried out according to Cochrane handbook 5.1.0, including randomization, allocation concealment, quality of blinding (participants and personal, and outcome assessment), withdrawal and loss to follow-up, and reporting bias. Quality evaluation of the above studies is shown in Figure 2.
Table 1.
Characteristics of randomized controlled trials included in the systemic review
| Included studies | Arm | Means of intervention | Male/female (n) | Age (years), range | Time (days) | Outcome |
|---|---|---|---|---|---|---|
| Xing et al. 2014[12] | EG | NVB 25 mg/m2 + DDP 80 mg/m2 + Aidi 50 ml | 36/24 | 62–78 | ≥42 | ①②③④⑧⑨ |
| CG | NVB 25 mg/m2 + DDP 80 mg/m2 | 38/22 | 64–77 | |||
| Huang et al. 2008[13] | EG | NVB 25 mg/m2 + DDP 30 mg/m2 + Aidi 50 ml | – | – | 56 | ①②③⑧ |
| CG | NVB 25 mg/m2 + DDP 30 mg/m2 | – | – | |||
| Xu et al. 2013[14] | EG | NVB 25 mg/m2 + DDP 80 mg/m2 + Aidi 100 ml | 27/8 | 35–75 | ≥42 | ①②③④⑤ |
| CG | NVB 25 mg/m2 + DDP 80 mg/m2 | 26/9 | 33–76 | |||
| Zhang et al. 2005[15] | EG | NVB 25 mg/m2 + DDP 30 mg/m2 + Aidi 50 ml | – | – | 21 | ①②⑧ |
| CG | NVB 25 mg/m2 + DDP 30 mg/m2 | – | – | |||
| Xu et al. 2007[16] | EG | NVB 25 mg/m2 + DDP 40 mg/m2 + Aidi 50 ml | – | – | 84 | ①③④ |
| CG | NVB 25 mg/m2 + DDP 40 mg/m2 | – | – | |||
| Cui and Wang 2005[17] | EG | NVB 25 mg/m2 + DDP 80 mg/m2 + Aidi 50 ml | – | – | ≥42 | ①②③④⑧⑨ |
| CG | NVB 25 mg/m2 + DDP 80 mg/m2 | – | – | |||
| Zhang et al. 2006[18] | EG | NVB 25 mg/m2 + DDP 30 mg/m2 + Aidi 50 ml | – | – | ≥84 | ①②③④ |
| CG | NVB 25 mg/m2 + DDP 30 mg/m2 | – | – | |||
| Xia et al. 2014[19] | EG | NVB 25 mg/m2 + DDP 25 mg/m2 + Aidi 50 ml | 15/8 | – | 21 | ①②③④ |
| CG | NVB 25 mg/m2 + DDP 25 mg/m2 | 14/9 | – | |||
| Zhao and Yang 2009[20] | EG | NVB 25 mg/m2 + DDP 35 mg/m2 + Aidi 50 ml | 28/12 | – | ≥30 | ①②③④ |
| CG | NVB 25 mg/m2 + DDP 35 mg/m2 | 30/13 | – | |||
| Zhang et al. 2003[21] | EG | NVB 25 mg/m2 + DDP 20–40 mg/m2 + Aidi 40–50 ml | 36/13 | 63.0 ± 7.8 | ≥42 | ①②③④ |
| CG | NVB 25 mg/m2 + DDP 20–40 mg/m2 | 38/11 | 63.0 ± 7.9 | |||
| Zhang and Lu 2014[22] | EG | NVB 25 mg/m2 + DDP 25 mg/m2 + Aidi 50 ml | 32/13 | 40–79 | 42 | ①②③④⑤ |
| CG | NVB 25 mg/m2 + DDP25 mg/m2 | 31/14 | 40–79 | |||
| Wang et al. 2004[23] | EG | NVB 30 mg/m2 + DDP 120 mg/m2 + Aidi 40 ml | – | – | 84 | ①②③⑧ |
| CG | NVB 30 mg/m2 + DDP 120 mg/m2 | – | – |
Values are n or mean ± SD or range. EG: Experimental group; CG: Control group; NVB: Vinorelbine; DDP: Cisplatin; –: Unclear. Outcome: ①efficacy rate; ②quality of life; ③myelosuppression; ④gastrointestinal reaction; ⑤damage of liver and kidney; ⑥neurotoxicity; ⑦alopecia; ⑧immune function; ⑨Phlebitis.
