Abstract
Liver transplantation (LT) is the treatment of choice for patients with cirrhosis and hepatocellular carcinoma (HCC) not amenable to resection. Locoregional therapies for HCC are often used to reduce tumor burden, bridge patients to LT, and down-stage HCC so that patients are eligible for LT. We hypothesized that prior endovascular antitumor therapy may increase the risk of hepatic artery (HA) and biliary complications after LT. The aim of this study was to compare HA and biliary complications in LT recipients with HCC who received transarterial chemoembolization (TACE) before LT with complications in LT recipients with HCC who did not receive TACE before LT. This was a retrospective cohort study of HCC patients at two transplant centers. The prevalence of HA complications (HA thrombosis, stenosis, or pseudoaneurysm) and biliary complications (nonanastomotic stricture, bile leak, and diffuse injury) were compared between patients treated with or without TACE. There were 456 HCC patients with a median age of 61 years (77% were male, and 63% had hepatitis C virus), and 328 (72%) received TACE before LT. The overall prevalence of HA complications was 4.7% in the no-TACE group and 7.9% in the TACE group (P = 0.22). All HA stenosis complications (n = 14) occurred in the TACE group (P = 0.018 versus the no-TACE group). An older donor age and a lower albumin level significantly increased the odds of HA complications. There was a nonstatistically significant increased odds of HA complications in the TACE group versus the no-TACE group according to an adjusted analysis (odds ratio = 2.02, 95% confidence interval = 0.79-5.16, P = 0.14). The overall prevalence of biliary complications was 16.4% in the no-TACE group and 19.8% in the TACE group (P = 0.40). In conclusion, a lower pre-LT albumin level and an older donor age were significantly associated with higher odds of HA complications after LT. TACE was not associated with higher odds of overall HA complications but was associated with a higher prevalence of HA stenosis. Further studies are warranted to confirm the HA stenosis findings and elucidate the pathogenesis.
Hepatocellular carcinoma (HCC) results in approximately 250,000 to 1 million deaths globally per year and now ranks as the third leading cause of cancer-related deaths in the world.1 Unlike most solid cancers, the incidence of HCC in the United States is only expected to rise because of the increasing prevalence of advanced liver disease due to chronic hepatitis C virus infection and the emerging epidemic of fatty liver disease.2,3
In the United States, curative therapies for HCC include resection, which is limited to patients with relatively preserved liver function, and liver transplantation (LT), which is the treatment of choice for patients with more advanced cirrhosis.4-7 For patients with HCC who are listed for LT, locoregional therapies are frequently used as a bridge to LT,8 particularly in regions where wait times are prolonged. The United Network for Organ Sharing regions have variable dropout rates ranging from 5% to 20% due to progression of liver disease or tumor.9 In some studies, this dropout rate has been reduced with locoregional therapy to control tumor progression.10 Additionally, locoregional therapy is used to down-stage patients with initially more advanced HCC to allow them to qualify for LT under the Milan criteria.11 Treatment algorithms for patients undergoing these locoregional therapies vary from center to center, but transarterial chemoembolization (TACE) is a widely accepted modality of therapy before LT.12
There are several recognized complications of TACE, including postembolization syndrome, infection such as abscess and biloma, biliary stricture, hepatic failure, access site injury, hepatic artery (HA) injury, nontarget embolization, and pulmonary embolism.13,14 Major complications are reported for approximately 5% of patients with a quoted risk of death of 1%.15,16 Very limited data exist on the incidence of biliary and arterial complications after LT among patients subjected to transarterial therapies before LT.16 Moreover, most existing data on late postprocedural complications due to TACE were derived from patients who otherwise would not have been LT candidates.17 Because TACE use is frequent in the pre-LT setting and arterial and biliary complications after LT can be associated with significant morbidity, the association between pre-LT TACE and these post-LT complications is an important question to address. We hypothesized that the HA could be injured during the instrumentation and administration of tumor-ablative substances, thereby predisposing the patient to HA-related complications after LT. We performed a retrospective 2-center study to determine the prevalence of post-LT HA and biliary complications in patients with and without prior TACE therapy.
