Abstract
Background:
The absolute lymphocyte counts (ALCs) have been reported as one of worse prognostic factors for hepatocellular carcinoma (HCC) patient after liver transplantation. The aim of this study was to assess the influence of ALCs on the outcomes of patients with hepatitis B virus (HBV)-related HCC within the Milan criteria following liver resection.
Methods:
Data of patients with HCC within the Milan criteria who received liver resection between January 2007 and June 2013 were reviewed, and perioperative ALCs were carefully monitored. All potential risk factors were statistically analyzed by uni- and multi-variate analyses. The receiver operating characteristic (ROC) curve was used to determine the optimal ALCs cut-off value to predict HCC recurrence after liver resection.
Results:
A total of 221 patients were enrolled in the current study. During the follow-up period, 106 patients experienced recurrence, and 38 patients died. Multivariate analysis suggested microvascular invasion (MVI), a tumor grade ≥2, and a low postoperative ALCs in the 1st postoperative month increased the incidence of postoperative recurrence, besides, MVI, intraoperative transfusion, and a low postoperative ALCs in the 1st postoperative month were associated with poor overall survival (OS). An ROC analysis showed that a cut-off value of 1.5 × 109/L for ALCs in the 1st postoperative month predicted postoperative recurrence. The 5-year recurrence-free survival (RFS) and OS rates of patients with low postoperative ALCs were 34.5% and 64.8%, respectively, which were significantly lower than those of patients with high postoperative ALC (58.5% for RFS and 86.5% for OS).
Conclusion:
Low ALCs in the 1st postoperative month may be associated with high recurrence incidence and poor OS for patients with HBV-related HCC within the Milan criteria after liver resection.
Keywords: Absolute Lymphocyte Counts, Hepatocellular Carcinoma, Liver Resection, Outcome
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most frequent malignancy and the third most common cancer-related cause of death worldwide. Chronic hepatitis B virus (HBV) infection is estimated to cause 55–60% of HCC worldwide, and it is the most important etiologic agent of HCC in Asia.[1] In China, approximately 7% of the population suffer from chronic HBV infection. Owing to this high prevalence, China alone accounts for approximately 55% of the HCC cases in the world.[2] Liver resection is a curative treatment for patients with HCC, but postoperative recurrence is still the main obstacle to the long-term survival of HCC patients. The incidence of postoperative recurrence for patients with small HCC can be as high as 50–70% after liver resection.[3]
Lymphopenia is advocated as a surrogate marker of malnutrition and poor anti-tumor immunomodulation abilities in patients with chronic liver disease.[4] Previous investigations confirmed that low absolute lymphocyte counts (ALCs) contributed to poor outcomes for many malignancies, such as leukemia, ovarian cancer, Merkel cell carcinoma, and others.[5,6] Recently, Nagai et al. investigation[7] indicated that perioperative ALCs were a prognostic factor in HCC recurrence after liver transplantation. They suggested that ALCs could reflect host cellular immunity because lymphocytes such as CD4+ and natural killer cells play key roles in antiviral immunity.[7] Li et al.[8] also suggested that low preoperative ALCs were powerful prognostic factors for HCC recurrence after thermal ablation. However, whether ALCs could predict HCC recurrence after liver resection for patients with HBV-related HCC was unclear. Accordingly, in this study, we aimed to clarify whether ALCs could predict outcomes for patients with HBV-related HCC within the Milan criteria following liver resection.
METHODS
Study group
Data of patients with HBV-related HCC within the Milan criteria who received liver resection at Department of General Surgery, West China Hospital, Sichuan University (China) between January 2007 and June 2013 were reviewed. Hepatitis B surface antigen was detected in all patients. HCC was confirmed by postoperative pathology. All patients had Child A liver function. Patients who fit any one of the following criteria were excluded from this study: (1) co-infection with hepatitis C virus; (2) simultaneous splenectomy; (3) ruptured HCC; (4) infections during the perioperative period; (5) re-resection; or (6) preoperative anti-tumor treatments. This study was approved by the Ethics Committee of West China Hospital.
