Abstract
Background:
The crush and the culotte stenting were both reported to be effective for complex bifurcation lesion treatment. However, their comparative performance remains elusive.
Methods:
A total of 300 patients with coronary bifurcation lesions were randomly assigned to crush (n = 150) and culotte (n = 150) treatment. The primary endpoint was the occurrence of major adverse cardiac events (MACEs) at 12 months including cardiac death, myocardial infarction, stent thrombosis, and target vessel revascularization. Index lesion restenosis at 12 months was a secondary endpoint. The surface integrals of time-averaged wall shear stress at bifurcation sites were also be quantified.
Results:
There were no significant differences in MACE rates between the two groups at 12-month follow-up: Crush 6.7%, culotte 5.3% (P = 0.48). The rates of index lesion restenosis were 12.7% versus 6.0% (P = 0.047) in the crush and the culotte groups, respectively. At 12-month follow-up, the surface integrals of time-averaged wall shear stress at bifurcation sites in the crush group were significantly lower than the culotte group ([5.01 ± 0.95] × 10−4 Newton and [6.08 ± 1.16] × 10−4 Newton, respectively; P = 0.003).
Conclusions:
Both the crush and the culotte bifurcation stenting techniques showed satisfying clinical and angiographic results at 12-month follow-up. Bifurcation lesions treated with the culotte technique tended to have lower restenosis rates and more favorable flow patterns.
Keywords: Bifurcation Lesions, Hemodynamics, Percutaneous Coronary Intervention, Stenting Technique
INTRODUCTION
Coronary bifurcation lesion was one of the most challenging subsets in the percutaneous coronary intervention (PCI) due to its lower angiographic success rates and higher risk of procedural complications.[1,2] Currently, single stent implantation in the main vessel with provisional side branch (SB) stenting has been considered the default approach in most coronary bifurcation lesions.[3] However, there is still a number of coronary bifurcation lesions need stent coverage both the main vessel and the SB. Bifurcation lesions with large SB, small bifurcation angle, or severe stenosis in SB may be assigned to double stenting group at the very beginning. Several double stenting techniques associated with promising angiographic and clinical outcome have been proposed. Among those, the crush and the culotte techniques are mostly used to provide stent coverage of the whole bifurcation region in clinical practice. However, the optimal double stenting technique is still under debate.
Abnormal blood flow patterns are known to be associated with cellular proliferation, platelet activation, and inflammation.[4,5] Altered local hemodynamic profile and associated blood flow disturbances caused by the stent implantation may influence restenosis and stent thrombosis. Both geometric deformation and introduction of stent struts participate in this process. Our previous work and other computational fluid dynamics (CFD) studies performed with ideal coronary bifurcation models have already shown that different stenting techniques bring different hemodynamic conditions and flow patterns.[6,7] To the best of our knowledge, the comparison of long-term local flow disturbances between the crush and the culottes stenting techniques have fewly been studied. The objective of this study was to provide insights into optimal double stent technique comparing clinical and hemodynamic results of the crush with the culotte bifurcation stenting techniques.
METHODS
Patient population and randomization
A total of 300 patients undergoing crush or culotte technique from June 2013 to August 2014 were enrolled in this study. The protocol was approved by the Ethics Committee and written informed consent was given to all participating patients. The inclusion criterion was de novo coronary true bifurcation lesion with an SB B2.25 mm in diameter. Exclusion criteria were ST-elevation acute myocardial infarction within 24 h, liver and/or renal dysfunction, left ventricular ejection fraction ≤30%, life expectancy <1 year, a platelet count ≤10 × 109/L, and suspected intolerance to any of the drugs used (aspirin, clopidogrel, and sirolimus).
The participants were randomly assigned in a 1:1 ratio to either the crush or culotte group.
