Abstract
Background:
The metastatic renal cell carcinoma (mRCC) patients treated with upfront cytoreductive nephrectomy combined with α-interferon yields additional overall survival (OS) benefits. It is unclear whether mRCC patients treated with vascular endothelial growth factor receptor-tyrosine kinase inhibitor (VEGFR-TKI) will benefit from such cytoreductive nephrectomy either. The aim of the study was to identify variables for selection of patients who would benefit from upfront cytoreductive nephrectomy for mRCC treated with VEGFR-TKI.
Methods:
Clinical data on 74 patients enrolled in 5 clinical trials conducted in Cancer Hospital (Institute), Chinese Academy of Medical Sciences from January 2006 to January 2014 were reviewed retrospectively. The survival analysis was performed by the Kaplan–Meier method. Comparisons between patient groups were performed by Chi-square test. A Cox regression model was adopted for analysis of multiple factors affecting survival, with a significance level of α = 0.05.
Results:
Fifty-one patients underwent cytoreductive nephrectomy followed by targeted therapy (cytoreductive nephrectomy group) and 23 patients were treated with targeted therapy alone (noncytoreductive nephrectomy group). The median OS was 32.2 months and 23.0 months in cytoreductive nephrectomy and noncytoreductive nephrectomy groups, respectively (P = 0.041). Age ≤45 years (P = 0.002), a low or high body mass index (BMI <19 or >30 kg/m2) (P = 0.008), a serum lactate dehydrogenase (LDH) concentration >1.5 × upper limit of normal (P = 0.025), a serum calcium concentration >10 mg/ml (P = 0.034), and 3 or more metastatic sites (P = 0.023) were independent preoperative risk factors for survival. The patients only with 0–2 risk factors benefited from upfront cytoreductive nephrectomy in terms of OS when compared with the patients treated with targeted therapy alone (40.0 months vs. 23.2 months, P = 0.042), while those with more than 2 risk factors did not.
Conclusions:
Five risk factors (age, BMI, LDH, serum calcium, and number of metastatic sites) seemed to be helpful for selecting patients who would benefit from undergoing upfront cytoreductive nephrectomy.
Keywords: Cytoreductive Nephrectomy, Metastatic Renal Cell Carcinoma, Targeted Therapy
INTRODUCTION
Renal cell carcinoma is the most common malignancy of kidney. Currently, the anti-angiogenic tyrosine kinase inhibitors (TKIs), such as sunitinib, sorafenib, and pazopanib, have been verified as the preferred treatment for metastatic renal cell carcinoma (mRCC). In the era of immunotherapy, it is well recognized that upfront cytoreductive nephrectomy combined with α-interferon yields additional overall survival (OS) benefits for mRCC patients with good performance status.[1] When it comes to the era of targeted therapy, there is a lack of prospective randomized controlled study on whether mRCC patients will benefit from such cytoreductive nephrectomy either. Retrospective studies showed that cytoreductive nephrectomy did not produce a universal effect on OS in mRCC patients receiving targeted therapy.[2] However, a few studies also showed that those patients with certain high-risk factors seemed to benefit from it. Such study is rare in China. In this paper, we retrospectively analyzed the data of mRCC patients treated with vascular endothelial growth factor receptor-TKIs (VEGFR-TKIs) as first-line therapy in 5 clinical trials in Cancer Institute and Hospital, Chinese Academy of Medical Sciences and try to evaluate whether cytoreductive nephrectomy affects the survival of mRCC patients.
