Abstract
Background:
Many studies have explored the diagnostic performance of soluble suppression of tumorigenicity-2 (sST2) for heart failure (HF), but the results are inconsistent. Here, we performed a meta-analysis to assess the role of sST2 in the diagnosis of HF.
Methods:
We searched PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure, and Wanfang Database from inception to April 2015. Studies that investigated the diagnostic role of sST2 for HF were reviewed. The numbers of true-positive, false-positive, false-negative, and true-negative results were extracted to calculate pooled diagnostic odds ratio (DOR) with 95% confidence interval (CI) and the summary receiver operating characteristic curve and area under the curve (AUC). The Spearman correlation coefficient was used to check the threshold effect. The Cochran Q statistic (P < 0.05) and the inconsistency index (I2 > 50%) were used to assess the nonthreshold effect. Meta-regression was conducted to explore the source of heterogeneity; subgroup analysis showed the results in different subgroups. Finally, the Deeks’ test was performed to assess the publication bias.
Results:
Nine articles including 10 studies were included in the meta-analysis. The pooled sensitivity was 0.84 (95% CI: 0.81–0.86), and pooled specificity was 0.74 (95% CI: 0.72–0.76). The summary DOR was 8.49 (95% CI: 4.54–15.86), and AUC was 0.81 (standard error: 0.03). The Spearman correlation coefficient identified the nonsignificant threshold effect (coefficient = 0.49, P = 0.148), but the nonthreshold effect heterogeneity was significant (Cochran Q = 58.52, P < 0.0001; I2 = 84.6%). Meta-regression found that characteristics of controls might be the suggestive source of nonthreshold effect heterogeneity (P = 0.095). Subgroup analysis found that DOR was 5.65 and 7.86, respectively for the controls of hospital patients and healthy populations. Deeks’ test demonstrated that there was no publication bias (P = 0.616).
Conclusion:
The meta-analysis illustrated that sST2 might play a role in diagnosing HF.
Keywords: Biomarker, Diagnosis, Heart Failure, Meta-analysis, Soluble Suppression of Tumorigenicity-2
INTRODUCTION
Heart failure (HF) is a life-threatening disease with a growing burden worldwide.[1] The annual global economic cost of HF was estimated nearly 108 billion dollars.[1] Although awareness of HF has been improved, the diagnosis and management remain difficult in clinical practice, since none of the signs and symptoms are specific or particularly sensitive.[2,3] Mostly, the diagnosis and prognosis of HF rely on biomarkers, because they can provide convenient, safe, and biologically relevant insight into the understanding of the complex disease status.[4,5] However, the natriuretic peptides have a “gray zone” between 100 and 499 pg/ml, therefore its diagnostic accuracy was limited,[6] and also the interpretation of its measurements must be taken in context of patients’ characteristics. Therefore, considerable efforts have been made to broaden the range of biomarkers, and there was a remarkable increase in the number of HF biomarkers after the year 2001.[7] Among all these candidate markers, soluble suppression of tumorigenicity-2 (sST2) had been regarded as the promising one.[8,9]
Suppression of tumorigenicity-2 (ST2) is a member of the interleukin (IL)-1 receptor family, with a soluble form (sST2) and a transmembrane form (ST2 ligand [ST2L]). Previous in vitro studies showed that ST2's production is stimulated by cardiomyocyte stretching,[10] and the expression is linked to cardiac hypertrophy, fibrosis, and ventricular dysfunction. The circulating sST2 has been recently considered as a novel biomarker of HF measurement in serum, its biological function might depend on the reduction of the potential cardioprotective effect of IL-33. IL-33 is the functional ligand for both sST2 and ST2L, and the beneficial effects of IL-33 are transduced through ST2L. The elevated circulating sST2 inhibits the mediation of IL-33 with ST2L, which may prevent the anti-remodeling effects on myocardium by promoting apoptosis, fibrosis, and hypertrophy.[11] This suggests that sST2 plays a role in regulating the critical biomechanically induced and cardioprotective signaling system, which may have biochemical and clinical correlates in patients with HF.
An increasing number of studies have demonstrated that the level of circulating sST2 is elevated in patients with HF;[12,13,14] therefore, sST2 is regarded as a potential biomarker for diagnosis of HF and gains wide attentions. However, up to nowadays, conflicting results have been obtained on the diagnostic value of sST2 for identifying HF patients. In some studies, the serum sST2 measurement provided diagnostic value for HF;[15,16] however, others suggested that sST2's diagnostic performance is inferior to established biomarkers and fails to show strong association with HF.[17,18,19] So far, to our best knowledge, few studies have determined the overall diagnostic performance in HF. Therefore, we performed this meta-analysis to summarize all the diagnostic tests to assess the role of sST2 in the diagnosis of HF.
METHODS
Search strategy
We searched for relevant studies in PubMed, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure, and Wanfang Database from their inception to April 2015. The search strategy was based on the following keywords: (“suppression of tumorigenicity-2” OR “ST2” OR “soluble ST2” OR “sST2”) and (“heart failure” OR “HF”). There were no restrictions in terms of publication year and language. Moreover, disagreement was resolved by discussion among DH, HS, and JS.