Medications and stent implantation
Patients were pretreated with a loading dose of clopidogrel 300 mg prior to the index procedure. Heparin was given as a bolus of 5000 U and a maintenance dose of 100 U/kg. Glycoprotein receptor antagonists were used at the discretion of the operator. After the intervention, aspirin was continued for life and clopidogrel for at least 12 months. The crush technique was typical crush technique performed as Colombo et al.[8] described. It consists of advancing two stents simultaneously into both the main vessel and SB. Crush stenting results in a triple layer of struts in the proximal main branch wall toward the branching vessel and a double layer of struts at the orifice of the SB. The culotte technique was performed as Chevalier et al.[9] described. It consists of implanting a first stent from the proximal to the distal segment of the main vessel. A second stent is then placed on the proximal main branch toward the SB through the struts of the first stent. Culotte stenting results in a double layer of struts in the proximal part of the main vessel and the presence of struts in the lumen of the main vessel at the bifurcation site.
Kissing balloon inflation was mandatory and additional stents to cover a possible dissection was allowed.
Follow-up
Clinical follow-up was achieved by means of an office visit or telephone contact every 3 months. Angiographic follow-up was scheduled for 12 months after the index procedure, unless there was a clear clinical evidence to perform the angiography earlier. There was no losing case in clinical follow-up. Angiographic follow-up was successfully performed in 127 of crush group and 125 of culotte group, respectively.
Study endpoints and definitions
The primary endpoint of the study was the occurrence of 1-year major adverse cardiac event (MACE) rate including cardiac death, myocardial infarction, stent thrombosis, and/or target vessel revascularization (TVR). The secondary endpoint was index lesion restenosis. Cardiac death, myocardial infarction, stent thrombosis, and TVR were defined according to the Academic Research Consortium definition.[10]
Quantitative coronary angiographic analysis
Coronary angiograms obtained at baseline, at poststenting, and after 12 months were analyzed offline with CAAS QCA 3D (Version 5.7.1, Pie Medical Imaging B.V., Maastricht, the Netherlands). Quantitative angiographic measurements of the proximal main vessel segment, the distal main vessel segment, and the SB were obtained. Measurements were obtained in the stents and in the margins 5 mm proximal and distal to the stents both main vessel and SB segment.
Three-dimensional reconstruction of the coronary bifurcations were done primarily with the commercial software CAAS QCA 3D from at least two different projection images with at least 30° difference. The results were then saved as STL files for further CFD analysis.
Computational fluid dynamics simulations and analysis
The simulations were conducted using the commercial software COMSOL Multiphysics (version 4.2, Comsol, Stockholm, Sweden). Defined boundary conditions are imposed, and the Navier–Stokes equations that describe the laminar motion of fluids are numerically solved using numeric grids. The artery walls were assumed rigid while no deformation was taken into account. The blood was considered as a Newtonian incompressible fluid with the density and the viscosity of 1.06 × 103 kg/m3 and 3.5 × 10−3 Pa·s based on documented data, respectively. Human left coronary artery pulsatile velocity measurements were applied at the inlet of the vessel. For the outlets, the downstream microcirculation resistance was considered, and the Murray's law was used to estimate the boundary conditions. The time average wall shear stress (TAWSS) and its surface integral at bifurcation sites were quantified.
Statistical analysis
We based our power calculations on an expected 1-year primary endpoint event rate of 20% in the crush group, alpha 5%, power 80%, and using a two-sided Chi-square test. To detect a reduction in primary endpoint rate to 10%, 125 patients would be needed in each group. Because of the considerable uncertainty, it was decided to include 150 patients in each group (20% increment).
Statistical analysis was done with the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as a mean and compared by the independent-samples t-test. Categorical variables were presented as frequencies with percentages and compared utilizing the Chi-square statistic or Fisher's exact test. Rate-free survival from events were generated by Kaplan–Meier analysis, and they were compared using the log-rank test. A P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics and procedural data
Baseline clinical characteristics and risk factors were well-balanced between 2 treatment groups [Table 1]. In four-fifths of the cases, the indication for treatment was unstable angina pectoris. The index lesion location was the left anterior descending artery in 66.0%, the circumflex artery in 20.3%, the left main stem in 10.7%, and the right coronary artery in 3.0%, with no difference between the two groups. SB angulation of <50° was seen in 42.8% of the lesions, with no difference between the groups. A final kissing balloon dilatation was performed in significantly less of the patients in the crush than in the culotte group [Table 2].
