Abstract
Objective:
To review the recent developments in the mechanisms of epithelium sodium channels (ENaCs) induced bone formation and regulation.
Data Sources:
Studies written in English or Chinese were searched using Medline, PubMed and the index of Chinese-language literature with time restriction from 2005 to 2014. Keywords included ENaC, bone, bone formation, osteonecrosis, estrogen, and osteoporosis. Data from published articles about the structure of ENaC, mechanism of ENaC in bone formation in recent domestic and foreign literature were selected.
Study Selection:
Abstract and full text of all studies were required to obtain. Studies those were not accessible and those did not focus on the keywords were excluded.
Results:
ENaCs are tripolymer ion channels which are assembled from homologous α, β, and γ subunits. Crystal structure of ENaCs suggests that ENaC has a central ion-channel located in the central symmetry axis of the three subunits. ENaCs are protease sensitive channels whose iron-channel activity is regulated by the proteolytic reaction. Channel opening probability of ENaCs is regulated by proteinases, mechanical force, and shear stress. Several molecules are involved in regulation of ENaCs in bone formation, including nitride oxide synthases, voltage-sensitive calcium channels, and cyclooxygenase-2.
Conclusion:
The pathway of ENaC involved in shear stress has an effect on stimulating osteoblasts even bone formation by estrogen interference.
Keywords: Bone Formation, Epithelium Sodium Channel, Estrogen, Osteoblasts, Shear Stress
INTRODUCTION
Bone formation, also known as osteogenesis, is a complicated process that underlies the whole life of a body.[1] The process of osteogenesis is comprised of maturation of osteoblasts and osteocytes, extracellular excretion, and ossification.[2] Osteoblasts are the most important cells in bone tissues which are critical for bone formation.[3] Ossification has a restrict order: First, collagen proteins accumulate in immature bone tissue; second, osteoblasts secrete alkaline phosphatase (ALP), osteopontin (OPN), and osteocalcin (OCN); and third, bone canaliculus forms finally.[4] Bone mesenchymal stem cells (BMSCs) are precursors of osteoblasts, which can differentiate into chondrocytes, adipocytes, and osteoblasts. The most cellular events involved in bone formation include the proliferation and differentiation of BMSCs.
BMSCs differentiation and osteoblasts maturation are regulated by several cytokines and signal pathways. Many studies have revealed that bone morphogenetic proteins (BMPs), belong to the transforming growth factor beta superfamily, stimulate proliferation and differentiation of BMSCs and enhance mineralization of skeleton tissue.[5,6,7] It is also known that Wnt proteins signals which regulate cell growth, differentiation, function, and death, are essential for bone mass regulation.[8,9,10] Prostaglandin (PG), mainly produced by cyclooxygenase-2 (COX-2), is reported with the significant role of bone formation by forming an important regulatory loop with BMPs.[11,12,13] Moreover, glucocorticoids (GCs) exert ambiguous effects to BMSCs and osteoblasts, low dose of GCs keeps hemostasis of bone tissues while high dose inhibits bone formation.[14,15,16] Recently, several researchers revealed that epithelium sodium channel (ENaC) may be the importance of regulation of BMSCs and osteoblasts.
The amiloride-sensitive ENaCs, first described in 1994, is a membrane-bound ion-channel that is permeable for Li+-ions, protons, and especially sodium ions. Meanwhile, this sodium channels are characterized primarily by their high affinity to the diuretic blocker amiloride.[17,18,19] Studies showed that ENaCs were abundantly expressed in the tissues of kidney, colon, lung, sweat glands, and reproductive ducts.[20,21] Furthermore, recent studies have discovered that ENaC may also be expressed in osteoblasts and MSCs, for sodium concentration in the extracellular fluid may affect proliferation and differentiation of osteoblasts.[20,22] Therefore, the study on ENaCs may provide a new sight to understand the process and regulation of bone formation. In this review, we retrospect researches within the last 5 years and try to illustrate the characteristics and regulation of ENaC as well as the function of ENaC during bone formation.
CHARACTERISTICS OF EPITHELIUM SODIUM CHANNEL
Structure of epithelium sodium channel
ENaCs are members of the ENaC/degenerin family of ion channels, which are assembled from homologous α, β, and γ subunits.[23] Each subunit consists of two transmembrane helices and an extracellular loop, and shares a similar secondary structure consisting of a large extracellular region linked to two membrane spanning domains (TM1 and TM2), and short intracellular N- and C-termini.[24,25] All three subunits are essential for transporting to the membrane assembly of functional channels on the membrane.[26] ENaC highly seems to be a heterotrimeric protein like the recently analyzed acid-sensing ion-channel 1 (ASIC1), which belongs to the same family.[27]
Crystal structure of ENaCs and site-directed mutagenesis studies suggest that ENaC has a central ion-channel located on the central symmetry axis of the three subunits.[28] Proteins that belong to this family consist of about 510–920 amino acid residues. They are made of an intracellular N-terminus region followed by a transmembrane domain, a large extracellular loop, a second transmembrane segment, and a C-terminal intracellular tail.[29] The extracellular region of ASIC1 has a highly ordered structure containing domains formed either by β strands or α helices. The structure resembles an outstretched hand containing a ball. Domains within the structure are aptly named wrist, finger, thumb, palm, knuckle, and β-ball [Figure 1].[30,31]
Figure 1.