Study selection
The inclusion criteria were as follows: (1) studies that investigated the diagnostic role of sST2 for HF; (2) studies with sufficient data to report or calculate the number of true-positive (TP), false-positive (FP), false-negative (FN), and true-negative (TN) results; and (3) >15 patients with HF and control individuals. In addition, we selected the most informative study if the studies involved overlapping populations. The following were excluded: (1) reviews, letters, comments, and meeting abstracts; (2) animal experiments; (3) duplicated publications; (4) studies with low-quality; and (5) studies in which data were insufficient to acquire or calculate the number of TP, FP, FN, and TN results.
Data extraction and quality assessment
The included studies were screened and extracted independently by two reviewers (DH and HS) and the following information was extracted: First author's name; publication year; language; numbers of patients and controls; age and sex distribution; study endpoints; biomarker assay method; reference standards and cut-off values; the diagnostic indices of sST2 including sensitivity and specificity; numbers of TP, FP, FN, and TN results. If the TP, FP, FN, and TN results were not provided directly, we calculated them according to the following formulas:
TP = number of HF patients × sensitivity;
FP = number of controls × (1 − specificity);
FN = number of HF patients × (1 − sensitivity);
TN = number of controls × specificity.
The revised quality assessment of diagnostic accuracy studies (QUADAS-2) criteria were used to assess the quality of the eligible studies.[20] The QUADAS-2 consists of four key domains (patients selection, index test, reference standard, and flow and timing), each domain is assessed in terms of the risk of bias, and the first 3 domains are also assessed in terms of concerns about applicability.[20] A total of 7 items were involved in the QUADAS-2 and each item of the risk of bias and applicability concerns are rated as “High,” “Unclear,” and “Low.” A study is considered low-quality if rated “High” ≥ 4 items. Discrepancies were resolved by a full discussion among the reviewers (DH, HS, and JS).
Statistical analysis
All statistical analyses were conducted using STATA version 11.0 (StataCorp LP, College Station, TX, USA) and Meta-Disc version 14.0 (Universidad Complutense, Madrid, Spain).[21] The graphic displays of QUADAS-2 assessment were conducted by Review Manager (RevMan, Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We used the numbers of TP, FP, FN, and TN results to calculate pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence interval (CI). The summary receiver operating characteristic curve (ROC) and the area under the curve (AUC) were also calculated. AUC close to 0.5 suggested a poor diagnostic test and close to 1.0 indicated good accuracy. The Spearman correlation coefficient was used to check whether the heterogeneity could be explained by the threshold effect. The Cochran Q statistic (P < 0.05) and inconsistency index (I2 > 50%) were used to assess the nonthreshold effect. Meta-regression was conducted to explore the source of heterogeneity; subgroup analysis was conducted to show the results in different groups. Finally, the Deeks’ test was performed to assess the publication bias.
RESULTS
Study selection
Figure 1 shows the flow diagram of the study selection process. We initially obtained 630 articles through our literature search strategy. After removal of duplicates and reading the titles and abstracts, 199 relevant articles were subjected to full-text review. Among the 11 potential studies, two studies were excluded because they have 4 items rated as “high” in terms of risk of bias and applicability concerns. Eventually, 9 articles fulfilled all the inclusion criteria, among them, Santhanakrishnan's et al. article comprised two studies;[22] therefore, there were 10 studies included in the finally meta-analysis.[15,16,18,22,23,24,25,26,27]
Figure 1.
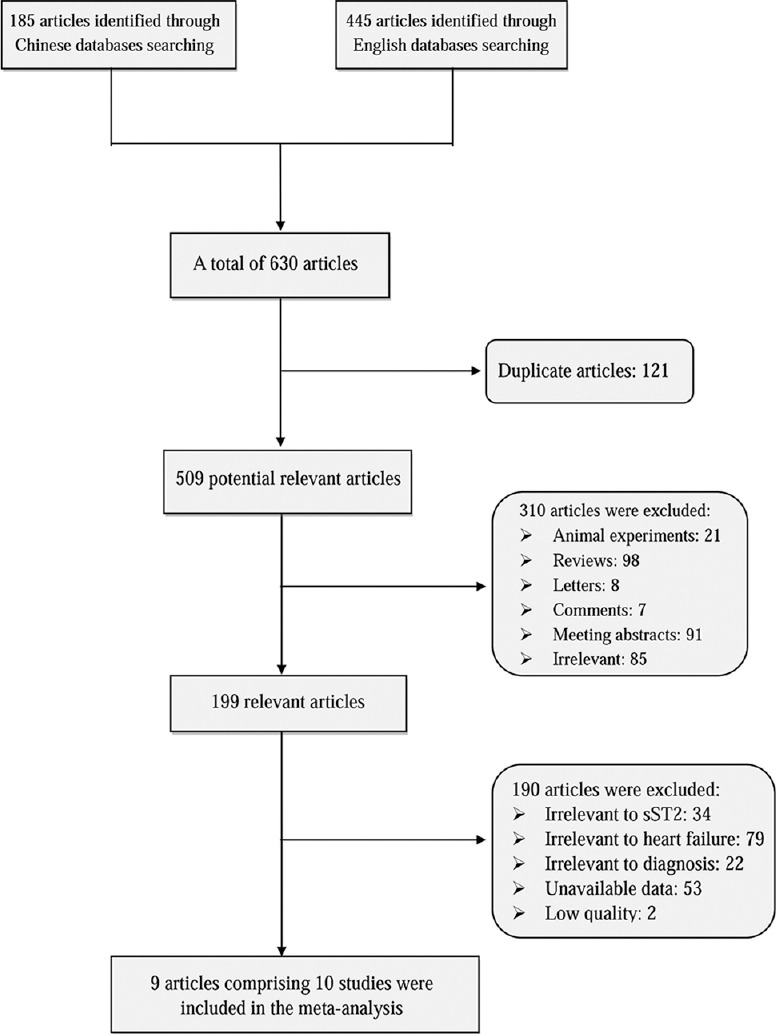
Flow diagram for study selection. sST2: Soluble suppression of tumorigenicity-2.
