Abstract
Background
Hydrolytic enzymes, such as cellulases and proteases, have various applications, including bioethanol production, extraction of fruit and vegetable juice, detergent formulation, and leather processing. Solid-substrate fermentation has been an emerging method to utilize low-cost agricultural residues for the production of these enzymes. Although the production of carboxy methyl cellulase (CMCase) and protease in solid state fermentation (SSF) have been studied extensively, research investigating multienzyme production in a single fermentation process is limited. The production of multienzymes from a single fermentation system could reduce the overall production cost of enzymes. In order to achieve enhanced production of enzymes, the response surface methodology (RSM) was applied.
Results
Bacillus subtilis IND19 utilized cow dung substrates for the production of CMCase and protease. A central composite design and a RSM were used to determine the optimal concentrations of peptone, NaH2PO4, and medium pH. Maximum productions of CMCase and protease were observed at 0.9 % peptone, 0.78 % NaH2PO4, and medium pH of 8.41, and 1 % peptone, 0.72 % NaH2PO4, and medium pH of 8.11, respectively. Under the optimized conditions, the experimental yield of CMCase and protease reached 473.01 and 4643 U/g, which were notably close to the predicted response (485.05 and 4710 U/g). These findings corresponded to an overall increase of 2.1- and 2.5-fold in CMCase and protease productions, respectively.
Conclusions
Utilization of cow dung for the production of enzymes is critical to producing multienzymes in a single fermentation step. Cow dung is available in large quantity throughout the year. This report is the first to describe simultaneous production of CMCase and protease using cow dung. This substrate could be directly used as the culture medium without any pretreatment for the production of these enzymes at an industrial scale.
Keywords: Cow dung, Solid-substrate fermentation, Carboxy methyl cellulase, Protease, Multienzymes, Response surface methodology
Background
Cellulases catalyze the hydrolysis of cellulose, and many microorganisms, including fungi, bacteria, and protozoans, to produce cellulase [1]. In recent years, cellulolytic enzymes from Saccharomyces cerevisiae [2], Talaromyces cellulolyticus [3], and S. cerevisiae TJ14 [4] have been identified and characterized for various biotechnological processes. These enzymes have many useful applications in the paper industry, bioethanol generation, extraction of fruit and vegetable juice, textiles, the detergent industry, and animal feed production [5–7]. Proteases are an important group of industrial enzymes and are widely used in the food, chemical, pharmaceutical, and leather processing industries [8]. The global market for these enzymes could reach $4.4 billion by the year 2015, and the maximum sales of industrial enzymes came from the leather and bioethanol market [9]. It was previously reported that the cost of growth medium covered approximately 30–40 % of production cost of industrial enzymes [10]. Hence, simultaneous production of cellulase and protease could help to reduce cost. Research examining novel substrates for the production of cellulase and protease has been a continuous effort.
SSF has been an emerging method to utilize the cost-effective agro-residues to produce cellulases and proteases [11, 12]. In the last two decades, SSF has attracted attention in Western countries due to its advantages in the production of secondary metabolites, enzymes, and novel foods [13]. In SSF, the cheap substrates, such as banana fruit stalk, wheat straw, paddy straw, apple pomace, sugarcane bagasse, oil palm empty fruit bunch, green gram husk, Imperata cylindrical grass and potato peel, and pigeon pea, have been utilized for the production of cellulase and protease [14–23]. Although these agro-residues were regarded as the potential substrates in SSF, their availability is largely seasonal. The ideal substrate should be available throughout the year and be cheap. Therefore, cow dung is a possible substrate. Cow dung is rich in cellulose (35.4 %), hemicelluloses (32.6 %), ash (13.3 %), nitrogen (1.4 %), and traces of minerals, such as nitrogen, potassium, and sulphur, and traces of phosphate, iron, cobalt, magnesium, potassium, chloride, and manganese [24].