METHODS
Patients
The data were obtained from 74 mRCC patients in five clinical trials conducted in Cancer Hospital (Institute), Chinese Academy of Medical Sciences from January 2006 to January 2014. All patients received a VEGFR-TKI as first-line treatment, either sunitinib (A6181132 [ClinicalTrials.gov Identifier: NCT00706706], VEG108844 [ClinicalTrials.gov Identifier: NCT00720941]), pazopanib (VEG113078 [ClinicalTrials.gov Identifier: NCT01147822]), sorafenib (Bayer-11559 [ClinicalTrials.gov Identifier: NCT00586105]), or famitinib (FMTN-II-MRCC [ClinicalTrials.gov Identifier: NCT01829841]).[3,4] Famitinib is a novel multi-targeting TKI against VEGFR, platelet-derived growth factor receptor, stem cell factor receptor (c-KIT) and FMS-like tyrosine kinase-3 receptor developed in China, which has found to be effective for clear cell renal cell carcinoma in the preliminary clinical trial. The patients had the following main common criteria from all studies: pathologically diagnosed as renal clear cell carcinoma, Eastern Cooperative Oncology Group score ≤1, age ≥18 years old, absolute neutrophil count ≥1.5 × 109/L, hemoglobin ≥100 g/L, blood platelet ≥100 × 109/L, total bilirubin ≤1.5 × upper limit of normal (ULN), alanine aminotransferase and aspartate aminotransferase ≤2.5 × ULN, and creatinine clearance rate ≥30 ml/min.
Treatment
Dosages of the various VEGFR-targeted TKIs administered were: sunitinib 50 mg once daily for 28 days, and then discontinued for 14 days; pazopanib 800 mg once daily; sorafenib 400 mg twice daily; and famitinib 25 mg once daily. According to the Common Terminology Criteria for Adverse Events v3.0 standard (U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Maryland, USA), if nonhematologic toxicity greater than Grade 3 or Grade 4 hematologic toxicity occurred, the treatment was discontinued, and when the toxicity had recovered to Grade 1 or less, the treatment was restarted at a lower dose. Two dosage reductions were allowed (sunitinib to 37.5 mg/d and 25 mg/d; pazopanib to 600 mg/d and 400 mg/d; sorafenib to 400 mg/d and 400 mg/2 days; and famitinib to 20 mg/d and 15 mg/d). If obvious toxic or adverse effects occurred after drug withdrawal and lasted for more than 14 days, or if they occurred after two dose reductions had been made, the treatment was stopped.
Evaluation and follow-up
The therapeutic evaluation was performed according to the Response Evaluation Criteria in Solid Tumors 1.0 (National Cancer Institute of the United States, National Cancer Institute of Canada), and included response rate, progression-free survival (PFS) time, and OS time. The curative effect was evaluated every 6 weeks during the 24-week treatment period, and every 12 weeks after 24 weeks of treatment. The evaluation was performed by spiral computed tomography with 5 mm thick slices.
The patients were followed up until March 31, 2015 (follow-up rate 100%). The median follow-up duration for the whole group was 38.3 months (range: 2.3–53.9 months).
Statistical analysis
Data analysis was performed using SPSS version 20.0 (SPSS Inc., IL, USA), and the data were shown as numbers and percentages. The survival analysis was performed by the Kaplan–Meier method, and the survival rates were compared by a log-rank test. Comparisons between patient groups were performed by the Chi-square test. A Cox regression model was adopted for analysis of multiple factors affecting survival, with a significance level of α = 0.05.
RESULTS
General data and survival
Median age of 74 patients was 51.4 years (range: 20.1–86.8 years), and the male to female ratio was about 3:1. Twenty-two patients aged ≤45 years, 52 aged >45 years. Eleven patients had body mass index (BMI) <19 kg/m2, 56 patients BMI 19–30 kg/m2; 7 patients BMI >30 kg/m2. Lung metastasis was the most common metastatic site in all cases. The median number of metastatic site was 2 (range: 1–7). Thirty-eight patients had 1–2 metastatic sites, and 36 had 3 or more. Of the 74 patients, 51 underwent upfront cytoreductive nephrectomy, followed by a VEGFR-TKI therapy (cytoreductive nephrectomy group) and 23 treated with a VEGFR-TKI therapy alone (noncytoreductive nephrectomy group). Thirty-three patients were treated with sunitinib, 5 with pazopanib, 22 with sorafenib and 14 with famitinib. No statistical difference exists between the cytoreductive and noncytoreductive nephrectomy groups in terms of age, gender, BMI, blood calcium, lactate dehydrogenase (LDH), anemia, T stage, number of metastatic sites, and lung metastasis [Table 1].
Table 1.