Figure 2.
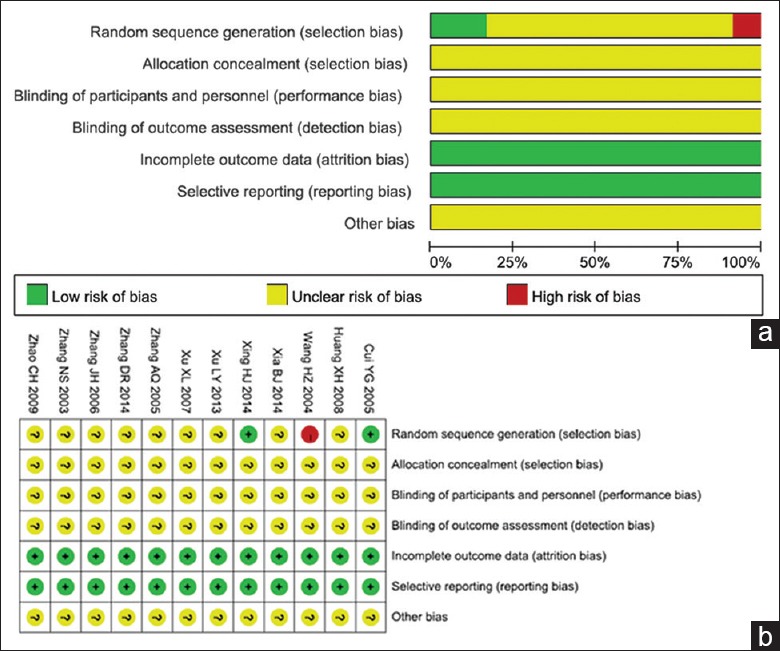
Methodological quality of the included studies assessing Aidi injection combined with NP chemotherapy for the treatment of nonsmall cell lung cancer. (a) Bias risk in clinical studies; (b) Summary of bias risk in clinical studies.
Results of the meta-analysis
Efficiency
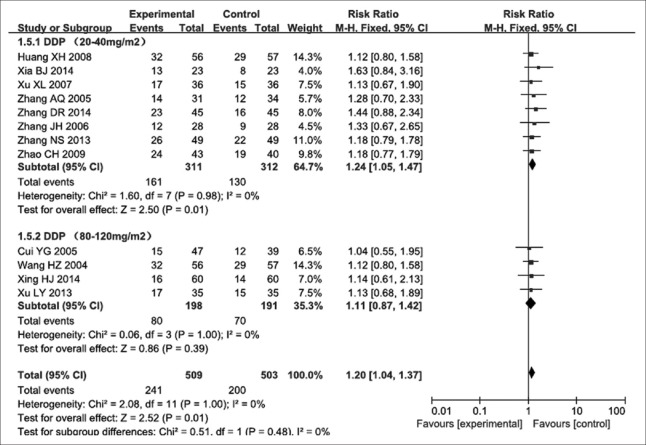
Data for efficiency were available from twelve trials,[12,13,14,15,16,17,18,19,20,21,22,23] which consisted of 1012 patients with advanced NSCLC, 509 and 503 in the experimental and control groups, respectively. According to our analysis, no significant heterogeneity (P = 1.000, I2 = 0) was found in these 12 studies. Therefore, we used a fixed-effects model to assess findings from these trials. The results of the meta-analysis showed that at cisplatin dose ranging from 20 to 40 mg/m2, efficiency of NP chemotherapy combined with Aidi injection was higher than that of NP chemotherapy alone for treating advanced NSCLC. The RR for efficiency was 1.24, with a 95% CI of 1.05–1.47 (P < 0.010) [Figure 3]. Meanwhile, when cisplatin was used at 80–120 mg/m2, the RR for efficiency was 1.11, with a 95% CI of 0.87–1.42 (P = 0.390) [Figure 3], showing no significant difference.
