Abstract
Background:
Inverted internal limiting membrane (ILM) flap technique has recently been reported in a limited number of studies as an effective surgical technique for the management of large macular holes (MHs) with fair MH closure rates as well as gains in visual acuity. In the current study, longitudinal changes in multi-focal electroretinogram (mfERG) responses, best-corrected visual acuity (BCVA) and spectral-domain optical coherence tomography (SD-OCT) were evaluated in eyes with large MHs managed by this technique.
Methods:
A prospective noncontrolled interventional study of eight patients (eight eyes) with large MHs (minimum diameter >400 μm) was conducted. All MHs were treated with pars plana vitrectomy and indocyanine green-assisted inverted ILM flap technique. SD-OCT images were used to assess the anatomical outcomes of surgery while BCVA and mfERG were used to evaluate the functional outcomes during a 3-month follow-up.
Results:
All patients underwent successful intended manipulation and translocation of the ILM flap without flap dislocation and achieved complete anatomical closure. Partial microstructural reconstruction, demonstrated on SD-OCT as restoration of the external limiting membrane and the ellipsoid zone, was observed in all cases as early as 1 month after surgery. Functionally, as compared to baseline, all patients showed improvements in BCVA and all but one in mfERG response during follow-up. However, Pearson's test revealed no significant correlations between BCVA and mfERG responses of the fovea and of the macular area at each evaluation time point.
Conclusions:
Inverted ILM flap technique appeares to be a safe and effective approach for the management of large idiopathic MHs with favorable short-term anatomical and functional results. Postoperative reconstruction of the microstructure generally shows good consistency with improvements in both BCVA and mfERG response, of which the latter might be a supplement for the former in postoperative functional follow-up.
Keywords: Inverted Internal Limiting Membrane Flap Technique, Large Macular Hole, Multi-focal Electroretinogram, Spectral-domain Optical Coherence Tomography
INTRODUCTION
A macular hole (MH) is a full-thickness neuroretinal defect or break of the fovea, most of which are considered idiopathic. MH is sight-threatening and not uncommon; however, not until 1991 did Kelly and Wendel reported it to be treatable via internal limiting membrane (ILM) peeling assisted pars plana vitrectomy (PPV), which was later established to be a standard surgical technique for this condition.[1] The second milestone of MH surgery was laid by Kadonosono et al. in 2000 when indocyanine green (ICG) was applied during surgery for staining of ILM before peeling,[2] thus providing a way to better visualize, easier, and more completely remove the ILM.[3] Other dyes, such as trypan blue[4] and brilliant blue G,[5] were later introduced as alternatives for ILM staining.
Despite the above-mentioned advances in surgical techniques, however, quite a proportion of the MHs did not achieve complete closure but were so-called “flat-open” with bare retinal pigment epithelium and absence of foveal neurosensory retina, especially in cases with large MH (minimum diameter >400 μm) or high myopia.[6,7] Although considered anatomically successful, flat-open closure of MH usually resulted in limited postoperative functional recovery.[8]
A continuing effort of refinements on vitreoretinal surgery led to another innovative technique established by Michalewska et al. in 2010, the “inverted ILM flap” technique, which was proved to be able to increase the rate of complete closure as well as the final visual outcome.[9] This technique was later used in patients with massive MH (minimum diameter >700 μm)[10] or high myopia[11,12,13] with satisfactory results except for high myopic patients complicated with retinal detachment.[12]
However, to the best of our knowledge, best corrected visual acuity (BCVA) was the only functional index used in the limited number of published studies applying the inverted ILM flap technique. In this study, multifocal electroretinogram (mfERG), which allows detailed topographical mapping of the retinal function in the central macula, was documented and compared before and after surgery in patients with large MHs (>400 μm) who underwent inverted ILM flap technique assisted PPV, trying to combine the mfERG results to the microstructural changes detected by spectral-domain optical coherence tomography (SD-OCT).
METHODS
This study is a prospective noncontrolled interventional study. It complied with the tenets of the Declaration of Helsinki (as revised in Brazil 2013) and was approved by Ethics Committee of our hospital. Patients who presented to our group with large (minimum diameter >400 μm) idiopathic stage III-IV (Gass classification) MHs from June to December 2014 were included. Informed consent was obtained before surgery in all patients. Exclusion criteria included: High myopia (>6 diopters), increased intraocular pressure (IOP, >21 mmHg) or glaucoma, moderate to severe cataract, severe systemic conditions that prevent surgery, and history of ocular trauma, intraocular inflammation, retinal vascular disease, or previous ocular surgery.