Clinical features of patients with metastatic renal cell carcinoma in this study, n (%)
| Factors | All patients (n = 74) | Cytoreductive nephrectomy group (n = 51) | Noncytoreductive nephrectomy group (n = 23) | χ2 | P |
|---|---|---|---|---|---|
| Gender | 0.860 | 0.354 | |||
| Male | 56 (75.7) | 37 (72.5) | 19 (82.6) | ||
| Female | 18 (24.3) | 14 (27.5) | 4 (17.4) | ||
| Age | 0.209 | 0.647 | |||
| >45 years | 52 (70.3) | 35 (68.6) | 17 (73.9) | ||
| ≤45 years | 22 (29.7) | 16 (31.4) | 6 (26.1) | ||
| BMI | 0.751 | 0.687 | |||
| <19 kg/m2 | 11 (14.9) | 7 (13.7) | 4 (17.4) | ||
| 19–30 kg/m2 | 56 (75.7) | 40 (78.4) | 16 (69.6) | ||
| >30 kg/m2 | 7 (9.5) | 4 (7.9) | 3 (13.0) | ||
| LDH | 1.383 | 0.240 | |||
| ≤1.5 × ULN | 49 (66.2) | 36 (70.6) | 13 (56.5) | ||
| >1.5 × ULN | 25 (33.8) | 15 (29.4) | 10 (39.1) | ||
| Anemia | 0.714 | 0.398 | |||
| No | 53 (71.6) | 35 (68.6) | 18 (78.3) | ||
| Yes | 21 (28.4) | 16 (31.4) | 5 (21.7) | ||
| Blood calcium | 0.267 | 0.605 | |||
| ≤10 mg/ml | 55 (74.3) | 37 (72.5) | 18 (78.3) | ||
| >10 mg/ml | 19 (25.7) | 14 (27.5) | 5 (21.7) | ||
| T stage | 4.046 | 0.257 | |||
| T1 | 27 (36.5) | 22 (43.1) | 5 (21.7) | ||
| T2 | 35 (47.3) | 22 (43.1) | 13 (56.6) | ||
| T3 | 11 (14.9) | 6 (11.8) | 5 (21.7) | ||
| T4 | 1 (1.3) | 1 (2.0) | 0 (0.0) | ||
| Number of metastatic sites | 3.618 | 0.057 | |||
| 1–2 | 38 (51.4) | 30 (58.8) | 8 (34.8) | ||
| ≥3 | 36 (48.6) | 21 (41.2) | 15 (65.2) | ||
| Pulmonary metastasis | 0.001 | 0.979 | |||
| No | 13 (17.6) | 9 (17.6) | 4 (17.4) | ||
| Yes | 61 (82.4) | 42 (82.4) | 19 (82.6) | ||
| Drugs | 5.595 | 0.140 | |||
| Sunitinib | 33 (44.6) | 24 (47.0) | 9 (39.1) | ||
| Pazopanib | 5 (6.8) | 3 (5.9) | 2 (8.7) | ||
| Sorafenib | 22 (29.7) | 19 (37.3) | 3 (13.1) | ||
| Famitinib | 14 (18.9) | 5 (9.8) | 9 (39.1) |
BMI: Body mass index; LDH: Lactate dehydrogenase; UNL: Upper limit of normal.
The median survival time for all 74 patients was 26.7 months (range: 4.0–52.7 months) [Figure 1]. The median OS times were 32.2 months and 23.0 months in the cytoreductive and noncytoreductive nephrectomy groups, respectively, with significant difference (P = 0.041) [Figure 2].
Figure 1.
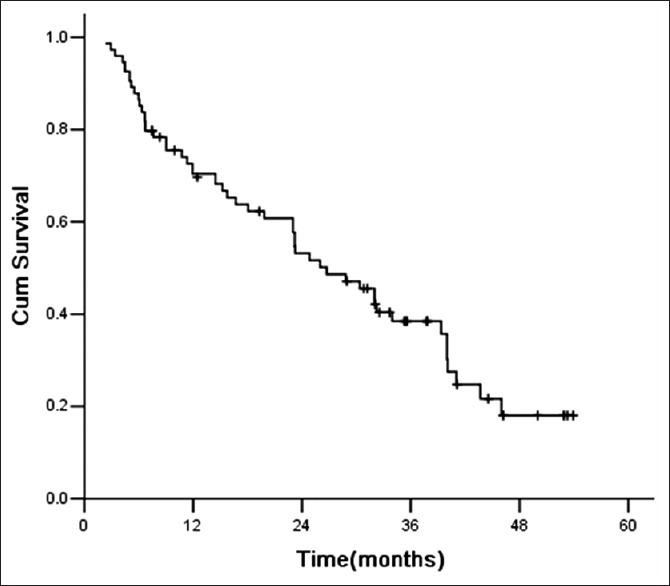
Survival curve for all patients.