Figure 3.
Forest plots of recent efficiency in advanced nonsmall cell lung cancer patients between NP chemotherapy and NP chemotherapy plus Aidi injection. DDP: Cisplatin.
Quality of life
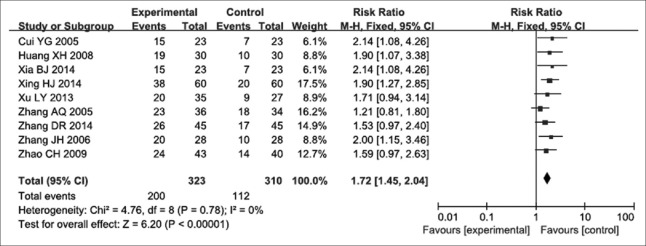
Nine of the RCTs[12,13,14,15,17,18,19,20,22] were fully compliant with the inclusion criteria. They contained 633 participants, including 323 and 310 cases in the experimental and control groups, respectively. With no significant heterogeneity (P = 0.780, I2 = 0) in the nine studies, a fixed-effects model was applied to assess their findings. The RR for quality of life was 1.72, with a 95% CI of 1.45–2.04 (P < 0.000) [Figure 4]. These results indicated a statistically significant difference in the quality of life of advanced NSCLC patients between NP chemotherapy alone and NP chemotherapy plus Aidi injection.
Figure 4.
Forest plots of the quality of life in advanced nonsmall cell lung cancer patients between NP chemotherapy and NP chemotherapy plus Aidi injection.
Toxicities
Grade II or above nausea and vomiting
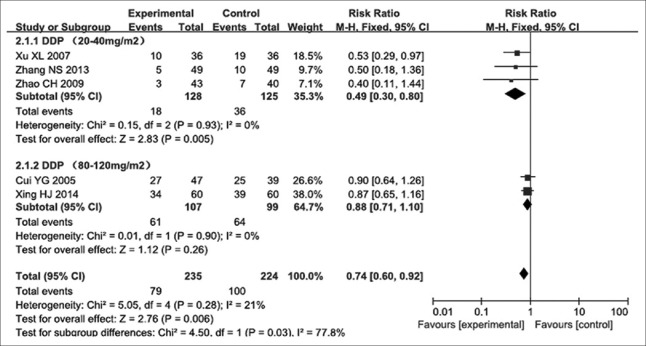
A total of five studies[12,16,17,20,21] assessed 459 participants, including 235 and 224 cases in the experimental and control groups, respectively. The heterogeneity between these trials was not significant (P = 0.28, I2 = 21%); thus, they were considered to be homogeneous, and a fixed-effects model was used for analysis. The results indicated that at cisplatin dose ranging from 20 to 40 mg/m2, the RR for Grade II or above nausea and vomiting was 0.49, with a 95% CI of 0.30–0.80 (P = 0.005), suggesting that the patients who received NP chemotherapy plus Aidi injection were more likely to show decreased incidence of Grade II or above nausea and vomiting in advanced NSCLC than those administered NP chemotherapy treatment alone. However, at cisplatin dose ranging from 80 to 120 mg/m2, the RR for Grade II or above nausea and vomiting was 0.88, with a 95% CI of 0.71–1.10 (P = 0.260) [Figure 5], showing no significant difference.
Figure 5.
Forest plots of Grade II and above nausea and vomiting in advanced nonsmall cell lung cancer patients between NP chemotherapy and NP chemotherapy plus Aidi injection. DDP: Cisplatin.