Characteristics of the eligible studies
The characteristics of the included studies are listed in [Table 1]. Six studies were performed in China, one in Austria, two in Singapore, and one in the Asia-Pacific region. Five studies were written in Chinese and five in English. Seven studies had taken HF as the endpoint, and the other three studies chose heart failure with a normal ejection fraction (HFNEF) or HF with reduced ejection fraction (HFREF) as the outcomes. The main findings of the eligible studies are shown in Table 2. Figure 2 summarizes QUADAS-2 assessments of the eligible studies.
Table 1.
Characteristics of the studies included in meta-analysis
| Studies (author, year) | Language | Sample size (n) | Age (years) | Male (%) | Endpoint | Characteristics of controls | Assay methods | Reference standard | Cut-off value |
|---|---|---|---|---|---|---|---|---|---|
| Dieplinger et al. (2009)[18] | English | 251 | 72.82 | 93.22 | HF | ED patients with dyspnea | ELISA (MBL ST2 assay) | Framingham score | 121 ng/L |
| Gong et al. (2011)[23] | Chinese | 179 | 72.35 | 58.66 | HF | Patients hospitalized in cardiology department | ELISA | ESC guidelines | 0.77 ng/L |
| Aldous et al. (2012)[16] | English | 995 | 66.00 | 59.40 | HF | ED patients with ischemic type pain | ELISA (presage ST2 assay) | Chest radiograph evidence of pulmonary edema or symptoms of HF with raised BNP | 34.3 U/ml |
| Santhanakrishnan et al. (2012)[22] | English | 100 | 66.00 | 52.00 | HFNEF | Community adults | ELISA (presage ST2 assay) | Framingham criteria | 26.47 ng/ml |
| Santhanakrishnan et al. (2012)[22] | English | 101 | 60.98 | 66.20 | HFREF | Community adults | ELISA (presage ST2 assay) | Framingham criteria | 30.32 ng/ml |
| Yuan et al. (2012)[24] | Chinese | 273 | 66.33 | 56.04 | HF | Healthy examined people | ELISA (MBL ST2 assay) | ESC guidelines | 0.2305 ng/ml |
| Wang et al. (2013)[15] | English | 107 | 65.08 | 53.27 | HFNEF | Outpatients with hypertension | ELISA (R&D ST2 assay) | Framingham criteria | 13.5 ng/ml |
| Di and Peng (2013)[25] | Chinese | 164 | 62.50 | 57.32 | HF | Patients with cardiac neurosis or PSVT | ELISA (MBL ST2 assay) | Framingham criteria | 0.85 ng/ml |
| Wen et al. (2013)[26] | Chinese | 404 | 58.03 | 52.23 | HF | Non-HF patients | ELISA (Shanghai Roche) | ESC guidelines | 0.15 μg/L |
| Dai et al. (2014)[27] | Chinese | 417 | – | – | HF | Community people | ELISA (presage ST2 assay) | Medical history, sign, and objective examination | Male: 41.0 μg/L Female: 28.1 μg/L |
–: Value unreported. HF: Heart failure; HFNEF: Heart failure with a normal ejection fraction; HFREF: Heart failure with reduced ejection fraction; ED: Emergency department; PSVT: Paroxysmal supraventricular tachycardia; ELISA: Enzyme-linked immune sorbent assay; MBL: Medical and Biological Laboratories; ESC: European society of cardiology; BNP: B-type natriuretic peptide.
Table 2.
Main findings of the included studies
| Studies (author, year) | Patients/controls (n) | Sensitivity (%) | Specificity (%) | TP (n) | FP (n) | FN (n) | TN (n) |
|---|---|---|---|---|---|---|---|
| Dieplinger et al. (2009)[18] | 137/114 | 90.00 | 22.00 | 123 | 89 | 14 | 25 |
| Gong et al. (2011)[23] | 84/95 | 77.80 | 55.30 | 65 | 42 | 19 | 53 |
| Aldous et al. (2012)[16] | 34/961 | 73.50 | 79.60 | 25 | 196 | 9 | 765 |
| Santhanakrishnan et al. (2012)[22] | 50/50 | 70.00 | 48.00 | 35 | 26 | 15 | 24 |
| Santhanakrishnan et al. (2012)[22] | 51/50 | 69.00 | 68.00 | 35 | 16 | 16 | 34 |
| Yuan et al. (2012)[24] | 192/81 | 88.20 | 77.80 | 169 | 18 | 23 | 63 |
| Wang et al. (2013)[15] | 68/39 | 74.00 | 74.00 | 50 | 10 | 18 | 29 |
| Di and Peng (2013)[25] | 129/35 | 79.00 | 66.00 | 102 | 12 | 27 | 23 |
| Wen et al. (2013)[26] | 354/50 | 99.40 | 54.00 | 352 | 23 | 2 | 27 |
| Dai et al. (2014)[27] | 117/300 | 51.20 | 92.70 | 60 | 22 | 57 | 278 |
TP: True-positive; FP: False-positive; FN: False-negative; TN: True-negative.