Table 1.
Base clinical characteristics of patients undergoing crush or culotte technique
| Characteristics | Crush group (n = 150) | Culotte group (n = 150) | P |
|---|---|---|---|
| Age, mean ± SD, years | 63 ± 8 | 64 ± 9 | 0.779 |
| Male, n (%) | 109 (72.7) | 111 (74.0) | 0.794 |
| Current smoker, n (%) | 58 (38.7) | 67 (44.7) | 0.292 |
| Hypertension, n (%) | 106 (70.7) | 109 (72.7) | 0.701 |
| Hypercholesterolemia, n (%) | 114 (76.0) | 105 (70.0) | 0.242 |
| Diabetes mellitus, n (%) | 33 (22.0) | 37 (24.7) | 0.585 |
| Family history, n (%) | 45 (30.0) | 52 (34.7) | 0.388 |
| Prior PCI, n (%) | 40 (26.7) | 34 (22.7) | 0.422 |
| Indication, n (%) | |||
| Unstable angina | 124 (82.7) | 129 (86.0) | 0.427 |
| Stable angina | 14 (9.3) | 12 (8.0) | 0.681 |
| Silent ischemia | 12 (8.0) | 9 (6.0) | 0.497 |
| Antiplatelet therapy, n (%) | |||
| Aspirin | 148 (98.7) | 150 (100.0) | 0.498 |
| Clopidogrel | 150 (100.0) | 149 (99.3) | 0.500 |
| GP IIb/IIIa inhibitors | 47 (31.3) | 41 (27.3) | 0.447 |
Values are n (%) or mean ± SD. Independent-samples t-test or Fisher's exact test were used. SD: Standard deviation; PCI: Percutaneous coronary intervention; GP: Glycoprotein.
Table 2.
Procedural characteristics of crush group and culotte group
| Characteristics | Crush group (n = 150) | Culotte group (n = 150) | P |
|---|---|---|---|
| Medina classification, n (%) | |||
| Medina 1,1,1 | 109 (72.7) | 111 (74.0) | 0.794 |
| Medina 0,1,1 | 14 (9.3) | 7 (4.7) | 0.113 |
| Medina 1,0,1 | 27 (18.0) | 32 (21.3) | 0.468 |
| Lesion location, n (%) | |||
| Left main | 13 (8.7) | 19 (12.7) | 0.262 |
| Left anterior descending artery | 96 (64.0) | 102 (68.0) | 0.465 |
| Circumflex artery | 35 (23.3) | 26 (17.3) | 0.197 |
| Right coronary artery | 6 (4.0) | 3 (2.0) | 0.498 |
| Lesion length, mean ± SD, mm | |||
| Main vessel | 16.1 ± 6.3 | 18.5 ± 7.6 | 0.278 |
| Side branch | 7.9 ± 4.1 | 7.4 ± 4.3 | 0.703 |
| Stent length, mm | |||
| Main vessel | 22.8 ± 7.5 | 24.6 ± 6.7 | 0.427 |
| Side branch | 10.4 ± 5.6 | 10.2 ± 5.8 | 0.914 |
| Proximal reference diameter, mean ± SD, mm | |||
| Main vessel | 3.4 ± 0.4 | 3.3 ± 0.5 | 0.424 |
| Side branch | 2.6 ± 0.3 | 2.7 ± 0.4 | 0.242 |
| SYNTAX score (points) | 21.6 ± 6.3 | 22.4 ± 5.8 | 0.628 |
| Final kissing balloon dilatation, n (%) | 107 (71.3) | 129 (86.0) | 0.002 |
| Angiographic success, n (%) | 145 (96.7) | 148 (98.7) | 0.444 |
| Procedural time, mean ± SD, min | 74 ± 20 | 70 ± 17 | 0.467 |
| Fluoroscopy time, mean ± SD, min | 25 ± 11 | 24 ± 9 | 0.628 |
| Contrast volume, mean ± SD, ml | 152 ± 37 | 138 ± 35 | 0.246 |
Independent-samples t-test or Fisher's exact test was used. SD: Standard deviation.