Grade II or above leukopenia and thrombocytopenia
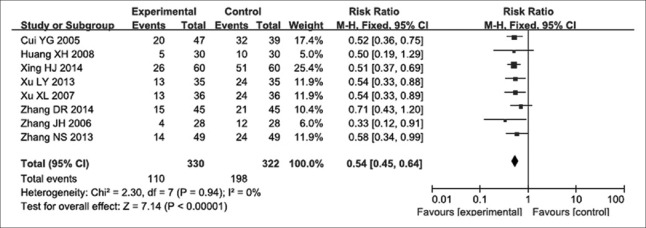
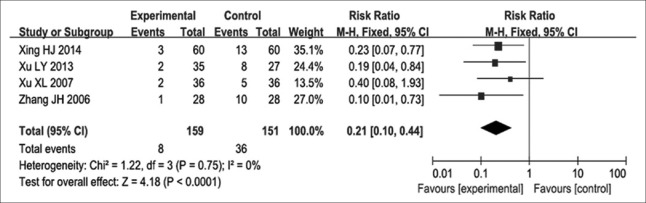
In eight studies[12,13,14,15,18,20,21,22] reporting Grade II or above leukopenia, 652 participants were evaluated, including 330 and 322 in the experimental and control groups, respectively. The eight studies had no heterogeneity (P = 0.940, I2 = 0). Therefore, a fixed-effects model was used to assess their findings. We found an RR for Grade II or above leukopenia of 0.54, with 95% CI of 0.45–0.64 (P < 0.000) [Figure 6], indicating that Aidi injection combined with NP chemotherapy decreased the incidence of Grade II or above leukopenia compared with NP chemotherapy alone. Four studies[12,14,16,18] including 310 participants were analyzed, of which 159 and 151 cases were in experimental and control groups, respectively. No heterogeneity was found in these studies (P = 0.750, I2 = 0), and the fixed-effects model was therefore used for analysis. The RR for Grade II or above thrombocytopenia was 0.21, with 95% CI of 0.10–0.44 (P < 0.000) [Figure 7], suggesting that NP chemotherapy combined with Aidi injection might decrease the incidence of Grade II or above thrombocytopenia in patients with advanced NSCLC.
Figure 6.
Forest plots of Grade II and above leukopenia in advanced nonsmall cell lung cancer patients between NP chemotherapy and NP chemotherapy plus Aidi injection.
Figure 7.
Forest plots of Grade II and above thrombocytopenia in advanced nonsmall cell lung cancer patients between NP chemotherapy and NP chemotherapy plus Aidi injection.
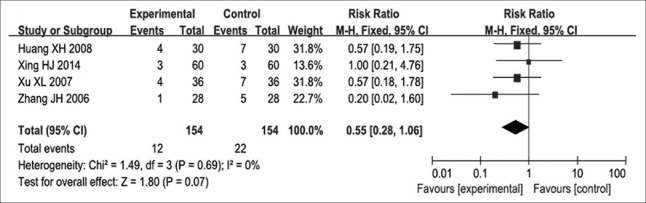
Grade II or above hemoglobin decrease
Four of the included RCTs[12,13,16,18] evaluated Grade II or above hemoglobin decrease. There were 308 patients in these trials, with 154 cases in the experimental group and 154 controls. These trials showed no significant heterogeneity (P = 0.690, I2 = 0) and were considered to be homogeneous; a fixed-effects model was used for analysis. The results indicated that an RR for Grade II or above hemoglobin of 0.55, with 95% CI of 0.28–1.06 (P = 0.070) [Figure 8]. Although no statistically significant differences were obtained, these results suggested that patients who received NP chemotherapy plus Aidi injection were more likely to show decreased incidence of Grade II or above hemoglobin after advanced NSCLC than those administered NP chemotherapy treatment alone.
Figure 8.
Forest plots of Grade II and above hemoglobin in advanced nonsmall cell lung cancer patients between NP chemotherapy and NP chemotherapy plus Aidi injection.
Sensitivity analysis
A sensitivity analysis was performed by sequentially omitting one single study to estimate the summary effect. The combined effect after exclusion was close to that before exclusion, with identical conclusions, suggesting that the stability of the combined analysis result was superior. Sensitivity analysis of efficiency is shown in Figure 9.
Figure 9.