Follow-up and definitions
All preoperative blood cell counts and differential counts were taken 2 days before the operation. After liver resection, all patients were regularly followed up in the 1st postoperative month and then every 3 months during the following 3 years and every 6 months in the subsequent years. Liver function, blood cell tests, serum alpha-fetoprotein (AFP), HBV-DNA, visceral ultrasonography, and computed tomography or magnetic resonance imaging and chest radiography were monitored for all patients. Bone scintigraphy was performed whenever HCC recurrence was suspected. Postoperative recurrence was defined as positive imaging findings compared with preoperative examination values and newly rising tumor marker (AFP) values or confirmation by biopsy or resection.[9] The neutrophil-to-lymphocyte ratio (NLR) was defined as absolute neutrophil counts divided by the lymphocyte counts. The platelet-to-lymphocyte ratio (PLR) was defined as the absolute platelet count divided by the lymphocyte counts. An HBV-DNA higher than 104 copies/ml was considered high.[10] An AFP >400 ng/ml was considered high.[9]
Statistical analysis
We used SPSS 21.0 for Windows (SPSS Company, Chicago, IL, USA) to perform statistical analyses. All continuous variables are presented as mean ± standard deviation (SD) and compared using one-way analysis of variance (ANOVA). Categorical variables were compared using the Chi-square test or Fisher's exact test. The independent risk factors for recurrence-free survival (RFS) and overall survival (OS) were identified by Cox regression. Factors those were significant at a P < 0.1 in the univariate analyses were included in the multivariate analysis. RFS and OS were determined using the Kaplan–Meier method, with comparisons using a log-rank test. A receiver operating characteristic (ROC) analysis was used to establish the cut-off value for postoperative ALCs in predicting recurrence. A P < 0.05 was considered statistically significant.
RESULTS
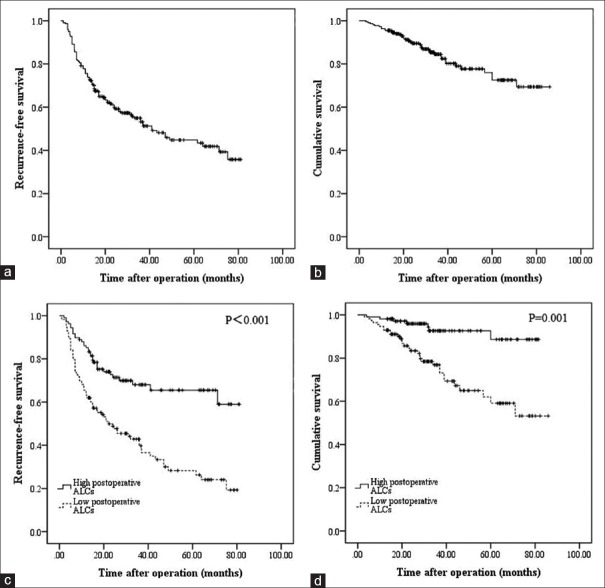
A total of 221 patients were included in the present study. With a mean age of 49.72 ± 11.84 years, there were 191 males and 30 females. The mean tumor size was 3.26 ± 1.04 cm. In total, 32 patients suffered from multiple tumors. Thirty-one patients received intraoperative blood transfusion. Sixty-one patients had a high preoperative HBV-DNA load, and high preoperative AFP levels were observed in 67 patients. Microvascular invasion (MVI) was observed in 42 patients. The mean follow-up time was 36.85 ± 20.65 months. During the follow-up periods, recurrence was observed in 106 patients, and 38 patients died. The 1-, 3-, and 5-year RFS of the whole cohort were 77.4%, 54.9%, and 44.7%, respectively [Figure 1a]. The 1-, 3-, and 5-year OS of the whole cohort were 95.5%, 84.5%, and 76.0%, respectively [Figure 1b].
Figure 1.
Recurrence-free survival (a) and overall survival (b) curves for all patients. Recurrence-free survival (c) and overall survival (d) comparisons of patients with high and low postoperative absolute lymphocyte counts.
Risk factors associated with postoperative recurrence
As shown in Table 1, univariate analyses suggested that MVI, intraoperative transfusion, a tumor grade ≥2, a high preoperative HBV-DNA load, postoperative ALCs, and postoperative NLR were all potential influences on RFS. However, only MVI (P< 0.001, hazard ratio [HR]= 2.270, 95% confidence interval [CI]= 1.468–3.509), a tumor grade ≥2 (P = 0.002, HR = 2.056, 95% CI = 1.289–3.280), and postoperative ALCs (P = 0.004, HR = 0.604, 95% CI = 0.427–0.854) increased the incidence of postoperative recurrence in the multivariate analysis [Table 2].