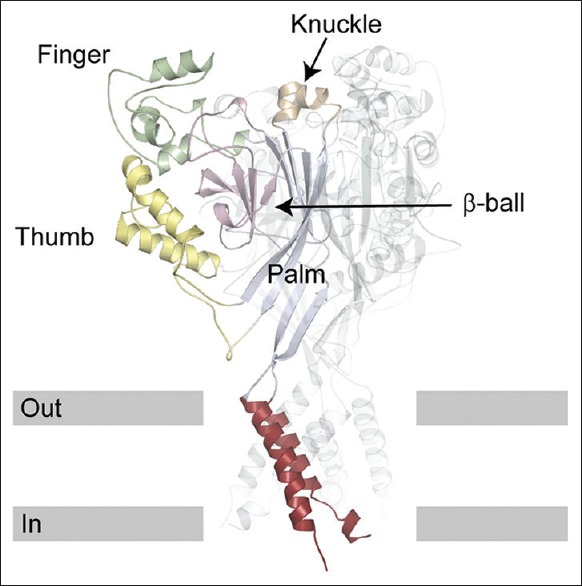
Structure of acid-sensing ion-channel 1. One subunit is highlighted and the others are transparent ribbons. The structure of Antiracism Study-Dialogue Circle 1 is similar to an outstretched hand containing a ball.[31].
ENaCs are protease sensitive channels whose iron-channel activity is regulated by the proteolytic reaction. Trypsin and chymotrypsin activate ENaCs in the epithelium of endometrium of the uterus to help fertilization of oocytes.[32,33] Contrastly, extracellular serine protease inhibitors, such as aprotinin and bikunin, have been shown to decrease channel activity.[34] In addition, furin, a proprotein convertase that cleaves latent precursor proteins into biologically active products, cleaves α and γ subunits of ENaCs during its maturation.[35] Furin cleaves α subunit twice and then release a 26 residue fragment.[35,36] Mutation on α subunit furin consensus sites dramatically reduces channel activity. The γ subunit is cleaved by furin only once and release 18,000 and 75,000 fragments of proteolytic peptides [Figure 2].[35]
Figure 2.
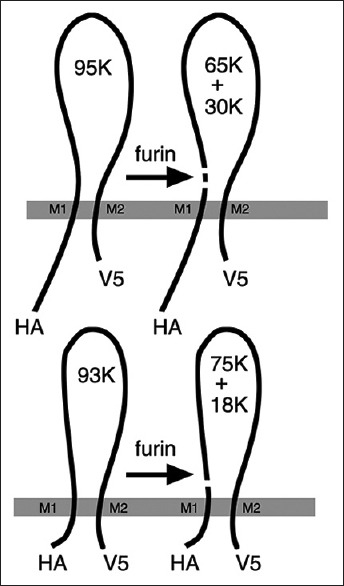
Remaining peptides after treatment of furin to α and γ subunits of epithelium sodium channels. Furin cleaves α subunit and releases 65,000 and 30,000 peptides and a 26 residue fragment. It cleaves γ subunits remaining 75,000 and 18,000 peptides.[35]
Activity of epithelium sodium channels
Channel opening probability (Po) of ENaCs is regulated by several physical and chemical factors. As mentioned, ENaCs are proteinase sensitive iron-channel, proteinase-treated ENaCs performing a high rate Po. Notably, the release of an inhibitory tract activates the channel by increasing its open Po, meanwhile, subunits must be cleaved at least twice at sites flanking the inhibitory tract to be activated.[37] Furin cleaves α subunit twice releasing a 65,000, a 30,000 peptide and a 26 residue fragment, enhance permeable of cations. However, as furin cleaves the γ subunit once, a second protease cleaving the γ subunit could release the inhibitory tract and improve iron permeability. Moreover, trypsin which is a serine protease activating ENaC induces an inward whole-cell current and the augment of the current could be abolished by amiloride, an ENaC inhibitor or by aprotinin.[32,38] In addition, renal tubule epithelia, especially apical membrane, which are regulated by aldosterone or tubular volume.[39,40] Prostasin is observed to have a role in γ subunit under the regulation of volume depletion and aldosterone excretion.[41]
Another factor that affects Po of ENaCs is a mechanical force. Studying ENaCs of Caenorhabditis elegans reveals a fact that ENaCs are members of a family of ion channels that own a character of mechanical-sensitive.[42] Hydrostatic pressure has been discovered to increase the activity of ENaCs. The negative pressure of collecting ducts which is up to 80 mmHg elicits an increase in channel Po.[43] An increased volume of the bladder, resulting in an augment of the hydrostatic pressure of endothelia of bladder, could induce improved inward whole-cell current which is partly abolished by amiloride. Therefore, it is indicated that increases in hydrostatic pressure enhance ENaC activity.[44]
Similar to mechanical force, ENaC is also regulated by shear stress. Renal tubule epithelia are exposed under the flow of urine which creates a condition of flow stress. An augment of sodium absorption was observed by the increase of collective duct perfusion.[45,46] Importantly, osteoblasts are observed to have an improved proliferation with shear flow mechanism.[47,48,49] It is still not unclear whether the enhanced effect of shear stress could be abrogated by amiloride, as ENaCs are abundantly expressed on osteoblasts. However, ENaC might play a significant role in shear stress further regulated osteoblast maturation.
SIGNAL INVOLVED IN EPITHELIUM SODIUM CHANNELS IN OSTEOBLASTS
ENaCs are critical in proliferation and differentiation of osteoblasts because ENaCs activate the expression of runt-related transcription factor 2 (RUNX2), a key transcriptional modulator of osteoblasts formation which plays a fundamental role in osteoblasts maturation and homeostasis.[50] Furthermore, RUNX2 controls transcription of ossify-specific genes including OCN, OPN, and collagenase-3 in BMSCs and osteoblasts. As osteoblasts are imbedded in periosteum and metaphysis of bones, different with reproductive tracts, the stimulation of ENaCs cannot be performed by protease release.[33,41,45]
Cyclic guanosine monophosphate and cyclic guanosine monophosphate-dependent protein kinase II
Recent studies have revealed that sensitization of ENaC in osteoblasts is mainly caused by cyclic guanosine monophosphate (cGMP). cGMP and cGMP signaling pathway play a positive role in bone formation.[51,52] Exogenous 8-pCPT-cGMP, a cell-permeable cGMP analog, is reported to stimulate the expression of RUNX2 via increased expression of ENaCs.[53] Consequently, the effect of stimulation could be abolished by either amiloride or small interfering RNA (siRNA) of ENaC. Furthermore, the effect of cGMP on ENaCs is due to sensitization of cGMP-dependent protein kinase II (PKG II) and knockdown of PKG II (via siRNA) blocked 8-pCPT-cGMP induced expression of ENaC.[53] Although 8-pCPT-cGMP stimulate expression of ENaC – an augment of ENaCs (see below), it is still under-investigated whether cGMP could improve the opening Po ENaC.
