Abstract
Background:
Renin-angiotensin system inhibitor and calcium channel blocker (CCB) are widely used in controlling blood pressure (BP) in patients with chronic kidney disease (CKD). We carried out a meta-analysis to compare the renoprotective effect of the combination of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) and CCB (i.e., ACEI/ARB + CCB) with ACEI/ARB monotherapy in patients with hypertension and CKD.
Methods:
Publications were identified from PubMed, Embase, Medline, and Cochrane databases. Only randomized controlled trials (RCTs) of BP lowering treatment for patients with hypertension and CKD were considered. The outcomes of end-stage renal disease (ESRD), cardiovascular events, BP, urinary protein measures, estimated glomerular filtration rate (GFR), and adverse events were extracted.
Results:
Based on seven RCTs with 628 patients, ACEI/ARB + CCB did not show additional benefit for the incidence of ESRD (risk ratio [RR] = 0.84; 95% confidence interval [CI]: 0.52–1.33) and cardiovascular events (RR = 0.58; 95% CI: 0.21–1.63) significantly, compared with ACEI/ARB monotherapy. There were no significant differences in change from baseline to the end points in diastolic BP (weighted mean difference [WMD] = −1.28 mmHg; 95% CI: −3.18 to −0.62), proteinuria (standard mean difference = −0.55; 95% CI: −1.41 to −0.30), GFR (WMD = −0.32 ml/min; 95% CI: −1.53 to −0.89), and occurrence of adverse events (RR = 1.05; 95% CI: 0.72–1.53). However, ACEI/ARB + CCB showed a greater reduction in systolic BP (WMD = −4.46 mmHg; 95% CI: −6.95 to −1.97), compared with ACEI/ARB monotherapy.
Conclusion:
ACEI/ARB + CCB had no additional renoprotective benefit beyond than what could be achieved with ACEI/ARB monotherapy.
Keywords: Calcium Channel Blocker, Chronic Kidney Disease, Hypertension, Renin-angiotensin System Inhibitor, Renoprotection, Therapy
INTRODUCTION
Hypertension, together with proteinuria, is one of the major factors contributing to the progression of chronic kidney disease (CKD) and cardiovascular events.[1,2] Aims of blood pressure (BP) control in CKD patients are to delay or prevent the progression of CKD to end-stage renal disease (ESRD) and inhibit the occurrence of cardiovascular events. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are effective in lowering BP, slowing the progression of diabetic and nondiabetic renal disease, reducing proteinuria, reducing the risk of overt nephropathy, and supporting initial antihypertensive therapy with an angiotensin-converting enzyme inhibitor (ACEI) or ARB in patients with CKD by improving kidney outcomes.[3] Calcium channel blockers (CCBs) are effective vasodilators and BP lowering agents; thus, they have been also extensively used in patients with CKD. The combination of an ACEI (or ARB) with a CCB (abbreviated to “ACEI/ARB + CCB”) has synergistic effects on BP control and target organ protection, whereas their effects are antagonistic for adverse events. For example, ACEI/ARB can reduce peripheral edema induced by CCB. Numerous randomized clinical trials have validated this treatment strategy.[4,5] However, it is controversial whether ACEI/ARB + CCB would produce greater renal protection than either ACEI/ARB or CCB alone. Treatment of hypertension in CKD patients has been shown to slow the progression of CKD, and the best antihypertensive regimen remains unclear.
In this study, we used the technique of meta-analysis to compare the renoprotective effect of ACEI/ARB + CCB with ACEI/ARB monotherapy in patients with hypertension and CKD.