Figure 2.
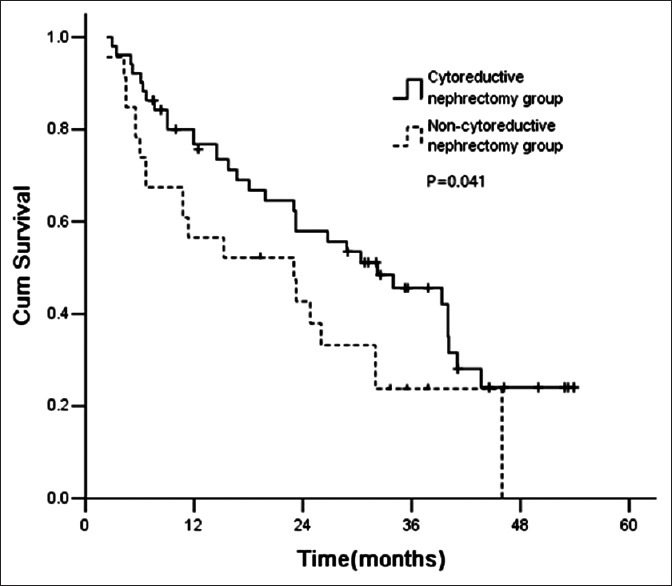
Survival curves for cytoreductive and noncytoreductive nephrectomy groups.
Univariate analysis of prognostic factors
Univariate analysis showed that the median survival time in patients aged ≤45 years was 19.8 months, which was significantly shorter than that of 40.0 months in those aged >45 years (P = 0.007). When analyzed according to the BMI grouping, the median survival time of patients with normal BMI (19–30 kg/m2) is 39.4 months, which was significantly longer than that of 9.0 months in those with lower (BMI <19 kg/m2) or higher (BMI >30 kg/m2) (P = 0.000). The number of metastatic sites also significantly affected the prognosis. With an increasing number of metastatic sites, the median survival time was decreased significantly. The OS time was 40.0 months for patients with 1–2 metastatic sites and 23.2 months for those with 3 or more metastatic sites (P = 0.047) [Table 2].
Table 2.
Analysis of prognostic factors for patients with metastatic renal clear cell carcinoma receiving cytoreductive nephrectomy
| Factors | Median survival time (months) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender | 1.453 (0.769–2.745) | 0.246 | 0.945 (0.373–2.397) | 0.906 | |
| Male | 39.4 | ||||
| Female | 23.2 | ||||
| Age | 2.291 (1.266–4.145) | 0.007 | 4.151 (1.681–10.251) | 0.002 | |
| >45 years | 40.0 | ||||
| ≤45 years | 19.8 | ||||
| BMI | 3.362 (1.827–6.187) | 0.000 | 4.247 (1.462–12.334) | 0.008 | |
| 19–30 kg/m2 | 39.4 | ||||
| <19 or >30 kg/m2 | 9.0 | ||||
| LDH | 2.865 (1.593–5.152) | 0.001 | 2.832 (1.313–6.110) | 0.025 | |
| ≤1.5 × UNL | 40.0 | ||||
| >1.5 × UNL | 14.5 | ||||
| Anemia | 3.001 (1.574–5.724) | 0.003 | 2.593 (0.968–6.946) | 0.058 | |
| No | 40.0 | ||||
| Yes | 15.7 | ||||
| Blood calcium | 1.998 (1.045–3.823) | 0.004 | 2.637 (1.076–6.462) | 0.034 | |
| ≤10 mg/ml | 39.4 | ||||
| >10 mg/ml | 18.0 | ||||
| T stage | 0.866 (0.593–1.264) | 0.746 | 0.962 (0.526–1.758) | 0.899 | |
| T1 | 32.2 | ||||
| T2 | 34.0 | ||||
| T3 | 7.6 | ||||
| T4 | 23.0 | ||||
| Number of metastatic sites | 2.913 (1.621–5.234) | 0.047 | 2.554 (1.135–5.747) | 0.023 | |
| 1–2 | 40.0 | ||||
| ≥3 | 23.2 | ||||
| Pulmonary metastasis | 0.829 (0.387–1.777) | 0.572 | 2.451 (0.728–8.246) | 0.148 | |
| No | 41.0 | ||||
| Yes | 30.4 | ||||
| Drugs | 1.097 (0.866–1.389) | 0.969 | 1.265 (0.848–1.887) | 0.250 | |
| Sunitinib | 34.0 | ||||
| Pazopanib | 39.4 | ||||
| Sorafenib | 26.7 | ||||
| Famitinib | 23.2 | ||||
BMI: Body mass index; LDH: Lactate dehydrogenase; UNL: Upper limit of normal; HR: Hazard ratio; CI: Confidence interval.