Most cellulolytic enzymes used in industry are of fungal origin; however, these enzymes lack stability at high temperatures. Because many industrial processes are carried out at high temperatures, there is a need for thermostable enzymes from other sources [25]. Cellulases of bacterial origin have potent activity with crystalline celluloses. These enzymes showed high activity and stability towards alkaline pH and are thermostable in nature compared with the fungal cellulases [26]. Cellulases produced by bacteria are notably high in quantity, whereas the fungal cellulases are mostly inducible in nature [27]. Likewise, a wide range of bacteria are known to produce proteases; a large proportion of the commercially available proteolytic enzymes are derived from the genus Bacillus because of their capacity to produce large amounts of alkaline proteases with significant activity and stability at high temperature and pH [8, 28].
The traditional method to evaluate the optimal conditions for enzyme production is based on one-variable-at-a-time approach. However, this approach fails to reflect the interactive effects among the selected factors or variables and it is a time-consuming process and requires multiple experimental runs. Additionally, this method does not guarantee to find accurate optimal conditions. However, statistical methods, such as response surface methodology (RSM), have been greatly used to determine the optimum level of factors in a bioprocess [29, 30]. RSM is a collection of statistical techniques for designing experiments, searching the significant factors, and evaluating optimum conditions, that has been successfully used in the optimization of many bioprocesses [31]. In RSM, 3D plots help to better identify the maximum response and interactions among the tested variables [32]. There have been many studies on RSM-mediated optimization of enzyme production from various microorganisms [33–36].
Well-established enzyme engineering is required for the effective and simultaneous production of multienzymes in a single fermentation [37, 38]. In a multienzyme production system, the supplement of various nutrients are critical, and not all nutrients may enhance the simultaneous production of all enzymes [39]. More than two or three enzymes have been produced in a particular environmental condition by microorganisms, specifically Bacillus sp. Multienzyme production is a complex process that is associated with complex patterns of repression and induction resulting from the mixed substrate environment, pH, moisture content, fermentation time, and inoculum concentration in SSF [40]. The interaction among these factors becomes the key aspect for investigation in the multienzyme production in SSF. Several reports are available for Bacillus sp. for the production of concomitant enzyme production, including lipase and protease [41], amylase and protease [42], proteases and amylases [43]. However, the reports on simultaneous production of CMCase and proteases from Bacillus sp. are limited and perhaps not available. Recently, cow dung was used as the solid substrate for the production of protease [12] and CMCase [36]. To the best of our knowledge, the current study is the first to report simultaneous production of CMCase and protease using cow dung substrate in SSF. Considering the production cost of CMCase and protease, this paper identified the optimum conditions for the production of these enzymes by Bacillus subtilis IND19. A statistical approach was employed to identify the significant factors and RSM was used to obtain the optimized conditions for CMCase and protease production in SSF utilizing cow dung substrate.
Results and discussion
Screening of B. subtilis IND19 for cellulolytic and proteolytic activity
In the present study, seven potential cellulolytic bacterial strains were used, which hydrolysed CMC with the zone range of 3.0–6.0 mm. The bacterial isolates, such as VA1, VA2, VA4, VA5, VA6, and VA7, hydrolysed 5, 3, 4, 5, 3, 3 mm, respectively, on CMC agar plates. The CMCase activity of B. subtilis IND19 was higher (6 mm) than the other screened bacterial isolates. Cellulase production of the bacterial strains from the genus Bacillus has been reported by various studies [44–46]. The cellulolytic enzyme-producing bacterial isolates, such as VA1, VA2, VA3, VA4, VA5, VA6, and VA7, were evaluated for protease production on skimmed milk agar plates. Among the tested bacterial strain, B. subtilis IND19 showed the maximum production of protease on skimmed milk agar plates (12 mm). The other tested isolates showed hydrolytic zone ranging from 3 to 11 mm. Hence, B. subtilis IND19 was selected for simultaneous production of CMCase and protease.
Cow dung is a substrate of choice for simultaneous production of CMCase and protease
In this paper, cow dung was explored as the low-cost substrate for the simultaneous production of CMCase and protease. This low-cost substrate could lower the production cost of enzymes. Because the production of hydrolytic enzymes using different fermentation processes is notably expensive, and the simultaneous production of several industrial enzymes in a single fermentation medium is a great challenge [47]. Cow dung was attempted for enzyme production. The selection of suitable solid waste for any enzyme production in an SSF process mainly depends on the cost and availability of the substrate material [48]. In recent years, many substrates have been reported for the production of CMCase and protease [17, 20, 23, 36, 49]. Considering availability and cost, cow dung is a suitable substrate for the production of cellulase and protease. Reports on SSF of cow dung for the simultaneous production of cellulolytic and proteolytic enzymes using bacteria are limited or perhaps not available. This report could be the first to describe the simultaneous production of CMCase and protease in SSF using cow dung substrate.