Figure 2.
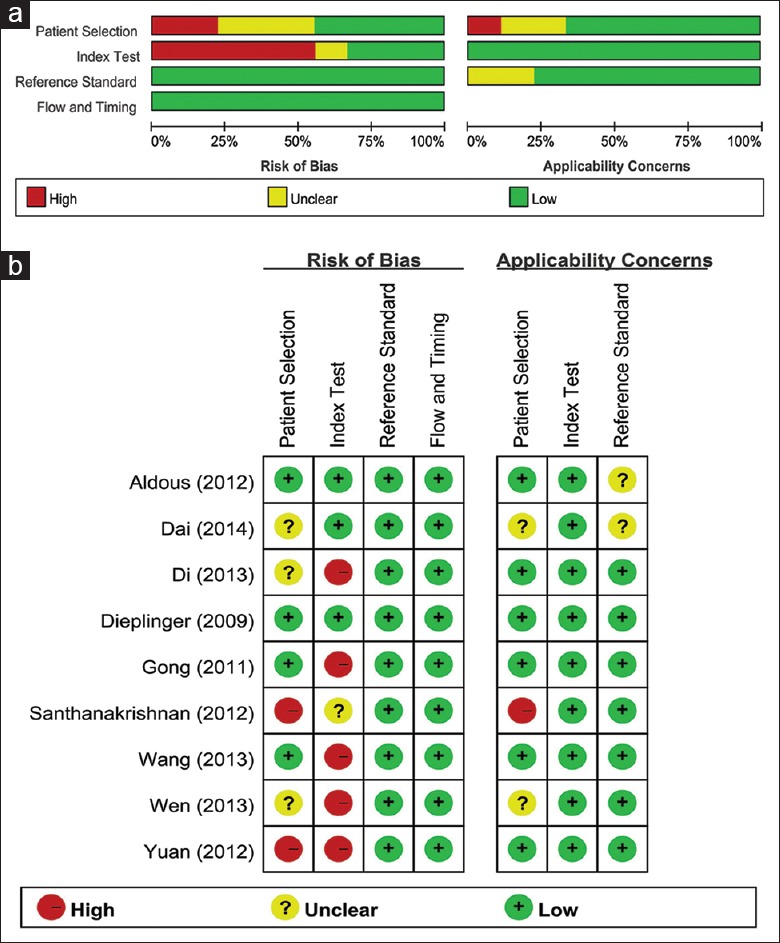
Quality assessment of the eligible studies. (a) Review authors’ judgments about each domain presented as percentages across included studies; (b) Review authors’ judgements about each domain for each included study.
Diagnostic role of soluble suppression of tumorigenicity-2
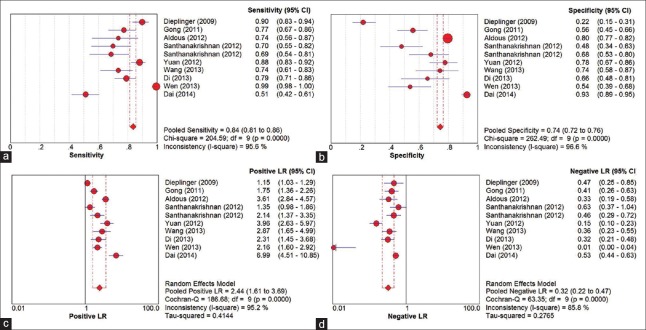
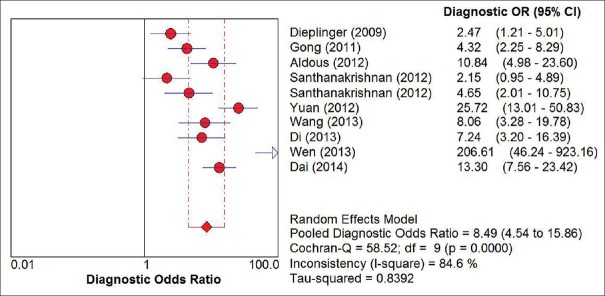
Figure 3 shows the forest plots for the pooled sensitivity, specificity, PLR, and NLR. The overall sensitivity and specificity values of sST2 for HF were 0.84 (95% CI: 0.81–0.86) and 0.74 (95% CI: 0.72–0.76), respectively. The pooled PLR and NLR were 2.44 (95% CI: 1.61–3.69) and 0.32 (95% CI: 0.22–0.47), respectively. In addition, summary DOR was 8.49 (95% CI: 4.54–15.86) and the AUC was 0.81 (standard error = 0.03). The forest plot of DOR is shown in Figure 4 and the details of the ROC curve are illustrated in Figure 5.
Figure 3.
The forest plots of pooled sensitivity (a); specificity (b); positive likelihood ratio (c); and negative likelihood ratio (d). CI: Confidence interval; df: Degree of freedom; LR: Likelihood ratio.
Figure 4.
The forest plot of pooled diagnostic odds ratio. OR: Odds ratio; CI: Confidence interval.
Figure 5.