METHODS
Study design
We considered all studies based on randomized controlled trials (RCTs), regardless of either parallel or cross-over design, that compared the effect of dual agents (ACEI/ARB and CCB) to that of single agent (ACEI/ARB only) after at least 4 weeks of follow-up, without language or publication year restrictions. Following the Preferred Reporting Items of Systematic Reviews and Meta-Analyses items, we observed the prespecified study protocol, conducted analyses, and reported results.[6]
Participants
Patients with hypertension and CKD were considered eligible for this study. The CKD was defined by Kidney Disease Outcomes Quality Initiative criteria.[7] That is, participants with any of the following conditions were considered as CKD patients:
Kidney damage for 3 or more months
Defined by structural or functional abnormalities of the kidney, with or without decreased glomerular filtration rate (GFR)
Manifested by either pathologic abnormalities or markers of kidney damage, including abnormalities in the composition of the blood or urine, or abnormalities in imaging tests
GFR < 60 ml·min–1·1.73 m–2 for 3 or more months, with or without kidney damage.
The inclusion criteria included patients with either systolic BP (SBP) ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg at baseline.
Outcome measures
The primary outcomes of interest were ESRD and cardiovascular events. Secondary outcomes were changes in SBP, DBP, urinary protein-related outcome, estimated GFR (eGFR) or GFR from baseline, and adverse events. ESRD referred to patients treated by dialysis or transplantation.[7] The urinary protein-related outcome was defined as any of the following measures: Urine albumin/creatinine ratio (UACR), urine protein/creatinine ratio (UPCR), urinary albumin excretion (UAE), or 24-h urine protein.
Information sources and search strategy
Publications were identified by searching electronic databases including PubMed, Embase, Medline and the Cochrane Library available up to July 2015. Search terms were CKD, chronic renal insufficiency, hypertension, hypertensive diseases, dual therapy, combination therapy, single therapy, monotherapy, ACEI, ARB, angiotensin receptor antagonist, calcium antagonists, CCBs, including the names of individual drugs. The use of combination therapy was defined as simultaneous treatment with both ACEI/ARB and CCB.
Two reviewers (Huang and Cheng) independently assessed the eligibility of each article and any disagreement was further reviewed by a third party. All authors reviewed the final list of articles and oversaw the overall search process.
Data extraction
For the articles in the final list, two investigators independently extracted data, and discrepancies were discussed with a third reviewer and resolved by consensus. The extracted data included publication date, study characteristics (sample size, follow-up period), patient characteristics (age, sex, diabetes mellitus), types of interventions (type, dosage, and duration of therapy), and intervention outcomes, including mean arterial pressure, SBP, DBP, target goal of BP, urinary protein related outcome (UAE, 24-h urine protein, UACR, or UPCR), and all-cause mortality, incidence of ESRD, cardiovascular events, and adverse events.
Quality assessment
We used Jadad scoring to assess the methodological quality of RCTs.[8] The Jadad scoring procedure is based on the degree of subject randomization, the application of the blinding method, and the description of study withdrawals and dropouts. A higher score indicates higher quality.
The risk of bias was assessed using the Cochrane Collaboration's tool.[9] The criteria include sequence generation, concealment of allocation, masking of subjects, staff and outcome assessors, completeness of data, selectiveness of outcome reporting, and other scores of bias.
Statistical analysis
The intervention of interest was ACEI/ARB + CCB vs. ACEI/ARB monotherapy. We used a random effects model to combine the studies since there existed significant heterogeneity (P < 0.1) in the treatment effects on some of the outcome measures.[10] The I2 statistic was used to assess the degree of heterogeneity, where I2 > 50% was considered to indicate a high heterogeneity.[11] Pooled relative risks (RRs) was used to compare treatment effects for dichotomous outcomes (including ESRD, cardiovascular events, and adverse events), and weighted mean difference (WMD) was used for continuous outcomes (including SBP and DBP, urinary protein-related outcome, and eGFR/GFR), along with their corresponding 95% confidence intervals (CIs). All statistical analyses were performed using Review Manager (RevMan), version 5.3 (Cochrane Collaboration, Oxford, UK). P < 0.05 was considered statistically significant, except for the test of heterogeneity where P < 0.1 was used.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were conducted to identify potential sources of heterogeneity by any of the following:
Combinations of medications, such as ACEI plus dihydropyridine CCB, ACEI plus nondihydropyridine CCB, ARB plus dihydropyridine CCB, and ARB plus nondihydropyridine CCB
Doses of treatment
Age distribution
Co-morbid condition: Diabetes
Baseline severity of hypertension, proteinuria and eGFR.