Clinical outcome
The rates of event-free survival for MACE after 12 months follow-up are shown in Figure 1. The individual endpoints after 12 months are shown in Table 3. The incidence of individual endpoints was low in both the two groups. Index lesion restenosis rates were found 12.7% versus 6.0% (P = 0.047) in the crush and culotte groups by 12 months, respectively.
Figure 1.

Major adverse cardiac event-free survival rate at 12 months. The rate was 93.3% in the crush group, and it was 94.7% in the culotte group (P = 0.48).
Table 3.
Individual endpoints after 12 months in crush group and culotte group
| Items | Crush group (n = 150) | Culotte group (n = 150) | P |
|---|---|---|---|
| Total death, n (%) | 2 (1.3) | 1 (0.7) | 0.624 |
| Cardiac death, n (%) | 2 (1.3) | 1 (0.7) | 0.624 |
| Myocardial infarction, n (%) | 7 (4.7) | 3 (2.0) | 0.335 |
| Stent thrombosis, n (%) | 4 (2.7) | 2 (1.3) | 0.684 |
| Target lesion revascularization, n (%) | 8 (5.3) | 6 (4.0) | 0.584 |
| Target vessel revascularization, n (%) | 9 (6.0) | 7 (4.7) | 0.607 |
| Index lesion restenosis, n (%) | 19 (12.7) | 9 (6.0) | 0.047 |
Values are n (%). Fisher's exact test was used.
Quantitative coronary angiography analysis
Minimal lumen diameter, acute gain, and late loss were similar in the two groups [Table 4]. There was 14 (9.3%) in-stent restenosis at SB in the crush group and 5 (3.3%) in the culotte group (P = 0.033), mostly located at the ostium of SB.
Table 4.
Quantitative coronary analysis for main vessel and side branch
| Items | Crush group (n = 150) | Culotte group (n = 150) | P |
|---|---|---|---|
| Proximal main vessel segment | |||
| Minimal luminal diameter, mean ± SD, mm | 1.85 ± 0.49 | 1.87 ± 0.46 | 0.882 |
| Acute gain | 1.89 ± 0.39 | 1.93 ± 0.42 | 0.792 |
| Late loss | 0.28 ± 0.18 | 0.25 ± 0.22 | 0.718 |
| Diameter stenosis, mean ± SD, % | |||
| Prior stenting | 56.06 ± 8.72 | 57.19 ± 10.13 | 0.708 |
| After PCI | 8.95 ± 5.31 | 8.43 ± 4.92 | 0.753 |
| Follow-up | 12.52 ± 7.60 | 11.90 ± 6.99 | 0.792 |
| Restenosis, n (%) | |||
| In-stent | 0 (0) | 0 (0) | NA |
| Edge | 2 (1.3) | 1 (0.7) | 0.624 |
| Distal main branch segment | |||
| Minimal luminal diameter, mean ± SD, mm | 1.42 ± 0.45 | 1.47 ± 0.38 | 0.664 |
| Acute gain | 1.71 ± 0.36 | 1.74 ± 0.31 | 0.788 |
| Late loss | 0.26 ± 0.24 | 0.22 ± 0.19 | 0.540 |
| Diameter stenosis, mean ± SD, % | |||
| Prior stenting | 63.93 ± 7.85 | 66.33 ± 9.37 | 0.384 |
| After PCI | 11.48 ± 7.93 | 11.26 ± 6.69 | 0.926 |
| Follow-up | 18.80 ± 10.78 | 16.45 ± 10.63 | 0.492 |
| Restenosis, n (%) | |||
| In-stent | 2 (1.3) | 1 (0.7) | 0.624 |
| Edge | 3 (2.0) | 1 (0.7) | 0.622 |
| Side branch | |||
| Minimal luminal diameter, mean ± SD, mm | 1.23 ± 0.34 | 1.32 ± 0.29 | 0.345 |
| Acute gain | 1.32 ± 0.36 | 1.35 ± 0.31 | 0.736 |
| Late loss | 0.23 ± 0.24 | 0.25 ± 0.24 | 0.848 |
| Diameter stenosis, mean ± SD, % | |||
| Prior stenting | 55.62 ± 10.42 | 56.25 ± 11.81 | 0.859 |
| After PCI | 14.26 ± 11.20 | 13.02 ± 10.45 | 0.720 |
| Follow-up | 20.94 ± 12.77 | 17.61 ± 10.57 | 0.375 |
| Restenosis, n (%) | |||
| In-stent | 14 (9.3) | 5 (3.3) | 0.033 |
| Edge | 4 (2.7) | 5 (3.3) | 0.735 |
Values are mean ± SD or n (%). Restenosis was defined as ≥50% diameter stenosis at 12-month follow-up. SD: Standard deviation; PCI: Percutaneous coronary intervention; NA: Not applicable.