PATIENTS AND METHODS
This is a retrospective cohort study of all HCC patients listed and transplanted at 2 northern California LT centers: the University of California San Francisco (UCSF) between 2002 and 2008 and the California Pacific Medical Center between 2002 and 2010. All first-time LT recipients with HCC who were older than 18 years were included. The diagnosis of HCC was made with pre-LT radiographic imaging or biopsy. Patients with an incidental finding of HCC on explant were excluded. The following patients were also excluded: patients with conditions known to have a higher risk of thrombotic complications, including a pre-LT diagnosis of hypercoagulability (protein C/S deficiency or factor V Leiden) and Budd-Chiari syndrome; patients treated with sirolimus within the first 3 months after LT; and patients with a pre-LT diagnosis of primary sclerosing cholangitis.
All pretransplant and ablative therapy data were obtained from patient medical records. Donor information, including the donor age, sex, ABO match level, and donation after cardiac death status, was obtained from the United Network for Organ Sharing. Surgery-specific characteristics such as the warm ischemia time (WIT), cold ischemia time (CIT), types of biliary and arterial anastomoses, and estimated blood loss were extracted from operative reports. Institutional review board approval for the study was obtained from both participating study sites. The informed consent requirement was waived.
Study Definitions
HCC was diagnosed with pre-LT multiphase, contrast-enhanced computed tomography or magnetic resonance imaging in accordance with American Association for the Study of Liver Diseases guidelines18; all patients were listed for LT and had HCC exception points requested. Locoregional therapies included TACE, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and hepatic resection. The dates and the number of TACE treatments were collected.
The primary outcomes were HA and biliary complications after LT. HA complications included HA thrombosis, stenosis, and pseudoaneurysm identified on angiography; ultrasonography alone was not considered diagnostic. Biliary complications included nonanastomotic strictures or bile leaks seen on endoscopic retrograde cholangiopancreatography, magnetic resonance cholangiopancreatography, or other abdominal imaging and diffuse biliary injury seen on histology. Diagnoses were based on procedure reports and not central reviews of studies. Complications were included only if they were severe enough to require a surgical revision or intervention such as balloon dilation, stenting, or anticoagulation; antiplatelet therapy alone (ie, aspirin) was not included as an intervention. HA and biliary complications in the same patient were recorded as separate outcomes.
Chemoembolization Technique
The technique for performing TACE involved the insertion of a catheter into the common femoral artery and an angiographic survey of the celiac and superior mesenteric arteries. After selective catheterization of the proper HA, right HA, and/or left HA, digital subtraction angiography was performed. Vessels supplying the affected hepatic segments were accessed with a coaxially placed microcatheter. Chemoembolization was performed with a suspension of doxorubicin hydrochloride, mitomycin C, cisplatin, and ethiodized oil. This was followed by embolization with a slurry of a gelatin sponge until stasis was achieved.19
Statistical Approach
On the basis of an estimated prevalence of HA complications of 15% among TACE patients,16 a sample size of 159 study patients in each group was sufficient to detect a difference between treated and untreated groups of 10% or more with a power of 80% and a 2-sided α value of 0.05.
The prevalence of HA and biliary complications (separate and combined) in LT recipients with HCC was determined. The association between the receipt of TACE and HA and biliary complications after LT was evaluated with logistic regression. Variables with a P value > 0.10 in univariate analysis were examined in adjusted models. For biliary complications, a sensitivity analysis was performed with the exclusion of donation after cardiac death and living related LT patients because these patients are recognized to be at higher risk of biliary strictures.20 The Cochran-Armitage trend test was used to determine whether there was a significant trend between HA complications and TACE. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported. Results were considered statistically significant when the P value was <0.05. All data analyses were performed with Stata 12.
RESULTS
Patient Characteristics
Four hundred fifty-six LT patients with HCC met all the inclusion and exclusion criteria and formed the study cohort; 328 (72%) received at least 1 TACE treatment. The median follow-up after LT was 4.01 years [interquartile range (IQR) = 2.32-5.91 years]. The recipient and donor characteristics of the TACE and no-TACE groups are shown in Table 1. The majority of patients were male with hepatitis C virus or hepatitis B virus as the etiology of cirrhosis; the median age was 61 years. Pretransplant tumor characteristics at the time of the initial HCC diagnosis were available for 258 patients and revealed that 220 patients (85%) were within the Milan criteria, 77% had 1 nodule, the median size of the largest tumor at the time of diagnosis was 2.8 cm (IQR = 2.1-3.8 cm), and the median pre-LT alpha-fetoprotein level was 15 ng/mL (IQR = 5-81 ng/mL). The median size of the largest tumor was 2.5 cm in the no-TACE group and 2.9 cm in the TACE group (P = 0.003). Patients outside the Milan criteria were more likely to undergo TACE.