Effect of carbon, nitrogen, and mineral sources on CMCase and protease production
Of the all of carbon sources that were tried, sucrose was the most promising, and the corresponding CMCase activity was 213 ± 34.5 U/g. CMCase productions were 181 ± 15.6, 174 ± 4.6, 148 ± 7.3, and 121 ± 4.8 U/g for maltose, fructose, xylose, and glucose, respectively. Among all carbon sources, sucrose enhanced protease production, and the enzyme activity was 1608 ± 28 U/g. Protease activity levels were 1412 ± 46.4, 1027 ± 46.9, 1092 ± 13.5, and 1358 ± 98 U/g, for maltose, fructose, xylose, and glucose, respectively. Of all nitrogen sources that were tested, peptone was the most promising, and the corresponding CMCase activity was 284 ± 32.7 U/g, and protease activity was 1831 ± 67.4 U/g. CMCase activity levels were 261.5 ± 12.8, 67.5 ± 7.3, 210.5 ± 12.8, and 44 ± 1.5 U/g, for yeast extract, oat meal, beef extract, and ammonium sulphate, respectively. Protease activity levels were 1412 ± 34.8, 913 ± 12.9, 1685 ± 121.5, and 819 ± 38.5 U/g for yeast extract, oat meal, beef extract, and ammonium sulphate. Among the mineral sources tested, sodium dihydrogen phosphate enhanced CMCase (248 ± 18.7 U/g) and protease activity (2113 ± 93 U/g). CMCase activity was 182 ± 7.5, 78 ± 0.6, 147 ± 8.4, 197 ± 18.3, and 136 ± 16.9 for ferrous sulphate, di-sodium hydrogen phosphate, ammonium chloride, sodium nitrate and calcium chloride, respectively. Protease activity was 641 ± 37, 1812 ± 29.5, 1741 ± 33, 1427 ± 20.5, and 1918 ± 33 U/g for ferrous sulphate, di-sodium hydrogen phosphate, ammonium chloride, sodium nitrate and calcium chloride, respectively.
Screening variables for the production of CMCase and protease by statistical approach
Initial screening of medium components indicated that carbon source (sucrose), nitrogen source (peptone), addition of salt solution (NaH2PO4), and variation of medium pH induced the CMCase and protease production. A statistical approach (25 full factorial design) was used to identify the most effective variables affecting CMCase and protease production. All experiments were carried out under SSF for 72 h at 37 °C in duplicates. The experimental values of two-level full factorial design for the production of CMCase and protease are given in Table 1. CMCase production varied between 41.5 and 497.4 U/g and protease yield varied from 206.5 to 4778.2 U/g. The variability in the yield of enzyme production in this paper provides space for the optimization of enzyme production. The F values of this model for CMCase and protease activities were 49.75 and 75.06 U/g, respectively, which were statistically significant at the 5 % level. In this paper, sucrose, peptone, NaH2PO4, and the initial pH and moisture content of the culture medium significantly influenced the production of both enzymes (Table 2). These results were in accordance with the observations made with Chaetomium sp. on cellulase production in SSF [50], suggesting that sucrose was the best carbon source for cellulase production. However, cellulose was demonstrated to be the best carbon source for cellulase production from Bacillus sp. [51]. Addition of peptone to the cow dung medium positively influenced both CMCase and protease production. Umikalsom et al. [52] recorded peptone as the suitable nitrogen source for the production of cellulase by Chaetomium globosum in SSF using delignified oil empty fruit bunch fibre as substrate. Likewise, another report also suggested peptone as the best nitrogen source for the cellulase production from Marinobacter sp. MSI032 [53]. In this paper, protease production was enhanced by the supplement of sucrose as the carbon source. This result was in accordance with the observations made with Yarrowia lipolytica [54] and Bacillus sp. [55]. The R2 of the model values for the production of CMCase and protease were 0.9970 and 0.9954, and the adjusted R2 was 0.977 and 0.9821, respectively. The regression equation coefficients of the 25 full factorial models were calculated and the data were well fitted.