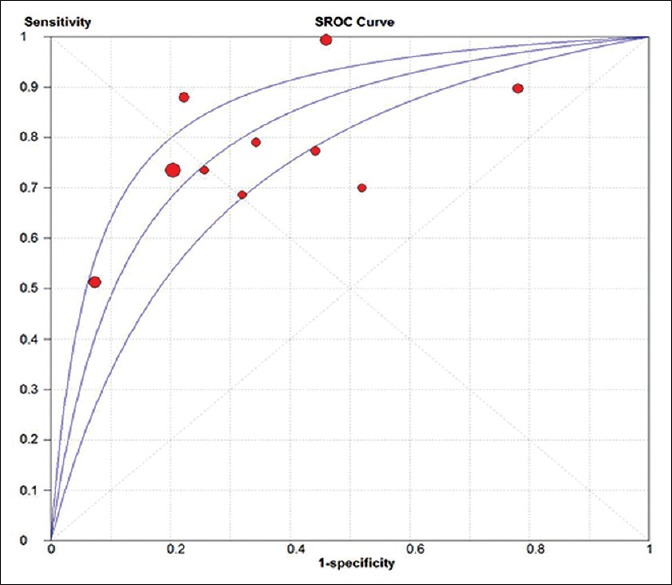
The summary receiver operating characteristic curve of all included studies. SROC: Summary receiver operating characteristic. The area under the summary receiver operating characteristic curve: 0.81; standard error: 0.03.
Threshold and nonthreshold effects
The Spearman correlation coefficient of 0.49 (P = 0.148) suggested a nonsignificant threshold effect in the meta-analysis. The Cochran Q (58.52, P < 0.0001) and I2 (84.6%) values illustrated the significant heterogeneity of the nonthreshold effect; therefore, the random effects model was used to assess the overall diagnostic performance of sST2 in HF.
Meta-regression
For exploring the source of heterogeneity, the covariates of language, assay methods, characteristics of controls, sample size, study quality, endpoints, and reference standards were all included in the meta-regression analysis. After excluding the covariate with the largest P value each time, characteristic of controls showed the suggestive but nonsignificant association with heterogeneity (P = 0.095).
Subgroup analysis
The subgroup analysis was based on language, endpoint, assay methods, study quality, and characteristics of controls [Table 3]. The DOR was 4.59 and 15.93 for the studies in English and Chinese languages, respectively. The DOR was 11.43, 4.11, and 4.65 for the endpoint of HF, HFNEF, and HFREF, respectively. There were mainly three enzyme-linked immunosorbent assays (ELISAs) used to determine sST2 concentrations, including presage ST2 assay, Medical and Biological Laboratories (MBL) ST2 assay, R&D ST2 assay, and other ELISA kits. The former three were the main assays in previously published studies; the latter ELISA kits were mostly made in China and not commonly used before. As shown, the DOR was 7.74, 6.35, 8.06, and 28.12 for the MBL ST2 assay, presage ST2 assay, R&D ST2 assay, and other ELISA kits. For the subgroups based on study quality, the DOR was respectively 11.84 and 5.36 for the low- (rated “Low” <6 items) and high-quality (rated “Low” ≥6 items) studies. Finally, the DOR was 5.65, 7.86, and 206.61 for the control hospital patients, healthy populations, and unclear controls, respectively.
Table 3.
Results of subgroup analysis
| Items | Number of studies (n) | Heterogeneity | DOR with 95% CI | ||||
|---|---|---|---|---|---|---|---|
| χ2 | P | I2 (%) | DOR | LL | UL | ||
| Language | |||||||
| English | 5 | 12.39 | 0.015 | 67.7 | 4.59 | 2.43 | 8.64 |
| Chinese | 5 | 29.74 | <0.001 | 86.5 | 15.93 | 6.31 | 40.19 |
| Endpoint | |||||||
| HF | 7 | 45.87 | <0.001 | 86.9 | 11.43 | 5.22 | 25.01 |
| HFNEF | 2 | 4.52 | 0.034 | 77.9 | 4.11 | 1.13 | 14.97 |
| HFREF | 1 | – | – | – | 4.65 | 2.01 | 10.75 |
| Assay methods | |||||||
| MBL ST2 assay | 3 | 21.95 | <0.001 | 90.9 | 7.74 | 1.90 | 31.48 |
| Presage ST2 assay | 4 | 14.97 | 0.002 | 80.0 | 6.35 | 2.78 | 14.54 |
| R&D ST2 assay | 1 | – | – | – | 8.06 | 3.28 | 19.78 |
| Other ELISA kits | 2 | 21.82 | <0.001 | 95.4 | 28.12 | 0.62 | 1272.00 |
| Study quality | |||||||
| Rated “Low” <6 items | 6 | 41.26 | <0.001 | 87.9 | 11.84 | 4.63 | 30.27 |
| Rated “Low” ≥6 items | 4 | 8.89 | 0.031 | 66.3 | 5.36 | 2.81 | 10.25 |
| Characteristic of controls | |||||||
| Hospital patients | 5 | 9.50 | 0.050 | 57.9 | 5.65 | 3.34 | 9.56 |
| Healthy populations | 4 | 24.99 | <0.001 | 88.0 | 7.86 | 2.80 | 22.05 |
| Unclear | 1 | – | – | – | 206.61 | 46.24 | 923.16 |
HF: Heart failure; HFNEF: Heart failure with a normal ejection fraction; HFREF: Heart failure with reduced ejection fraction; MBL: Medical and Biological Laboratories; ELISA: Enzyme-linked immune sorbent assay; DOR: Diagnostic odds ratio; CI: Confidence interval; LL: Lower limit; UL: Upper limit.