Computational fluid dynamics analysis
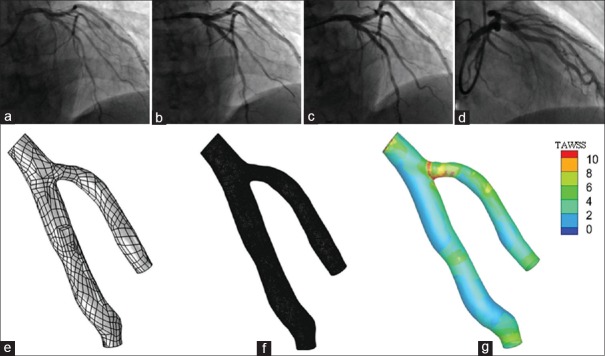
Figure 2 showed coronary angiography pre-PCI, post-PCI, and at 12-month follow-up of a representative bifurcation lesion and its corresponding CFD results. The surface integral of TAWSS was both similar in the two groups at baseline and post-PCI (P > 0.05). However, at 12-month follow-up, the surface integral of TAWSS of the bifurcation site in the crush group was significantly lower than that in the culotte group ([5.01 ± 0.95] × 10−4 Newton and [6.08 ± 1.16] × 10−4 Newton, respectively; P = 0.003).
Figure 2.
(a) Angiography left anterior oblique cranial view of a left anterior descending-diagonal true bifurcation lesion pre percutaneous coronary intervention. (b) Left anterior oblique cranial view after culotte stenting technique. (c) Left anterior oblique cranial view (12-month follow-up). (d) Right anterior oblique cranial view (12-month follow-up). (e) Three-dimensional geometry at 12-month follow-up. (f) Mesh model at 12-month follow-up. (g) Scalar of time-averaged wall shears stress at 12-month follow-up.
DISCUSSION
We compared the crush with the culotte bifurcation stenting technique in this randomized study and made several novel observations which as follows: (1) low and similar 12-month MACE rates were found in two study groups. Both the crush and the culotte bifurcation stenting techniques showed satisfying clinical and angiographic results by 12 months. (2) There was a trend toward less restenosis in patients treated with the culotte stenting technique. (3) Compared with the crush stenting technique, the culotte stenting technique was associated with more favorable flow profile by 12 months follow-up.
Based on the results of several randomized clinical trials, the provisional SB stenting technique is considered the default strategy for bifurcation lesions at present.[4,11] However, despite the current consensus on the single stenting technique, some complex bifurcation lesions, especially with a large SB or serious stenosis at the ostium of the SB may still need double stenting techniques in order to provide full stent coverage of the bifurcation area. We evaluated the crush and the culotte bifurcation techniques which are most commonly double stenting techniques in clinical practice in this study. The 12-month follow-up data showed that both the crush and the culotte stenting technique were associated with low MACE rates (6.7% and 5.3%, respectively) and satisfying clinical results. The definite stent thrombosis rates were 2.7% and 1.3% (P = 0.68) in the crush and culotte groups, respectively. This result is in line with previous studies which suggested that the rate of ST is 3% more or less for bifurcation lesions treated with a double stenting technique. Kaplan et al.[12] compared the culotte with the T-stenting technique in a nonrandomized study. After 9-month follow-up, they found that there was a trend toward lower MACE rate in the culotte group than the T-stenting group (13.3% and 27.3%, respectively). The stent thrombosis rate of the culotte group was 2.2% at 9 months in this study. In another multicenter prospective clinical trail, 134 bifurcation lesions in 132 patients were treated with the culotte technique and the incidence of stent thrombosis was 1.5% at 12 months.[13] Regarding the crush stenting technique, a single center prospective registry study enrolled 100 patients treated with the crush technique. After 3-year follow-up, the stent thrombosis rate was 3% and the TVR rate was 11%.[14] Similar to these clinical trials, the low stent thrombosis rates in the present study further convince the fact that both the crush and the culotte stenting techniques are safe for the treatment of complex bifurcation lesions.
