Abstract
Biofilm formation is a complex process involving various signaling pathways and changes in gene expression. Many of the sensory mechanisms and regulatory cascades involved have been defined for biofilms formed by diverse organisms attached to solid surfaces. By comparison, our knowledge of the basic mechanisms underlying the formation of biofilms at air-liquid interfaces, i.e. pellicles, is much less complete. In particular, the roles of flagella have been studied in multiple solid-surface biofilm models, but remain largely undefined for pellicles. In this work, we characterize the contributions of flagellum-based motility, chemotaxis and oxygen sensing to pellicle formation in the Gram-positive Bacillus subtilis. We confirm that flagellum-based motility is involved in, but is not absolutely essential for, B. subtilis pellicle formation. Further, we show that flagellum-based motility, chemotaxis, and oxygen sensing are important for successful competition during B. subtilis pellicle formation. We report that flagellum-based motility similarly contributes to pellicle formation and fitness in competition assays in the Gram-negative Pseudomonas aeruginosa. Time-lapse imaging of static liquid cultures demonstrates that in both B. subtilis and P. aeruginosa, a turbulent flow forms in the tube and a zone of clearing appears below the air-liquid interface just before the formation of the pellicle, but only in strains that have flagella.
Keywords: Bacillus subtilis, Pseudomonas aeruginosa, biofilm, pellicle development, motility
Introduction
Research over the last several decades has shown that natural bacterial communities exist mainly as biofilms, cellular aggregates encased in self-produced matrices that often form on surfaces [1-3]. The term for a biofilm can depend on the type of interface at which it is found: colonies form on solid surfaces in air, submerged or flow cell biofilms form on solid surfaces in liquid, and pellicle biofilms form on liquid in air [4]. Pellicle formation has been described for various Gram-negative and Gram-positive bacteria [5-11] and proceeds through several stages. In early stages, a thin layer of cells appears at the air-liquid interface. This layer originates either from cells attached to the wall of the vessel that spread over the liquid or from cell clusters in the middle of the medium surface that spread outward [6, 12]. In later stages, three-dimensional structures can develop as the pellicle grows and thickens, resulting in the formation of wrinkles [4, 5, 13].
The exact mechanisms underpinning the initial stages of pellicle formation (i.e. how cells reach the interface) are not well-characterized. The production of an extracellular matrix is essential for pellicle formation in all species examined, while most cells of the biofilm are non-motile [6, 14]. Different types of motility, Brownian motion, or buoyancy could all influence whether or not cells are close to the air-liquid interface during the initiation stage [12]. As cells in a biofilm are sessile, biofilm formation and motility are considered opposing mechanisms [15]. This is supported by the fact that in many investigated organisms genes required for biofilm formation and flagellar motility are inversely regulated, often with c-di-GMP being involved [15-18]. However, many bacteria show defects in pellicle formation when genes involved in flagellum synthesis are mutated and in some species the ability to form a pellicle is completely abolished [7, 9, 12, 19, 20].
The air-liquid interface represents a favourable environment especially for aerobic microorganisms, since access to oxygen in high concentrations as well as nutrients from the medium are provided. The presence of oxygen as an electron acceptor in the atmospheric phase is important for pellicle formation in bacteria such as Escherichia coli and Shewanella oneidensis, which do not form pellicles under anaerobic conditions when alternative electron acceptors are provided in the medium [10, 19]. Further, the movement towards oxygen, i.e. positive aerotaxis, is proposed to control movement of the cells towards the air-liquid interface in S. oneidensis [7] and regulates biofilm formation on abiotic surfaces in Ralstonia solanacearum [21]. Oxygen has been shown to be a crucial factor for survival and growth of coexisting strains of Brevibacillus sp. and Pseudoxanthomonas sp. at the air-liquid interface [22]. In P. aeruginosa, decreased availability of oxygen in the atmosphere has been shown to be detrimental to pellicle integrity, and provision of an alternative electron acceptor in the medium promoted growth in the liquid phase below the pellicle [23].
Bacillus subtilis is a highly tractable Gram-positive model for biofilm formation. The regulation of B. subtilis pellicle formation and morphogenesis has been well characterized in undomesticated strains [5, 12, 16, 24-26], but the roles of flagellar motility and aerotaxis in this process have not been fully elucidated. Domesticated, laboratory strains of B. subtilis have mostly reduced biofilm forming ability [27]. Using the undomesticated strain ATCC 6051, Kobayashi showed that flagellum-deficient mutants exhibit a delay in pellicle formation [12]. However, this effect was not observed in the minimal medium MSgg, which is generally used to investigate both colony biofilms and pellicles of B. subtilis wild isolates. Based on these results it was concluded that flagella may rather play an indirect regulatory role in pellicle formation [12]. It has been hypothesized that B. subtilis cells migrate to the air-liquid interface due to aerotaxis [5] but this has not been demonstrated experimentally. Further, lack of flagellar motility did not alter colony biofilm spreading on agar medium as demonstrated by Seminara and colleagues [28]. B. subtilis can also colonize a semisolid surface via multicellular movements [29]. While hyperflagellated cells move in rafts [30], flagellum-less isolates of B. subtilis are able to slide in a surfactin dependent manner [31]. Notably, the flagellum serves as a transmitter of mechanical signals during surface attachment (i.e. initiation of biofilm formation) to activate gene expression in B. subtilis via the DegS-DegU two component system [32]. Throughout this paper, we will refer to individual cell based movement as motility.
Motility also plays an important role in Pseudomonas aeruginosa biofilms as the structures of flow cell biofilms are altered when motility and its switch-off during biofilm formation is not tightly controlled [9, 15, 33-35]. For example, evolved hyperswarmers are outcompeted by the less motile ancestral strain in surface attached biofilms indicating a trade-off between biofilm formation and flagellar motility [36]. In P. aeruginosa, flagella and pili contribute to specific types of motility (swimming/swarming and twitching, respectively) [37]. It was also investigated whether flagella and pili are involved in the formation of pellicles of P. aeruginosa PAO1. Yamamoto et al. observed that a flagellum-deficient mutant fails to produce a normally structured pellicle and pellicle formation is delayed. On the contrary, pili-deficient mutants as well as mutants deficient in both pili and flagella formed proper pellicles [9].
In this work, we characterized the roles of flagellum-dependent motility, chemotaxis and oxygen sensing in pellicle development of B. subtilis. We found that, although flagellar motility was not strictly required for pellicle formation, it conferred a competitive advantage over non-motile mutants in static competition assays. Mutants defective in flagellar motility, chemotaxis, or oxygen sensing were also at a disadvantage when competed against the parent strain under these conditions. P. aeruginosa PA14 mutants lacking flagella showed a partial defect in pellicle formation and a disadvantage in competition assays. We reasoned that flagella might facilitate localization near the surface and thereby contribute to increased turbidity near the air-liquid interface in static cultures. Time-lapse imaging revealed formation of an intriguing transparent zone near the air-liquid interface in wild-type B. subtilis and P. aeruginosa static cultures during the time preceding pellicle formation. Non-flagellated mutants showed more culture turbidity in this region.