Nitride oxide synthases (NOS), which synthesis nitride oxide (NO), are a series of proteins expressed widely on nearly all cells. It has been revealed that NOS has three isoforms, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). These three isoforms are of significance in the regulation of bone formation. It also shown that anabolic effect of estrogen is partly mediated by eNOS isoform, as deletion of the eNOS leads to an osteoblast-driven mild osteoporotic bone, and finally shows a blunted response to estrogen.[54] Moreover, NO synthesized by iNOS activates osteoclasts in inflammatory bone disease. iNOS-derived NO also stimulates fracture healing as well as recovery of bone mass.[55,56] NO derived from nNOS negatively regulates osteogenesis since nNOS knocked-out osteoblasts show an increased level of ALP.[57] In addition, cGMP pathway is a classical target for NO.[58] NO derived from NOS triggers PKG II by cGMP which is synthesized by soluble guanylate cyclase (sGC) and, therefore, up-regulates the expression of ENaC. It is also reported that fluid shear stress increased osteoblast NO synthesis, leading to activation of PKG II.[58] This means ENaC may partly mediate the influence of shear stress and the PKG II and ENaC might have a regulation loop in shear stress which activates osteoblast proliferation and differentiation.
Voltage-sensitive calcium channel
As discussed above, the activation of ENaCs triggers influx of sodium iron leading to a consequence that membrane potential is elevated temporarily. The underlying mechanism of elevating potential is velocity and quantity of influx sodium irons pass through opening ENaCs. Importantly, for osteoblasts and oocytes, which are different from duct cells, two factors are related to depolarization of cell membrane – number (N) of ENaCs localized on membrane and activity (Po) of an individual ENaC.[59] The result of patch clamps shows that potential of oocytes is declined by 22.24 mV and potential of SMC-C6 cells, an immoral epithelium cell line, is dropped by 12.1 mV, both boosting potential abrogated by amiloride.[32,50] These results consequently suggest that the summarized effects of both N and Po of ENaC in different cells are diverged.
Voltage-sensitive calcium channels (VSCCs) respond to the elevated potential through ENaC activity. In details, serine protease-induced, ENaC-mediated membrane depolarization could result in Ca2+ mobilization. Moreover then, the Ca2+ level rise was largely lessened in Ca2+-free solutions, while nifedipine, a blocker of the VSCC, abolished the trypsin-induced Ca2+ level rise.[32] As investigated, VSCCs are classified into two types, T-type and L-type VSCCs. Osteoblasts predominantly express L-type VGCCs, whereas osteocytes in mature bone tissues which are derived from osteoblasts, express T-type VSCCs and a small amount of L-type.[60] Both types of cells in calcium-free medium showed no response to fluid flow. For example, amlodipine, an L-type VSCC blocker, is used to treat MC3T3-E1 (immoral osteoblast cell line) and MLO-Y4 (immoral osteocyte cell line) cells showing a prolonged declining of calcium in both types of cells, while treatment of NNC55-0396 (T-type VSCC blocker) can discontinue the repetitive influx calcium in MLO-Y4 cells after inducing an immediate spike, and it had no observable effects on MC3T3-E1 cells.[60]
It is reported that influx calcium irons resulted in physiology change of osteoblasts. In details, increased intracellular calcium leads to activation of constitutively expressed NO synthases, and increased NO production in osteoblasts.[61,62] NO further activates PKG II, which phosphorylates substrates make the MEK/Erk pathway activated.[63,64,65] Furthermore, the MEK/Erk pathway increases transcription of c-fos, fra-1, fra-2, and fosB,[66,67] which is via the following activation of cAMP-response element binding protein (CREB), direct phosphorylation of Elk and recruitment of AP-1 complexes.[68,69,70] These fos family genes encode members of the AP-1 transcription factor family that regulate osteoblast proliferation and differentiation.[71]
For another, elevated intracellular calcium has been reported to facilitate COX2 induced production of PGE2[72] and calcium promotes COX transcription via activating of CREB.[73] PGE2 derived from COX2 enhances the expression of ALP, an indicator of bone formation, and could be impeded by RU486, an inhibitor of COX2.[74] Therefore, the absence of COX2 would be expected to decrease both bone formation and bone resorption.[75] Moreover, COX2 may form an important regulatory loop with BMP/Smad family in BMSCs and osteoblasts and further have an influence on RUNX2, a key transcriptional modulator in bone formation.[11,76] Signal transduction that ENaCs involves in[77,78] is presented in Figure 3.
Figure 3.