Similarly, elevation of LDH, anemia and hypercalcemia were adverse prognosis factors for patients undergoing cytoreductive nephrectomy. The median survival of those patients with LDH ≤1.5 × UNL and LDH >1.5 × UNL was 40.0 months and 14.5 months, respectively (P = 0.001). The median survival time were 40.0 months and 15.7 months respectively for patients without and with anemia (P = 0.003), 39.4 months and 18.0 months respectively for patients with blood calcium ≤10 mg/ml and >10 mg/ml (P = 0.004) [Table 2].
Multivariate analysis of prognosis factors
After applying Cox risk model calculations, 5 factors were found to be independent predictors of shorter survival: age ≤45 years (P = 0.002), lower or higher BMI (BMI <19 kg/m2 or BMI >30 kg/m2) (P = 0.008), LDH >1.5 × ULN (P = 0.025), blood calcium >10 mg/ml (P = 0.034) and number of metastatic sites ≥3 (P = 0.023) [Table 2].
Correlation between survival and cytoreductive nephrectomy
Among patients, with 0–2 risk factors before therapy, those received cytoreductive nephrectomy had significantly longer survival than those did not. The median survival time was 40.0 months and 23.2 months for cytoreductive nephrectomy and noncytoreductive nephrectomy groups, respectively (P = 0.042) [Figure 3]. However, the patients with 3–5 factors before therapy did not benefit from cytoreductive nephrectomy [Figure 4]. The median survival time was 6.7 and 6.0 months for cytoreductive nephrectomy and noncytoreductive nephrectomy groups, respectively (P = 0.535) [Figure 3].
Figure 3.
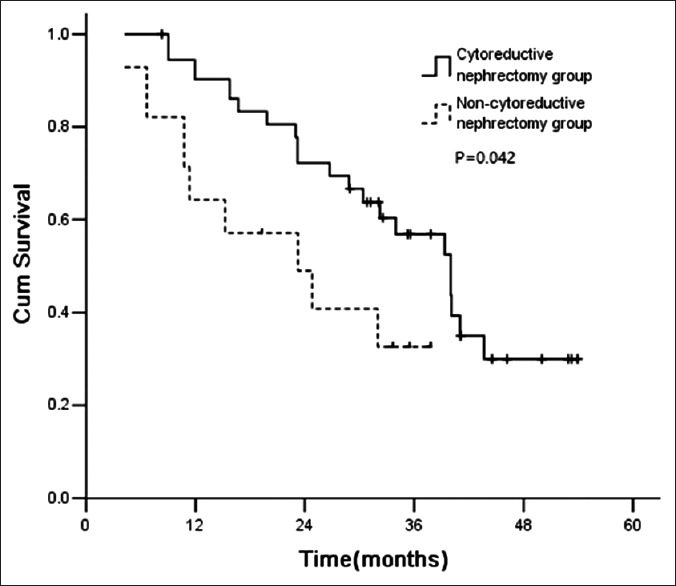
Survival curve for patients with 0–2 risk factors.
Figure 4.