Results
Flagellar motility is required for wild-type pellicle maturation dynamics in Bacillus subtilis
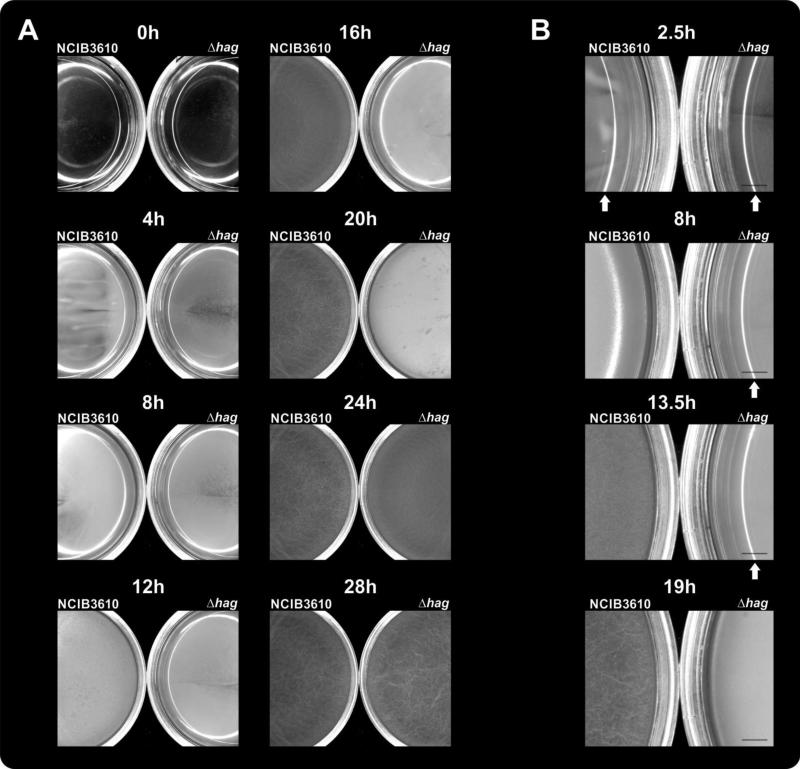
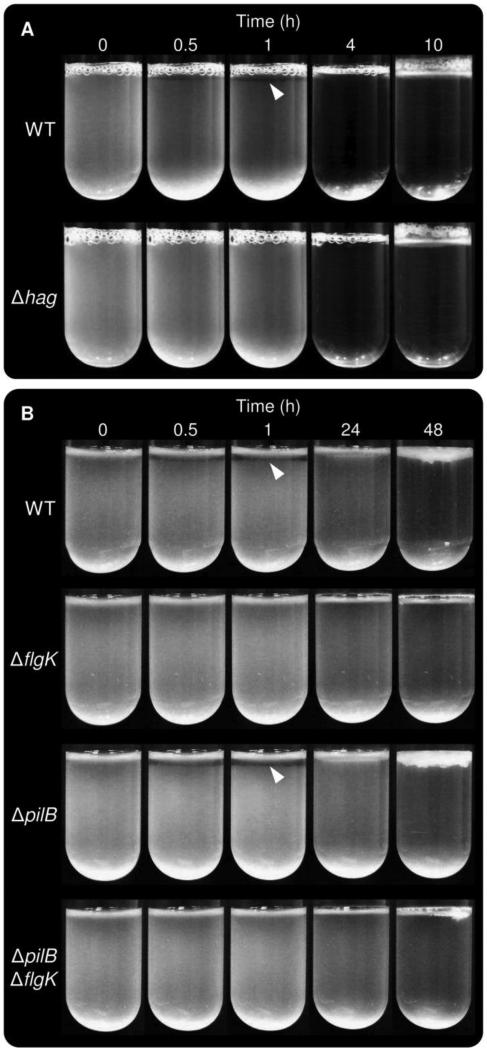
To investigate the role and costs of flagellar motility during pellicle formation, the flagellin-deficient and therefore immotile mutant Δhag of B. subtilis strain NCIB3610 was used. First, pellicle formation of Δhag and the wild-type was compared in the biofilm-promoting medium MSgg to test whether the previously described delay of the Δhag mutant in rich medium would also be present in this minimal medium. As shown in Fig. 1A and Video S1, planktonic growth of both strains could be noticed at the same time (i.e. cultures became turbid). Subsequently, the wild type formed a thick layer of pellicle after 8 hours of incubation (observable as reduced light reflectivity of the medium surface on Fig. 1B), and wrinkled after 19 hours. The Δhag mutant exhibited delayed pellicle formation, with initiation at 15 hours after inoculation and wrinkle formation after 25 hours. The wild type-like appearance of the Δhag mutant pellicle demonstrated that pellicle formation and morphogenesis per se was not impeded by lack of flagella. Further, when the wild-type and the Δhag mutant were inoculated at high starting cell densities (~OD600 of 1.0), no delay in pellicle formation was observed (data not shown). The delay of the Δhag mutant was not due to disadvantages in growth as the Δhag mutant reached higher OD values and therefore showed enhanced growth in shaken cultures when compared to the wild type (Fig. S1). The latter result suggests that the production of flagellin is accompanied by energetic costs for the wild type. The same growth advantage was observed for mutants lacking components of the flagellum indicating that both flagellum production and flagellar activity are costly for the cell (Fig. S1).
Fig. 1.
A: Pellicle formation of B. subtilis wild type (left petri-dish) and its Δhag derivative (right petri-dish) is shown in 35 mm diameter petri dishes containing MSgg medium. B: Magnified section of the petri dishes at selected time point mentioned in the text. Scale bar indicates 2 mm. Arrows point to the reflected light on the medium surface before a thin layer of pellicle is formed. The time points are presented from Video S1.
The Δhag mutant is outcompeted by wild-type cells during pellicle formation
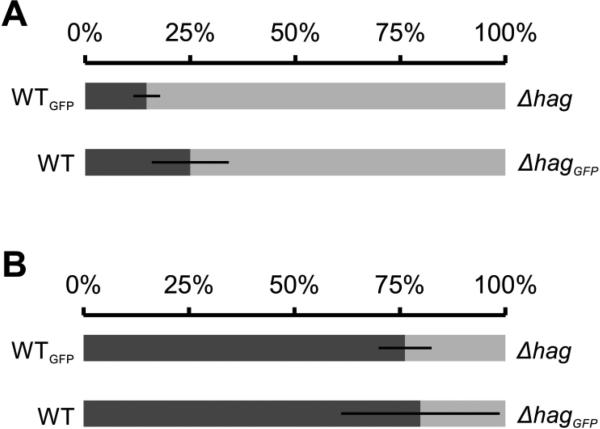
To assay whether the cost of flagellin production influences the competitiveness of the B. subtilis wild-type strain, a competition with the Δhag mutant was performed under pellicle promoting as well as non-promoting conditions. The wild type and the Δhag mutant were inoculated together in MSgg medium in a 1:1 ratio and incubated under static (pellicle promoting) or shaking (pellicle non-promoting) conditions. One of the strains was labelled with constitutively expressed GFP and the final ratio of the two strains was determined by CFU counting. The experiment was repeated with a switched label to exclude any effects caused by the production of GFP (NCIB3610 vs. ΔhagGFP and NCIB3610GFP vs. Δhag). Under shaken, well aerated conditions, the wild type was outcompeted by the Δhag mutant indicating that the cost of flagellin production leads to disadvantages for the wild type under this condition (Fig. 2A). However, under pellicle promoting conditions the Δhag mutant was strongly outcompeted by the wild type (Fig. 2B). This finding indicates that flagellar motility is important in the competition for the favourable niche at the air-liquid interface.
Fig. 2.
Relative abundance of B. subtilis wild-type (dark grey) and Δhag (light grey) strains in competition experiments performed under shaken (A, n=3, Student's t-Test p<0.05) or static (B, n=6, Student's t-Test p<0.01) conditions. Under planktonic conditions (A), the cultures reached stationary phase (20 hours of incubation) and grew for about 5.8 generations. Bars represent percentages of strains in the pellicles. Error bars indicate standard deviation.
To verify the results, the pellicle competition experiment was repeated with strains labelled with different constitutively produced fluorescent markers for green and red fluorescence (GFP and mKATE2, respectively). The fluorescent strains were followed in competition experiments (Video S2) and fluorescence intensities were measured after 3 days and normalised against the fluorescence intensities of control competitions (see Materials and methods for normalisation details). The fluorescent signals in the pellicles were analysed by both fluorescence microscopy and a microplate fluorometer. In pellicles the Δhag mutant was outcompeted by the wild type regardless of the fluorescent label as determined by quantitative fluorescence intensity measurements (Fig. 3A) and stereomicroscopy (Fig. 3B).