Table 1.
Response of two-level full factorial design for screening of variables for CMCase and protease production
| Run | Sucrose | Peptone | NaH2PO4 | pH | Moisture | CMCase activity (U/g) | Protease activity (U/g) |
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| 1 | −1 | 1 | 1 | −1 | 1 | 403.8 | 206.5 |
| 2 | −1 | −1 | −1 | −1 | −1 | 85.3 | 1143.8 |
| 3 | 1 | 1 | −1 | −1 | 1 | 134.21 | 1547.3 |
| 4 | 1 | 1 | −1 | 1 | −1 | 252.84 | 930.6 |
| 5 | −1 | −1 | −1 | 1 | −1 | 130.7 | 922.9 |
| 6 | 1 | 1 | 1 | −1 | −1 | 41.5 | 2030.5 |
| 7 | 1 | −1 | −1 | −1 | −1 | 135.07 | 2084.8 |
| 8 | 1 | 1 | −1 | −1 | 1 | 298.53 | 1875.9 |
| 9 | 1 | −1 | −1 | −1 | −1 | 88.78 | 1154.7 |
| 10 | 1 | −1 | −1 | −1 | 1 | 133.2 | 916.4 |
| 11 | 1 | −1 | −1 | 1 | −1 | 103.9 | 1143.9 |
| 12 | −1 | 1 | 1 | 1 | 1 | 110.74 | 2775.8 |
| 13 | −1 | 1 | −1 | −1 | −1 | 129.56 | 1152.4 |
| 14 | −1 | 1 | −1 | −1 | 1 | 228.5 | 925.3 |
| 15 | 1 | 1 | −1 | 1 | 1 | 399.6 | 4375.9 |
| 16 | 1 | −1 | −1 | −1 | 1 | 123.2 | 2753.9 |
| 17 | −1 | 1 | −1 | 1 | 1 | 219.5 | 1401.6 |
| 18 | 1 | 1 | 1 | −1 | −1 | 145.12 | 1170.5 |
| 19 | −1 | 1 | 1 | 1 | −1 | 441.45 | 920.5 |
| 20 | −1 | −1 | 1 | −1 | 1 | 309.3 | 1382.7 |
| 21 | 1 | −1 | 1 | 1 | 1 | 150.09 | 3640.4 |
| 22 | −1 | −1 | −1 | −1 | 1 | 125.8 | 1106.1 |
| 23 | 1 | 1 | 1 | 1 | −1 | 375.5 | 915.6 |
| 24 | 1 | 1 | 1 | 1 | 1 | 259.6 | 1210.3 |
| 25 | −1 | −1 | 1 | −1 | −1 | 346.3 | 1826.6 |
| 26 | −1 | −1 | 1 | 1 | 1 | 171.5 | 1154.6 |
| 27 | 1 | −1 | 1 | −1 | 1 | 108.5 | 1163.7 |
| 28 | −1 | −1 | 1 | 1 | −1 | 66.7 | 2982.6 |
| 29 | −1 | 1 | 1 | −1 | −1 | 90.73 | 1844.9 |
| 30 | −1 | −1 | −1 | 1 | 1 | 145.74 | 1867.4 |
| 31 | −1 | 1 | −1 | 1 | −1 | 497.4 | 1133.8 |
| 32 | 1 | −1 | 1 | 1 | −1 | 78.93 | 4778.2 |
Table 2.