Each patient received a complete ophthalmological examination and the following clinical parameters were obtained at baseline and 2 weeks, 1–3 months and ad-lib postoperatively: BCVA (recorded in decimals and was converted to logarithm of the minimum angle of resolution units for statistical analysis), IOP (Auto-tonometer TX-20, Canon, Tokyo, Japan), minimum and base MH diameter on SD-OCT (3D-OCT 1000, Topcon, Tokyo, Japan). mfERG (VERIS-EDI System, Califronia, USA) was not conducted until 1 month after surgery for the sake of avoiding infection and was monitored during the following visits. The ERG protocol complied with the standards published by the International Society for Clinical Electrophysiology of Vision.[14]
One-hundred and three hexagonal stimulus elements that scaled concentrically and covered the central 50° of the fundus were applied to each patient when examine mfERG. The central hexagon (representing foveal area) corresponded to approximately 2.8°, and the central seven hexagons (representing macula, including foveal and perifoveal areas) to approximately 5°. Three-dimensional plots and retinal response density ([RD], defined as amplitude per retinal area, nV/deg2) value plots were used to demonstrate the topographical retinal electrical activities.
A standard 3-port PPV (23-gauge) (Stellaris, Bausch and Lomb, New York, USA) with inverted ILM flap technique was performed in all patients by a single experienced surgeon. After a core vitrectomy, posterior vitreous detachment was created, followed by removal of the residual premacular posterior vitreous cortex. Then, ICG (0.125% solution) was slowly injected toward the ILM around the MH instead of directly toward it to minimize retinal toxicity, and let the ILM be stained for about 30 s. After that, the ILM along with any epiretinal membrane if present was grasped and peeled off in a circumferential pattern for about 1–1.5 disk diameter around the MH using ILM forceps, leaving only the innermost narrow circle of ILM attached to the macular border by a pedicel. Then, this remnant of ILM was gently turned upside-down toward the bottom of the MH and was carefully flattened to make sure it was properly positioned rather than packed irregularly. The perfusion pressure was set at the lowest level (30 cmH2O) that the vitrectomy machine allowed when covering the MH with the inverted ILM flap and during the air-fluid exchange to avoid the flimsy flap being washed away. At the end of surgery, 14% C3F8 gas tamponade was applied to secure the position of the inverted ILM flap and the postoperative face-down position was instructed for at least 1 week.
Statistical analysis
Statistical analysis was performed by SPSS 21.0 for windows (SPSS, Inc., Chicago, IL, USA). Numerical variables were presented as mean ± standard deviation (SD). BCVAs and RD of the central hexagon and the central seven hexagons on mfERG at different time points (baseline, 1 and 3 months after surgery) were compared pairwisely by post-hoc tests after analysis of variance. Pearson tests were conducted to evaluate the correlation between BCVAs and mfERG responses at each measurement point. A value of P < 0.01 was considered to be statistically significant.
RESULTS
A total of eight eyes in eight consecutive patients (four men and four women) were enrolled. The postoperative follow-up period ranged from 3 to 10 months (mean 6.0 ± 2.7 months). The detailed clinical data are summarized in Table 1.
Table 1.