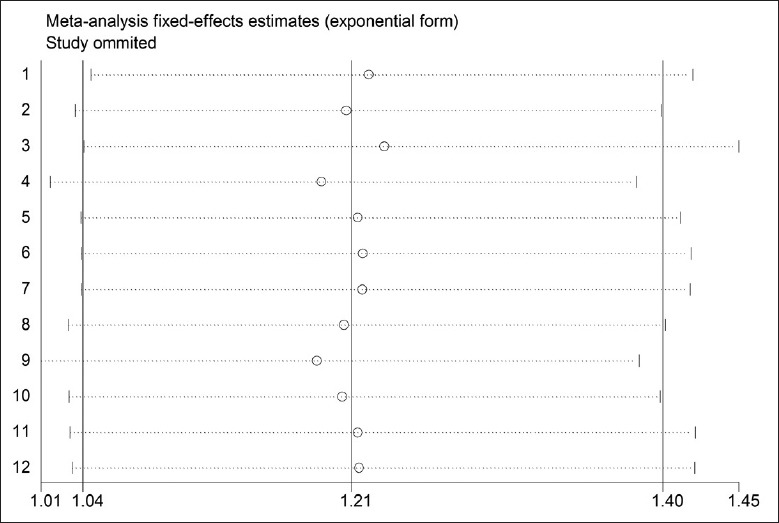
Sensitivity analysis of efficiency in advanced nonsmall cell lung cancer patients between NP chemotherapy and NP chemotherapy plus Aidi injection. X-axis: Sensitivity (95% confidence intervals); Y-axis: Study ID.
Publication bias
Begg's and Egger's tests were performed to examine potential publication bias among the included studies, and no evidence of publication bias was found for outcomes of efficiency (P = 0.304 and P = 0.194 for Begg's and Egger's tests, respectively), quality of life (P = 0.076 and P = 0.095 for Begg's and Egger's tests, respectively), Grade II or above nausea and vomiting (P = 0.086 and P = 0.096 for Begg's and Egger's tests, respectively), Grade II or above leukopenia (P = 0.902 and P = 0.138 for Begg's and Egger's tests, respectively), Grade II or above thrombocytopenia (P = 0.734 and P = 0.553 for Begg's and Egger's tests, respectively), and Grade II or above hemoglobin decrease (P = 0.734 and P = 0.504 for Begg's and Egger's tests, respectively).
DISCUSSION
The combination of integrated traditional Chinese and Western medicine is a common strategy in tumor clinical therapy. Aidi injection, as a broad-spectrum anti-tumor proprietary Chinese medicine, is widely applied in combination with various chemotherapies. To evaluate the role of Aidi injection in combination therapy, this study included randomized controlled clinical trials assessing Aidi injection combined with NP chemotherapy in the treatment of advanced NSCLC. A total of twelve studies (n = 1012) were identified and analyzed comprehensively. As shown above, at a cisplatin dose of 20–40 mg/m2, Aidi injection combined with NP chemotherapy could increase efficiency and alleviate Grade II or above nausea and vomiting. However, at a cisplatin dose of 80–120 mg/m2, there was no significant difference between the two groups. Besides, Aidi injection combined with NP chemotherapy could also enhance the quality of life of patients and reduce the incidence of Grade II or above leukopenia and thrombocytopenia. These results broadly corroborate other studies demonstrating that Aidi injection is important in the treatment of advanced NSCLC.[10,11]
Of note, baselines of the included studies were not consistent. For example, doses and treatment durations for Aidi injection and cisplatin were quite different. Therefore, we identified the factors affecting the baselines. First of all, according to articles, Aidi injection dose was 40–100 ml, which was consistent with the 50–100 ml dose mentioned in dosage instructions. In the sensitivity analysis, the articles were excluded one by one, and the results were relatively stable, suggesting that Aidi injection dose has no significant effect on efficiency. In terms of cisplatin dose, this study evaluated the general (20–40 mg/m2) and large (80–120 mg/m2) dose groups and carried out a stratified analysis for all included studies. The results showed that when cisplatin was applied at a large dose, no significant difference (P = 0.390) was obtained between Aidi injection combined with NP chemotherapy and NP chemotherapy alone. Meanwhile, a significant difference was found between these treatments with cisplatin used at a small dose, suggesting that cisplatin dose should be taken into consideration when combining Aidi injection with NP chemotherapy clinically. In addition, treatment durations of the included studies ranged from 21 to 84 days. In general, a treatment cycle for chemotherapy is 21 days, and two cycles are needed to evaluate efficacy; thus, this study set two groups: one with less than two treatment cycles and the other with more than two cycles. No significant difference (P = 0.090) was obtained with less than two treatment cycles, in efficiency between the combination chemotherapy and NP chemotherapy alone, suggesting that observation time should be taken into consideration when supplementing Aidi injection to NP chemotherapy. Besides, we evaluated the effects of cisplatin dose and treatment duration on quality life, Grade II or above nausea and vomiting, leukopenia, thrombocytopenia and hemoglobin, and no significant differences were observed.