Fig. 3.
Competition of GFP or mKATE2 labelled wild-type and Δhag strains of B. subtilis strains during pellicle formation. Relative fluorescence level (arbitrary units) quantified in a plate reader (A, n=4, Student's T-test p<0.001) or visualized using a stereomicroscope (B). Error bars indicate standard deviation. Certain wells (16 mm diameter) of a 24-well plate are shown in the green- or red-fluorescence channels (false coloured in green and red) together with its bright light images.
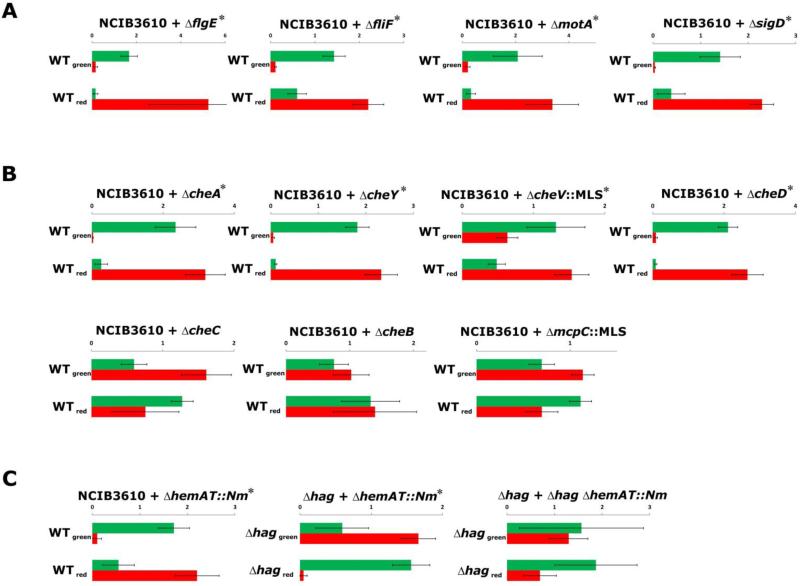
To investigate whether other strains with defects in flagellar motility would show disadvantages in pellicle competitions with the wild type, we engineered mutants with deletions in motA (encoding part of the motor complex), fliF (encoding the cell wall anchor), flgE (encoding the hook protein) and sigD (encoding the sigma factor regulating flagella, motility, chemotaxis and autolysis) and added the fluorescent tag constructs described above. Mutants defective in swimming motility - namely ΔmotA, ΔfliF, ΔflgE, and ΔsigD - were outcompeted by the wild type in 1:1 pellicle competitions (Fig. 4A, Fig. S2 and Table S1). A disadvantage was observed regardless of whether the mutants lacked a specific flagellar component or exhibited reduced gene expression of the whole apparatus (i.e. in the ΔsigD strain). Importantly, all mutant strains formed pellicle structures comparable to those of the wild type when grown as monocultures (Fig. S2).
Fig. 4.
Competition of GFP- or mKATE2-labelled B. subtilis strains during pellicle formation. Mutants are categorized by the locus’ involvement in (A) flagellum biosynthesis and regulation, (B) chemotaxis, or (C) aerotaxis. Relative fluorescence levels (arbitrary units are shown on the axis) were quantified in a fluorescence plate reader and are shown with bar charts (n=4). Green and red bars indicate relative GFP and mKATE2 levels, respectively. In each graph, the upper bars represent the mix of NCIB3610GFP and mutantmKATE2 strains (WTgreen), while the down bars represent the mix of NCIB3610mKATE2 and mutantGFP strains (WTred). Error bars indicate standard deviation. Significant differences (Student's T-test p<0.05, see Materials and Methods) are indicated with a star next to the strains used for competitions.
Chemotaxis and oxygen sensing contribute to B. subtilis competitiveness in pellicles
As chemotaxis and oxygen sensing have been proposed to play roles in the initial stages of pellicle formation in various bacterial species, we tested a collection of B. subtilis NCIB3610 mutants defective in chemotaxis for their competitiveness against the wild type in pellicles. In B. subtilis, most of the chemotaxis genes are located in the same operon as the genes encoding flagellar proteins, namely the fla/che-operon. The chemotaxis machinery includes a two-component system that consists of the receptor-coupled kinase CheA and the response regulator CheY. Phosphorylated CheY binds to a flagellar motor component leading to counterclockwise rotation of the flagellum and therefore swimming. Additional proteins (e.g. CheV, CheR-CheB, and CheC-CheD circuits) are involved in adaptation, the process by which the signalling state of the chemotaxis pathway is reset to a background concentration [38]. The mutants tested were ΔmcpC (receptor involved in amino acid chemotaxis), ΔcheA (receptor-coupled kinase), ΔcheB (involved in methylation of receptors), ΔcheC and ΔcheD (both regulating CheA autophosphorylation), ΔcheV (link between receptors and CheA) and ΔcheY (response regulator) [30, 39]. All mutants that retained some form of (albeit reduced) chemotaxis, i.e. ΔmcpC, ΔcheC, and ΔcheB were able to successfully compete with the wild type as demonstrated by high intensities of the corresponding fluorescence (Fig. 4B and Table S1). In contrast, chemotaxis-null strains (ΔcheV, ΔcheY, and ΔcheA) were outcompeted by the wild type (Fig. 4B, Fig. S2, and Table S1) suggesting that the ability to sense attractants or repellents provides benefits for colonisation of the air-liquid interface.
Aerotaxis is a special form of chemotaxis in which oxygen functions as the attractant or repellent. In pellicle formation of aerobic bacteria, oxygen has been suggested to be an important factor mediating active movement towards the surface of the medium, where the highest oxygen concentrations are present [6]. A strain with a knock-out of the hemAT gene, encoding the B. subtilis oxygen sensor protein [40], was constructed and competed against the wild type. As can be seen in Fig. 4C and Fig. S2, the ΔhemAT mutant was outcompeted by the wild type. Further, the ΔhemAT mutant was competed against the Δhag mutant to investigate which mutation leads to greater disadvantage. Here, the Δhag mutant was outcompeted by the ΔhemAT mutant showing that immobility is more harmful than lack of oxygen sensing for the individual cells during pellicle co-colonisation. However, during the competition of a ΔhemAT Δhag double mutant with the Δhag mutant, both were present in a more or less equal ratio in the pellicle. This finding indicates that ability to sense oxygen is not providing an advantage when the bacteria are not able to actively swim. These results suggest that oxygen sensing provides an advantage during competition for the air-liquid interface.
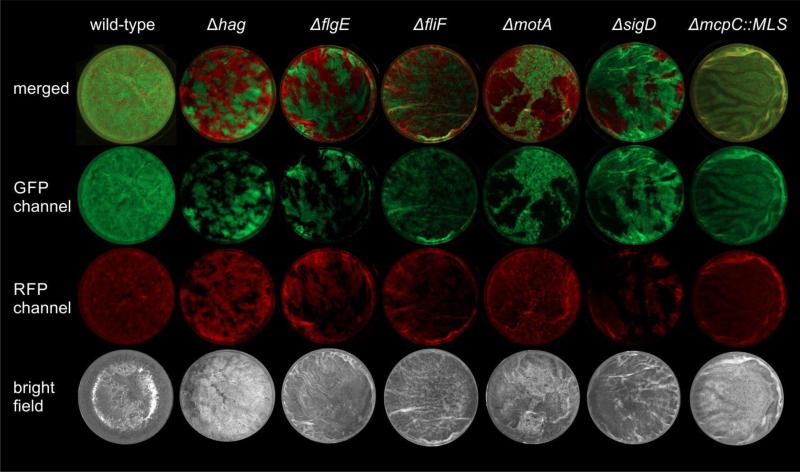
Pellicle intermixing depends on motility, but not on chemotaxis
In the competition experiments described above, strains were differentiated by their constitutive expression of the fluorescent proteins GFP or mKATE2. Control experiments included genetically identical strains that were only distinguished by their labelling (e.g. WTGFP versus WTmKATE2 or ΔhagGFP versus ΔhagmKATE2). Using stereomicroscopy, we observed that the differentially labelled but otherwise wild-type strains mixed well during pellicle formation (Fig. 5, first column), similar to mutants lacking certain chemotaxis- or aerotaxis-related genes e.g. ΔmcpC::MLS (Fig. 5, last column). However, strains that lacked functional flagella (Δhag, ΔmotA, ΔfliF, ΔflgE, and ΔsigD strains) showed increased spatial assortment, i.e. green- and red-fluorescent patches of individual strains. These results suggest that active flagellar motility is not only important for B. subtilis to reach the air-medium interface, but flagellar motility could be required for mixing of cells during the initiation of the pellicle.