Basic demographic data, related biometric measurements, visual acuity and mfERG response of patients with large macular holes
| Number | Gender | Age (years) | Axial length (mm) | Preoperative MH diameter (µm) | BCVA in logMAR | RD on mfERG (nV/deg2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Preoperative | Postoperative 1 month | Postoperative 3 months | Preoperative | Postoperative 1 month | Postoperative 3 months | |||||||
| Fovea | Macula | Fovea | Macula | Fovea | Macula | |||||||||
| 1 | Male | 83 | 23.3 | 725 | 1148 | 2.0 | 1.4 | 0.8 | 3.5 | 2.2 | 4.1 | 2.7 | 5.4 | 4.3 |
| 2 | Male | 66 | 23.9 | 400 | 648 | 1.0 | 0.5 | 0.3 | 5.9 | 5.9 | 9.9 | 8.3 | 12.3 | 9.6 |
| 3 | Female | 53 | 22.9 | 734 | 965 | 1.4 | 0.9 | 0.7 | 8.5 | 8.1 | 12.3 | 9.5 | 12.5 | 11.1 |
| 4 | Female | 66 | 23.0 | 905 | 1213 | 1.1 | 0.8 | 0.5 | 4.2 | 3.6 | 7.3 | 6.4 | 12.9 | 8.0 |
| 5 | Female | 67 | 23.1 | 478 | 854 | 1.7 | 1.3 | 1.0 | 10.1 | 8.0 | 6.0 | 5.0 | 7.5 | 5.8 |
| 6 | Female | 57 | 23.5 | 450 | 750 | 0.9 | 1.0 | 0.5 | 4.6 | 4.0 | 7.6 | 6.5 | 13.4 | 11.0 |
| 7 | Male | 70 | 24.5 | 694 | 1516 | 1.1 | 0.7 | 0.5 | 11.8 | 9.5 | 12.5 | 11.5 | 18.3 | 12.8 |
| 8 | Male | 64 | 23.0 | 645 | 2020 | 1.0 | 0.8 | 0.7 | 4.6 | 4.6 | 6.7 | 5.4 | 7.8 | 6.7 |
| Mean ± SD | 65.8 ± 8.9 | 23.4 ± 0.6 | 629.0 ± 172.0 | 1139.0 ± 452.0 | 1.3 ± 0.4 | 0.9 ± 0.3 | 0.6 ± 0.2 | 6.7 ± 3.1 | 5.7 ± 2.6 | 8.3 ± 3.0 | 6.9 ± 2.8 | 11.3 ± 4.1 | 8.7 ± 3.0 | |
MH: Macular hole; BCVA: Best corrected visual acuity; logMAR: Logarithm of the minimum angle of resolution; RD: Response density; mfERG: Multifocal electroretinography; Min: Minimal; Max: Maximal or basal; “Fovea” and “Macula” represents the most central and the central 7 hexagonal areas on mfERG, respectively.
All patients underwent successful ILM peeling, intended manipulation, and translocation of the ILM flap. None of the eight patients had flap dislocation during surgery. Complete closure (defined as no neurosensory defect at the fovea on SD-OCT) was observed in all eyes 2 weeks after surgery (although the tissue filling the original MH appeared more likely to be sheets of inverted ILM flap), followed by a gradual microstructural reconstruction afterward marked by partial restoration of the back-reflection lines representing the external limiting membrane (ELM) and ellipsoid zone (EZ) on SD-OCT [Figure 1]. Encouragingly and in consistency with the reconstruction process of the