TABLE 1.
Demographic and Liver-Specific Characteristics of HCC Transplant Recipients
| Characteristic | No TACE (n = 128) | TACE (n= 328) |
|---|---|---|
| Recipient sex: male (%) | 73 | 79 |
| Recipient age (years)* | 60.8 (55.0-64.5) | 60.8 (55.7-66.6) |
| Study site (%) | ||
| 1 | 57 | 60 |
| 2 | 43 | 40 |
| Etiology of liver disease (%) | ||
| Hepatitis C | 63 | 63 |
| Hepatitis B | 20 | 24 |
| Alcohol | 20 | 14 |
| Autoimmune† | 1.5 | <1 |
| NAFLD | 3 | 2 |
| Other‡ | 5 | 3 |
| Laboratory values at transplant* | ||
| Creatinine (mg/dL) | 0.9 (0.7-1.4) | 0.9 (0.8-1.2) |
| Total bilirubin (mg/dL) | 1.9 (1-4.4) | 1.4 (0.9-2.4) |
| International normalized ratio | 1.4 (1.2-1.8) | 1.2 (1.1-1.5) |
| Albumin (g/dL) | 3.0 (2.6-3.8) | 3.3 (2.8-3.8) |
| MELD score | 13 (9-21) | 11 (8-16) |
| HCC at diagnosis (n = 258) | ||
| Size (cm)* | 2.5 (2.0-3.0) | 2.9 (2.2-4.1) |
| Number of nodules* | 1 (1-1) | 1 (1-1) |
| Alpha-fetoprotein at LT (ng/mL)* | 11 (5-59) | 17 (5-92) |
| Within Milan criteria (%) | 98 | 82 |
| Within UCSF criteria (%) | 98 | 94 |
| Outside UCSF criteria (%) | 2 | 6 |
| HCC treatment (%) | ||
| TACE | 0 | 100 |
| RFA | 41 | 33 |
| PEI | 2 | 4 |
| Resection | 6 | 4 |
| Surgery characteristics* | ||
| WIT (minutes) | 43 (37-50) | 45 (39-52) |
| CIT (hours) | 8.0 (6.2-9.7) | 8.1 (6.6-10.2) |
| Donor characteristics | ||
| Split transplant (%) | 0 | 3 |
| Living related (%) | 4 | 4 |
| Donor sex: male (%) | 66 | 57 |
| Donor age (years)* | 46.0 (27.0-54.5) | 42.0 (25.3-53.0) |
| Donation after cardiac death (%) | 3 | 3 |
| ABO mismatch (%) | 2 | 1 |
The data are presented as medians and IQRs.
Autoimmune include autoimmune hepatitis and primary biliary cirrhosis.
Other includes fulminant hepatic failure, cryptogenic cirrhosis, and other etiologies of liver disease not otherwise specified.
For the 328 patients who underwent TACE, the median treatment number was 1 (IQR = 1-2), with 104 (32%) receiving more than 1 treatment. Before 2006 at UCSF, patients with HCC commonly received prophylactic whole liver TACE on the day of LT to reduce the risk of posttransplant HCC recurrence21; 66 patients (20%) underwent TACE within 24 hours of surgery. The median number of days between TACE and LT for all patients was 70 days (IQR = 15-139 days), and it was 98.5 days (IQR = 50-154 days) when patients who underwent TACE within 24 hours of LT were excluded. Aspirin was used for post-LT prophylaxis in patients with a small-vessel-caliber, complex arterial anatomy or reconstruction.