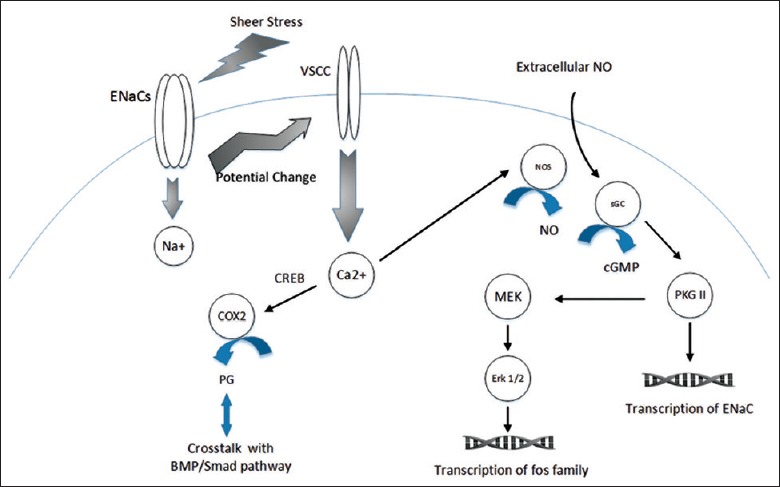
Signal transduction that epithelium sodium channels involved in. Osteoblast exposure to fluid shear stress leads to influx of sodium. Transmembrane potential is decreased leading to stimulation of voltage-sensitive calcium channel accompanying with calcium influx. An initiation of nitride oxide synthesis may require calcium activation of nitride oxide synthases. For one thing, nitride oxide activates soluble guanylate cyclase and the resulting cyclic guanosine monophosphate activates cyclic guanosine monophosphate-dependent protein kinase II, which phosphorylates substrates leading to activation of the MEK/Erk pathway.[77,78] Extracellular nitride oxide from endothelium e.g., may also stimulate MEK/Erk pathway. For another, activated cyclic guanosine monophosphate-dependent protein kinase II may also enhance expression of epithelium sodium channels. PGE2 derived from cyclooxygenase-2 forms a regulation loop with bone morphogenetic protein/Smad family.
ESTROGEN REGULATING BONE FORMATION PARTLY VIA EPITHELIUM SODIUM CHANNELS
The regulation of ENaCs is still not fully illustrated now. Although ENaCs are regulated by angiotensin, vasopressin, and aldosterone in various organs, it is still unclear whether ENaCs expression in osteoblasts is related to these hormone.[79,80,81,82,83,84] Interestingly, estrogen regulates BMSCs, osteoblasts and osteocytes via several different signal pathways, mainly by Wnt and RANKL signals.[85,86,87,88] These may probably answer the question why estrogen protects premenopausal women. However, it has been investigated that estrogen has maintained bone density via ENaCs[89] because estrogen regulates the expression of ENaC α and γ subunits in osteoblasts. Patch clamps result shows that ENaC activity changes and increases influx current of whole cells as estrogen is added to the medium. Therefore, both results suggest that estrogen not only improves Po of ENaC but also increases NO of ENaCs in an individual cell. In addition, research of Tang et al. presents a consistent result that raloxifene, the selective estrogen receptor modulator, collaborates with the effect of shear stress on ENaCs, enhancing proliferation of osteoblasts.[47]
Further studies focusing on the mechanism of estrogen on ENaCs show that NO may act as a mediator of estrogen role in bone formation.[86,90] For example, mice with NOS deficiency lost bone normally following ovariectomy but exhibited a significantly blunted anabolic response to high-dose exogenous estrogen. However, osteoblasts with NOS knocked-out had reduced rates of growth.[54] As discussed above, NO derived up-regulates the expression of ENaC via the cGMP-PKG pathway, the number of ENaCs on an individual osteoblast is increased by estrogen partly via NO synthesis.
CHALLENGES
Although we partly illustrate the role of ENaCs on bone formation, the effect and regulations mechanism of ENaCs are still not quite clear. The main doubt is whether ENaCs, which are regulated via cGMP/PKG II pathway, can stimulate the synthesis of cGMP via NOS stimulation. As a fact, the regulation of ENaC is not out of control; thus, more mechanisms to be unveiled may participate in the regulation of ENaCs. Moreover, estrogen regulates bone formation via various pathways, including ENaCs stimulation. Furthermore, it is still not well known about the pathways that estrogen participates in. Last but not the least, high-salt dietary may cause osteoporosis and the hypothesis raised is that the calciuria is partly due to salt-induced volume expansion which causes excretion of parathyroid hormone leading to bone remodeling.[91] In fact, the adult human body contains 90–130 g sodium, roughly half of that stored in bone. As fluctuate of plasma and intracellular sodium may affect the function (Po) of ENaCs, a bold suspicion is whether high-salt dietary affects bone remodeling via ENaCs expressed on the surface of osteoblasts and osteocytes.
Financial support and sponsorship
This work was supported by a grant of the National Natural Science Foundation of China (No. 81271969).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank J Biol Chem for the permission of using their published figures in the present paper.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Caetano-Lopes J, Canhão H, Fonseca JE. Osteoblasts and bone formation. Acta Reumatol Port. 2007;32:103–10. doi: 10.1016/S1569-2590(08)60130-5. [PubMed] [Google Scholar]
- 2.Seeman E, Delmas PD. Bone quality –The material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–61. doi: 10.1056/NEJMra053077. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 3.Ducy P, Schinke T, Karsenty G. The osteoblast: A sophisticated fibroblast under central surveillance. Science. 2000;289:1501–4. doi: 10.1126/science.289.5484.1501. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 4.Lo YC, Chang YH, Wei BL, Huang YL, Chiou WF. Betulinic acid stimulates the differentiation and mineralization of osteoblastic MC3T3-E1 cells: Involvement of BMP/Runx2 and beta-catenin signals. J Agric Food Chem. 2010;58:6643–9. doi: 10.1021/jf904158k. doi: 10.1021/jf904158k. [DOI] [PubMed] [Google Scholar]