The numerically higher restenosis rate in the crush stenting technique group is in keeping with results of other large clinical trials. In the Nordic Stent Technique Study, 424 patients were enrolled and in-stent restenosis rates after 8 months were found 10.5% versus 4.5% (P = 0.046) in the crush and culotte groups, respectively.[15] In another prospective registry study, 100 patients were treated with the crush stenting technique and target lesion revascularization rate was 11% by 3-year follow-up.[14] These results may be explained by the lower success rate of final kissing balloon dilatation in the operation process and more disturbed flow pattern after the treatment in the crush-treated group.
Final kissing balloon dilatation are generally recommended in double stenting techniques to improve clinical outcome and prevent SB reocclusion.[16,17] In the crush-treated patients, three layers of struts covering the SB ostium make the rewiring and balloon insertion through stent struts laborious. Nevertheless, the observed lower success rate of final kissing balloon dilatation did not result in high target lesion revascularization rates in the present study. This may partly be explained by the fact that malapposed struts at the SB ostium are not always angiographically visible and become apparent only when neointima and fibrin deposition cover the struts left jailing the ostium. On the other hand, early follow-up results in patients treated with a drug-eluting stent and antiplatelet therapies may underestimate the risk associated with leaving malapposed struts at the SB ostium. Thus, final kissing balloon dilation should be performed, whenever possible, in complex bifurcation lesions requiring double stenting technique.
Regarding flow pattern alteration after stent implantation, previous studies showed neointima hyperplasia and fibrin deposition tend to form in areas where frequently exposed to low and oscillatory shear stress. Act as a media increasing the expression of platelet-derived growth factor, endothelin-1, and vascular endothelial growth factor, low shear stress can promote the activation, proliferation, and migration of smooth muscle cells.[18] In addition, low shear stress upregulates proinflammatory genes, including chemoattractant chemokines, adhesion molecules, and cytokines, thereby enhancing injury-induced inflammation.[19] Thus, in theory, the coronary intervention was aimed to restore laminar flow with a high shear stress so that fibrous tissue could not proliferate. Improved hemodynamic performance-guided stent implantation may translate to improved clinical outcomes. Several studies have assessed flow patterns after stent implantation at bifurcation sites. Katritsis et al.[6] studied TAWSS alterations by simulating different stenting techniques that commonly used in bifurcation lesions in idealized bifurcation models and found that crush stenting gave the most favorable results among the double stenting techniques. However, on the contrary, in another in vitro study, Foin et al.[20] compared the results of different double stenting strategies and found that the crush technique resulted in a higher risk of malapposition and a more disturbed flow than either the culotte or T with protrusion stenting technique. In the present study, we calculated the surface integrals of TAWSS at the subregion of bifurcation site. Our CFD results suggested that the crush and the culotte stenting techniques do not produce similar hemodynamic disturbances at bifurcations. Lower surface integrals of TAWSS were found in the crush-treated patients than in the culotte group ([5.01 ± 0.95] × 10−4 Newton and [6.08 ± 1.16] × 10−4 Newton, respectively; P = 0.003). Despite we cannot directly link hemodynamic disturbances with the risk of restenosis and stent thrombosis, it is plausible that the risk of these complications would be higher if the subregion of bifurcation sites are continuously exposed to unfavorable hemodynamic conditions. However, the observed lower TAWSS in the crush group did not result in a high target lesion revascularization rates in the present study. Except for the short follow-up time, this may also partly be explained by the fact that drug-eluting stents and antiplatelet therapies alleviate the unfavorable hemodynamic effect of disturbed flow by inhibiting the neointima hyperplasia process.