Fig. 5.
Stereomicroscopy images of B. subtilis wild-type or mutant strains competed against its genetically identical, but differently labelled derivative. Wells (16 mm diameter) of a 24-well plate are shown in the green- or red-fluorescence channels (false-coloured in green and red) together with merged and bright light images. Representative examples for each strain are shown.
P. aeruginosa pellicle formation is also influenced by motility
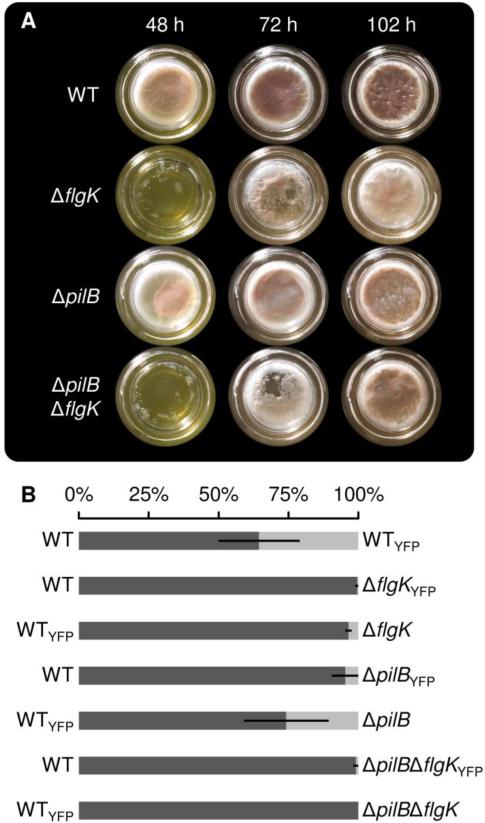
To evaluate the broader relevance of our observations, we next examined the role of motility in pellicle development of the Gram-negative biofilm model organism P. aeruginosa. Pellicle formation of P. aeruginosa PAO1 was previously shown to require flagellar motility [9]. However, this work suggested that cells lacking both flagella and pili form pellicles that are comparable to the wild-type strain. Here, we tested the importance of the flagellum and the type IV pilus for pellicle development in P. aeruginosa PA14. Pellicles of strains lacking the genes flgK (flagellar hook-associated protein), pilB (type IV pilus motor protein) or both (ΔpilB ΔflgK) were investigated. The ΔflgK mutation prevents swimming and swarming, while ΔpilB prevents twitching in P. aeruginosa PA14 [36, 41]. Mutants lacking the flgK gene showed delayed pellicle development when compared to the wild type, while the ΔpilB mutant showed pellicle development that was similar to that of the wild type (Fig. 6A). Interestingly, pellicle formation is not abolished in the mutants as after 102 h, a robust pellicle comparable with that of the wild type was observed. Patchy structures of the pellicles were observed in early pellicle formation stages of the ΔflgK and ΔpilB ΔflgK mutants (Fig. 6A, after 48 h and 72 h) similar to a previous description [9] but were not detected in later stages of pellicle development.
Fig. 6.
Pellicle formation of P. aeruginosa wild-type and mutant strains in borosilicate scintillation vials (28 mm diameter) containing LB medium (A). Relative abundance of P. aeruginosa wild-type (dark grey) and mutant (light grey) strains in competition experiments performed under static conditions (B, WT vs. WTGFP: n=6; others: n=3). Bars represent percentages of strains indicated on the side of the bar. Error bars indicate standard deviation.
Additionally, competition experiments similar to those conducted with B. subtilis were performed with P. aeruginosa PA14. The ΔpilB, ΔflgK and ΔpilB ΔflgK mutants were competed against the wild type in pellicles with initial ratios of 1:1. To distinguish the competing strains, one was fluorescently labelled with YFP. A fluorescence label swap indicated a slight reduction in fitness for labelled strains. In these experiments, all motility mutants were outcompeted by the wild type (Fig. 6B). While the ΔflgK and the ΔpilB ΔflgK mutant were strongly outcompeted (<4% and <1% of the pellicle, respectively), the ΔpilB mutant showed a less severe disadvantage (5-26% of the pellicle) when grown together with the wild type. These results demonstrate that in P. aeruginosa motility is not required for pellicle formation but provides a benefit against a non-motile strain during the colonization of the air-liquid interface.
Pellicle formation is preceded by population mixing and the appearance of a cleared zone
The involvement of motility in the formation of pellicles by diverse bacteria led us to hypothesize that it enables cells to counteract sedimentation and stay at the surface. Cells with impaired motility might therefore be expected to sink, leading to decreased culture turbidity. To evaluate this we recorded the side view of static liquid cultures over several days. We were surprised to find that a zone of clearing appeared specifically in strains capable of flagellum-dependent motility, while flagellum-null mutants showed homogenous turbidity over time (Fig. 7 and Videos S3-S6). The clear zone was visible relatively early after inoculation and eventually disappeared when the pellicle started to thicken. The fact that the transparent zone appears in strains that exhibit earlier pellicle formation suggests that cells in this zone abruptly exit and swim toward the surface of the liquid to access more oxygen. However, the fact that the B. subtilis ΔhemAT static cultures inoculated at high cell density exhibit clear zones similar to those formed by the wild type (Video S7) suggests that oxygen is not the signal that stimulates exit from the region just below the pellicle. Alternative potential explanations are that a gradient of another nutrient attracts cells away from this zone, or that a waste product/chemorepellant accumulates in the region due to the high cell density of the pellicle immediately above. Emergence of the zone of clearing coincided with the appearance of a turbulent flow in the lower portion of the culture tube, both at high and low cell density (Videos S3-S6). This flagellum-dependent turbulent flow is particularly intriguing as it could serve to facilitate the mixing of oxygen into the medium and represent a population-level strategy for resource acquisition. Our time-lapse observation of B. subtilis and P. aeruginosa pellicle development indicates that motility plays a central role in defining population dynamics and conferring a growth advantage in this model biofilm system.
Fig. 7.
Pellicle formation of B. subtilis wild-type and its Δhag derivative in MSgg medium, shown from the side (A). Pellicle formation of P. aeruginosa wild-type, ΔflgK, ΔpilB and ΔflgK ΔpilB strains in LB medium, shown from the side (B). Selected time points are presented from Videos S3 and S5.