- 5.Carreira AC, Alves GG, Zambuzzi WF, Sogayar MC, Granjeiro JM. Bone morphogenetic proteins: Structure, biological function and therapeutic applications. Arch Biochem Biophys. 2014;561:64–73. doi: 10.1016/j.abb.2014.07.011. doi: 10.1016/j.abb.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Ogura N, Aonuma H, Ito K, Ishigami D, Kamino Y, et al. Bone morphogenetic protein 6 stimulates mineralization in human dental follicle cells without dexamethasone. Arch Oral Biol. 2013;58:690–8. doi: 10.1016/j.archoralbio.2012.10.018. doi: 10.1021/jf904158k. [DOI] [PubMed] [Google Scholar]
- 7.Lysdahl H, Baatrup A, Foldager CB, Bünger C. Preconditioning human mesenchymal stem cells with a low concentration of BMP2 stimulates proliferation and osteogenic differentiation in vitro. Biores Open Access. 2014;3:278–85. doi: 10.1089/biores.2014.0044. doi: 10.1089/biores.2014.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9. doi: 10.1172/JCI28551. doi: 10.1172/jci28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laudes M. Role of Wnt signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol. 2011;46:R65–72. doi: 10.1530/JME-10-0169. doi: 10.1530/jme-10-0169. [DOI] [PubMed] [Google Scholar]
- 10.Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O’Rahilly S, et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119(Pt 12):2613–20. doi: 10.1242/jcs.02975. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JH, Liu YZ, Yin LJ, Chen L, Huang J, Liu Y, et al. BMP9 and COX-2 form an important regulatory loop in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Bone. 2013;57:311–21. doi: 10.1016/j.bone.2013.08.015. doi: 10.1016/j.bone.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Welting TJ, Caron MM, Emans PJ, Janssen MP, Sanen K, Coolsen MM, et al. Inhibition of cyclooxygenase-2 impacts chondrocyte hypertrophic differentiation during endochondral ossification. Eur Cell Mater. 2011;22:420–36. doi: 10.22203/ecm.v022a31. [DOI] [PubMed] [Google Scholar]
- 13.Haversath M, Catelas I, Li X, Tassemeier T, Jäger M. PGE2 and BMP-2 in bone and cartilage metabolism:2 intertwining pathways. Can J Physiol Pharmacol. 2012;90:1434–45. doi: 10.1139/y2012-123. doi: 10.1139/y2012-123. [DOI] [PubMed] [Google Scholar]
- 14.Lane NE, Yao W. Glucocorticoid-induced bone fragility. Ann N Y Acad Sci. 2010;1192:81–3. doi: 10.1111/j.1749-6632.2009.05228.x. doi: 10.1111/j.1749-6632.2009.05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalak R, Zhou H, Street J, Day RE, Modzelewski JR, Spies CM, et al. Endogenous glucocorticoid signalling in osteoblasts is necessary to maintain normal bone structure in mice. Bone. 2009;45:61–7. doi: 10.1016/j.bone.2009.03.673. doi: 10.1016/j.bone.2009.03.673. [DOI] [PubMed] [Google Scholar]
- 16.Azuma K, Urano T, Ouchi Y, Inoue S. Glucocorticoid-induced gene tripartite motif-containing 63 (TRIM63) promotes differentiation of osteoblastic cells. Endocr J. 2010;57:455–62. doi: 10.1507/endocrj.k09e-290. doi: 10.1507/endocrj.K09E-290. [DOI] [PubMed] [Google Scholar]
- 17.Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+channel. J Biol Chem. 1995;270:27411–4. doi: 10.1074/jbc.270.46.27411. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- 18.Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A. Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochem Cell Biol. 2012;137:339–53. doi: 10.1007/s00418-011-0904-1. doi: 10.1007/s00418-011-0904-1. [DOI] [PubMed] [Google Scholar]
- 19.Garty H. Molecular properties of epithelial, amiloride-blockable Na+channels. FASEB J. 1994;8:522–8. doi: 10.1096/fasebj.8.8.8181670. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Wu L, Jia H, Li Y, Chen J, Xu D, et al. The epithelial sodium channel is involved in dexamethasone-induced osteoblast differentiation and mineralization. Cell Biol Toxicol. 2012;28:279–89. doi: 10.1007/s10565-012-9222-1. doi: 10.1007/s10565-012-9222-1. [DOI] [PubMed] [Google Scholar]
- 21.Chan LN, Tsang LL, Rowlands DK, Rochelle LG, Boucher RC, Liu CQ, et al. Distribution and regulation of ENaC subunit and CFTR mRNA expression in murine female reproductive tract. J Membr Biol. 2002;185:165–76. doi: 10.1007/s00232-001-0117-y. doi: 10.1007/s00232-001-0117-y. [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Wu L, Chen J, Lin XH, Wan C, Li QN. Effects of sodium on rat osteoblast and the role of epithelial sodium channel (in Chinese) J Southern Med Univ. 2011;31:1871–4. [PubMed] [Google Scholar]
- 23.Loffing J, Schild L. Functional domains of the epithelial sodium channel. J Am Soc Nephrol. 2005;16:3175–81. doi: 10.1681/ASN.2005050456. doi: 10.1681/asn.2005050456. [DOI] [PubMed] [Google Scholar]
- 24.Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member. Am J Physiol Renal Physiol. 2011;301:F684–96. doi: 10.1152/ajprenal.00259.2011. doi: 10.1152/ajprenal.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008;19:1845–54. doi: 10.1681/ASN.2008020225. doi: 10.1681/asn.2008020225. [DOI] [PubMed] [Google Scholar]
- 26.Edelheit O, Hanukoglu I, Dascal N, Hanukoglu A. Identification of the roles of conserved charged residues in the extracellular domain of an epithelial sodium channel (ENaC) subunit by alanine mutagenesis. Am J Physiol Renal Physiol. 2011;300:F887–97. doi: 10.1152/ajprenal.00648.2010. doi: 10.1152/ajprenal.00648.2010. [DOI] [PubMed] [Google Scholar]
- 27.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–23. doi: 10.1038/nature06163. doi: 10.1038/naturne06163. [DOI] [PubMed] [Google Scholar]