Sensitivity analysis
To evaluate the robustness of the meta-analysis results, we carried out two sensitivity analyses: (1) compare results with and without the low-quality studies, and (2) compare results with and without the studies with small sample sizes.
RESULTS
Study characteristics
Of the 157 articles identified, 106 articles were excluded by the abstract review, and 51 articles were excluded by the full paper review, leading to data pooling of seven studies [Figure 1].[12,13,14,15,16,17,18] The main reason for the exclusion of 44 articles was a comparison between combination therapy versus combination therapy rather than combination therapy versus monotherapy.
Figure 1.
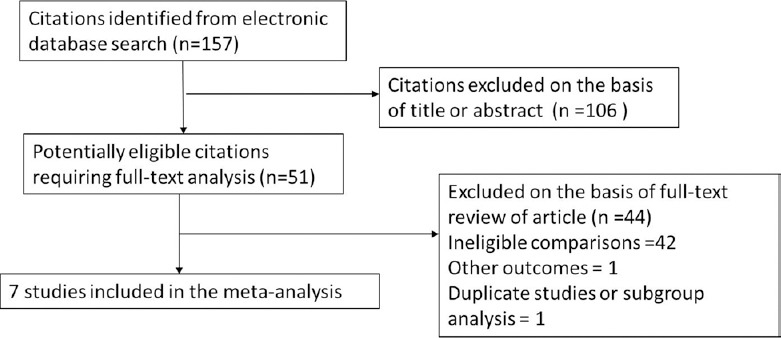
Flow diagram for study selection.
The final seven studies were all parallel RCTs, comparing the renoprotective effect of ACEI/ARB + CCB with ACEI/ARB monotherapy, leading to the total of 628 hypertensive patients who were followed up for 3–66 months. Two RCTs used the same dose of ACEI/ARB in both combination therapy and monotherapy arms; four RCTs compared single-dose combination therapy with double-dose monotherapy; one RCT compared combination therapy with monotherapy using 1.5 times doses of candesartan. Regarding types of medications used for the combination therapies, four RCTs combined ACEI with dihydropyridine calcium antagonist, one RCT combined ACEI with nondihydropyridine calcium antagonist (verapamil), and two RCTs combined ARB with dihydropyridine calcium antagonist. Three RCTs recruited only diabetic patients, whereas two RCTs recruited only nondiabetic patients.
The assessments of quality and risk of bias are summarized in Table 1 and Figure 2. The qualities of two studies were considered low (Jadad score 1–2) while those of the other five studies were considered high (Jadad score 3–5). The Cochrane Collaboration's assessment suggested that three studies were at low risk of bias while the other four studies were at high risk of bias.
Table 1.
Characteristics of randomized controlled trials included in this meta-analysis of trials of combination therapy versus monotherapy
| Studies | Combined therapy versus monotherapy | Medication dosage (mg/d) | Number of patients | Number of diabetes mellitus | Follow-up (months) | Mean age (years) | Mean eGFR (ml·min−1·1.73 m−2) | Mean BP (mmHg) | Jadad score |
|---|---|---|---|---|---|---|---|---|---|
| MacGregor et al., 2005[17] | Quinapril/amlodipine versus quninapril | 2.5+5 versus 5 | 45 | 0 | 48 | 50 | 20 | 149/88 | 3 |
| Nakagawa et al., 2011[13] | Candesartan/nifedipine versus candesartan | 8+20 versus 12 | 86 | NR | 4 | 58 | 67.7 | 157/98 | 3 |
| Petersen et al., 2001[16] | Spirapril/isradipine versus spirapril | 3+2.5 versus 6 | 40 | 9 | 21 | 58 | 31.4 | 151/88 | 3 |
| Ruggenenti et al., 2011[18] | Delapril/manidipine versus delapril | 30+10 versus 30 | 253 | 253 | 66 | 61 | 99.5 | 148/87 | 5 |
| Bakris et al., 1998[12] | Trandolapril/verapamil versus trandolapril | 2.9+219 versus 5.5 | 26 | 26 | 12 | 59 | 73 | 170/104 | 2 |
| Yilmaz et al., 2010[14] | Valsartan/amlodipine versus valsartan | 160+10 versus 160 | 73 | 73 | 3 | 48 | 112 | 150/82 | 1 |
| Herlitz et al., 2001[15] | Ramipril/felodipine versus ramipril | 5+5 versus 10 | 105 | 0 | 24 | 53 | 44 | 157/100 | 3 |
eGFR: Estimated glomerular filtration rate; BP: Blood pressure.