Table 1.
Univariate analyses of factors associated with postoperative recurrence
| Variables | Recurrence group (n = 106) | Non-recurrence group (n = 115) | Statistics | P |
|---|---|---|---|---|
| Gender (male/female) | 98/8 | 95/20 | 4.831* | 0.084 |
| Age (years) | 49.91 ± 12.40 | 49.55 ± 11.35 | 0.224† | 0.823 |
| Tumor size (cm) | 3.23 ± 1.06 | 3.29 ± 1.03 | 0.377† | 0.707 |
| Differentiation (well/moderate/poor, n) | 8/63/35 | 15/72/28 | 3.147* | 0.207 |
| Tumor number ≥2 (n) | 23 | 9 | 8.572* | 0.003 |
| MVI (n) | 29 | 13 | 9.236* | 0.002 |
| TB (μmol/ml) | 16.03 ± 7.25 | 15.14 ± 6.57 | 0.951† | 0.343 |
| Platelet (×103/L) | 108.4 ± 43.7 | 119.7 ± 47.1 | 0.065† | 0.097 |
| Transfusion (n) | 20 | 11 | 3.958* | 0.047 |
| High HBV-DNA load (n) | 39 | 22 | 8.572* | 0.003 |
| High AFP (n) | 38 | 29 | 2.951* | 0.086 |
| Preoperative NLR | 1.87 ± 1.07 | 1.62 ± 1.41 | 1.440† | 0.151 |
| Postoperative NLR | 1.99 ± 1.66 | 1.36 ± 1.04 | 3.441† | 0.001 |
| Preoperative PLR | 76.44 ± 38.08 | 88.14 ± 59.30 | 1.729† | 0.085 |
| Postoperative PLR | 76.90 ± 35.27 | 79.45 ± 46.57 | 0.456† | 0.649 |
| Preoperative ALCs (×109/L) | 1.62 ± 0.62 | 1.69 ± 0.61 | 0.845† | 0.623 |
| Postoperative ALCs (×109/L) | 1.38 ± 0.69 | 1.81 ± 0.57 | 5.065† | <0.001 |
*χ2 values; †t values. ALCs: Absolute lymphocyte counts; AFP: Alpha-fetoprotein; HBV: Hepatitis B virus; MVI: Microvascular invasion; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; TB: Total bilirubin.
Table 2.
Multivariate analyses of factors associated with postoperative recurrence
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Tumor number ≥2 | 2.056 | 1.289–3.280 | 0.002 |
| MVI | 2.270 | 1.468–3.509 | <0.001 |
| Postoperative ALCs | 0.604 | 0.427–0.854 | 0.004 |
ALCs: Absolute lymphocyte counts; MVI: Microvascular invasion; HR: Hazard ratio; CI: Confidence interval.
Risk factors associated with overall survival
Additional analyses were also performed to identify risk factors associated with postoperative survival by univariate and multivariate Cox regression analyses. In the univariate analyses, age, MVI, intraoperative transfusion, platelet, high preoperative HBV-DNA load, and preoperative and postoperative ALCs and NLRs were associated with mortality after liver resection for patients with HCC within the Milan criteria [Table 3]. However, only MVI (P = 0.002, HR = 3.030, 95% CI = 1.519–6.044), intraoperative transfusion (P = 0.002, HR = 3.139, 95% CI = 1.536–6.415), and postoperative ALCs (P < 0.001, HR = 0.210, 95% CI = 0.116–0.380) were demonstrated as independent prognostic factors for poor OS in the multivariate analysis [Table 4].
Table 3.