- 28.Edelheit O, Ben-Shahar R, Dascal N, Hanukoglu A, Hanukoglu I. Conserved charged residues at the surface and interface of epithelial sodium channel subunits –Roles in cell surface expression and the sodium self-inhibition response. FEBS J. 2014;281:2097–111. doi: 10.1111/febs.12765. doi: 10.1111/febs.12765. [DOI] [PubMed] [Google Scholar]
- 29.Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem. 1994;269:24379–83. [PubMed] [Google Scholar]
- 30.Shi S, Carattino MD, Hughey RP, Kleyman TR. ENaC regulation by proteases and shear stress. Curr Mol Pharmacol. 2013;6:28–34. doi: 10.2174/18744672112059990027. doi: 10.2174/18744672112059990027#sthash.PlYDFg9l.dpuf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashlan OB, Boyd CR, Argyropoulos C, Okumura S, Hughey RP, Grabe M, et al. Allosteric inhibition of the epithelial Na+channel through peptide binding at peripheral finger and thumb domains. J Biol Chem. 2010;285:35216–23. doi: 10.1074/jbc.M110.167064. doi: 10.1074/jbc.M110.167064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan YC, Guo JH, Liu X, Zhang R, Tsang LL, Dong JD, et al. Activation of the epithelial Na+channel triggers prostaglandin E2 release and production required for embryo implantation. Nat Med. 2012;18:1112–7. doi: 10.1038/nm.2771. doi: 10.1038/nm.2771. [DOI] [PubMed] [Google Scholar]
- 33.Chraïbi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111:127–38. doi: 10.1085/jgp.111.1.127. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+channels. Am J Physiol Cell Physiol. 2004;286:C190–4. doi: 10.1152/ajpcell.00342.2003. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 35.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, et al. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–4. doi: 10.1074/jbc.C400080200. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 36.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem. 2006;281:18901–7. doi: 10.1074/jbc.M604109200. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- 37.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: Regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–51. doi: 10.1074/jbc.R800083200. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nesterov V, Dahlmann A, Bertog M, Korbmacher C. Trypsin can activate the epithelial sodium channel (ENaC) in microdissected mouse distal nephron. Am J Physiol Renal Physiol. 2008;295:F1052–62. doi: 10.1152/ajprenal.00031.2008. doi: 10.1152/ajprenal.00031.2008. [DOI] [PubMed] [Google Scholar]
- 39.Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, et al. Regulation of prostasin by aldosterone in the kidney. J Clin Invest. 2002;109:401–8. doi: 10.1172/JCI13229. doi: 10.1172/jci13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nesterov V, Dahlmann A, Krueger B, Bertog M, Loffing J, Korbmacher C. Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol. 2012;303:F1289–99. doi: 10.1152/ajprenal.00247.2012. doi: 10.1152/ajprenal.00247.2012. [DOI] [PubMed] [Google Scholar]
- 41.Uchimura K, Kakizoe Y, Onoue T, Hayata M, Morinaga J, Yamazoe R, et al. In vivo contribution of serine proteases to the proteolytic activation of gENaC in aldosterone-infused rats. Am J Physiol Renal Physiol. 2012;303:F939–43. doi: 10.1152/ajprenal.00705.2011. doi: 10.1152/ajprenal.00705.2011. [DOI] [PubMed] [Google Scholar]
- 42.Mano I, Driscoll M. DEG/ENaC channels: A touchy superfamily that watches its salt. Bioessays. 1999;21:568–78. doi: 10.1002/(SICI)1521-1878(199907)21:7<568::AID-BIES5>3.0.CO;2-L. doi: 10.1002/(sici).1521-1878(199907)21:7<568:aid-bies5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: Effects of voltage and membrane stretch. J Gen Physiol. 1996;107:35–45. doi: 10.1085/jgp.107.1.35. doi: 10.1085/jgp.107.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang EC, Lee JM, Johnson JP, Kleyman TR, Bridges R, Apodaca G. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol. 2003;285:F651–63. doi: 10.1152/ajprenal.00403.2002. doi: 10.1152/ajprenal.00403.2002. [DOI] [PubMed] [Google Scholar]
- 45.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol. 2006;291:F923–31. doi: 10.1152/ajprenal.00192.2006. doi: 10.1152/ajprenal.00192.2006. [DOI] [PubMed] [Google Scholar]
- 46.Carattino MD, Sheng S, Kleyman TR. Epithelial Na+channels are activated by laminar shear stress. J Biol Chem. 2004;279:4120–6. doi: 10.1074/jbc.M311783200. doi: 10.1074/jbc.M311783200. [DOI] [PubMed] [Google Scholar]
- 47.Tang WR, Liu Y, Li LH, Wu ZB, He Y. Fluid shear stress and raloxifene stimulates the proliferation of osteoblast through regulating the expresstion of β-catenin and estrogen receptor α(in Chinese) J Sichuan Univ (Med Sci Ed) 2014;45:913–8. doi: 10.13464/j.scuxbyxb.2014.06.007. [PubMed] [Google Scholar]
- 48.Shivaram GM, Kim CH, Batra NN, Yang W, Harris SE, Jacobs CR. Novel early response genes in osteoblasts exposed to dynamic fluid flow. Philos Trans A Math Phys Eng Sci. 2010;368:605–16. doi: 10.1098/rsta.2009.0231. doi: 10.1098/rsta.2009.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of β-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2:Implications for the study of mechanosensation in bone. Bone. 2010;47:872–81. doi: 10.1016/j.bone.2010.08.007. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vasquez MM, Mustafa SB, Choudary A, Seidner SR, Castro R. Regulation of epithelial Na+channel (ENaC) in the salivary cell line SMG-C6. Exp Biol Med (Maywood) 2009;234:522–31. doi: 10.3181/0806-RM-209. doi: 10.3181/0806-rm-209. [DOI] [PubMed] [Google Scholar]