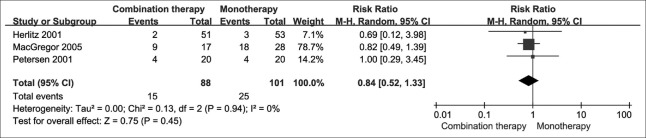
Figure 2.
The number of end-stage renal disease patients by treatment group.
Primary outcomes
Incidence of end-stage renal disease
Three studies directly compared ACEI/ARB + CCB with ACEI/ARB monotherapy and reported that there was no significant difference in the risk of ESRD. This result was consistent with our founding using meta-analysis [RR = 0.84; 95% CI: 0.52–1.33; P = 0.450; Figure 2]. The treatment effects were homogeneous (I2 = 0; P = 0.940).
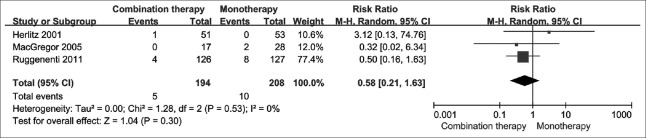
Cardiovascular events
In three studies, there were 15 cardiovascular events in total; five of them occurred in the combination therapy arm, and ten of them occurred in the monotherapy arm. In our meta-analysis, combination therapy did not significantly reduce the risk of cardiovascular events, compared with monotherapy [RR = 0.58; 95% CI: 0.21–1.63; P = 0.300; Figure 3]. The treatment effects were homogeneous (I2 = 0; P = 0.530).
Figure 3.
The count of cardiovascular events by treatment group.
Secondary outcomes
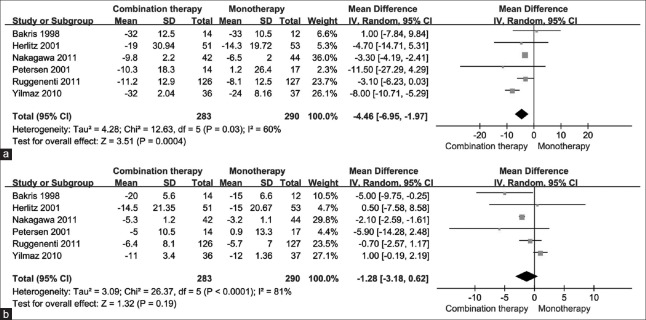
Systolic blood pressure and diastolic blood pressure
In six studies reporting the changes of SBP and DBP, there was a significant decrease in SBP with combination therapy [WMD = −4.46 mmHg; 95% CI: −6.95 to −1.97; P < 0.001; Figure 4a], while there was no significant difference in DBP (WMD = −1.28 mmHg; 95% CI: −3.18 to −0.62; P = 0.190), comparing to monotherapy. The treatment effects were heterogeneous with I2 = 60% (P = 0.030) for SBP and I2 = 81% (P < 0.001) for DBP [Figure 4b].
Figure 4.
The changes in blood pressure by treatment group. (a) For systolic blood pressure. (b) For diastolic blood pressure.
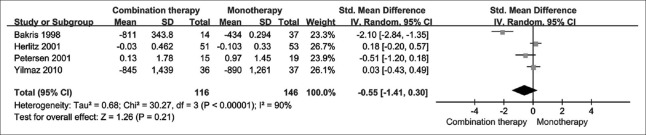
Urinary protein related outcome
Two studies reported 24-h urine protein, and another two studies reported UAE. We used standard mean difference (SMD) to overcome the use of different units of measurement. Our meta-analysis found that the change in urinary protein-related outcome was not significantly different between the two treatment arms [SMD = −0.55; 95% CI: −1.41–0.30; P = 0.210; I2 = 90%; Figure 5].