Discussion
This study examined the contributions of motility to fitness during bacterial pellicle biofilm development of B. subtilis and P. aeruginosa, and the relevance of oxygen availability to the behavior of B. subtilis static cultures. We observed that in both organisms, pellicle formation was delayed in motility-deficient mutants. Despite this delay, the mutant strains exhibited wild-type morphology demonstrating that motility is not essential for robust pellicle formation. We hypothesise that in the non-motile strains pellicle formation is delayed because of a lack of directed movement towards the air-liquid interface. The cell number may increase mainly by division of the few cells that reached the air-liquid interface by passive, non-directional Brownian motion, slowing down the process of pellicle formation. This is supported by the fact that at high initial cell density, the delay of the B. subtilis Δhag and P. aeruginosa ΔflgK mutants was abolished. Further, we observed that motility-deficient strains of B. subtilis and P. aeruginosa were outcompeted in pellicle competition experiments with their respective wild-type counterparts. This result can also be explained by our hypothesis that the motility-deficient cells make it to the liquid surface only by chance and therefore only inefficiently contribute to formation of a mixed pellicle. On the contrary, the wild-type cells can move towards the air-liquid interface in a directed manner at initial stages of pellicle formation; these cells are therefore present in the pellicle in higher abundance. These results suggest that it is important for successful competition with other strains to reach the air-liquid interface efficiently. We conclude that motility contributes to successful competition for residence at the air-liquid interface although it is not essential for pellicle formation per se. It is also important to note that motility is a costly trait, which was shown for B. subtilis in competition experiments performed under conditions that do not promote pellicle formation (Fig. 2A).
Most probably, cells perceive the location of the air-liquid interface by the sensing of an attractant, which is then translated into directed movement by the chemotaxis system [38]. B. subtilis mutants lacking core components of the chemotaxis machinery were also outcompeted by wild-type cells during pellicle development. This suggests that sensing of certain signals is important for successful pellicle co-colonisation. In these chemotaxis mutant strains, even if the cells are able to move and sense an attractant, they lack the translation of signals into directed movement. Thus the cells move around randomly and only the cells that are located at the air-liquid interface by chance can participate in pellicle formation. In B. subtilis, we identified oxygen as a putative trigger for active movement towards the air-liquid interface since the ΔhemAT mutant, which is not able to sense oxygen [40], was outcompeted by the wild-type in static co-cultures. Thus it was able to move but lacked information regarding the direction of the most favorable conditions in its surroundings, resulting again in random distribution in the liquid. Yamamoto et al. showed that in P. aeruginosa PAO1 pellicle formation was reduced under oxygen-depleted conditions [23], suggesting that aerotaxis might be important for localization to the air-liquid interface in this organism as well. In S. oneidensis, aerotaxis was also found to be important since without oxygen, pellicle initiation was abolished [7]. Further, pellicle maturation of S. oneidensis was blocked in anoxic conditions in cultures where pellicle formation was allowed to be initiated in the presence of oxygen. Similarly, E. coli does not form biofilms at the air-liquid interface in anoxic conditions [10]. These findings indicate that oxygen and aerotaxis might be generally important for locating the air-liquid interface and proper initiation of pellicle formation in diverse bacterial species. Armitano et al. also concluded that chemotaxis and a functional flagellum are essential for pellicle formation in S. oneidensis since the respective mutants did not form a pellicle [7]. However, the pellicle formation ability was investigated after only 20 hours; it is therefore possible that pellicle formation is just delayed in both mutants. Nevertheless, their results show that in diverse bacteria, functional flagella and the chemotaxis system are important for normal progress of at least the initial stages of pellicle formation.
Interestingly, flagellar motility, but not chemotaxis, also influences population intermixing in B. subtilis pellicles. Spatial segregation has been shown to facilitate the stabilization of cooperative traits in B. subtilis colony biofilms [42] and also influences interaction in microbial populations [43]. Non-motile strains show increased spatial segregation. This elevated assortment could be due to reduced flagellar motility within a newly formed and still dynamic layer of pellicle or, alternatively, it could be caused by a dissimilar surface colonization mechanism of the motile and non-motile strains. Kobayashi previously proposed a model in which B. subtilis cell clusters float to the surface of a biofilm-promoting, nutrient-rich medium during pellicle formation [12]. While our experiments highlight the importance of an active signal-driven motility, non-motile strains might colonize the air-medium interface via clusters of cells that expand to form a convergent layer. This could then be observed as spatially segregated clusters of cells initiated by two strains with distinct fluorescent labels, as we present here. We hypothesize that the two putative mechanisms, single-cell and cluster-based pellicle initiation, might coexist in the wild-type strain or depend on the culturing conditions.
We further observed a zone of clearing below the initial pellicle layer in wild-type B. subtilis, wild-type P. aeruginosa, and the P. aeruginosa ΔpilB mutant, but not in B. subtilis or P. aeruginosa flagellum-deficient mutants. The formation of this zone may be triggered by the higher abundance of oxygen close to the air-liquid interface. However, time-lapse experiments of the ΔhemAT B. subtilis strain initiated with high cell densities showed the presence of the clearing zone suggesting that under such conditions, sensing of oxygen plays no significant role. In contrast, the B. subtilis Δhag strain was not delayed in pellicle formation when a high initial cell density was used. As the clearing occurs in the Gram-positive B. subtilis and the Gram-negative P. aeruginosa, we speculate that the occurrence of this clear zone may be a general property of pellicle-forming, facultative aerobes. The mechanism underlying the formation of the clear zone remains to be investigated.
Materials and methods
Bacterial strains, plasmids, and materials
All strains used in this study are listed in Table 1. The Bacillus subtilis strains are derivatives of the wild isolate NCIB3610 (referred to as the wild type) otherwise indicated. The Pseudomonas aeruginosa strains are derivatives of strain PA14 (referred to as the wild type) and were created as described below. B. subtilis fluorescently labelled strains were created by phage transduction. E. coli MC1061 and UQ950 strains were used for cloning.
Table 1.
Strains and plasmids used in this study. For phage transduction, strains 168hyGFP, 168hymKATE2, and TB239 were used as donor.
| Strains/plasmids | Characteristics | Source or reference |
|---|---|---|
| Bacillus subtilis | ||
| NCIB3610 | prototroph | [5] |
| 168hyGFP | 168 amyE::Phyperspank-GFP (CmR) | [42] |