According to the present results, both the crush and the culotte technique are efficient and safe for complex bifurcation lesions. The operator may choose technique on the basis of the lesion characteristics and personal experience. Compared to the crush technique, the possible hemodynamic advantages of the culotte technique still require longer follow-up and more clinical trials to further confirm.
Several limitations of the current study should be acknowledged. First, since it was an open trial, operators and patients were aware of the technique used. This may introduce information bias when to collect and analyze data at follow-up. Second, despite the rates of stent thrombosis and MACE were low after 12 months clinical and angiographic follow-up in the present study, the durability of these results on a long-term basis is not known. Finally, the accuracy of CFD analysis largely depends on the quality and precision of reconstruction. The quality of reconstruction view needs to be improved by giving more precise angiographic images of coronary bifurcation at an angle of more than 30°.
In conclusion, both the crush and the culotte technique were associated with an excellent clinical and angiographic results at 12-month follow-up. Bifurcation lesions treated with the culotte technique tended to have lower restenosis rates and more favorable flow patterns.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Lassen JF, Holm NR, Stankovic G, Lefèvre T, Chieffo A, Hildick-Smith D, et al. Percutaneous coronary intervention for coronary bifurcation disease: Consensus from the first 10 years of the European Bifurcation Club meetings. EuroIntervention. 2014;10:545–60. doi: 10.4244/EIJV10I5A97. doi: 10.4244/EIJV10I5A97. [DOI] [PubMed] [Google Scholar]
- 2.Kornowski R. The complexity of stenting in bifurcation coronary lesions. JACC Cardiovasc Interv. 2013;6:696–7. doi: 10.1016/j.jcin.2013.04.005. doi: 10.1016/j.jcin.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Maeng M, Holm NR, Erglis A, Kumsars I, Niemelä M, Kervinen K, et al. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions: Nordic Bifurcation Study 5-year follow-up results. J Am Coll Cardiol. 2013;62:30–4. doi: 10.1016/j.jacc.2013.04.015. doi: 10.1016/j.jacc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Chatzizisis YS, Giannoglou GD. Shear stress and inflammation: Are we getting closer to the prediction of vulnerable plaque? Expert Rev Cardiovasc Ther. 2010;8:1351–3. doi: 10.1586/erc.10.126. doi: 10.1586/erc.10.126. [DOI] [PubMed] [Google Scholar]
- 5.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–53. doi: 10.1161/CIRCULATIONAHA.105.590018. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 6.Katritsis DG, Theodorakakos A, Pantos I, Gavaises M, Karcanias N, Efstathopoulos EP. Flow patterns at stented coronary bifurcations: Computational fluid dynamics analysis. Circ Cardiovasc Interv. 2012;5:530–9. doi: 10.1161/CIRCINTERVENTIONS.112.968347. doi: 10.1161/CIRCINTERVENTIONS.112.968347. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Liu J, Zheng X, Rong X, Zheng X, Peng H, et al. Three-dimensional virtual surgery models for percutaneous coronary intervention (PCI) optimization strategies. Sci Rep. 2015;5:10945. doi: 10.1038/srep10945. doi: 10.1038/srep10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo A, Stankovic G, Orlic D, Corvaja N, Liistro F, Airoldi F, et al. Modified T-stenting technique with crushing for bifurcation lesions: Immediate results and 30-day outcome. Catheter Cardiovasc Interv. 2003;60:145–51. doi: 10.1002/ccd.10622. doi: 10.1002/ccd.10622. [DOI] [PubMed] [Google Scholar]