Figure 5.
The change in urinary protein related outcome by treatment group.
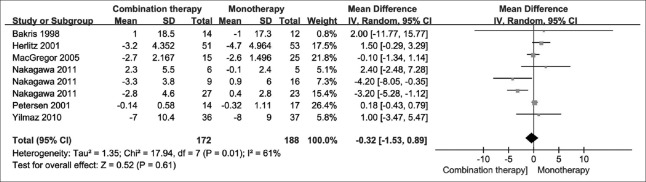
Estimated glomerular filtration rate/glomerular filtration rate
Six studies reported overall eGFR/GFR outcomes. No significant difference was found when combination therapy was compared with monotherapy [WMD = −0.32 ml/min; 95% CI: −1.53–0.89; P = 0.610; I2 = 61%; Figure 6].
Figure 6.
Glomerular filtration rate by treatment group.
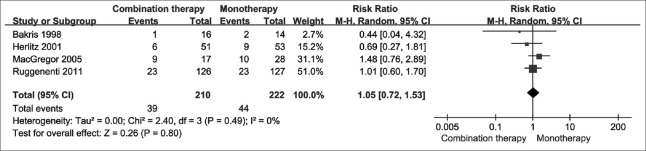
Adverse events
Four studies reported adverse events. In combination therapy arm, 39 of 210 patients (18.6%) suffered adverse events, while 44 of 222 patients (19.8%) did in the monotherapy arm. There was no significant difference between two treatment strategies [RR = 1.05; 95% CI: 0.72–1.53; P = 0.800; I2 = 0%; Figure 7].
Figure 7.
Adverse events by treatment group.
Subgroup and sensitivity analyses
Different combinations of medications
Four trials directly compared ACEI plus dihydropyridine CCB with ACEI only. The results in this subgroup were consistent with the meta-analysis results mentioned above. One trial compared ACEI plus nondihydropyridine CCB (verapamil) with ACEI only, in which the change in SBP was not significantly different between two treatment strategies, and the combination therapy reduce proteinuria to a greater extent than monotherapy, both opposite from the meta-analysis results. In the subgroup analyses, comparing ARB plus dihydropyridine CCB with ARB only, neither trials reported primary outcomes nor adverse events. The results for SBP, DBP, proteinuria, and eGFR were similar to the meta-analysis results.
Different doses of medication
If the same dose of ACEI (or ARB) was used in both monotherapy and combination therapy, the same results came out as the meta-analysis results. Four RCTs compared double-dose monotherapy with single-dose combination therapy and reported that combination therapy was associated with a greater reduction in DBP, though not in SBP.
Age and severity of disease
Only one study reported data stratified by eGFR and age, where changes in UAE and eGFR were examined in patients stratified at an eGFR of 60 ml·min–1·1.73 m–2.[13] The change in UAE was significantly lower in the combination therapy group than in the up-titrated monotherapy group (P < 0.05) at the end of double-blind treatment in subjects with eGFR ≥ 60, but similar in the combination therapy group and the up-titrated monotherapy group (P = 0.252) in subjects with eGFR < 60. No significant change in eGFR was found in patients stratified by eGFR ≥ 60 and < 60. In addition, examination of changes in eGFR by age group revealed no significant difference between treatment groups. We were unable to carry out the subgroup analysis for baseline severity of hypertension or proteinuria since there were no studies available.
Co-morbid condition: Diabetes
Three trials recruited only diabetes patients. Our findings for cardiovascular events and all secondary outcomes except SBP were consistent with the overall results. Two trials in patients with no diabetes reported that combination therapy and monotherapy had no significantly different effects on any of the primary or secondary outcomes.