Analysis of variance (ANOVA) for the CMCase and protease activity of B. subtilis IND19
| Source | Sum of squares | df | Mean square | F value | p value | |
|---|---|---|---|---|---|---|
| Analysis of variance (ANOVA) for the CMCase activity of B. subtilis IND19 | ||||||
| Model | 4.72E+05 | 27 | 1.75E+04 | 49.75 | 0.0008 | Significant |
| A-Sucrose | 1.42E+04 | 1 | 1.42E+04 | 40.46 | 0.0031 | |
| B-Peptone | 93049.74 | 1 | 93049.74 | 264.84 | <0.0001 | |
| C-NaH2PO4 | 3.27E+03 | 1 | 3.27E+03 | 9.31 | 0.038 | |
| D-pH | 1.73E+04 | 1 | 1.73E+04 | 49.13 | 0.0022 | |
| E-Moisture | 3.24E+03 | 1 | 3.24E+03 | 8.66 | 0.0423 | |
| AB | 1.87E+03 | 1 | 1.87E+03 | 5.33 | 0.0821 | |
| AC | 5.85E+03 | 1 | 5.85E+03 | 16.64 | 0.0151 | |
| AD | 1.18E+04 | 1 | 1.18E+04 | 33.57 | 0.0044 | |
| AE | 6.57E+03 | 1 | 6.57E+03 | 18.7 | 0.0124 | |
| BC | 8.98E+03 | 1 | 8.98E+03 | 25.56 | 0.0072 | |
| BD | 6.36E+04 | 1 | 6.36E+04 | 180.91 | 0.0002 | |
| CD | 1.90E+04 | 1 | 1.90E+04 | 54.15 | 0.0018 | |
| CE | 4.42E+03 | 1 | 4.42E+03 | 12.58 | 0.0239 | |
| DE | 3.30E+04 | 1 | 3.30E+04 | 93.8 | 0.0006 | |
| ABC | 3.46E+03 | 1 | 3.46E+03 | 9.86 | 0.0348 | |
| ABE | 7.46E+03 | 1 | 7.46E+03 | 21.23 | 0.01 | |
| ACD | 1.94E+04 | 1 | 1.94E+04 | 55.17 | 0.0018 | |
| ACE | 1.63E+04 | 1 | 1.63E+04 | 46.53 | 0.0024 | |
| ADE | 1.91E+04 | 1 | 1.91E+04 | 54.34 | 0.0018 | |
| BCD | 2.19E+03 | 1 | 2.19E+03 | 6.23 | 0.0671 | |
| BCE | 4.10E+03 | 1 | 4.10E+03 | 11.68 | 0.0268 | |
| BDE | 6.52E+04 | 1 | 6.52E+04 | 185.67 | 0.0002 | |
| ABCE | 1.36E+04 | 1 | 1.36E+04 | 38.81 | 0.0034 | |
| ABDE | 2.15E+04 | 1 | 2.15E+04 | 61.14 | 0.0014 | |
| ACDE | 1.35E+03 | 1 | 1.35E+03 | 3.84 | 0.1216 | |
| BCDE | 8.84E+03 | 1 | 8.84E+03 | 25.15 | 0.0074 | |
| ABCDE | 3.54E+03 | 1 | 3.54E+03 | 10.08 | 0.0337 | |
| Residual | 1.41E+03 | 4 | 1.41E+03 | |||
| Cor Total | 4.73E+05 | 31 | 4.73E+05 | |||
| ANOVA for the protease activity of B. subtilis IND19 | ||||||
| Model | 3.36E+07 | 23 | 1.46E+06 | 75.06 | <0.0001 | Significant |
| A-Sucrose | 3.93E+05 | 1 | 3.93E+05 | 20.22 | 0.002 | |
| B-Peptone | 2.84E+06 | 1 | 2.84E+06 | 145.89 | <0.0001 | |
| C-NaH2PO4 | 3.63E+06 | 1 | 3.63E+06 | 186.53 | <0.0001 | |
| D-pH | 2.79E+06 | 1 | 2.79E+06 | 143.25 | <0.0001 | |
| E-Moisture | 2.02E+05 | 1 | 2.02E+05 | 10.37 | 0.0122 | |
| AB | 1.65E+06 | 1 | 1.65E+06 | 84.73 | <0.0001 | |
| AC | 2.17E+05 | 1 | 2.17E+05 | 11.14 | 0.0103 | |
| AD | 8.21E+05 | 1 | 8.21E+05 | 42.21 | 0.0002 | |
| AE | 1.27E+05 | 1 | 1.27E+05 | 6.55 | 0.0337 | |
| BC | 9.99E+04 | 1 | 9.99E+04 | 5.14 | 0.0532 | |
| BD | 3.81E+06 | 1 | 3.81E+06 | 195.77 | <0.0001 | |
| BE | 2.28E+06 | 1 | 2.28E+06 | 117.25 | <0.0001 | |
| CE | 1.05E+05 | 1 | 1.05E+05 | 5.38 | 0.0489 | |
| ABD | 1.11E+06 | 1 | 1.11E+06 | 57.04 | <0.0001 | |
| ABE | 4.54E+06 | 1 | 4.54E+06 | 233.39 | <0.0001 | |
| ACE | 2.27E+05 | 1 | 2.27E+05 | 11.68 | 0.0091 | |