Prevalence of HA and Biliary Complications
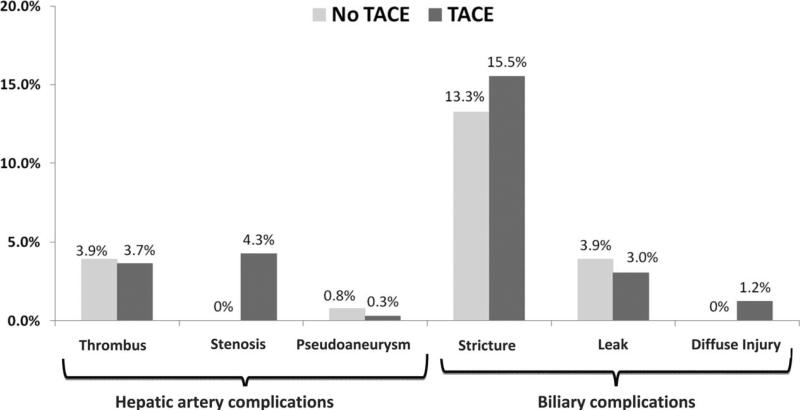
Overall, 105 patients (23%) had either a HA complication or a biliary complication: 32 (7%) had a HA complication, 86 (19%) had a biliary complication, and 13 (3%) had both. In 8 of the 13 patients with both complications (including 9 biliary strictures, 1 stricture with a bile leak, and 3 diffuse biliary injuries), the HA complication preceded the biliary complication. The overall combined prevalence of HA and biliary complications was 19.5% for the no-TACE group and 24% for the TACE group (P = 0.27). The HA complication rate was 4.7% in the no-TACE group and 7.9% in the TACE group (P = 0.22), and the biliary complication rate was 16.4% for the no-TACE group and 19.8% for the TACE group (P = 0.40). The prevalence and types of HA and biliary complications in the no-TACE and TACE groups are shown in Fig. 1. The type of biliary anastomosis (duct-to-duct anastomosis versus choledochojejunostomy) was not associated with either type of complication (P = 0.28 for biliary complications, P = 0.55 for HA complications), although data were available from only 1 site. No bile leak events occurred in living donor LT. All HA stenosis events occurred in patients who had undergone TACE (P = 0.018 versus the no-TACE group).
Figure 1.
Comparison of post-LT HA and biliary complications in HCC patients who underwent TACE before LT and HCC patients who did not.
HCC treatment–related factors in patients with HA and biliary complications are shown in Table 2. The timing of TACE with respect to LT (≤24 versus >24 hours), the number of TACE treatments (1 versus >1), and the use of other ablative modalities were not associated with either type of complication. When excluding patients treated with resection from the outcome analysis, the results were unchanged.
TABLE 2.
HCC Treatment Factors and Frequency of HA and Biliary Complications
| HA Complications |
Biliary Complications |
||||
|---|---|---|---|---|---|
| Treatment Characteristics | n | Yes | P Value | Yes | P Value |
| TACE (%) | 328 | 8 | 0.22 | 20 | 0.40 |
| No TACE (%) | 128 | 5 | 16 | ||
| Number of TACE treatments (%) | 0.23 | 0.31 | |||
| 1 | 224 | 7 | 18 | ||
| >1 | 104 | 11 | 23 | ||
| TACE timing (%) | 0.55 | 0.31 | |||
| ≤24 hours before LT | 66 | 6 | 15 | ||
| >24 hours before LT | 262 | 8 | 21 | ||
| Other HCC treatment (%) | |||||
| RFA (versus no RFA) | 162 | 6 | 0.36 | 18 | 0.70 |
| PEI (versus no PEI) | 16 | 14 | 0.38 | 6 | 0.19 |
| Resection (versus no resection) | 21 | 0 | 0.20 | 10 | 0.26 |
Factors Associated With HA Complications
In univariate analysis, TACE was associated with nonsignificantly increased odds of HA complications (OR = 1.75, 95% CI = 0.70-4.36, P = 0.23). After adjustments for pre-LT serum albumin level and older donor age, the odds of HA complications increased further to 2.02 (95% CI = 0.79-5.16), but was still not statistically significant (P = 0.14). A lower pre-LT serum albumin level (OR = 0.54, 95% CI = 0.33-0.90, P = 0.02) and an older donor age (OR = 1.02, 95% CI = 1.00-1.05, P = 0.03) remained significantly associated with the risk of HA complications. Only 1 of these 32 patients with HA complications received an infrarenal aortic jump graft.
The overall rate of HA stenosis was 3.1% (95% CI = 0.02-0.06), and all occurred in the TACE group. The odds of HA stenosis were increased in older recipients (OR = 1.06, 95% CI = 0.99-1.14, P = 0.09), those with female donors (OR = 2.63, 95% CI = 0.87-7.98, P = 0.09), and those with older donors (OR = 1.03, 95% CI = 0.99-1.07, P = 0.09), but these associations were not statistically significant. A multivariate analysis could not be performed because of the collinear relationship between HA stenosis and the receipt of TACE.
Factors Associated With Biliary Complications (Table 4)
TABLE 4.