Limitations of this study should be mentioned. First, in some studies, randomization and double-blinding were not strictly developed and implemented. Second, a potential drawback is the relatively limited number of studies and sample sizes involved in this meta-analysis. Third, most studies did not mention detailed characteristics regarding NSCLC types, and patient age and gender distribution. Therefore, Aidi injection combined with NP chemotherapy in treating advanced NSCLC should be further analyzed, for detailed description of the randomization method and allocation concealment.
In conclusion, this study systemically analyzed Aidi injection combined with NP chemotherapy for the treatment of advanced NSCLC, and preliminarily validated its efficacy and safety. Well-designed RCTs with lager sample sizes are still needed to further evaluate the effects of cisplatin dose and treatment duration on efficacy and alleviation of side effects when treating advanced NSCLC.
Financial support and sponsorship
This work was supported by a grant from the Independent Subject of Beijing University of Chinese Medicine (No. 2015-JYB-XS087).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Jeremic B, Milicic B, Milisavljevic S. Clinical prognostic factors in patients with locally advanced (stage III) nonsmall cell lung cancer treated with hyperfractionated radiation therapy with and without concurrent chemotherapy: Single-institution experience in 600 patients. Cancer. 2011;117:2995–3003. doi: 10.1002/cncr.25910. doi:10.1002/cncr.25910. [DOI] [PubMed] [Google Scholar]
- 2.Xi Q, Wang JQ, Chang XH. The research progress of Aidi injection in the lung cancer treatment (In Chinese) China Med Her. 2014;11:163–5. [Google Scholar]
- 3.Zhang MM, Liu YL, Chen Z, Li XR, Xu QM, Yang SL. Studies on chemical constituents from Aidi injection (In Chinese) Chin Tradit Herbal Drugs. 2012;8:1462–70. [Google Scholar]
- 4.Xu J, Ju WZ, Tan HS. Study of pharmacological effects and clinical application of Aidi injection (In Chinese) Pharm Clin Res. 2012;1:48–51. [Google Scholar]
- 5.Zhang H, Jiang H, Hu X, Jia Z. Aidi injection combined with radiation in the treatment of non-small cell lung cancer: A meta-analysis evaluation the efficacy and side effects. J Cancer Res Ther. 2015;11(Suppl 1):C118–21. doi: 10.4103/0973-1482.163864. doi:10.4103/0973-1482.163864. [DOI] [PubMed] [Google Scholar]
- 6.Yuan WL, Qian B, Chang J, Zhou LL, Zhang RM. Aidi injection plus treatment arterial chemoembolization and chemotherapy for hepatocellular carcinoma: A systematic review and mata-analysis (In Chinese) West China Med J. 2010;1:144–8. [Google Scholar]
- 7.Ji B, Yuan J. Meta-analysis of the clinical efficacy and safety about Aidi injection in the treatment of colorectal cancer (In Chinese) China Pharm. 2011;40:3797–9. [Google Scholar]
- 8.Jiancheng W, Long G, Ye Z, Jinlong L, Pan Z, Lei M, et al. Effect of Aidi injection plus chemotherapy on gastric carcinoma: A Meta-analysis of randomized controlled trials. J Tradit Chin Med. 2015;35:361–74. doi: 10.1016/s0254-6272(15)30111-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YS, Li Q, Sun FL, Zhao L. Meta-analysis of Aidi injection treatment combining CHOP chemotherapy in treatment of malignant lymphoma (In Chinese) Chin New Drugs Remedies. 2014;11:807–12. [Google Scholar]