- 51.Cheng G, Zhai Y, Chen K, Zhou J, Han G, Zhu R, et al. Sinusoidal electromagnetic field stimulates rat osteoblast differentiation and maturation via activation of NO-cGMP-PKG pathway. Nitric Oxide. 2011;25:316–25. doi: 10.1016/j.niox.2011.05.009. doi: 10.1016/j.niox.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Hagiwara H, Inoue A, Yamaguchi A, Yokose S, Furuya M, Tanaka S, et al. cGMP produced in response to ANP and CNP regulates proliferation and differentiation of osteoblastic cells. Am J Physiol. 1996;270(5 Pt 1):C1311–8. doi: 10.1152/ajpcell.1996.270.5.C1311. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Zhang H, Zhang X, Yang G, Lu L, Lu X, et al. Epithelial sodium channel enhanced osteogenesis via cGMP/PKGII/ENaC signaling in rat osteoblast. Mol Biol Rep. 2014;41:2161–9. doi: 10.1007/s11033-014-3065-1. doi: 10.1007/s11033-014-3065-1. [DOI] [PubMed] [Google Scholar]
- 54.Armour KE, Armour KJ, Gallagher ME, Gödecke A, Helfrich MH, Reid DM, et al. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142:760–6. doi: 10.1210/endo.142.2.7977. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- 55.van’t Hof RJ, Armour KJ, Smith LM, Armour KE, Wei XQ, Liew FY, et al. Requirement of the inducible nitric oxide synthase pathway for IL-1-induced osteoclastic bone resorption. Proc Natl Acad Sci U S A. 2000;97:7993–8. doi: 10.1073/pnas.130511497. doi: 10.1073/pnas.130511497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aguirre J, Buttery L, O’Shaughnessy M, Afzal F, Fernandez de Marticorena I, Hukkanen M, et al. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol. 2001;158:247–57. doi: 10.1016/S0002-9440(10)63963-6. doi: 10.1016/s0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van’t Hof RJ, Macphee J, Libouban H, Helfrich MH, Ralston SH. Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology. 2004;145:5068–74. doi: 10.1210/en.2004-0205. doi: 10.1210/en.2004-0205. [DOI] [PubMed] [Google Scholar]
- 58.Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Frangos JA, et al. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem. 2009;284:14796–808. doi: 10.1074/jbc.M806486200. doi: 10.1074/jbc.M806486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marunaka Y. Characteristics and pharmacological regulation of epithelial Na+channel (ENaC) and epithelial Na+transport. J Pharmacol Sci. 2014;126:21–36. doi: 10.1254/jphs.14R01SR. [PubMed] [Google Scholar]
- 60.Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res. 2012;27:563–74. doi: 10.1002/jbmr.1474. doi: 10.1002/jbmr.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res. 1999;14:930–6. doi: 10.1359/jbmr.1999.14.6.930. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- 62.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 63.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: Role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–508. doi: 10.1152/ajpcell.00122.2003. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, Zhuang S, Cassenaer S, Casteel DE, Gudi T, Boss GR, et al. Synergism between calcium and cyclic GMP in cyclic AMP response element-dependent transcriptional regulation requires cooperation between CREB and C/EBP-beta. Mol Cell Biol. 2003;23:4066–82. doi: 10.1128/MCB.23.12.4066-4082.2003. doi: 10.1128/MCB.23.12.4066-4082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao X, Zhuang S, Chen Y, Boss GR, Pilz RB. Cyclic GMP-dependent protein kinase regulates CCAAT enhancer-binding protein beta functions through inhibition of glycogen synthase kinase-3. J Biol Chem. 2005;280:32683–92. doi: 10.1074/jbc.M505486200. doi: 10.1074/jbc.M505486200. [DOI] [PubMed] [Google Scholar]
- 66.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–40. doi: 10.1111/j.0105-2896.2005.00332.x. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 67.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–90. doi: 10.1038/79683. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 68.Adiseshaiah P, Li J, Vaz M, Kalvakolanu DV, Reddy SP. ERK signaling regulates tumor promoter induced c-Jun recruitment at the Fra-1 promoter. Biochem Biophys Res Commun. 2008;371:304–8. doi: 10.1016/j.bbrc.2008.04.063. doi: 10.1016/j.bbrc.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adiseshaiah P, Papaiahgari SR, Vuong H, Kalvakolanu DV, Reddy SP. Multiple cis-elements mediate the transcriptional activation of human fra-1 by 12-O-tetradecanoylphorbol-13-acetate in bronchial epithelial cells. J Biol Chem. 2003;278:47423–33. doi: 10.1074/jbc.M303505200. doi: 10.1074/jbc.M303505200. [DOI] [PubMed] [Google Scholar]
- 70.Adiseshaiah P, Peddakama S, Zhang Q, Kalvakolanu DV, Reddy SP. Mitogen regulated induction of FRA-1 proto-oncogene is controlled by the transcription factors binding to both serum and TPA response elements. Oncogene. 2005;24:4193–205. doi: 10.1038/sj.onc.1208583. doi: 10.1038/sj.onc.1208583. [DOI] [PubMed] [Google Scholar]