Univariate Analyses of Factors Associated With Biliary Complications
| Univariate OR | 95% CI | P Value | |
|---|---|---|---|
| TACE | 1.26 | 0.73-2.16 | 0.40 |
| Recipient female | 1.12 | 0.65-1.93 | 0.69 |
| Recipient age (per year) | 1.02 | 0.99-1.05 | 0.18 |
| Center | 1.12 | 0.70-1.80 | 0.64 |
| Hepatitis C | 0.92 | 0.57-1.50 | 0.74 |
| MELD | 0.99 | 0.97-1.02 | 0.83 |
| Albumin (per 1 g/dL) | 0.99 | 0.71-1.40 | 0.93 |
| WIT (minutes)* | 1.00 | 0.99-1.01 | 0.56 |
| CIT (hours)* | 0.94 | 0.87-1.02 | 0.16 |
| Donor age | 1.01 | 0.99-1.02 | 0.17 |
| Donor female | 1.30 | 0.81-2.10 | 0.28 |
| DCD donor | 1.92 | 0.73-5.06 | 0.19 |
| >1 TACE | 1.34 | 0.76-2.36 | 0.31 |
| Early TACE | 0.66 | 0.32-1.38 | 0.27 |
| Other HCC treatment | 0.80 | 0.49-1.30 | 0.37 |
Bivariate logistic regression.
TACE was associated with numerically higher odds of biliary complications in univariate analysis (OR = 1.26, 95% CI = 0.73-2.16, P = 0.40), but this was not statistically significant. A sensitivity analysis after the exclusion of living donor LT and donation after cardiac death donors revealed similar odds of biliary complications in TACE patients (OR = 1.22, 95% CI = 0.70-2.14, P = 0.48).
DISCUSSION
LT is the treatment of choice for patients with small HCCs not amenable to resection.4 Locoregional therapies for HCC are often used to reduce tumor burden, bridge the period between listing and transplantation, and down-stage HCC so that patients can meet transplant listing criteria.5,8,9,11 Our results suggest that pretransplant TACE may be associated with a higher prevalence of HA complications (specifically HA stenosis). All HA stenosis events included in our study required treatment with stenting, balloon dilation, anticoagulation, or surgical revision. Vasculitis is a reported complication of chemoembolization, especially in patients subjected to repeated TACE sessions.22 Direct trauma to arterial vessels, local toxicity from chemotherapeutic agents, and a systemic immune response related to chemoembolization are potential factors underlying this risk for stenosis. Although our study does not confirm a specific pathophysiology, it does imply that stenosis is not confined to intrahepatic branches and that a risk of HA stenosis extends to the post-LT period. Previous studies evaluating posttransplant HA stenosis have identified surgical trauma to vessels, differences in vessel calibers, and excessive vessel length as risk factors for arterial stenosis.23 The use of infrarenal aortic jump grafts for arterial reconstruction during LT has also been associated with early HA thrombosis and poor graft survival.24 We acknowledge that the lack of information on surgery-specific factors such as vessel caliber and length are limiting, but because of the frequency of this finding (approximately 1 in every 20 TACE patients) and the frequency of TACE use in wait-listed patients with HCC, our finding warrants confirmation in a larger multicenter study.
The prevalence of HA complications in our cohort was numerically lower than the prevalence reported in the only other study evaluating complications after LT in TACE patients. Richard et al.16 reported a 13% HA complication rate after LT in their smaller single-center cohort of 47 patients who had undergone TACE. Notably, however, the lower bound of the 95% CI for their estimate (95% CI = 5.6%-25.6%) overlapp with that of our study. The inclusion of 2 centers in our study provides a more robust point estimate of complications and also allows for greater generalizability.
Since 2000, more centers have adopted TACE as a standard method of treating HCC.12 Perhaps with improved TACE and surgical techniques, the overall prevalence of HA complications after LT has decreased. Although these aspects may have improved, the use of high-risk donors has increased at high-volume centers in an effort to get more patients to LT, which may be a factor leading to the higher risk of hepatic and biliary complications.25 An older donor age, an important factor in the donor risk index, was associated with higher odds of HA complications in our study.26 A lower recipient albumin level at the time of transplantation was also associated with higher odds of HA complications, perhaps indicating that those with preserved liver function have a lower risk of these post-LT complications.27 It will be important to account for these factors in future studies of the effects of TACE on post-LT complications.