| 168hymKATE2 | 168 amyE::Phyperspank-mKATE2 (CmR) | [42] |
| DS1677 | NCIB3610 Δhag | [50] |
| TB201 | DS1677 transduced with Phy-mKATE2 | This study |
| TB185 | DS1677 transduced with Phy-GFP | This study |
| DS4681 | NCIB3610 ΔflgE | [51] |
| TB203 | DS4681 transduced with Phy-mKATE2 | This study |
| TB187 | DS4681 transduced with Phy-GFP | This study |
| DS7080 | NCIB3610 ΔfliF | [52] |
| TB208 | DS7080 transduced with Phy-mKATE2 | This study |
| TB192 | DS7080 transduced with Phy-GFP | This study |
| DS7498 | NCIB3610 ΔmotA | [52] |
| TB210 | DS7498 transduced with Phy-mKATE2 | This study |
| TB193 | DS7498 transduced with Phy-GFP | This study |
| DS6420 | NCIB3610 ΔsigD | [53] |
| TB204 | DS6420 transduced with Phy-mKATE2 | This study |
| TB188 | DS6420 transduced with Phy-GFP | This study |
| DS6887 | NCIB3610 ΔcheA | [39] |
| TB213 | DS6887 transduced with Phy-mKATE2 | This study |
| TB197 | DS6887 transduced with Phy-GFP | This study |
| DS7306 | NCIB3610 ΔcheB | [39] |
| TB209 | DS7306 transduced with Phy-mKATE2 | This study |
| TB194 | DS7306 transduced with Phy-GFP | This study |
| DS6867 | NCIB3610 ΔcheC | [39] |
| TB205 | DS6867 transduced with Phy-mKATE2 | This study |
| TB189 | DS6867 transduced with Phy-GFP | This study |
| DS6868 | NCIB3610 ΔcheD | [39] |
| TB206 | DS6868 transduced with Phy-mKATE2 | This study |
| TB190 | DS6868 transduced with Phy-GFP | This study |
| DS70 | NCIB3610 ΔcheV::MLS | [30] |
| TB199 | DS70 transduced with Phy-mKATE2 | This study |
| TB183 | DS70 transduced with Phy-GFP | This study |
| DS6870 | NCIB3610 ΔcheY | [39] |
| TB207 | DS6870 transduced with Phy-mKATE2 | This study |
| TB191 | DS6870 transduced with Phy-GFP | This study |
| DS180 | NCIB3610 ΔmcpC::MLS | [30] |
| TB200 | DS180 transduced with Phy-mKATE2 | This study |
| TB184 | DS180 transduced with Phy-GFP | This study |
| TB239 | 168 ΔhemAT::Nm | This study |
| TB241 | 3610 transduced with hemAT::Nm | This study |
| TB243 | TB241 transduced with Phy-mKATE2 | This study |
| TB244 | TB241 transduced with Phy-GFP | This study |
| TB242 | DS1677 transduced with hemAT::Nm | This study |
| TB245 | TB242 transduced with Phy-mKATE2 | This study |
| TB246 | TB242 transduced with Phy-GFP | This study |
| Pseudomonas aeruginosa | ||
| PA14 | Clinical isolate UCBPP-PA14 | [54] |
| LD592 | PA14 with chromosomally integrated constitutive eYFP | [55] |
| LD371 | PA14 ΔflgK | This study |
| LD2221 | PA14 ΔflgK (LD371) with chromosomally integrated constitutive eYFP | This study |
| LD369 | PA14 ΔpilB | This study |
| LD2222 | PA14 ΔpilB (LD369) with chromosomally integrated constitutive eYFP | This study |
| LD384 | PA14 ΔpilB ΔflgK | This study |
| LD2151 | PA14 ΔpilB ΔflgK (LD384) with chromosomally integrated constitutive eYFP | This study |
| Escherichia coli | ||
| MC1061 | host for cloning; araD139, Δ(ara, leu)7694, ΔlacX74, galU−, galK−, hsr−, hsm−, strA | [56] |
| UQ950 | DH5α λ (pir) host for cloning; F− (argF-lac)169 φ80dlacZ58(ΔM15) glnV44(AS) rfbD1 gyrA96(Nalr) recA1 endA1 spoT1 thi-1 hsdR17 deoR λpir+ | D. Lies |
| BW29427 | donor strain for conjugation; thrB1004 pro thi rpsL hsdS lacZ ΔM15RP4–1360 Δ(araBAD)567 ΔdapA1341::[erm pir(wt)] | B. Wanner |
| β-2155 | donor strain for conjugation; thrB1004 pro thi strA hsdS lacZΔM15 ΔdapA::erm (Ermr) pir::RP4 [::kan (Kmr) from SM10] | [57] |
| Saccharomyces cerevisiae | ||
| InvSc1 | MA Ta/MA Tα leu2/leu2 trp1-289/trp1-289 ura3-52/ura3-52 his3-Δ1/his3-Δ1 | [48] |
| Plasmids | ||
| pBEST501 | PrepU-neo, AmpR | [58] |
| pBluescript SK+ | cloning vector, AmpR | Stratagene |
| pTB120 | NmR cassette cloned into pBluescript SK+ | This study |
| pTB235 | NmR cassette with upstream and downstream regions of B. subtilis hemAT | This study |
| pMQ30 | Yeast-based allelic-exchange vector; sacBa CEN/ARSH URA3+ Genr | [48] |
| pLD348 | flgK deletion fragments cloned into pMQ30 | This study |
| pLD349 | pilB deletion fragments cloned into pMQ30 | This study |
| pAKN69 | Mini-Tn7 derived fluorescent labelling vector; mini-Tn7(Gm)PA1/04/03::eyfp | [49] |
| pUX-BF13 | R6K replicon-based helper plasmid providing the Tn7 transposition function in trans; Ampr | [59] |
Growth conditions, biofilm conditions, and competition experiments
B. subtilis overnight cultures were grown aerobically in 3 mL Lenox broth (Carl-Roth GmbH, Karlsruhe, Germany) medium at 37°C, shaking at 225 rpm. For routine liquid cultures of P. aeruginosa PA14, the cells were grown in 2 mL lysogeny broth [44] in 12- by 100-mm tubes at 37°C with shaking at 250 rpm. Growth conditions for pellicles are described below.
Construction of B. subtilis strains
GFP and mKATE labelled B. subtilis strains and ΔhemAT mutants in B. subtilis NCIB3610 were obtained via phage transduction. Constructs were then transferred to selected NCIB3610 derivatives using SPP1-mediated generalized phage transduction [45]. For constructing the ΔhemAT mutant, first a PCR fragment containing a neomycin resistance cassette was amplified with primer pair oRGM2 and oRGM7 (see primer sequences in Table 2) from pBEST501 and cloned into the SmaI site of pBluescriptSK+ to create pTB120. Upstream and downstream regions of hemAT gene were PCR amplified with primer pairs oTB78-oTB79 and oTB80-oTB81, and sequentially cloned into the XhoI-PstI and BamHI-SacI sites of pTB120, respectively, resulting in pTB235. Plasmid pTB235 was confirmed through sequencing. The Eam1101I linearized pTB235 was transformed into B. subtilis 168 with natural competence [46] and transformants were selected on LB agar medium with 5 μg/mL kanamycin. Deletion in the hemAT gene was confirmed by PCR with primer pair oTB82-oTB83 and by sequencing.
Table 2.
Primers used in this study
| Primers | Sequence (5’ to 3’) |
|---|---|
| for B. subtilis constructs | |
| oRGM2 | TACCGTTCGTATAATGTATGCTATACGAAGTTATAGATCAATTTGATAATTACTAATAC |
| oRGM7 | TACCGTTCGTATAGCATACATTATACGAAGTTATTAGAGCTTGGGTTACAGGCATGG |
| oTB78 | GATCCTCGAGAAGCCGGCACGCCATTAAG |
| oTB79 | TTATCGGCCAAGGGAAAC |
| oTB80 | GATCGGATCCAAACCGGTCTGCCATACG |
| oTB81 | CACGGAGCTCATGGGAATGGCCGTACATC |
| oTB82 | GGCCGAATTTATGAAGAGAC |
| oTB83 | AAGATCCGCATTGCTTATGG |
| for P. aeruginosa constructs | |
| ΔflgK 5’ flank-1 | GGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAGGTGATGAAGTCGCTGGTC |
| ΔflgK 5’ flank-2 | ACTGACCCATGTCCGACCTACCAACCTGATCCAGTTCCAG |
| ΔflgK 3’ flank-1 | CTGGAACTGGATCAGGTTGGTAGGTCGGACATGGGTCAGT |
| ΔflgK 3’ flank-2 | CCAGGCAAATTCTGTTTTATCAGACCGCTTCTGCGTTCTGATCACCAAGCAGTACCAGGACA |
| ΔpilB 5’ flank-1 | GGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTTGCCATCCTCCTGCTATTTC |
| ΔpilB 5’ flank-2 | GCAGATCGTTGAAGCCTTCTGCCTTTTCATCGAGAAGTTCA |
| ΔpilB 3’ flank-1 | TGAACTTCTCGATGAAAAGGCAGAAGGCTTCAACGATCTGC |
| ΔpilB 3’ flank-2 | CCAGGCAAATTCTGTTTTATCAGACCGCTTCTGCGTTCTGATGCTGGACACGTCTTGTTTGA |
Construction of P. aeruginosa strains
Construction of deletion and complementation plasmids: Unmarked deletions were generated for the genes flgK (PA14_50360) and pilB (PA14_58750) in wild-type PA14 as described [47]. Deletion plasmids were generated using yeast gap repair cloning. Flanking regions (~1 kb in length) for flgK and pilB were generated using primers listed in Table 3. The flanking regions and the linearized allelic-replacement vector pMQ30 were assembled by gap repair cloning using the yeast strain InvSc1 [48]. The resulting deletion plasmid was transformed into E. coli BW29427 and mobilized into PA14 using biparental conjugation. PA14 single recombinants were selected on LB agar containing 100 μg/mL gentamicin. Potential flgK or pilB deletion mutants were generated by selecting for double recombinants that grew in the presence of 10% sucrose. These candidates were further analyzed by PCR to detect the desired deletion.