foveal microstructure, all patients except one (7/8) showed incremental improvements of visual function (both in BCVA and mfERG responses) over time during the first 3 months after surgery [Figure 2]. The exceptional patient, however, demonstrated depressed mfERG responses at 1 month after surgery with limited recovery detected at 3 months, although a contemporary slow increase in BCVA was observed [Figure 3]. Interestingly, in accordance with the mfERG changes, the central defects of the ELM and the EZ appeared widened at the 1-month postoperative visit as compared to baseline, followed by partial restoration observed at 3 months after surgery; in addition, the temporal inner macular surface appeared rugged after surgery, suggesting the possibility of nerve fiber layer injury during ILM peeling [Figure 3].
Figure 1.
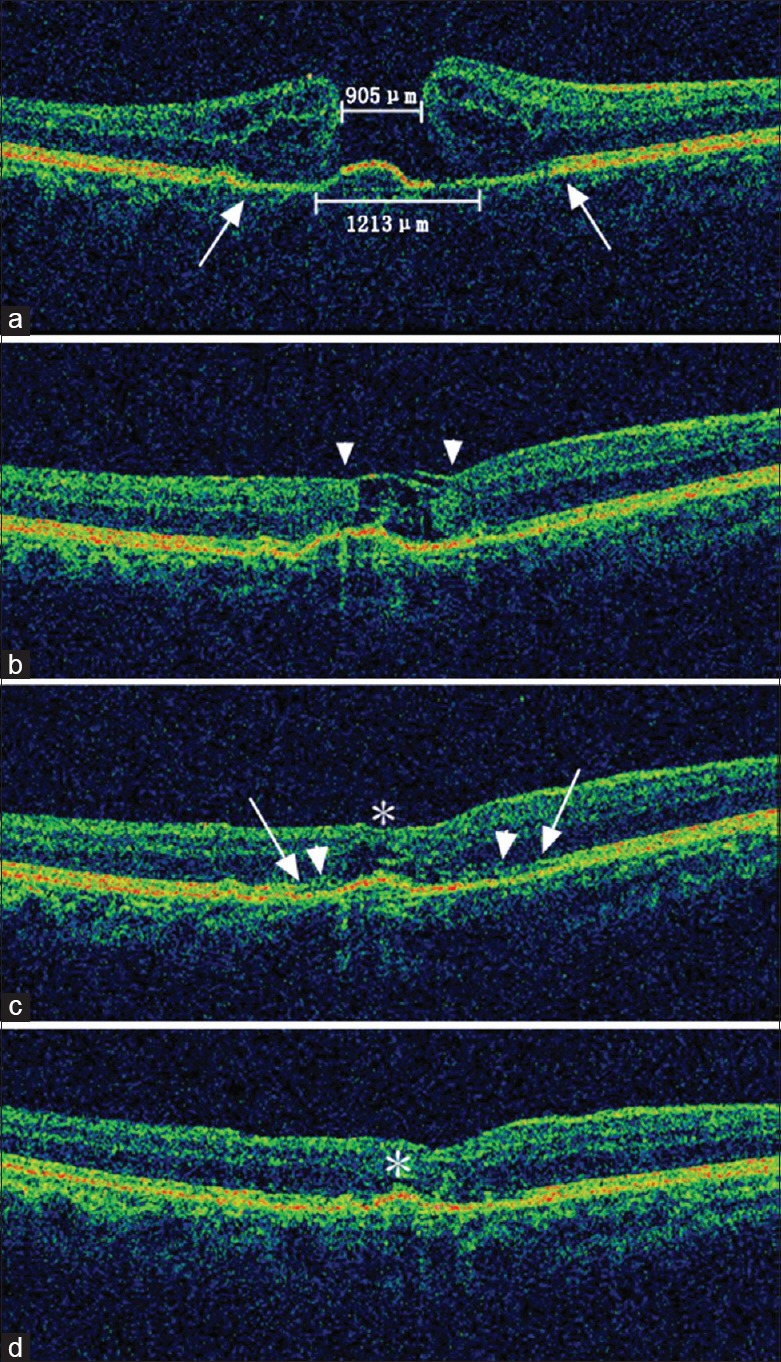
Spectral-domain optical coherence tomography images of the microstructural reconstruction process of a large macular hole in a 66-year-old female patient. (a) The preoperative minimum and base diameter of the macular hole was 905 μm and 1213 μm, respectively. Note the cystoid edema of the elevated margin and the disrupted external limiting membrane/ellipsoid zone (between the two arrows). The initial best corrected visual acuity was 0.08 (20/250). (b) Closure of the macular hole was observed 2 weeks after surgery, although the tissue filling the macular hole appeared to be sheets of inverted internal limiting membrane (flap closure, arrowheads). The best corrected visual acuity improved to 0.10 (20/200). (c) One month after surgery, the inner layers of the neurosensory retina thickened along the internal limiting membrane scaffold, leaving only a small defect of the outer retina (asterisk). The leading edge of the reconstructing external limiting membrane (arrowheads) and the ellipsoid zone (arrows) were both growing towards the center of the macular hole compared with the original sites before surgery. The best corrected visual acuity at this time was 0.16 (20/125). (d) Three months after surgery, the macular contour appeared further normalized, and the neurosensory retinal tissue above the fovea further thickened, leaving only a cleft (asterisk). Although not fully recovered at this stage, the external limiting membrane and the ellipsoid zone appeared more regular and clear. The best corrected visual acuity was 0.32 (20/63).
Figure 2.