Sensitivity analysis
Two included trials got Jadad score below three points, representing low quality. We redid the meta-analysis after excluding those two trials and found that most of the results were similar to what we found before the exclusion. Only one trial had a small sample size and meta-analysis results with and without the one trial were very similar.
DISCUSSION
Summary of main results
Our meta-analysis showed that ACEI/ARB + CCB treatment did not have significant benefit of reducing the risk of ESRD and cardiovascular mortality, compared with ACEI/ARB monotherapy. In addition, there were no significant differences in change of DBP, proteinuria, GFR, and occurrence of adverse events between the two treatment strategies. However, our meta-analysis found ACEI/ARB + CCB was more effective in reduced SBP than ACEI/ARB monotherapy (WMD = −4.46 mmHg). Based on these findings, we concluded that additional use of a CCB did not significantly improve the beneficial renoprotective effect of using ACEI/ARB only.
Overall completeness and applicability of evidence
The review was limited to studies with patients with both hypertension and CKD and comparison of two treatment strategies. Therefore our findings may not be applicable to the general population of hypertensive patients without kidney injury. The renoprotective effects of other medications (such as diuretics, alpha-blockers, beta-blockers, etc.) have not been discussed in this article.
Quality of the evidence
Table 1 (characteristics of included studies) and Table 2 (risk of bias for each study) provided an assessment of the quality of this review. This review included seven studies examining two therapeutic strategies applied in 628 patients with hypertension and CKD, and hence the sample size was considered to be sufficient to draw a reliable conclusion.
Table 2.
Risk of bias summary: Review authors’ judgments about each risk of bias item for each included study
| Studies | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Risk of bias |
|---|---|---|---|---|---|---|---|
| MacGregor et al., 2005 | ? | ⊕ | ⊖ | ⊖ | ⊖ | ⊖ | High |
| Nakagawa et al., 2011 | ? | ⊖ | ⊕ | ⊖ | ⊕ | ⊕ | Low |
| Petersen et al., 2001 | ? | ⊖ | ⊕ | ⊖ | ⊖ | ⊕ | High |
| Ruggenenti et al., 2011 | ? | ⊖ | ⊕ | ⊕ | ⊕ | ⊖ | Low |
| Bakris et al., 1998 | ? | ⊖ | ⊖ | ⊖ | ⊕ | ⊖ | High |
| Yilmaz et al., 2010 | ? | ⊖ | ⊖ | ⊕ | ⊕ | ⊕ | Low |
| Herlitz et al., 2001 | ⊕ | ⊕ | ⊖ | ⊖ | ⊕ | ⊖ | High |
⊕: Yes (low bias); ⊖: No (high bias); ?: Not clear.
Potential biases in the review process
Using rigid inclusion criteria minimized the potential of bias during the selection process. Multiple reviewers independently assessed studies, resolved any disagreement through discussion, and included a third reviewer if needed.
However, like other meta-analyses, our review was limited by the data (both quantity and data type) available and accessible. The description of data type was unclear or not mentioned. For urinary protein related outcome, some studies reported data with arithmetic mean, some with geometric mean, some with median and some with quartile. A major difficulty in conducting a meta-analysis was to unify various types of measure. Hence, this process might lower the quality of the evidence. Most of our subgroups had small sample sizes, from which the results were incomplete and not generalizable. Given the lack of data in each trial, we were unable to clarify relationships between BP, proteinuria and eGFR. We noticed several earlier trials recruited subjects with serum creatine over 30 mg/L, for whom ACEI or ARB is not suitable.
Agreements and disagreements with other studies or reviews
Since Bakris et al. first reported that the combination of a nondihydropyridine CCB with an ACEI produced greater reductions in proteinuria, compared to higher doses of an ACEI alone in diabetic nephropathy.[12] Many studies focused on the renoprotective effect of the combination of an ACEI (or an ARB) and a calcium antagonist in patients with hypertension and CKD. The NICE-Combi study also suggested ARB plus CCB treatment was superior in renal protection than monotherapy with ARB in patients with hypertension and microalbuminuria.[13] However, the REIN-2 study showed that in patients with nondiabetic proteinuric nephropathies being treated with ACEI, felodipine failed to additionally reduce proteinuria or renal outcomes although additional BP reduction was achieved.[19] Other trials presented that the use of ACEI plus calcium antagonist and ACEI alone decreased proteinuria to a similar extent,[15,16,17,18] which means there is no clear blunting or enhancement of the effect of ACEIs by CCBs. This conclusion was consistent with our results.