- 10.Wang Q, He X, Tian J, Wang X, Ru P, Ruan Z, et al. A meta analysis of aidi injection plus taxotere and cisplatin in the treatment of non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2010;13:1027–34. doi: 10.3779/j.issn.1009-3419.2010.11.06. doi:10.3779/j.issn.1009-3419.2010.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JJ, Ding M. Eddie combined with gemcitabine and cisplatin for advanced non-small cell lung cancer: Meta-analysis (In Chinese) Chin Gen Pract. 2012;8:2794–8. doi:10.3969/j.issn.1007-9572.2012.08.100. [Google Scholar]
- 12.Xing HJ, Wang LL, Lv HY, Zhou QH, Qi XH. The application of Aidi injection plus NP chemotherapy in the treatment of non-small cell lung cancer in the elderly (In Chinese) Chin J Prim Med Pharm. 2014;21:280–1. doi:10.3760/cma.j.issn.1008-6706.2014.02.056. [Google Scholar]
- 13.Huang XH, Wang CJ, Lu QH, Liao ZJ. Treatment of 60 cases of non-small cell lung cancer in the elderly with Aidi injection plus NP chemotherapy (In Chinese) JETCM. 2008;17:20–1. [Google Scholar]
- 14.Xu LY, Wu B, Hong W. Effect of Aidi combined with vinorelbine and cisplatin in patients with non-small cell lung cancer (In Chinese) Chin Remedies Clin. 2013;10:77–9. [Google Scholar]
- 15.Zhang AQ, Sun ZD, Shu QJ, Tan P. Clinical observation of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer (In Chinese) Pract Clin J Integr Tradit Chin West Med. 2005;5:26–7. [Google Scholar]
- 16.Xu XL, Zhang Y, Fu ZH. Clinical study on efficacy of Aidi injection plus NP regimen in treatment of non-small cell lung cancer (In Chinese) Her Med. 2007;26:244–5. [Google Scholar]
- 17.Cui YG, Wang WB. Clinical observation of Aidi-injection combining vinorelbine and cisplation in the treatment of advanced non-small cell lung cancer (In Chinese) Chin J Cancer Prev Treat. 2005;6:456–8. [Google Scholar]
- 18.Zhang JH, Zhu J, Zhang L. Clinical observation of Aidi injection plus NP regimen in treatment of non-small cell lung cancer (In Chinese) Med J Commun. 2006;20:528–9. [Google Scholar]
- 19.Xia BJ, Huang ZH, Zhang FL, Ren B. Treatment of 23 cases of Aidi injection combined with chemotherapy in the treatment of non-small cell lung cancer (In Chinese) Jiangxi J Tradit Chin Med. 2014;45:27–8. [Google Scholar]
- 20.Zhao CH, Yang ZP. The recent curative effect observation of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer (In Chinese) China Pract Med. 2009;2:126–7. [Google Scholar]
- 21.Zhang NS, Yang DZ, Niu HG, Pan T. Treatment of 98 cases of Aidi injection combined with chemotherapy in the treatment of Middle-late stage non-small cell lung cancer (In Chinese) Chin Arch Tradit Chin Med. 2003;21:1599–600. [Google Scholar]
- 22.Zhang DR, Lu XD. Clinical observation of late non-small cell lung cancer with Aidi injection (In Chinese) J Mod Med Health. 2014;30:578–9. doi:10.3969/j.issn.1009-5519.2014.04.048. [Google Scholar]
- 23.Wang HZ, Wang CH, Wang RX. Clinical observation of chemotherapies combined with Aidi injection for treatment of the advanced non-small cell lung cancer (In Chinese) Chin J Clin Oncol Rehabil. 2004;4:358–60. [Google Scholar]