Univariate analyses of factors associated with postoperative survival
| Variables | Died (n = 38) | Survival (n = 183) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 46.79 ± 10.60 | 50.33 ± 12.01 | 1.684† | 0.094 |
| Gender (male/female) | 35/3 | 158/25 | 0.946* | 0.331 |
| Tumor size (cm) | 3.08 ± 1.05 | 3.30 ± 1.04 | 1.146† | 0.253 |
| Differentiation (well/moderate/poor, n) | 3/21/14 | 20/114/49 | 1.652* | 0.438 |
| Tumor number ≥2 (n) | 5 | 27 | 0.065* | 0.799 |
| MVI (n) | 14/24 | 28/155 | 9.486* | 0.002 |
| TB (μmol/ml) | 15.92 ± 7.54 | 15.49 ± 6.78 | 0.344† | 0.731 |
| Platelet (×103/L) | 104.2 ± 42.4 | 116.4 ± 46.2 | 1.503† | 0.095 |
| Transfusion (n) | 12 | 19 | 11.723* | 0.001 |
| High HBV-DNA load (n) | 16 | 45 | 4.831* | 0.028 |
| High AFP (n) | 15 | 52 | 1.821* | 0.177 |
| Preoperative NLR | 2.19 ± 1.07 | 1.65 ± 1.28 | 2.449† | 0.015 |
| Postoperative NLR | 2.85 ± 2.24 | 1.42 ± 1.00 | 6.167† | <0.001 |
| Preoperative PLR | 73.41 ± 29.25 | 84.43 ± 53.72 | 1.225† | 0.222 |
| Postoperative PLR | 79.27 ± 32.32 | 78.01 ± 43.19 | 0.170† | 0.865 |
| Preoperative ALCs (×109/L) | 1.43 ± 0.52 | 1.70 ± 0.62 | 2.547† | 0.006 |
| Postoperative ALCs (×109/L) | 1.06 ± 0.41 | 1.71 ± 0.65 | 5.952† | <0.001 |
*χ2 values; †t values. ALCs: Absolute lymphocyte counts; AFP: Alphafetoprotein; HBV: Hepatitis B virus; MVI: Microvascular invasion; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; TB: Total bilirubin.
Table 4.
Multivariate analyses of factors associated with postoperative survival
| Variables | HR | 95% CI | P |
|---|---|---|---|
| MVI | 3.030 | 1.519–6.044 | 0.002 |
| Transfusion | 3.139 | 1.536–6.415 | 0.002 |
| Postoperative ALCs | 0.210 | 0.116–0.380 | <0.001 |
ALCs: Absolute lymphocyte counts; MVI: Microvascular invasion; HR: Hazard ratio; CI: Confidence interval.
Comparative outcomes of patients with high versus low postoperative absolute lymphocyte counts
An ROC analysis showed that the cut-off value of 1.5 × 109/L for postoperative ALCs predicted recurrence with a sensitivity of 68.9% and a specificity of 65.2% [Figure 2]. The area under the ROC curve was 0.716 [Figure 2]. The 1-, 3-, and 5-year RFS rates for patients with low (≤1.5 × 109/L, n = 113) and high (>1.5 × 109/L, n = 108) postoperative ALCs were 68.9%, 45.0%, and 34.5%; and 87.9%, 63.5%, and 58.6%, respectively. Significant differences were observed [Figure 1c, P = 0.001]. The 1-, 3-, and 5-year OS rates of patients with low postoperative ALCs were also significantly lower than were those for patients with high postoperative ALCs [93.4%, 77.5%, and 64.8% vs. 98.0%, 93.2%, and 86.5%, respectively; Figure 1d, P = 0.001].
Figure 2.
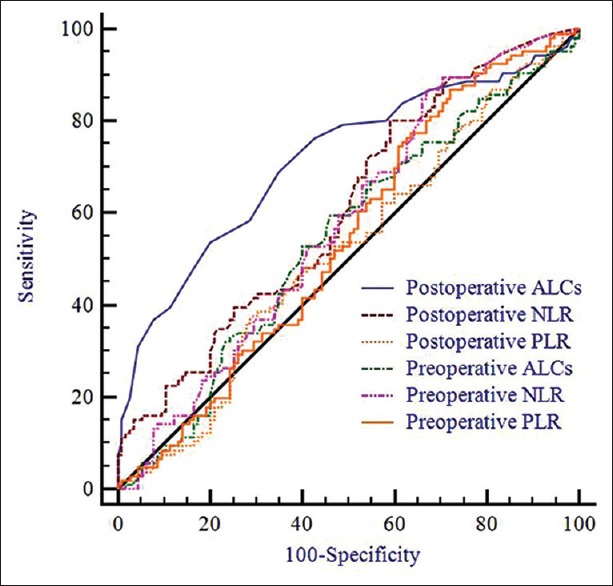
Comparison of the area under the receiver operating characteristics of different inflammation-based scores for recurrence prediction.