Constitutive eYFP expressing strains: The construct containing the eyfp gene under a constitutive promoter was genomically inserted through a Tn7 transposon-based system [49]. The eyfp-containing plasmid pAKN69 was mobilized into PA14 via triparental conjugation, which involves (1) donor strain E. coli BW29427 harboring pAKN69, (2) donor strain E. coli β-2155 harboring the helper plasmid pUX-BF13 and (3) the recipient PA14 strains. YFP-tagged PA14 clones were selected on LB agar containing 100 μg/mL gentamicin.
Biofilm growth and pellicle competition experiments in B. subtilis
For pellicle formation of B. subtilis, 20 μL of overnight culture were mixed with 2 mL of biofilm promoting minimal medium MSgg [5] in a well (16 mm diameter) of a 24-well plate. The culture was statically incubated at 30°C for 3 days. For pellicle competition experiments with CFU determination, each competitor was inoculated to a final OD of 0.05 in 2 mL MSgg in a well of a 24-well plate and incubated statically at 30 °C. After incubation, the pellicles were harvested, disrupted by sonication (2x 12 pulses of 1s with 30% amplitude; Ultrasonic Processor VCX-130, Zinsser Analytics, Frankfurt am Main, Germany) and plated on LB and chloramphenicol containing (5 μg/mL) agar plates in dilutions. The CFU count was recorded and the relative percentage of each competitor in the pellicle was calculated based on their antibiotic resistance. For competition experiments under shaking conditions, each competitor was inoculated to a final OD of 0.05 in 5 mL MSgg in a 100 mL bottle and incubated at 30°C and 225 rpm shaking for 20 h. The cultures reached the stationary phase and grew for about 5.8 generations. Afterwards, the CFU count was determined as described for the pellicle competition (see above). A two sided Student's T-test was performed.
For fluorescence competition experiments, 10 μL of overnight cultures of each competitor were mixed with 2 mL MSgg in a well of a 24-well plate and incubated as described above. The fluorescence intensity was measured using an infinite F200PRO plate reader (TECAN Group Ltd, Männedorf, Switzerland). Fluorescence intensities of mutant strains against wild type competitions (“assay competitions”) measured with the plate reader were normalised against the fluorescence intensities of competitions of wild typeGFP against wild typemKATE2 (“wild-type control competition”) or mutantGFP against mutantmKATE2 (“mutant control competition”). Wild-type signals were normalised based on the “wild type against wild type” control competition. Analogously, signals of mutant fluorescence were normalised, resulting in percentage numbers. Each competition was performed in four replicates, if not stated otherwise. A two-sided Student's T-test was applied to check whether obtained fluorescence intensity measurements were statistically significantly different (significance level α=0.05; number of replicates n=4; degrees of freedom f=3; critical value c=3.182). Results were deemed significantly different if the calculated t-value was outside of the critical interval -c ≤ t ≥ c.
Biofilm growth and pellicle competition experiments in P. aeruginosa
Overnight cultures were diluted 100x, subcultured for ~2.5 hours to OD500 of 0.5-0.7, and then diluted to OD500 of 0.5. For the pellicle morphology assay, 230 μl of the exponential-phase culture (OD500 of 0.5) was diluted in 23 mL of LB in a borosilicate scintillation vial (Thomas Scientific, 9718G12) and grown at 37°C for 2 days to form pellicles. For competition assays, cells were mixed at 500 μl : 500 μl, and 50 μl of each mix was then diluted in 5 mL of LB in a borosilicate tube (18- by 150-mm) and incubated without shaking at 37°C for 2 days. For CFU counts of samples from competition experiments, each pellicle was transferred to 1 mL of 1% tryptone in a bead beating tube containing 0.5 g of zirconium beads (1.4 mm, OPS Diagnostics). Pellicles were homogenized at 400 rpm for 5 min at 4°C. 10−7 of the total amount of cells from each pellicle was plated on 1% tryptone agar medium for CFU counting. YFP-tagged strains were identified using a Typhoon FLA 7000 scanner (GE Healthcare).
Fluorescence microscopy
Bright field, green- and red-fluorescence images of the pellicles were taken with an Axio Zoom V16 stereomicroscope (Carl Zeiss, Jena, Germany) at 3.5x magnification equipped with a Zeiss CL 9000 LED light source, HE eGFP filter set (excitation at 470/40 nm and emission at 525/50 nm), HE mRFP filter set (excitation at 572/25 nm and emission at 629/62 nm), and an AxioCam MRm monochrome camera (Carl Zeiss, Jena, Germany). The exposure times were set to 0.1 s, 0.85 s and 3 s for bright field, green- and red-fluorescence, respectively. ImageJ (National Institute of Health, Bethesda, MD, USA) was used for background subtraction and channel merging. For time-lapse experiments, cultures in 35 mm diameter Falcon petri dishes (VWR, Darmstadt, Germany) were incubated in INUL-MS2-F1 incubators (Tokai Hit, Shizuoka, Japan) at 30 °C and images were recorded every 30 min.
Time-lapse experiments (side view)
For P. aeruginosa time-lapse videos, the growth conditions were the same as those described for competition experiments, starting at OD (500 nm) = 0.005 or 2.0 at 37°C. For B. subtilis, pellicles were grown in MSgg medium, starting at OD = 0.005 or 1.2 at 30°C. Images were recorded every minute using an iPod touch (Apple) or a customized recording system (Logitech HD Webcam C525) under LED illumination. Image acquisition and lighting were synchronized with LabVIEW (National Instruments).
Supplementary Material
Highlights.
motility facilitates pellicle formation in B. subtilis and P. aeruginosa
non-motile mutants show a competitive disadvantage in pellicles
production of flagella is a costly process in B. subtilis
oxygen sensing contributes to pellicle formation in B. subtilis
Acknowledgements
We thank Daniel Kearns for sharing B. subtilis strains and his comments.
T.H. and R.G.-M. are supported by IMPRS (International Max Planck Research School) and CONACyT-DAAD, respectively. Work in the laboratory of A.T.K. is supported by a Marie Curie Career Integration Grant (PheHetBacBiofilm), Grant KO4741/2-1 from the Deutsche Forschungsgemeinschaft (DFG) within the framework of the DFG Priority Programme SPP1617, and a JSMC (Jena School for Microbial Communication) startup fund. The Dietrich lab is supported by grant R01-AI103369 from NIAID/NIH.