- 9.Chevalier B, Glatt B, Royer T, Guyon P. Placement of coronary stents in bifurcation lesions by the “culotte” technique. Am J Cardiol. 1998;82:943–9. doi: 10.1016/s0002-9149(98)00510-4. doi: 10.1016/S0002-9149(98)00510-4. [DOI] [PubMed] [Google Scholar]
- 10.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 11.Koh YS, Kim PJ, Chang K, Park HJ, Jeong MH, Kim HS, et al. Long-term clinical outcomes of the one-stent technique versus the two-stent technique for non-left main true coronary bifurcation disease in the era of drug-eluting stents. J Interv Cardiol. 2013;26:245–53. doi: 10.1111/joic.12025. doi: 10.1111/joic.12025. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan S, Barlis P, Dimopoulos K, La Manna A, Goktekin O, Galassi A, et al. Culotte versus T-stenting in bifurcation lesions: Immediate clinical and angiographic results and midterm clinical follow-up. Am Heart J. 2007;154:336–43. doi: 10.1016/j.ahj.2007.04.019. doi: 10.1016/j.ahj.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Adriaenssens T, Byrne RA, Dibra A, Iijima R, Mehilli J, Bruskina O, et al. Culotte stenting technique in coronary bifurcation disease: Angiographic follow-up using dedicated quantitative coronary angiographic analysis and 12-month clinical outcomes. Eur Heart J. 2008;29:2868–76. doi: 10.1093/eurheartj/ehn512. doi: 10.1093/eurheartj/ehn512. [DOI] [PubMed] [Google Scholar]
- 14.Chue CD, Routledge HC, Ludman PF, Townend JN, Epstein AC, Buller NP, et al. 3-year follow-up of 100 consecutive coronary bifurcation lesions treated with Taxus stents and the crush technique. Catheter Cardiovasc Interv. 2010;75:605–13. doi: 10.1002/ccd.22252. doi: 10.1002/ccd.22252. [DOI] [PubMed] [Google Scholar]
- 15.Erglis A, Kumsars I, Niemelä M, Kervinen K, Maeng M, Lassen JF, et al. Randomized comparison of coronary bifurcation stenting with the crush versus the culotte technique using sirolimus eluting stents: The Nordic stent technique study. Circ Cardiovasc Interv. 2009;2:27–34. doi: 10.1161/CIRCINTERVENTIONS.108.804658. doi: 10.1161/CIRCINTERVENTIONS.108.804658. [DOI] [PubMed] [Google Scholar]
- 16.Biondi-Zoccai G, Sheiban I, De Servi S, Tamburino C, Sangiorgi G, Romagnoli E. To kiss or not to kiss?Impact of final kissing-balloon inflation on early and long-term results of percutaneous coronary intervention for bifurcation lesions. Heart Vessels. 2014;29:732–42. doi: 10.1007/s00380-013-0416-0. doi: 10.1007/s00380-013-0416-0. [DOI] [PubMed] [Google Scholar]
- 17.Foin N, Torii R, Mortier P, De Beule M, Viceconte N, Chan PH, et al. Kissing balloon or sequential dilation of the side branch and main vessel for provisional stenting of bifurcations: Lessons from micro-computed tomography and computational simulations. JACC Cardiovasc Interv. 2012;5:47–56. doi: 10.1016/j.jcin.2011.08.019. doi: 10.1016/j.jcin.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Redmond EM, Cullen JP, Cahill PA, Sitzmann JV, Stefansson S, Lawrence DA, et al. Endothelial cells inhibit flow-induced smooth muscle cell migration: Role of plasminogen activator inhibitor-1. Circulation. 2001;103:597–603. doi: 10.1161/01.cir.103.4.597. doi: 10.1161/01.CIR.103.4.597. [DOI] [PubMed] [Google Scholar]
- 19.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–93. doi: 10.1016/j.jacc.2007.02.059. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 20.Foin N, Alegria-Barrero E, Torii R, Chan PH, Viceconte N, Davies JE, et al. Crush, culotte, T and protrusion: Which 2-stent technique for treatment of true bifurcation lesions? – Insights from in vitro experiments and micro-computed tomography. Circ J. 2013;77:73–80. doi: 10.1253/circj.cj-12-0272. doi: 10.1253/circj.CJ-12-0272. [DOI] [PubMed] [Google Scholar]