CCBs do not always show renoprotective effects possibly because of their glomerular pressure-increasing action. Most antihypertensive CCBs suppress L-type calcium channels that exist in glomerular afferent but not efferent arterioles, and their afferent arteriole-specific vasodilation causes glomerular hypertension.[20] Some experimental and clinical evidence indicates that for any given BP reduction, CCBs are less effective than other antihypertensives in reducing proteinuria or protecting glomerular capillaries in diabetic and nondiabetic proteinuric nephropathies.[21]
However, CCBs are useful agents to prevent cardiovascular disease and mortality, as shown by a meta-analysis of very large clinical trials.[22] Thus, in hypertensive patients with CKD, CCBs are effective antihypertensive drugs to be considered in combination with an ACEI or an ARB. Our meta-analysis demonstrated that the combination of an ACEI or ARB and a CCB produced a greater reduction in SBP than ACEI/ARB monotherapy. Previous trails found fixed-dose ARB/CCB combinations offered a powerful reduction of SBP. As SBP is a better predictor of cardiovascular risk than DBP, the combination treatments are likely more effective in reducing the risk of cardiovascular events in hypertensive patients.[6] However, our results did not support this idea.
Considering there is no evidence-based on well-designed RCTs that compare these strategies and assess their effects on important health outcomes, the 8th Joint National Committee guidelines equally recommend to increase the dose of the initial drug (an ACEI or ARB) or to add a second drug (such as a CCB), if the targeted BP is not achieved in adults with CKD.[23] Thus, better quality trials with larger samples are proposed to learn more about the renoprotective effect of the combination of ACEI/ARB and CCB. More clinical research is needed to explore how new antihypertensive CCBs, which can suppress different type calcium channels, would affect kidney function.
In conclusion, this meta-analysis of RCTs with hypertension and CKD patients examined the effect of ACEI/ARB + CCB versus ACEI/ARB monotherapy on kidney-related outcomes. Despite the effectiveness of lowering SBP, ACEI/ARB + CCB may have no additional benefit for any other kidney-related outcomes than what can be achieved with ACEI/ARB monotherapy. In some cases where we need to lower BP to the levels recommended by current guidelines (and perhaps even further), CCBs are indispensable tools to achieve these high targets.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Dr. Yi Li and his team from Kidney Epidemiology and Cost Center, University of Michigan, School of Public Health for data analysis and language support.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Locatelli F, Marcelli D, Comelli M, Alberti D, Graziani G, Buccianti G, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant. 1996;11:461–7. doi: 10.1093/ndt/11.3.461. doi: 10.1093/oxfordjournals.ndt.a027312. [DOI] [PubMed] [Google Scholar]
- 2.Culleton BF, Larson MG, Parfrey PS, Kannel WB, Levy D. Proteinuria as a risk factor for cardiovascular disease and mortality in older people:A prospective study. Am J Med. 2000;109:1–8. doi: 10.1016/s0002-9343(00)00444-7. doi: 10.1016/S0002-9343(00)00444-7. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel RR. Renal protection in hypertensive patients: Selection of antihypertensive therapy. Drugs. 2005;65(Suppl 2):29–39. doi: 10.2165/00003495-200565002-00005. doi: 10.2165/00003495-200565002-00005. [DOI] [PubMed] [Google Scholar]
- 4.Boutouyrie P. Angiotensin converting enzyme inhibitors and calcium antagonists: A synergistic action for a better prevention of cardiovascular events. Therapie. 2009;64:241–8. doi: 10.2515/therapie/2009047. doi: 10.2515/therapie/2009047. [DOI] [PubMed] [Google Scholar]
- 5.Mourad JJ. The evolution of systolic blood pressure as a strong predictor of cardiovascular risk and the effectiveness of fixed-dose ARB/CCB combinations in lowering levels of this preferential target. Vasc Health Risk Manag. 2008;4:1315–25. doi: 10.2147/vhrm.s4073. doi: 10.2147/VHRM.S4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S46–75. doi: 10.1053/ajkd.2002.30943. [PubMed] [Google Scholar]
- 8.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. Available from: http//www.cochrane-handbook.org .