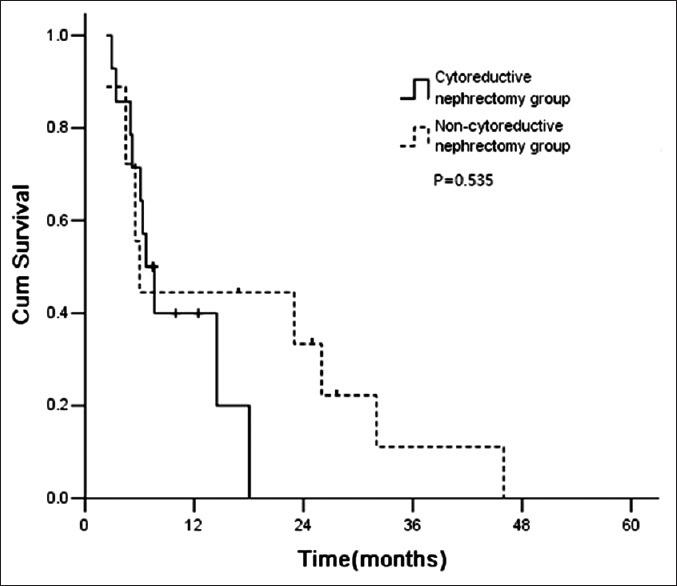
Survival curve of patients with 3–5 risk factors.
DISCUSSION
In the era of immunotherapy, two prospective randomized controlled studies demonstrated that the median survival time of mRCC patients treated with cytoreductive nephrectomy followed by α-interferon is superior to that of patients treated with α-interferon alone. In the study reported by Flanigan et al.,[5] 241 mRCC patients were randomized to receive cytoreductive nephrectomy followed by α-interferon or α-interferon alone. The median survival times were 11.1 and 8.1 months, respectively (P = 0.05). Mickisch et al.[6] also reported that 85 mRCC patients were randomized to receive upfront cytoreductive nephrectomy followed by α-interferon or α-interferon alone. Time to progression was 5 months and 3 months, respectively (hazard ratio [HR]: 0.60, 95% confidence interval [CI]: 0.36–0.97); median survival time was 17 months and 7 months, respectively (HR: 0.54, 95% CI: 0.31–0.94). Therefore, it has been recommending to remove the primary kidney lesion in patients with mRCC before immunotherapy in order to further improve survival.
When it comes to the era of targeted therapy, whether mRCC patients treated with targeted agents will still benefit from upfront cytoreductive nephrectomy remains unclear. No final conclusion has yet been reached due to the lack of prospective randomized controlled studies. A large retrospective study analyzed the clinical data of 1658 mRCC patients from the combined international database of mRCC from 20 countries and showed that patients treated with cytoreductive nephrectomy combined with targeted therapy had longer survival than those treated with target therapy alone, with median OS times of 20.6 months versus 9.5 months (P < 0.0001) and median PFS times of 7.6 months and 4.5 months, respectively (P < 0.001). Further analysis found that the OS was significantly correlated with 6 risk prognostic factors (hemoglobin below the lower limit of normal value; blood calcium higher than the ULN; neutrophil higher than the ULN; blood platelet higher than the ULN; Karnofsky performance status <80 scores; and time interval from diagnosis to treatment <1 year). For patients with ≤3 risk factors, the OS were significantly prolonged with cytoreductive nephrectomy when compared to those without; Those patients with ≥4 risk factors did not benefit from cytoreductive nephrectomy (P = 0.0005).[7] You et al.[2] retrospectively analyzed the data of 171 mRCC patients, of whom, 96 received cytoreductive nephrectomy followed by targeted therapy (sunitinib 63%, sorafenib 27%, pazopanib 4%, and temsirolimus 6%), and 75 were treated with targeted therapy alone (sunitinib 80%, sorafenib 9%, pazopanib 4%, and temsirolimus 7%). The median survival times of the two groups were 19.9 and 11.7 months respectively (P < 0.001). The authors also found that Karnofsky performance status score <80, anemia, neutropenia, and N2 stage were independent poor prognosis factors for mRCC patients receiving cytoreductive nephrectomy. The survival benefited from cytoreductive nephrectomy was obvious for patients with less two risk factors (28.2 months vs. 18.4 months, P = 0.018).[2] However, there is still lack of such retrospective study in China.