Publication bias
The publications bias of the included studies was evaluated by the Deeks’ funnel plot asymmetry test. As showed in Figure 6, the symmetrical funnel shape indicated the absence of publication bias. In addition, the slope coefficient of 10 studies had a P value of 0.616, which also demonstrated the absence of publication bias in the meta-analysis.
Figure 6.
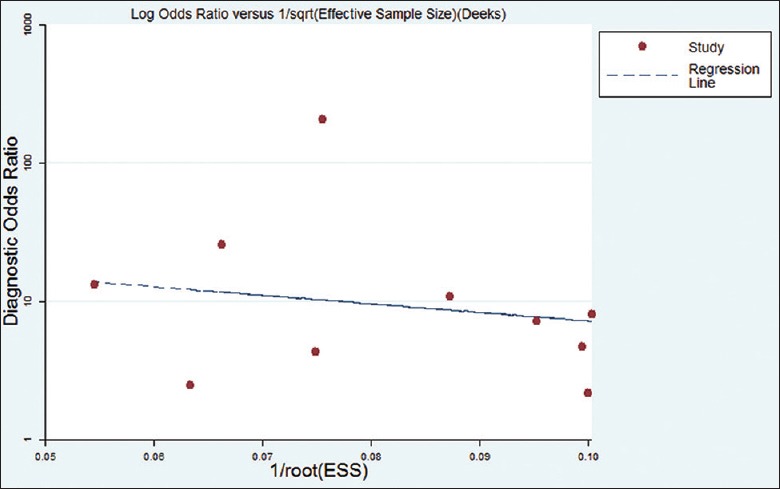
The funnel plot of publication bias. ESS: Effective sample size.
DISCUSSION
This meta-analysis aimed to evaluate the diagnostic value of sST2 for HF. Nonsignificant threshold effect suggested that the pooled sensitivity, specificity, and DOR could indicate the diagnostic accuracy of sST2. Moreover, in this meta-analysis, the pooled sensitivity and specificity was 0.84 and 0.74, respectively, and the pooled DOR was 8.49. These results indicated that a promising performance of biomarker sST2 for diagnosing HF. Furthermore, we chose the indices of pooled ROC and AUC, which were stable for varied cut-off values, to evaluate the overall diagnostic value of sST2. Finally, the AUC of 0.81 demonstrated moderate accuracy of sST2 in diagnosing HF. Moreover, as the heterogeneity in the nonthreshold effect might limit our conclusion, we conducted meta-regression to explore the source and finally identified the characteristics of controls as the suggestive but nonsignificant source with a P = 0.095. Subgroup analysis based on the characteristics of controls indicated that I2 had reduced to 57.9% for the control group of hospital patients, which suggested the different controls might be associated with the heterogeneity. Results from other subgroup analysis suggested the significant diagnostic value of sST2 even though heterogeneity existed in the study. Finally, no publication bias was observed among the included studies, which suggested a robust result of this meta-analysis.
The sST2 is a truncated soluble form of ST2 that lacks the transmembrane and intracellular domains. Its mechanism of association with HF remains unclear, but its potential biological basis has been proposed. The level of circulating sST2 in patients with HF is associated with neurohormonal and sympathetic activation.[9] Sánchez-Más et al.[28] also demonstrated the correlation between sST2 and the ongoing processes of fibrosis and inflammation; therefore, it is suggested that the inflammatory biomarker sST2 is correlated with disease severity and prognosis of HF.[29] sST2 also functions as a decoy receptor to inhibit IL-33/ST2L signaling.[11] The interaction between ST2L and IL-33 is induced by myocardial overload or stretch. Therefore, elevated sST2 blocks the modulating responses of T-helper 2 cells and inhibits the inflammatory and immunological responses produced by IL-33/ST2L signaling, thus aggravating disease progression. sST2 is mechanically induced by the stimulation of various proliferation-inducing agents, and in vitro biomechanical stimulation of sST2 is similar to the mechanical induction of brain natriuretic peptide (BNP), which is a useful diagnostic and prognostic marker in human HF. All these biological findings have raised the hypothesis that sST2 has the potential to diagnose HF, but the mechanisms need further exploration.
As an emerging biomarker, sST2 has revealed its superiority in many respects. sST2 can be detected in the blood using several assay methods, and it provides additional information beyond the current established biomarkers.[30] As previously suggested, measurement of multiple markers might be effective at improving diagnostic capacity and thus tailoring patient management.[31] Therefore, it is necessary to determine the additional diagnostic information of sST2 beyond that of the current established biomarkers, including BNP and N-terminal pro-BNP.[3] Our subgroup analysis showed that sST2 was of significance for diagnosis of HF, HFNEF, and HFREF, and it played a role in the prediction of long-term outcome of HF, including mortality.[32,33,34] Measurement of sST2 was able to provide comprehensive information on diagnostic and prognostic aspects of HF, which could help clinicians to improve risk stratification and tailor management of patients with HF. With the growing number of studies showing that the prognostic value of sST2 is superior to its diagnostic performance,[35,36] further studies should focus on the optimal utility of sST2 and incorporate it into clinical practice.