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis:Q statistic or I2 index?Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 12.Bakris GL, Weir MR, DeQuattro V, McMahon FG. Effects of an ACE inhibitor/calcium antagonist combination on proteinuria in diabetic nephropathy. Kidney Int. 1998;54:1283–9. doi: 10.1046/j.1523-1755.1998.00083.x. doi: 10.1046/j.1523-1755.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa N, Fujino T, Kabara M, Matsuki M, Chinda J, Kikuchi K, et al. Angiotensin II receptor blocker and long-acting calcium channel blocker combination therapy decreases urinary albumin excretion while maintaining glomerular filtration rate. Hypertens Res. 2011;34:1121–6. doi: 10.1038/hr.2011.101. doi: 10.1038/hr.2011.101. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz MI, Carrero JJ, Martín-Ventura JL, Sonmez A, Saglam M, Celik T, et al. Combined therapy with renin-angiotensin system and calcium channel blockers in type 2 diabetic hypertensive patients with proteinuria: Effects on soluble TWEAK, PTX3, and flow-mediated dilation. Clin J Am Soc Nephrol. 2010;5:1174–81. doi: 10.2215/CJN.01110210. doi: 10.2215/cjn.01110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herlitz H, Harris K, Risler T, Boner G, Bernheim J, Chanard J, et al. The effects of an ACE inhibitor and a calcium antagonist on the progression of renal disease: The nephros study. Nephrol Dial Transplant. 2001;16:2158–65. doi: 10.1093/ndt/16.11.2158. doi: 10.1093/ndt/16.11.2158. [DOI] [PubMed] [Google Scholar]
- 16.Petersen LJ, Petersen JR, Talleruphuus U, Møller ML, Ladefoged SD, Mehlsen J, et al. A randomized and double-blind comparison of isradipine and spirapril as monotherapy and in combination on the decline in renal function in patients with chronic renal failure and hypertension. Clin Nephrol. 2001;55:375–83. [PubMed] [Google Scholar]
- 17.MacGregor MS, Deighan CJ, Rodger RS, Boulton-Jones JM. A prospective open-label randomised trial of quinapril and/or amlodipine in progressive non-diabetic renal failure. Nephron Clin Pract. 2005;101:c139–49. doi: 10.1159/000086714. doi: 10.1159/000086714. [DOI] [PubMed] [Google Scholar]
- 18.Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S, et al. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: The delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension. 2011;58:776–83. doi: 10.1161/HYPERTENSIONAHA.111.174474. doi: 10.1161/hypertensionaha.111.174474. [DOI] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2):Multicentre, randomised controlled trial. Lancet. 2005;365:939–46. doi: 10.1016/S0140-6736(05)71082-5. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 20.Griffin KA, Picken MM, Bakris GL, Bidani AK. Class differences in the effects of calcium channel blockers in the rat remnant kidney model. Kidney Int. 1999;55:1849–60. doi: 10.1046/j.1523-1755.1999.00434.x. doi: 10.1046/j.1523-1755.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 21.Griffin KA, Bidani AK. Potential risks of calcium channel blockers in chronic kidney disease. Curr Cardiol Rep. 2008;10:448–55. doi: 10.1007/s11886-008-0071-8. doi: 10.1007/s11886-008-0071-8. [DOI] [PubMed] [Google Scholar]
- 22.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: A meta-analysis. Lancet. 2001;358:1305–15. doi: 10.1016/S0140-6736(01)06411-X. doi: 10.1016/S0140-6736(01)06411-X. [DOI] [PubMed] [Google Scholar]
- 23.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]