Comparison of predictive ability of inflammation-based prognostic scores
ROC curves were constructed for postoperative recurrence, and the areas under the ROC curve (AUCs) were compared to assess the discrimination ability of each inflammation-based prognostic score. As shown in Figure 2, postoperative ALC had the highest AUC (0.716), followed by postoperative NLR (0.609), preoperative NLR (0.575), preoperative ALC (0.545), preoperative PLR (0.541), and postoperative PLR (0.516).
DISCUSSION
In the current study, we found an association between the postoperative prognosis of patients with HCC following liver resection and postoperative ALCs. Our results suggested that low postoperative ALCs in the 1st postoperative month may be a simple and effective marker to use in determining the risks of recurrence and mortality for patients with HCC after liver resection.
T lymphocytes play a key role in anti-tumor immune responses. Previous investigations indicated that tumor-infiltrating lymphocytes represent the host immune response to cancer and further suggested that CD8(+) cytotoxic T-cells have a central role in eliminating tumors and that regulatory T-cells may suppress the immune reaction.[11] Fu et al. study[12] confirmed that the functional impairment of CD8(+) T-cells may promote HCC progression. Recently, the same group further confirmed that the impairment of CD4(+) cytotoxic T-cells was also associated with poor survival rates and high recurrence of HCC after liver resection.[13] Yang et al.[14] reported that CD4+ CD25+ T-cells in the marginal region of HCC may play a critical role in controlling CD8+ cytotoxic T-cell activity and, thereby, contribute to the progression of HCC. Unitt et al. study[15] suggested that for patients who underwent liver transplantation as well as who received liver resection and with a reduced lymphocyte infiltration and CD4 to CD8 ratio also played an important role in overcoming HCC recurrence. Our study suggested that patients with low postoperative ALC had poor outcomes after liver resection. One possible explanation for this result was that the anti-tumor immune system was weakened during the perioperative period. Our results also indicated that we should pay more attention to the changes in the anti-tumor immune system after liver resection because strengthening anti-tumor immune responses may improve outcomes. However, this hypothesis needs additional studies for confirmation.
Many previous studies suggested that a high NLR was an independent risk factor for HCC recurrence after liver resection.[16,17] However, our study found that both pre- and post-operative NLR had no prognostic power in predicting RFS and OS after liver resection. The potential explanations for NLR in predicting HCC patients’ outcomes may be as follows: first, patients with high NLRs had high absolute neutrophil counts and low ALCs; however, some published investigations confirmed that neutrophils could secret vascular endothelial growth factor (VEGF) which could lead to angiogenesis and increase tumor growth;[18,19] second, patients’ anti-tumor responses mainly depend on lymphocytes. Patients with low ALCs had poor anti-tumor responses.[20] However, Motomura et al.[21] confirmed that the VEGF, interleukin-8 (IL-8), IL-17, CD68, and CD163 levels were similar between patients with high and low preoperative NLRs who underwent liver transplantation. A high level of IL-17 in the serum and peritumoral tissues was observed in patients with a high NLR. According to Motomura et al. results, the NLRs ability to predict tumor recurrence may not be through neutrophil-induced angiogenesis but via the inflammatory tumor microenvironment.[21] Wu et al.[22] confirmed that both tumors and IL-17 counts from liver infiltrated T-cells were associated with early HCC recurrence and progression after liver resection.
Similar to NLR, some investigations also suggested that high PLR was associated with high risk of postoperative recurrence.[23] Activated platelets could help tumor cells escape from immune elimination.[23] Platelet-released factors, such as platelet-derived growth factor, enhance tumor progression and metastasis.[24] Experimental research even suggested that platelets could stimulate the growth and invasion of a number of HCC cell lines.[25]
Our study suggested that intraoperative transfusion negatively impacted the OS of patients with HCC after liver resection. Blood loss and secondary transfusion remain major concerns during liver resection. The potential mechanisms that underlie the negative influence of transfusion on postoperative outcomes have been assumed to be associated with suppressive effects of transfusion on the host immune system. Although the exact mechanism is still not well established, a number of investigations have suggested that intraoperative blood transfusion could weaken the function of host natural killer cells, cytotoxic T-cells, macrophages, and monocytes and increase the number of suppressor T-cells.[26] This may explain why blood transfusion was an independent risk factor of OS after liver resection.