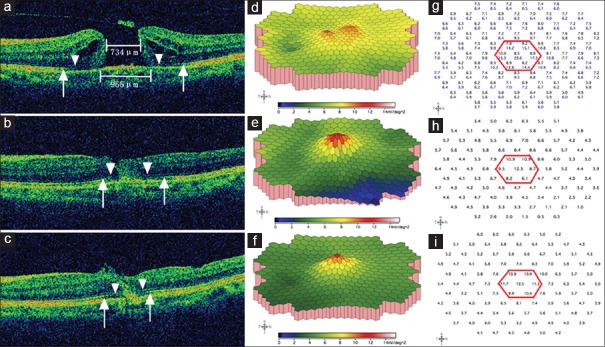
Representative spectral-domain optical coherence tomography and multi-focal electroretinogram changes of a 53-year-old female patient preoperative minimum and base diameters were 734 μm and 965 μm, respectively; defects of the external limiting membrane and the ellipsoid zone were indicated by arrow heads and arrows, respectively. Cystoid edema and elevation of the margin were demonstrated (a). On three-dimensional plot of multi-focal electroretinogram, the foveal and perifoveal area showed two separate small “peaks” (d), which reflected marked depression of the macular area and poor fixation due to low central vision. The response density of the central seven hexagons in this patient (g, the black numbers within the red hexagon) were much lower than a normal subject (g, the blue numbers). The initial best corrected visual acuity was 0.04 (20/500). One month after surgery, the macular hole closed, with partial restoration of both the external limiting membrane and the ellipsoid zone (b). The corresponding multi-focal electroretinogram showed a confluent blunt single peak (e), and the average retinal response density of the central seven hexagons and the fovea had increased from preoperative 8.5 and 8.8 nV/deg2 to 9.5 and 12.3 nV/deg2, respectively (h). The best corrected visual acuity was 0.13 (20/160) at this visit. Three months postoperatively, a further inward reconstruction of the external limiting membrane and the ellipsoid zone were observed (c). On three-dimensional plot of multi-focal electroretinogram, the peak became more sharp, with higher elevated immediate surrounding area (f); the corresponding average retinal response density of the central seven hexagons and the fovea had increased to 11.1 and 12.5 nV/deg2, respectively (i). The 3-month postoperative best corrected visual acuity was 0.20 (20/100).
Figure 3.
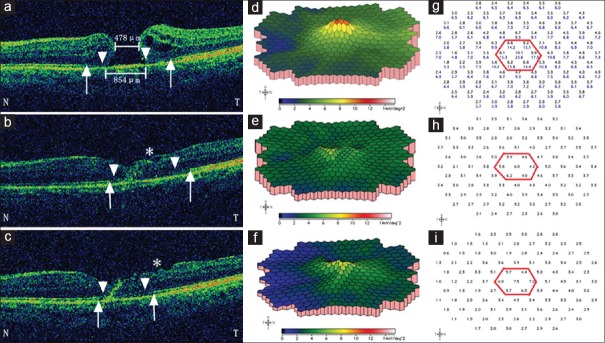
Representative spectral-domain optical coherence tomography and multi-focal electroretinogram changes in a 67-year-old female patient preoperative minimum and base diameters were 478 μm and 854 μm, respectively; defects of the external limiting membrane were indicated by arrow heads and the ellipsoid zone by arrows. Letter “N” and “T” represent “nasal” and “temporal” side of the macula, respectively. At baseline, on spectral-domain optical coherence tomography, cystoid edema was more prominent on the temporal side of the macular hole (a), and there was a single small peak on three-dimensional plot of multi-focal electroretinogram (d) and the average retinal response density of the central seven hexagons and the fovea (g, the black numbers within the red hexagon) were 8.4 and 10.1 nV/deg2, respectively; the initial best corrected visual acuity was 0.02 (20/1000). One-month postoperatively, the macular hole had closed, but the defects of the external limiting membrane and the ellipsoid zone were larger than baseline levels, especially on the temporal side; note also the rugged inner surface of the temporal macula (asterisk) (b); the central peak on the three-dimensional plot of multi-focal electroretinogram was further depressed, (e) with average retinal response density of the central seven hexagons and the fovea decreased to 5.0 and 6.0 nV/deg2, respectively (h); however, the best-corrected visual acuity still increased to 0.05 (20/400). Three months after surgery, defects of the external limiting membrane and the ellipsoid zone were partially restored although the inner surface of the temporal macula became even more rugged (asterisk) (c); a mild recovery of the central peak on the three-dimensional plot of multi-focal electroretinogram was detected (f) with average retinal response density of the central seven hexagons and the fovea increased to 5.8 and 7.5 nV/deg2, respectively (i); the best corrected visual acuity increased to 0.10 (20/200).