The results of this meta-analysis improve our understanding of sST2 and have implications for further study. First, although the endpoint was HF, there were two studies with an endpoint of HFNEF and one with HFREF included in the meta-analysis. Subgroup analysis found that sST2 had a stronger diagnostic value than HFNEF and HFREF, which suggests a weak correlation between sST2 and ejection fraction. However, the exact target population and detailed mechanism need further study. Second, measurement of sST2 seemed to be of diagnostic value for hospital patients and healthy populations. The lower DOR in hospital patients compared with the healthy population might be related to the complex disease status of patients. Third, the eligible studies in the meta-analysis were mostly from Asian countries, and there were five studies in Chinese. As subgroup analysis showed, the Chinese studies might have overstated the diagnostic value of sST2. Therefore, there is a need to conduct further well-designed studies in a wide range of regions and populations to confirm our conclusions. Finally, the methods for assaying sST2 were not uniform, and the cut-off values varied accordingly. The variability in the assay methods might have led to some bias in the pooled effect; thus, it was important to determine the optimal method for sST2 measurement and to set an exact cut-off point for identifying HF.
The present meta-analysis revealed the diagnostic value of sST2 for HF, but there were limitations when interpreting the results. Heterogeneity was important in our meta-analysis; although the threshold effect was nonsignificant, the heterogeneity in nonthreshold effects had an influence on the final results. Furthermore, the limited number of eligible studies had limited the ability of meta-regression to explore the source of nonthreshold heterogeneity, so additional studies need to be included in this meta-analysis. Moreover, almost all eligible studies had design deficiencies, which resulted in suboptimal quality of the studies involved in the meta-analysis and limited the final conclusions. As shown in subgroup analysis, the studies with low-quality might have overstated the diagnostic performance of sST2 for HF, so additional well-designed studies with large samples are necessary to strengthen our conclusions.
In conclusion, the results of our meta-analysis show that sST2 might have a moderate value in diagnosing HF. A well-designed prospective study with a large sample should be performed to further validate the diagnostic value of sST2.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Acknowledgments
We would like to thank the entire team of Cardiovascular Diseases Institute and Evidence Based Medicine Center of the First Affiliated Hospital of China Medical University. We are grateful to those participants in this study who had been generous with their time and assistance.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–76. doi: 10.1016/j.ijcard.2013.12.028. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. 2012:The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 4.Gaggin HK, Januzzi JL., Jr Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. 2013;1832:2442–50. doi: 10.1016/j.bbadis.2012.12.014. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Taub PR, Gabbai-Saldate P, Maisel A. Biomarkers of heart failure. Congest Heart Fail. 2010;16(Suppl 1):S19–24. doi: 10.1111/j.1751-7133.2010.00168.x. doi: 10.1111/j.1751-7133.2010.00168.x. [DOI] [PubMed] [Google Scholar]
- 6.Maisel AS, Peacock WF, Shah KS, Clopton P, Diercks D, Hiestand B, et al. Acoustic cardiography S3 detection use in problematic subgroups and B-type natriuretic peptide “gray zone” :Secondary results from the heart failure and audicor technology for rapid diagnosis and initial treatment multinational investigation. Am J Emerg Med. 2011;29:924–31. doi: 10.1016/j.ajem.2010.03.032. doi: 10.1016/j.ajem.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 7.van Kimmenade RR, Januzzi JL., Jr Emerging biomarkers in heart failure. Clin Chem. 2012;58:127–38. doi: 10.1373/clinchem.2011.165720. doi: 10.1373/clinchem.2011.165720. [DOI] [PubMed] [Google Scholar]
- 8.Shah RV, Januzzi JL., Jr ST2:A novel remodeling biomarker in acute and chronic heart failure. Curr Heart Fail Rep. 2010;7:9–14. doi: 10.1007/s11897-010-0005-9. doi: 10.1007/s11897-010-0005-9. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–6. doi: 10.1161/01.cir.0000047274.66749.fe. doi: 10.1161/01.cir.0000047274.66743.fe. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–49. doi: 10.1172/JCI30634. doi: 10.1172/jci30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A. doi: 10.1161/01.cir.0000127958.21003.5a. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–44. doi: 10.1161/CIRCULATIONAHA.107.728022. doi: 10.1161/circulationaha.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble ST2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin Chim Acta. 2015;445:155–60. doi: 10.1016/j.cca.2015.03.033. doi: 10.1016/j.cca.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Wang YC, Yu CC, Chiu FC, Tsai CT, Lai LP, Hwang JJ, et al. Soluble ST2 as a biomarker for detecting stable heart failure with a normal ejection fraction in hypertensive patients. J Card Fail. 2013;19:163–8. doi: 10.1016/j.cardfail.2013.01.010. doi: 10.1016/j.cardfail.