- 71.Eferl R, Hoebertz A, Schilling AF, Rath M, Karreth F, Kenner L, et al. The fos-related antigen Fra-1 is an activator of bone matrix formation. EMBO J. 2004;23:2789–99. doi: 10.1038/sj.emboj.7600282. doi: 10.1038/sj.emboj.7600282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruan YC, Wang Z, Du JY, Zuo WL, Guo JH, Zhang J, et al. Regulation of smooth muscle contractility by the epithelium in rat vas deferens: Role of ATP-induced release of PGE2. J Physiol. 2008;586(Pt 20):4843–57. doi: 10.1113/jphysiol.2008.154096. doi: 10.1113/jphysiol.2008.154096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun X, Ruan YC, Guo J, Chen H, Tsang LL, Zhang X, et al. Regulation of miR-101/miR-199a-3p by the epithelial sodium channel during embryo implantation: Involvement of CREB phosphorylation. Reproduction. 2014;148:559–68. doi: 10.1530/REP-14-0386. doi: 10.1530/rep-14-0386. [DOI] [PubMed] [Google Scholar]
- 74.Xu Z, Choudhary S, Okada Y, Voznesensky O, Alander C, Raisz L, et al. Cyclooxygenase-2 gene disruption promotes proliferation of murine calvarial osteoblasts in vitro. Bone. 2007;41:68–76. doi: 10.1016/j.bone.2007.03.009. doi: 10.1016/j.bone.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okada Y, Lorenzo JA, Freeman AM, Tomita M, Morham SG, Raisz LG, et al. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J Clin Invest. 2000;105:823–32. doi: 10.1172/JCI8195. doi: 10.1172/jci8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Susperregui AR, Gamell C, Rodríguez-Carballo E, Ortuño MJ, Bartrons R, Rosa JL, et al. Noncanonical BMP signaling regulates cyclooxygenase-2 transcription. Mol Endocrinol. 2011;25:1006–17. doi: 10.1210/me.2010-0515. doi: 10.1210/me.2010-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: A continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Prywes R. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: The c-fos AP-1 and p62TCF sites. Oncogene. 2000;19:1379–85. doi: 10.1038/sj.onc.1203443. doi: 10.1038/sj.onc.1203443. [DOI] [PubMed] [Google Scholar]
- 79.Tazaki M, Endoh T, Kobayashi H, Ohkubo M, Sueishi K. Angiotensin II induces modulation of calcium channel currents in osteoblasts. Bull Tokyo Dent Coll. 2013;54:275–8. doi: 10.2209/tdcpublication.54.275. doi: http://doi.org/10.2209/tdcpublication.54.275. [DOI] [PubMed] [Google Scholar]
- 80.Lienhard D, Lauterburg M, Escher G, Frey FJ, Frey BM. High salt intake down-regulates colonic mineralocorticoid receptors, epithelial sodium channels and 11α-hydroxysteroid dehydrogenase type 2. PLoS One. 2012;7:e37898. doi: 10.1371/journal.pone.0037898. doi: 10.1371/journal.pone.0037898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hernández-Díaz I, Giraldez T, Morales S, Hernandez G, Salido E, Canessa CM, et al. Heterogeneous nuclear ribonucleoprotein A2/B1 is a tissue-specific aldosterone target gene with prominent induction in the rat distal colon. Am J Physiol Gastrointest Liver Physiol. 2013;304:G122–31. doi: 10.1152/ajpgi.00130.2012. doi: 10.1152/ajpgi.00130.2012. [DOI] [PubMed] [Google Scholar]
- 82.Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: Differential regulation of ion transport in distal nephron. Physiology (Bethesda) 2011;26:115–23. doi: 10.1152/physiol.00049.2010. doi: 10.1152/physiol.00049.2010. [DOI] [PubMed] [Google Scholar]
- 83.Gaeggeler HP, Guillod Y, Loffing-Cueni D, Loffing J, Rossier BC. Vasopressin-dependent coupling between sodium transport and water flow in a mouse cortical collecting duct cell line. Kidney Int. 2011;79:843–52. doi: 10.1038/ki.2010.486. doi: 10.1038/ki.2010.486. [DOI] [PubMed] [Google Scholar]
- 84.Bankir L, Bichet DG, Bouby N. Vasopressin V2 receptors, ENaC, and sodium reabsorption: A risk factor for hypertension? Am J Physiol Renal Physiol. 2010;299:F917–28. doi: 10.1152/ajprenal.00413.2010. doi: 10.1152/ajprenal.00413.2010. [DOI] [PubMed] [Google Scholar]
- 85.Kim RY, Yang HJ, Song YM, Kim IS, Hwang SJ. Estrogen modulates bone morphogenetic protein-induced sclerostin expression through the Wnt signaling pathway. Tissue Eng Part A. 2015;21:2076–88. doi: 10.1089/ten.TEA.2014.0585. doi: 10.1089/ten.TEA.2014.0585. [DOI] [PubMed] [Google Scholar]
- 86.Joshua J, Kalyanaraman H, Marathe N, Pilz RB. Nitric oxide as a mediator of estrogen effects in osteocytes. Vitam Horm. 2014;96:247–63. doi: 10.1016/B978-0-12-800254-4.00010-6. doi: 10.1016/b978-0-12-800254-4.00010-6. [DOI] [PubMed] [Google Scholar]
- 87.Martin A, Xiong J, Koromila T, Ji JS, Chang S, Song YS, et al. Estrogens antagonize RUNX2-mediated osteoblast-driven osteoclastogenesis through regulating RANKL membrane association. Bone. 2015;75:96–104. doi: 10.1016/j.bone.2015.02.007. doi: 10.1016/j.bone.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu SM, Shih LH, Lee JY, Shen YJ, Lee HH. Estrogen enhances activity of Wnt signaling during osteogenesis by inducing Fhl1 expression. J Cell Biochem. 2015;116:1419–30. doi: 10.1002/jcb.25102. doi: 10.1002/jcb.25102. [DOI] [PubMed] [Google Scholar]
- 89.Yang GZ, Nie HG, Lu L, Chen J, Lu XY, Ji HL, et al. Estrogen regulates the expression and activity of epithelial sodium channel in mouse osteoblasts. Cell Mol Biol (Noisy-le-grand) 2011;57(Suppl):OL1480–6. doi: 10.1170/170. [PMC free article] [PubMed] [Google Scholar]
- 90.Joshua J, Kalyanaraman H, Marathe N, Pilz RB. Nitric oxide as a mediator of estrogen effects in osteocytes. In: Gerald L, editor. Vitamins and Hormones. Ch.10. Vol. 96. San Diego: Academic Press; 2014. pp. 247–63. doi: http://dx.doi.org/10.1016/B978-0-12-800254-4.00010-6. [DOI] [PubMed] [Google Scholar]
- 91.Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr. 2006;25(3 Suppl):271S–6S. doi: 10.1080/07315724.2006.10719577. doi: 10.1080/07315724.2006.10719577. [DOI] [PubMed] [Google Scholar]