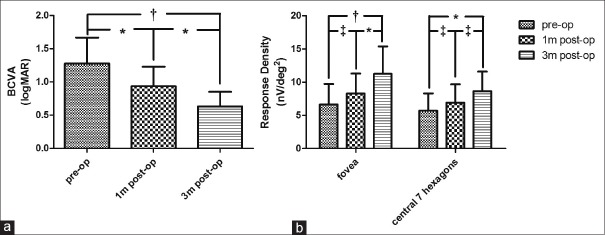
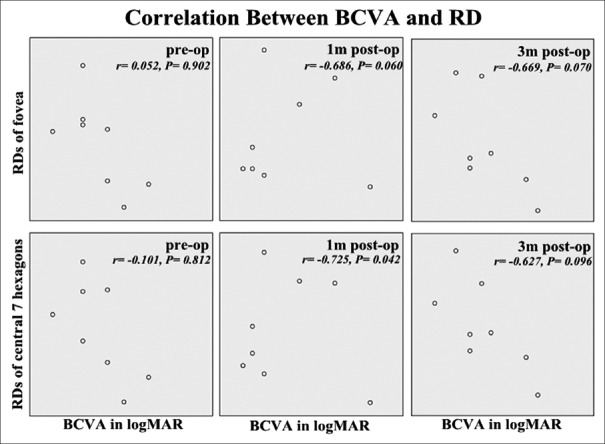
From a statistical point of view, significant improvements in BCVA were observed as early as 1 month after surgery [Figure 4a], while for RD of central seven hexagons and the fovea, not until 3 months after surgery did their increase reach “significance” [Figure 4b]. BCVA neither “significantly” correlated with mfERG RD of fovea nor with RD of the central seven hexagons at each evaluation time point [Figure 5], despite their similar incremental tendency after surgery [Figure 4].
Figure 4.
Longitudinal evaluations of best corrected visual acuity and multi-focal electroretinogram responses. As shown in figure, increasing trends in best corrected visual acuity (a) and multi-focal electroretinogram responses (b) were observed during the first 3 months after srugery. One-way analysis of variance and Tukey's multiple post-hoc comparison tests were used for analysis for visual acuity (a), and two-way analysis of variance with Bonferroni post-hoc tests were used for comparions between multi-focal electroretinogram responses at different time points (b). Results of statistical analysis were indicated (P < 0.01 and P < 0.001 were defined as significant [*] and very significant [†], respectively; [‡] means not significant). BCVA: Best corrected visual acuity, logMAR: Logarithm of the minimum angle of resolution. pre-op: Preoperative; 1m post-op: Postoperative 1 month; 3m post-op: Postoperative 3 months.
Figure 5.
Associations between best corrected visual acuity in logarithm of the minimum angle of resolution and response densities of fovea or central 7 hexagons at base line (pre-op), 1 month after surgery (1 m post-op) and 3 months after surgery (3 m post-op). Pearson test coefficients (r) and P values were demonstrated in each plot. Although improvement could be observed with time for both paremeters, there was no clear correlation between best corrected visual acuity and multi-focal electroretinogram responses at each evaluation time point, and retinal response densities varied in patients even with the same best corrected visual acuity (P < 0.01 was defined as significant). BCVA: Best corrected visual acuity, RD:Response density, logMAR: Logarithm of the minimum angle of resolution.
Two eyes had a transient elevation of IOP and were well controlled by topical antiglaucoma medications. None of the included eyes developed severe cataract and any other complications that might compromise visual function, interfere with the examinations or necessitate surgery during follow-up.
DISCUSSION
The closure rate of MHs was 68% when ILM peeling assisted PPV was first introduced to manage idiopathic MH,[1] and have recently improved to 86–100% with the aid of ILM staining technique.[15] Large MHs (minimum diameter >400 μm), however, usually had an increased risk of surgical failure, and the closure rate of MHs with high myopia, reported between 83% and 87%, appeared to be lower than the idiopathic forms.[16,17] In addition, about 19–39% of the so-called anatomically closed large MHs were actually flat-open.[6,7,8] This is not hard to understand considering that the traditional ILM peeling might only release the tangential traction, but cannot compensate for tissue shortening in large MHs. Encouragingly, the recent advent of the inverted ILM flap technique had increased the rate of complete closure to 98% in the original study for large idiopathic MHs,[9] and to 100% in a report for high myopic MHs.[13] In this study, with all MHs achieved complete closure, further supported the reliability of this technique for the management of large MHs. The inverted ILM flap technique, however, is not without limitations. In the original report, spontaneous detachment of the flap occurred in seven of fifty eyes during the air-fluid exchange.[9] To address this, Shin et al. described a modified technique in which perfluoro-n-octane was used to assist the covering of the MH with a single sheet of ILM flap.[18] In our experience, we found that lowering the perfusion pressure when inverting the flap and during air-fluid exchange might help to avoid the flap from being washed away, and indeed no dislocation of the ILM flap occurred during surgery in the current study. Another notable modification of this technique reported recently by the original authors was to peel only the temporal side of the ILM instead of the whole area around the fovea, which was found to be associated with a reduction in the frequency of having a dissociated optic nerve fiber layer appearance.[19]
The proposed mechanisms for tissue repair in the MHs following inverted ILM flap technique assisted PPV were as follows: The inverted ILM which contained Müller cell fragments not only provoked gliosis via activating tissue necrosis growth factor-α but also served as a scaffold and basement membrane for tissue proliferation, thus providing an environment to instruct the photoreceptors to assume correct position during the reconstruction process and finally to improve the postoperative vision.[9] The microstructural recovery process of the MHs after surgery revealed by SD-OCT in our study was in line with the above-mentioned speculation [Figure 1]: A membranous structure (which we believe was the inverted ILM) bridging over the macula defect was observed 2 weeks after surgery and seemed to serve as a scaffold for the repairing tissue because the subsequent repair appeared to proceed in an inner-to-outer manner. In addition, based on the observations in our study and as proposed previously,[20] restoration of the ELM might be important for visual recovery as the leading edge of the reconstructing ELM was found to precede that of the EZ in all patients in our study. Unintended injury of the nerve fiber layer during ILM peeling, however, might compromise recovery of BCVA and mfERG [Figure 3].