There is no universal consensus regarding the low ALCs cut-off level in predicting the prognoses of patients with HCC after liver resection. In Nagai et al. study, they proposed that 1.0 × 109/L was considered a low ALCs for liver transplant recipients.[7] Li et al. study suggested 1.64 × 109/L as the optimal ALCs cut-off value to determine OS of patients with recurrent HCC following thermal ablation.[8] In our study, the optimal ALCs cut-off value in predicting postoperative recurrence were 1.5 × 109/L. We acknowledge that additional studies are needed to determine the optimal ALCs cut-off point in predicting the outcomes of patients who underwent liver resection for HCC. However, our study confirmed the fact that low postoperative ALCs are associated with a high incidence of postoperative recurrence and poor OS.
There are also some limitations to the current study. This is a single-center retrospective study and, also, it did not exclude patients with portal hypertension. There is no doubt that ALCs may be very low in patients with portal hypertension. We thought the lymphopenia secondary to portal hypertension could be related to poor anti-tumor immunity. Hidaka et al.[27] confirmed that high portal vein pressure was associated with poor long-term outcomes after liver resection for HCC. Chen et al.[28] even suggested that synchronic liver resection and splenectomy could improve the long-term survival of patients with HCC and severe hypersplenism. We also acknowledge that ALC can be affected by numerous factors such as liver function and anti-tumor medicines. In our center, hepatectomy was only performed on patients with Child-Pugh A liver function, and the liver function of most patients who received liver resection recovered during the 1st postoperative month; thus, we think that the influence of liver function on the duration of survival in this study was limited. Moreover, during the 1st postoperative month, the patients in our center did not receive any anti-tumor medicines. Accordingly, we used ALCs measured in the 1st month after liver resection in this study.
In conclusion, our study suggested that low ALCs in the 1st month after liver resection for HBV-related HCC within the Milan criteria were associated with poor outcomes. Low postoperative ALCs might be a surrogate marker for better stratification and management of HCC patients after liver resection. This result also indicated that we should pay more attention to the changes in anti-tumor immune responses in patients with HCC after liver resection.
Financial support and sponsorship
This work was supported by a grant of the Scientific and Technological Support Project of Sichuan Province (No. 2013SZ0032).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B:Special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52. doi: 10.1016/j.jhep.2007.11.011. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. doi: 10.1111/jgh.12220. doi: 10.1111/jgh.12220. [DOI] [PubMed] [Google Scholar]
- 3.Tateishi R, Shiina S, Akahane M, Sato J, Kondo Y, Masuzaki R, et al. Frequency, risk factors and survival associated with an intrasubsegmental recurrence after radiofrequency ablation for hepatocellular carcinoma. PLoS One. 2013;8:e59040. doi: 10.1371/journal.pone.0059040. doi: 10.1371/journal.pone.0059040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 5.Johnson ME, Zhu F, Li T, Wu H, Galloway TJ, Farma JM, et al. Absolute lymphocyte count: A potential prognostic factor for Merkel cell carcinoma. J Am Acad Dermatol. 2014;70:1028–35. doi: 10.1016/j.jaad.2014.01.890. doi: 10.1016/j.jaad.2014.01.890. [DOI] [PubMed] [Google Scholar]
- 6.Shen HQ, Feng JH, Tang YM, Song H, Yang SL, Shi SW, et al. Absolute lymphocyte count is associated with minimal residual disease level in childhood B-cell precursor acute lymphoblastic leukemia. Leuk Res. 2013;37:671–4. doi: 10.1016/j.leukres.2013.02.002. doi: 10.1016/j.leukres.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Nagai S, Abouljoud MS, Kazimi M, Brown KA, Moonka D, Yoshida A. Peritransplant lymphopenia is a novel prognostic factor in recurrence of hepatocellular carcinoma after liver transplantation. Transplantation. 2014;97:694–701. doi: 10.1097/01.TP.0000437426.15890.1d. doi: 10.1097/01.TP.0000437426.15890.1d. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Han Z, Cheng Z, Yu J, Yu X, Liang P. Prognostic value of preoperative absolute lymphocyte count in recurrent hepatocellular carcinoma following thermal ablation: A retrospective analysis. Onco Targets Ther. 2014;7:1829–35. doi: 10.2147/OTT.S69227. doi: 10.2147/OTT.S69227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–32. doi: 10.1097/TP.0b013e31816b67e4. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 10.Zhou JY, Zhang L, Li L, Gu GY, Zhou YH, Chen JH. High hepatitis B virus load is associated with hepatocellular carcinomas development in Chinese chronic hepatitis B patients: A case control study. Virol J. 2012;9:16. doi: 10.1186/1743-422X-9-16. doi: 10.1186/1743-422X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, Ma Z, et al. Intrahepatic interleukin-17+T cells and FoxP3+regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:851–9. doi: 10.1111/jgh.12418. doi: 10.1111/jgh.12418. [DOI] [PubMed] [Google Scholar]
- 12.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39. doi: 10.1053/j.gastro.2007.03.102. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 13.Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, et al. Impairment of CD4+cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58:139–49. doi: 10.1002/hep.26054. doi: 10.1002/hep.26054. [DOI] [PubMed] [Google Scholar]
- 14.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, et al. Increase of CD4+CD25+regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–62. doi: 10.1016/j.jhep.2006.01.036. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246–53. doi: 10.1016/j.jhep.2005.12.027. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Yamamura K, Sugimoto H, Kanda M, Yamada S, Nomoto S, Nakayama G, et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci. 2014;21:682–8. doi: 10.1002/jhbp.114. doi: 10.1002/jhbp.114. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan KM, Groeschl RT, Turaga KK, Tsai S, Christians KK, White SB, et al. Neutrophil-to-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma: A western perspective. J Surg Oncol. 2014;109:95–7. doi: 10.1002/jso.23448. doi: 10.1002/jso.23448. [DOI] [PubMed] [Google Scholar]
- 18.Phan VT, Wu X, Cheng JH, Sheng RX, Chung AS, Zhuang G, et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc Natl Acad Sci U S A. 2013;110:6079–84. doi: 10.1073/pnas.1303302110. doi: 10.1073/pnas.1303302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S, Lamers GE, Beenakker JW, Cui C, Ghotra VP, Danen EH, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J Pathol. 2012;227:431–45. doi: 10.1002/path.4013. doi: 10.1002/path.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halazun KJ, Hardy MA, Rana AA, Woodland DC, 4th, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–51. doi: 10.1097/SLA.0b013e3181a77e59. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 21.Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64. doi: 10.1016/j.jhep.2012.08.017. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Du J, Liu L, Li Q, Rong W, Wang L, et al. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS One. 2012;7:e50035. doi: 10.1371/journal.pone.0050035. doi: 10.1371/journal.pone.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Chen ZH, Xing YF, Wang TT, Wu DH, Wen JY, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2015;36:2263–9. doi: 10.1007/s13277-014-2833-9. doi: 10.1007/s13277-014-2833-9. [DOI] [PubMed] [Google Scholar]
- 24.Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30:95–108. doi: 10.1055/s-2004-822974. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
- 25.Carr BI, Cavallini A, D’Alessandro R, Refolo MG, Lippolis C, Mazzocca A, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer. 2014;14:43. doi: 10.1186/1471-2407-14-43. doi: 10.1186/1471-2407-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259–64. doi: 10.1159/000103656. doi: 10.1159/000103656. [DOI] [PubMed] [Google Scholar]
- 27.Hidaka M, Takatsuki M, Soyama A, Tanaka T, Muraoka I, Hara T, et al. Intraoperative portal venous pressure and long-term outcome after curative resection for hepatocellular carcinoma. Br J Surg. 2012;99:1284–9. doi: 10.1002/bjs.8861. doi: 10.1002/bjs.8861. [DOI] [PubMed] [Google Scholar]
- 28.Chen XP, Wu ZD, Huang ZY, Qiu FZ. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg. 2005;92:334–9. doi: 10.1002/bjs.4776. doi: 10.1002/bjs.4776. [DOI] [PubMed] [Google Scholar]