References
- 1.Kolter R, Greenberg EP. Microbial sciences: the superficial life of microbes. Nature. 2006;441:300–2. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 3.Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemon KP, Earl AM, Vlamakis HC, Aguilar C, Kolter R. Biofilm development with an emphasis on Bacillus subtilis. Curr Top Microbiol Immunol. 2008;322:1–16. doi: 10.1007/978-3-540-75418-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A. 2001;98:11621–6. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitano J, Méjean V, Jourlin-Castelli C. Gram-negative bacteria can also form pellicles. Environ Microbiol Rep. 2014;6:534–44. doi: 10.1111/1758-2229.12171. [DOI] [PubMed] [Google Scholar]
- 7.Armitano J, Méjean V, Jourlin-Castelli C. Aerotaxis governs floating biofilm formation in Shewanella oneidensis. Environ Microbiol. 2013 doi: 10.1111/1462-2920.12158. [DOI] [PubMed] [Google Scholar]
- 8.Nait Chabane Y, Marti S, Rihouey C, Alexandre S, Hardouin J, Lesouhaitier O, et al. Characterisation of pellicles formed by Acinetobacter baumannii at the air-liquid interface. PLoS One. 2014;9:e111660. doi: 10.1371/journal.pone.0111660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto K, Arai H, Ishii M, Igarashi Y. Involvement of flagella-driven motility and pili in Pseudomonas aeruginosa colonization at the air-liquid interface. Microbes Environ. 2012;27:320–3. doi: 10.1264/jsme2.ME11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colon-Gonzalez M, Mendez-Ortiz MM, Membrillo-Hernandez J. Anaerobic growth does not support biofilm formation in Escherichia coli K-12. Res Microbiol. 2004;155:514–21. doi: 10.1016/j.resmic.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Rainey PB, Travisano M. Adaptive radiation in a heterogeneous environment. Nature. 1998;394:69–72. doi: 10.1038/27900. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J Bacteriol. 2007;189:4920–31. doi: 10.1128/JB.00157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Abee T, Kovács ÁT. Kuipers OP, van der Veen S. Biofilm formation and dispersal in Gram-positive bacteria. Curr Opin Biotechnol. 2011;22:172–9. doi: 10.1016/j.copbio.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Guttenplan SB, Kearns DB. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev. 2013;37:849–71. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol. 2013;11:157–68. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttenplan SB, Blair KM, Kearns DB. The EpsE flagellar clutch is bifunctional and synergizes with EPS biosynthesis to promote Bacillus subtilis biofilm formation. PLoS Genet. 2010;6:e1001243. doi: 10.1371/journal.pgen.1001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrova OE, Cherny KE, Sauer K. The Pseudomonas aeruginosa diguanylate cyclase GcbA, a homolog of P. fluorescens GcbA, promotes initial attachment to surfaces, but not biofilm formation, via regulation of motility. J Bacteriol. 2014;196:2827–41. doi: 10.1128/JB.01628-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y, Gao H, Chen J, Dong Y, Wu L, He Z, et al. Pellicle formation in Shewanella oneidensis. BMC Microbiol. 2010;10:291. doi: 10.1186/1471-2180-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemon KP, Higgins DE, Kolter R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J Bacteriol. 2007;189:4418–24. doi: 10.1128/JB.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao J, Allen C. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J Bacteriol. 2007;189:6415–24. doi: 10.1128/JB.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K, Haruta S, Kato S, Ishii M, Igarashi Y. Determinative factors of competitive advantage between aerobic bacteria for niches at the air-liquid interface. Microbes Environ. 2010;25:317–20. doi: 10.1264/jsme2.me10147. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Arai H, Ishii M, Igarashi Y. Trade-off between oxygen and iron acquisition in bacterial cells at the air-liquid interface. FEMS Microbiol Ecol. 2011;77:83–94. doi: 10.1111/j.1574-6941.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;69:1399–410. doi: 10.1111/j.1365-2958.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- 25.Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–49. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 26.Mhatre E, Monterrosa RG, Kovács ÁT. From environmental signals to regulators: modulation of biofilm development in Gram-positive bacteria. J Basic Microbiol. 2014;54:616–32. doi: 10.1002/jobm.201400175. [DOI] [PubMed] [Google Scholar]
- 27.McLoon AL, Guttenplan SB, Kearns DB, Kolter R, Losick R. Tracing the domestication of a biofilm-forming bacterium. J Bacteriol. 2011;193:2027–34. doi: 10.1128/JB.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seminara A, Angelini TE, Wilking JN, Vlamakis H, Ebrahim S, Kolter R, et al. Osmotic spreading of Bacillus subtilis biofilms driven by an extracellular matrix. Proc Natl Acad Sci U S A. 2012;109:1116–21. doi: 10.1073/pnas.1109261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–44. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearns DB, Losick R. Swarming motility in undomesticated Bacillus subtilis. Mol Microbiol. 2003;49:581–90. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- 31.Kinsinger RF, Shirk MC, Fall R. Rapid surface motility in Bacillus subtilis is dependent on extracellular surfactin and potassium ion. J Bacteriol. 2003;185:5627–31. doi: 10.1128/JB.185.18.5627-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cairns LS, Marlow VL, Bissett E, Ostrowski A, Stanley-Wall NR. A mechanical signal transmitted by the flagellum controls signalling in Bacillus subtilis. Mol Microbiol. 2013;90:6–21. doi: 10.1111/mmi.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–4. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 34.Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 2003;48:1511–24. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- 35.O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 36.van Ditmarsch D, Boyle KE, Sakhtah H, Oyler JE, Nadell CD, Deziel E, et al. Convergent evolution of hyperswarming leads to impaired biofilm formation in pathogenic bacteria. Cell Rep. 2013;4:697–708. doi: 10.1016/j.celrep.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Köhler T, Curty LK, Barja F, van Delden C, Pechere JC. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol. 2000;182:5990–6. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter SL, Wadhams GH, Armitage JP. Signal processing in complex chemotaxis pathways. Nat Rev Microbiol. 2011;9:153–65. doi: 10.1038/nrmicro2505. [DOI] [PubMed] [Google Scholar]
- 39.Calvo RA, Kearns DB. FlgM is secreted by the flagellar export apparatus in Bacillus subtilis. J Bacteriol. 2015;197:81–91. doi: 10.1128/JB.02324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou S, Larsen RW, Boudko D, Riley CW, Karatan E, Zimmer M, et al. Myoglobin-like aerotaxis transducers in Archaea and Bacteria. Nature. 2000;403:540–4. doi: 10.1038/35000570. [DOI] [PubMed] [Google Scholar]
- 41.Kuchma SL, Delalez NJ, Filkins LM, Snavely EA, Armitage JP, O'Toole GA. Cyclic di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa PA14 requires the MotAB stator. J Bacteriol. 2015;197:420–30. doi: 10.1128/JB.02130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Gestel J, Weissing FJ, Kuipers OP, Kovács ÁT. Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms. ISME J. 2014;8:2069–79. doi: 10.1038/ismej.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovács ÁT. Impact of spatial distribution on the development of mutualism in microbes. Front Microbiol. 2014;5:649. doi: 10.3389/fmicb.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasbin RE, Young FE. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol. 1974;14:1343–8. doi: 10.1128/jvi.14.6.1343-1348.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anagnostopoulos C, Spizizen J. Requirements for Transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–6. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dietrich LE, Okegbe C, Price-Whelan A, Sakhtah H, Hunter RC, Newman DK. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J Bacteriol. 2013;195:1371–80. doi: 10.1128/JB.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O'Toole GA. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol. 2006;72:5027–36. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambertsen L, Sternberg C, Molin S. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ Microbiol. 2004;6:726–32. doi: 10.1111/j.1462-2920.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 50.Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science. 2008;320:1636–8. doi: 10.1126/science.1157877. [DOI] [PubMed] [Google Scholar]
- 51.Courtney CR, Cozy LM, Kearns DB. Molecular characterization of the flagellar hook in Bacillus subtilis. J Bacteriol. 2012;194:4619–29. doi: 10.1128/JB.00444-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan JM, Guttenplan SB, Kearns DB. Defects in the flagellar motor increase synthesis of poly-gamma-glutamate in Bacillus subtilis. J Bacteriol. 2014;196:740–53. doi: 10.1128/JB.01217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cozy LM, Phillips AM, Calvo RA, Bate AR, Hsueh YH, Bonneau R, et al. SlrA/SinR/SlrR inhibits motility gene expression upstream of a hypersensitive and hysteretic switch at the level of sigma(D) in Bacillus subtilis. Mol Microbiol. 2012;83:1210–28. doi: 10.1111/j.1365-2958.2012.08003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 55.Ramos I, Dietrich LE, Price-Whelan A, Newman DK. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res Microbiol. 2010;161:187–91. doi: 10.1016/j.resmic.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casadaban MJ, Cohen SN. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 57.Dehio C, Meyer M. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J Bacteriol. 1997;179:538–40. doi: 10.1128/jb.179.2.538-540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Itaya M, Kondo K, Tanaka T. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 1989;17:4410. doi: 10.1093/nar/17.11.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao Y, Lies DP, Fu H, Roberts GP. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene. 1991;109:167–8. doi: 10.1016/0378-1119(91)90604-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.