Although both BCVA and mfERG responses improved incrementally after surgery [Figure 4], they were not found to be statistically correlated at each evaluation time point of our short-term observation [Figure 5] as well as in previous longer term studies concerning conventional surgical technique (just ILM peeling but without inverting) in treatment of MHs.[21,22,23] The lack of correlation between BCVA and mfERG responses suggested that these two functional evaluation modalities could not adequately represent, but might be supplemental to, each other in the clinical setting of MHs, and might be explained by the following differences between them: BCVA is a subjective measurement and only measure the function of the fixation point under optimal condition, while mfERG is an objective measurement and provides a topographic electrophysiological mapping of the central retina. Further studies combining BCVA, OCT, mfERG, and subjective functional mapping of the central retina, such as microperimetry, might provide a more comprehensive evaluation of the patients with MHs.
This study is a prospective study in large idiopathic MHs treated with inverted ILM flap assisted PPV that combined the microstructural recovery process with changes of two aspects of functional evaluations (BCVA and mfERG response) and analyzed the association between BCVA and mfERG. The following major limitations of this study should be kept in mind when interpreting the results: (1) the absence of a control group, (2) the limited sample size, and (3) the limited length of follow-up. However, we would like to point out that, no control group was designed considering the rareness of large macular holes (minimum diameter >400 μm), and no other effective treating methods had been verified and reported in the literature by far. Further studies with more cases and longer term observations could be conducted, although it still would be very time consuming.
In conclusion, inverted ILM flap technique appeared to be a safe and successful approach for the management of large idiopathic MHs with promising short-term anatomical and functional results. Lowering perfusion pressure when inverting the ILM flap and during air-fluid exchange might help to avoid dislocation of the ILM flap during surgery. Postoperative reconstruction of the foveal microstructure generally showed good consistency with improvements in both BCVA and mfERG response. And although not significantly correlated, mfERG response might be a good supplement for BCVA to evaluate postoperative functional recovery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Welly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–9. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 2.Kadonosono K, Itoh N, Uchio E, Nakamura S, Ohno S. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118:1116–8. doi: 10.1001/archopht.118.8.1116. [DOI] [PubMed] [Google Scholar]
- 3.Ando F, Sasano K, Ohba N, Hirose H, Yasui O. Anatomic and visual outcomes after indocyanine green-assisted peeling of the retinal internal limiting membrane in idiopathic macular hole surgery. Am J Ophthalmol. 2004;137:609–14. doi: 10.1016/j.ajo.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Beutel J, Dahmen G, Ziegler A, Hoerauf H. Internal limiting membrane peeling with indocyanine green or trypan blue in macular hole surgery: A randomized trial. Arch Ophthalmol. 2007;125:326–32. doi: 10.1001/archopht.125.3.326. [DOI] [PubMed] [Google Scholar]
- 5.Henrich PB, Haritoglou C, Meyer P, Ferreira PR, Schötzau A, Katamay R, et al. Anatomical and functional outcome in brilliant blue G assisted chromovitrectomy. Acta Ophthalmol. 2010;88:588–93. doi: 10.1111/j.1755-3768.2008.01477.x. doi: 10.1111/j.1755-3768.2008.01477.x. [DOI] [PubMed] [Google Scholar]