The prevalence of biliary complications in post-LT patients who have undergone TACE has not previously been reported. Biliary complications immediately after TACE include bile leaks, biliary strictures, and bile duct necrosis; the prevalence of these complications varies from 1% to 12% in published studies.14 Our study shows that TACE does not increase the odds of biliary complications. It is possible that the prompt recognition and intervention of HA complications prevent subsequent biliary complications.
There are several limitations to this study. First, because this is a retrospective cohort study, the causal relationship between TACE and HA and biliary complications cannot be confirmed. However, a randomized study of TACE and no-TACE patients would be challenging if not impossible to execute. Although we report a strong association between post-transplant HA stenosis and TACE, there may be other unmeasured variables contributing to the increased odds of this specific HA complication. Surgical techniques, including use of jump grafts to reconstruct the HA, aberrant arterial anatomy, and the types of biliary and arterial anastomoses may contribute to complications, and this information was not comprehensively captured in our study. Second, algorithms for the treatment of HCC vary by transplant center. Although the HCC treatment algorithms used by the 2 centers in this study are quite similar, other transplant programs may have different surgical or locoregional management algorithms. This could potentially diminish the generalizability of our results. Third, the overall frequency of HA complications was low; hence, the ability to detect small to modest differences between groups and to adjust for all potential confounders was limited. Lastly, the overall clinical morbidity and mortality due to these complications were not assessed in our study. This would be important to delineate in future studies, with the ultimate goal of identifying ways to reduce the incidence of these complications.
In summary, although it is known that TACE can cause HA injury and vasculitis, the downstream effects of such treatment (ie, post-LT risks) remain relatively unknown. Our results suggest that the risk of HA stenosis may be heightened in patients treated with TACE. Because of the importance of this complication to graft health and survival after transplantation, further studies are warranted to confirm our findings and to determine the pathogenetic underpinnings of this association.
TABLE 3.
Univariate and Adjusted Analyses of Factors Associated With HA Complications
| Univariate OR | 95% CI | P Value | Adjusted OR* | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| TACE | 1.75 | 0.70-4.36 | 0.23 | 2.02 | 0.79-5.16 | 0.14 |
| Recipient sex: female | 0.94 | 0.40-2.25 | 0.90 | — | — | — |
| Recipient age (per year) | 1.02 | 0.98-1.07 | 0.37 | — | — | — |
| Center | 1.14 | 0.55-2.35 | 0.72 | — | — | — |
| Hepatitis C | 0.73 | 0.35-1.52 | 0.40 | — | — | — |
| MELD score | 1.00 | 0.96-1.04 | 0.96 | — | — | — |
| Albumin (per 1 g/dL) | 0.54 | 0.33-0.90 | 0.02 | 0.53 | 0.31-0.89 | 0.02 |
| WIT (minutes) | 0.99 | 0.98-1.02 | 0.98 | — | — | — |
| CIT (hours) | 0.91 | 0.80-1.04 | 0.17 | — | — | — |
| Donor age (per year) | 1.02 | 1.00-1.05 | 0.03 | 1.02 | 1.00-1.05 | 0.04 |
| Donor sex: female | 1.37 | 0.66-2.85 | 0.40 | — | — | — |
| Donation after cardiac death donor | 0.92 | 0.20-4.15 | 0.91 | — | — | — |
| >1 TACE treatment | 1.65 | 0.73-3.72 | 0.23 | — | — | — |
| Early TACE | 0.70 | 0.23-2.09 | 0.52 | — | — | — |
| Other HCC treatment | 0.56 | 0.25-1.23 | 0.15 | — | — | — |
Trivariate logistic regression.