In our series, patients received cytoreductive nephrectomy had significantly longer median survival than those did not (32.2 months vs. 23.0 months, P = 0.041). This results was similar to those reported in literatures. As for whether different TKI drugs have impact on survival, the study results showed that there was no significant difference in the median survival among patients treated with either sunitinib, pazopanib, sorafenib orfamitinib, respectively after cytoreductive nephrectomy. Further analysis of this study showed that the survival benefited from upfront cytoreductive nephrectomy depended on the number of risk factors. Age ≤45 years, lower or higher BMI (BMI <19 kg/m2 or BMI >30 kg/m2), LDH >1.5 × ULN, blood calcium >10 mg/ml and ≥3 metastatic sites were independent poor prognostic factors. The survival benefited from cytoreductive nephrectomy was significant in patients with 0 to 2 risk factors (P = 0.042). With an increasing number of risk factors, the benefit from cytoreductive nephrectomy diminished. In general, skinny patients may have significant tumor consumption with poor physical condition and organ function reserve, while in obese patients, the heart, lungs, liver, and other organs are overburdened and have poor compensatory ability during drug treatment. All these factors may cause patients to exhibit resistance to the drug treatment administered, contributing to the overall treatment results. Albiges et al.[8] investigated the effect of BMI on treatment outcome in 1975 patients treated with targeted therapy for mRCC. Overweight/obese (BMI ≥25 kg/m2) patients had a longer median OS than underweight/normal weight (BMI <25 kg/m2) patients (25.6 months vs. 17.1 months, P < 0.0001). LDH had been used in wide variety of tumor prognostic factor models, LDH elevation commonly indicated poor prognosis.[9]
Furthermore in this study, the survival time of the patients treated with targeted therapy combined with cytoreductive nephrectomy was slightly longer than that reported in the literatures. This may partly due to the fact that these cases were all from clinical trials and were well selected with relative good performance status. However, the data were objective and completely recorded. Therefore, it has some reference values on determining whether patients with mRCC need cytoreductive nephrectomy in addition to target therapy and also provides reference for developing prospective randomized study plan in future.
In conclusion, five risk factors (age, BMI, LDH, serum calcium, and number of metastatic sites) seemed to be helpful for selecting patients who would benefit from undergoing upfront cytoreductive nephrectomy, that need to be further warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: A combined analysis. J Urol. 2004;171:1071–6. doi: 10.1097/01.ju.0000110610.61545.ae. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 2.You D, Jeong IG, Song C, Lee JL, Hong B, Hong JH, et al. Analysis of pre-operative variables for identifying patients who might benefit from upfront cytoreductive nephrectomy for metastatic renal cell carcinoma in the targeted therapy era. Jpn J Clin Oncol. 2015;45:96–102. doi: 10.1093/jjco/hyu171. doi: 10.1093/jjco/hyu171. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Zhou AP, Qin Q, Chang CX, Jiang HY, Ma JH, et al. Famitinib in metastatic renal cell carcinoma: A single center study. Chin Med J. 2013;126:4277–81. doi: 10.3760/cma.j.issn.0366-6999.20131757. [PubMed] [Google Scholar]
- 4.Zhou A, Zhang W, Chang C, Chen X, Zhong D, Qin Q, et al. Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemother Pharmacol. 2013;72:1043–53. doi: 10.1007/s00280-013-2282-y. doi: 10.1007/s00280-013-2282-y. [DOI] [PubMed] [Google Scholar]
- 5.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 6.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R European Organisation for Research, and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/s0140-6736(01)06103-7. doi: 10.1016/S0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 7.Heng DY, Wells JC, Rini BI, Beuselinck B, Lee JL, Knox JJ, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: Results from the international metastatic renal cell carcinoma database consortium. Eur Urol. 2014;66:704–10. doi: 10.1016/j.eururo.2014.05.034. doi: 10.1016/j.eururo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Albiges L, Xie WL, Lee JL, Rini BI, Srinivas S, Bjarnason GA, et al. The impact of body mass index (BMI) on treatment outcome of targeted therapy in metastatic renal cell carcinoma (mRCC):Results from the international metastatic renal cell cancer database consortium. J Clin Oncol. 2014;32:5s. Suppl;abstr 4576. doi: 10.1016/j.eururo.2013.10.013. [Google Scholar]
- 9.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]