- 6.Imai M, Iijima H, Gotoh T, Tsukahara S. Optical coherence tomography of successfully repaired idiopathic macular holes. Am J Ophthalmol. 1999;128:621–7. doi: 10.1016/s0002-9394(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 7.Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87:1015–9. doi: 10.1136/bjo.87.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalewska Z, Michalewski J, Cisiecki S, Adelman R, Nawrocki J. Correlation between foveal structure and visual outcome following macular hole surgery: A spectral optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2008;246:823–30. doi: 10.1007/s00417-007-0764-5. doi: 10.1007/s00417-007-0764-5. [DOI] [PubMed] [Google Scholar]
- 9.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–25. doi: 10.1016/j.ophtha.2010.02.011. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Mahalingam P, Sambhav K. Surgical outcomes of inverted internal limiting membrane flap technique for large macular hole. Indian J Ophthalmol. 2013;61:601–3. doi: 10.4103/0301-4738.121090. doi: 10.4103/0301-4738.121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, Matsumoto M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 2013;156:125–31.e1. doi: 10.1016/j.ajo.2013.02.014. doi: 10.1016/j.ajo.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Kuriyama S. Foveal microstructure in macular holes surgically closed by inverted internal limiting membrane flap technique. Retina. 2014;34:2444–50. doi: 10.1097/IAE.0000000000000252. doi: 10.1097/IAE.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 13.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 2014;34:664–9. doi: 10.1097/IAE.0000000000000042. doi: 10.1097/IAE.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 14.Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition) Doc Ophthalmol. 2012;124:1–13. doi: 10.1007/s10633-011-9296-8. doi: 10.1007/s10633-011-9296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla D, Kalliath J, Neelakantan N, Naresh KB, Ramasamy K. A comparison of brilliant blue G, trypan blue, and indocyanine green dyes to assist internal limiting membrane peeling during macular hole surgery. Retina. 2011;31:2021–5. doi: 10.1097/IAE.0b013e318213618c. doi: 10.1097/IAE.0b013e318213618c. [DOI] [PubMed] [Google Scholar]
- 16.Chuang LH, Chen YP, Wang NK, Yeung L, Chen KJ, Hwang YS, et al. Macular hole repair by vitrectomy and internal limiting membrane peeling in highly myopic eyes. Retina. 2014;34:2021–7. doi: 10.1097/IAE.0000000000000183. doi: 10.1097/IAE.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 17.Conart JB, Selton J, Hubert I, Trechot F, El Adssi H, Creuzot-Garcher C, et al. Outcomes of macular hole surgery with short-duration positioning in highly myopic eyes: A case-control study. Ophthalmology. 2014;121:1263–8. doi: 10.1016/j.ophtha.2013.12.005. doi: 10.1016/j.ophtha.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Shin MK, Park KH, Park SW, Byon IS, Lee JE. Perfluoro-n-octane-assisted single-layered inverted internal limiting membrane flap technique for macular hole surgery. Retina. 2014;34:1905–10. doi: 10.1097/IAE.0000000000000339. doi: 10.1097/IAE.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 19.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Adelman RA, Nawrocki J. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: A Comparative Study. Retina. 2015;35:1844–50. doi: 10.1097/IAE.0000000000000555. doi: 10.1097/IAE.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: Spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117:1815–24. doi: 10.1016/j.ophtha.2010.01.017. doi: 10.1016/j.ophtha.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Si YJ, Kishi S, Aoyagi K. Assessment of macular function by multifocal electroretinogram before and after macular hole surgery. Br J Ophthalmol. 1999;83:420–4. doi: 10.1136/bjo.83.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moschos M, Apostolopoulos M, Ladas J, Theodossiadis P, Malias J, Moschou M, et al. Multifocal ERG changes before and after macular hole surgery. Doc Ophthalmol. 2001;102:31–40. doi: 10.1023/a:1017507220510. [DOI] [PubMed] [Google Scholar]
- 23.Apostolopoulos MN, Koutsandrea CN, Moschos MN, Alonistiotis DA, Papaspyrou AE, Mallias JA, et al. Evaluation of successful macular hole surgery by optical coherence tomography and multifocal electroretinography. Am J Ophthalmol. 2002;134:667–74. doi: 10.1016/s0002-9394(02)01700-2. [DOI] [PubMed] [Google Scholar]