Abbreviations
- CI
confidence interval
- CIT
cold ischemia time
- HA
hepatic artery
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- PEI
percutaneous ethanol injection
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
- UCSF
University of California San Francisco
- WIT
warm ischemia time
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009. 27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 3.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, for Practice Guidelines Committee of the American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 6.Oldhafer KJ, Chavan A, Frühauf NR, Flemming P, Schlitt HJ, Kubicka S, et al. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: marked tumor necrosis, but no survival benefit? J Hepatol. 1998;29:953–959. doi: 10.1016/s0168-8278(98)80123-2. [DOI] [PubMed] [Google Scholar]
- 7.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–883. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 8.Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169–3177. doi: 10.1245/s10434-008-0071-3. [DOI] [PubMed] [Google Scholar]
- 9.Kadry Z, Schaefer EW, Uemura T, Shah AR, Schreibman I, Riley TR III. Impact of geographic disparity on liver allocation for hepatocellular cancer in the United States. J Hepatol. 2012;56:618–625. doi: 10.1016/j.jhep.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, et al. Chemoembolization followed by liver transplantation for hepato-cellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557–563. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 11.Yao FY, Kerlan RK, Jr, Hirose R, Davern TJ III, Bass NM, Feng S, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, for American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda N, Osuga K, Mikami K, Higashihara H, Onishi H, Nakaya Y, et al. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat Med. 2008;26:206–212. doi: 10.1007/s11604-007-0216-5. [DOI] [PubMed] [Google Scholar]
- 14.Sun Z, Li G, Ai X, Luo B, Wen Y, Zhao Z, et al. Hepatic and biliary damage after transarterial chemoembolization for malignant hepatic tumors: incidence, diagnosis, treatment, outcome and mechanism. Crit Rev Oncol Hematol. 2011;79:164–174. doi: 10.1016/j.critrevonc.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006;23:119–125. doi: 10.1055/s-2006-941442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richard HM III, Silberzweig JE, Mitty HA, Lou WY, Ahn J, Cooper JM. Hepatic arterial complications in liver transplant recipients treated with pretransplantation chemoembolization for hepatocellular carcinoma. Radiology. 2000;214:775–779. doi: 10.1148/radiology.214.3.r00mr31775. [DOI] [PubMed] [Google Scholar]
- 17.Prajapati HJ, Dhanasekaran R, El-Rayes BF, Kauh JS, Maithel SK, Chen Z, Kim HS. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24:307–315. doi: 10.1016/j.jvir.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Sherman M, Bruix J, Porayko M, Tran T, for AASLD Practice Guidelines Committee Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology. 2012;56:793–796. doi: 10.1002/hep.25869. [DOI] [PubMed] [Google Scholar]
- 19.Kwan SW, Fidelman N, Ma E, Kerlan RK, Jr, Yao FY. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18:727–736. doi: 10.1002/lt.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tashiro H, Itamoto T, Sasaki T, Ohdan H, Fudaba Y, Amano H, et al. Biliary complications after duct-to-duct biliary reconstruction in living-donor liver transplantation: causes and treatment. World J Surg. 2007;31:2222–2229. doi: 10.1007/s00268-007-9217-x. [DOI] [PubMed] [Google Scholar]
- 21.Stockland AH, Walser EM, Paz-Fumagalli R, McKinney JM, May GR. Preoperative chemoembolization in patients with hepatocellular carcinoma undergoing liver transplantation: influence of emergent versus elective procedures on patient survival and tumor recurrence rate. Cardiovasc Intervent Radiol. 2007;30:888–893. doi: 10.1007/s00270-007-9111-9. [DOI] [PubMed] [Google Scholar]
- 22.Bismuth H, Morino M, Sherlock D, Castaing D, Miglietta C, Cauquil P, Roche A. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163:387–394. doi: 10.1016/0002-9610(92)90039-t. [DOI] [PubMed] [Google Scholar]
- 23.Abad J, Hidalgo EG, Cantarero JM, Parga G, Fernandez R, Gomez M, et al. Hepatic artery anastomotic stenosis after transplantation: treatment with percutaneous transluminal angioplasty. Radiology. 1989;171:661–662. doi: 10.1148/radiology.171.3.2524086. [DOI] [PubMed] [Google Scholar]
- 24.Del Gaudio M, Grazi GL, Ercolani G, Ravaioli M, Varotti G, Cescon M, et al. Outcome of hepatic artery reconstruction in liver transplantation with an iliac arterial interposition graft. Clin Transplant. 2005;19:399–405. doi: 10.1111/j.1399-0012.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 25.Ozhathil DK, Li YF, Smith JK, Tseng JF, Saidi RF, Bozorgzadeh A, Shah SA. Impact of center volume on outcomes of increased-risk liver transplants. Liver Transpl. 2011;17:1191–1199. doi: 10.1002/lt.22343. [DOI] [PubMed] [Google Scholar]
- 26.Akkina SK, Asrani SK, Peng Y, Stock P, Kim WR, Israni AK. Development of organ-specific donor risk indices. Liver Transpl. 2012;18:395–404. doi: 10.1002/lt.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannou GN. Development and validation of a model predicting graft survival after liver transplantation. Liver Transpl. 2006;12:1594–1606. doi: 10.1002/lt.20764. [DOI] [PubMed] [Google Scholar]