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Aldous SJ, Richards AM, Troughton R, Than M. ST2 has diagnostic and prognostic utility for all-cause mortality and heart failure in patients presenting to the emergency department with chest pain. J Card Fail. 2012;18:304–10. doi: 10.1016/j.cardfail.2012.01.008. doi: 10.1016/j.cardfail.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Januzzi JL, Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-brain natriuretic peptide investigation of dyspnea in the emergency department) study. J Am Coll Cardiol. 2007;50:607–13. doi: 10.1016/j.jacc.2007.05.014. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Dieplinger B, Gegenhuber A, Haltmayer M, Mueller T. Evaluation of novel biomarkers for the diagnosis of acute destabilised heart failure in patients with shortness of breath. Heart. 2009;95:1508–13. doi: 10.1136/hrt.2009.170696. doi: 10.1136/hrt.2009.170696. [DOI] [PubMed] [Google Scholar]
- 19.Henry-Okafor Q, Collins SP, Jenkins CA, Miller KF, Maron DJ, Naftilan AJ, et al. Soluble ST2 as a diagnostic and prognostic marker for acute heart failure syndromes. Open Biomark J. 2012;2012:1–8. doi: 10.2174/1875318301205010001. doi: 10.2174/1875318301205010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2:A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 21.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc:A software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santhanakrishnan R, Chong JP, Ng TP, Ling LH, Sim D, Leong KT, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2012;14:1338–47. doi: 10.1093/eurjhf/hfs130. doi: 10.1093/eurjhf/hfs130. [DOI] [PubMed] [Google Scholar]
- 23.Gong GH, Zhang W, Luo Y, Yang Y. Clinical research about three myocardial cell stress biomarkers in patients with heart failure (In Chinese) Chin J Misdiagnosis. 2011;11:261–3. [Google Scholar]
- 24.Yuan W, Gu YY, Zhang DF. Measurement of serum soluble ST2 in patients with heart failure and its diagnostic value (In Chinese) Acad J Sec Mil Med Univ. 2012;33:175–8. doi: 10.3724/SP.J.1008.2012.00175. [Google Scholar]
- 25.Di YQ, Peng DQ. Role of soluble ST2 in the diagnosis of heart failure (In Chinese) Chin J Cardiovasc Med. 2013;18:334–8. doi: 10.3969/j.issn.1007-5410.2013.05.003. [Google Scholar]
- 26.Wen JL, Cui XY, Chen FY. Correlation analysis of N-terminal brain natriuretic peptide, brain natriuretic peptide and ST2 with heart failure severity (In Chinese) China Med Her. 2013;10:76–80. doi: 10.3969/j.issn.1673-7210.2013.02.030. [Google Scholar]
- 27.Dai Q, Wu J, Guo W, Zhang CY, Pan BS. The performance evaluation of soluble ST2 detection kit and the clinical application of sST2 in diagnosing heart failure (In Chinese) Chin J Lab Med. 2014;37:394–8. doi: 10.3760/cma.j.issn.1009-9158.2014.05.019. [Google Scholar]
- 28.Sánchez-Más J, Lax A, Asensio-López Mdel C, Fernandez-Del Palacio MJ, Caballero L, Santarelli G, et al. Modulation of IL-33/ST2 system in postinfarction heart failure: Correlation with cardiac remodelling markers. Eur J Clin Invest. 2014;44:643–51. doi: 10.1111/eci.12282. doi: 10.1111/eci.12282. [DOI] [PubMed] [Google Scholar]
- 29.Hartupee J, Mann DL. Positioning of inflammatory biomarkers in the heart failure landscape. J Cardiovasc Transl Res. 2013;6:485–92. doi: 10.1007/s12265-013-9467-y. doi: 10.1007/s12265-013-9467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanavitski M, Givertz MM. Novel biomarkers in acute heart failure. Curr Heart Fail Rep. 2011;8:206–11. doi: 10.1007/s11897-011-0065-5. doi: 10.1007/s11897-011-0065-5. [DOI] [PubMed] [Google Scholar]
- 31.Kossaify A, Garcia A, Succar S, Ibrahim A, Moussallem N, Kossaify M, et al. Perspectives on the value of biomarkers in acute cardiac care and implications for strategic management. Biomark Insights. 2013;8:115–26. doi: 10.4137/BMI.S12703. doi: 10.4137/bmi.s12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu AH, Wians F, Jaffe A. Biological variation of galectin-3 and soluble ST2 for chronic heart failure: Implication on interpretation of test results. Am Heart J. 2013;165:995–9. doi: 10.1016/j.ahj.2013.02.029. doi: 10.1016/j.ahj.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, et al. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57:874–82. doi: 10.1373/clinchem.2010.159277. doi: 10.1373/clinchem.2010.159277. [DOI] [PubMed] [Google Scholar]
- 34.Anand IS, Rector TS, Kuskowski M, Snider J, Cohn JN. Prognostic value of soluble ST2 in the valsartan heart failure trial. Circ Heart Fail. 2014;7:418–26. doi: 10.1161/CIRCHEARTFAILURE.113.001036. doi: 10.1161/circheartfailure.113.001036. [DOI] [PubMed] [Google Scholar]
- 35.Bayes-Genis A, Zhang Y, Ky B. ST2 and patient prognosis in chronic heart failure. Am J Cardiol. 2015;115(7 Suppl):64B–9B. doi: 10.1016/j.amjcard.2015.01.043. doi: 10.1016/j.amjcard.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 36.Dieplinger B, Mueller T. Soluble ST2 in heart failure. Clin Chim Acta. 2015;443:57–70. doi: 10.1016/j.cca.2014.09.021. doi: 10.1016/j.cca.2014.09.021. [DOI] [PubMed] [Google Scholar]