| ADE | 2.25E+06 | 1 | 2.25E+06 | 115.41 | <0.0001 | |
| BCD | 1.42E+06 | 1 | 1.42E+06 | 72.82 | <0.0001 | |
| BCE | 5.99E+05 | 1 | 5.99E+05 | 30.8 | 0.0005 | |
| ABCD | 6.30E+05 | 1 | 6.30E+05 | 32.38 | 0.0005 | |
| ABCE | 1.25E+06 | 1 | 1.25E+06 | 64.02 | <0.0001 | |
| ACDE | 2.42E+06 | 1 | 2.42E+06 | 124.24 | <0.0001 | |
| ABCDE | 1.98E+05 | 1 | 1.98E+05 | 10.17 | 0.0128 | |
| Residual | 1.56E+05 | 8 | ||||
| Cor Total | 3.37E+07 | 31 | ||||
Final equations in terms of coded factors.
CMCase activity
Enzyme activity = +197.86 − 21.08A + 53.92B + 10.11C + 23.22D + 9.75E + 7.65AB − 13.52AC + 19.2AD + 14.33AE − 16.75BC + 44.57BD − 24.38CD − 11.75CE − 32.09DE + 10.4ABC + 15.27ABE + 24.61ACD − 22.6ACE + 24.43ADE + 8.27BCD − 11.33BCE − 45.15BDE − 20.64ABCE + 25.91ABDE + 6.49ACDE − 16.62BCDE + 10.52ABCDE.
Protease activity
Enzyme activity = +1700.69 + 110.85A + 297.78B + 336.7C + 295.07D + 79.4E + 226.93AB − 82.29AC + 160.17AD + 63.11AE + 55.88BC + 344.94BD − 266.95BE − 57.21CE + 186.2ABD + 376.63ABE + 84.25ACE + 264.85ADE + 210.38BCD − 136.82BCE − 140.29ABCD + 197.26ABCE + 274.8ACDE + 78.62ABCDEwhere A is sucrose, B is peptone, C is NaH2PO4, D is pH, and E is moisture.
Central composite design
Optimizing process parameters was carried out using RSM. The factors—namely, pH, peptone, and NaH2PO4, which significantly influenced both CMCase and protease production—were selected for further optimization using central composite design (CCD) to maximize the CMCase and protease production. Our findings showed that peptone, NaH2PO4, and pH positively influenced CMCase and protease production. However, an excessive concentration of NaH2PO4 had a negative effect on protease production. Most cellulases and proteases are inducible enzymes and addition of carbon sources, such as sucrose, mannitol, and maltose, enhanced the production of cellulolytic and proteolytic enzymes [56, 57]. It was previously reported that the production of protease was enhanced by the addition of nitrogen sources, such as tryptone, peptone, yeast extract, skimmed milk, and soybean meal [58]. The observed response in the production of CMCase and protease is shown in Table 3. As shown in Table 4, the p value of the model generated was <0.05, suggesting the CMCase and protease activity could be well-described by this model. Analysis of variance (ANOVA) was carried out to establish a response surface quadratic model. The model F values of 67.14 and 197.54 implied that both quadratic models for the production of CMCase and protease were significant. The model terms, such as A, B, C, AB, A2, B2, and C2, were significant for the production of CMCase; B, C, AB, AC, BC, A2, and B2 were significant for protease production. For CMCase production, the R2 of the model was 0.9837, indicating that the experimental data agreed well with the model prediction. The model could explain 98.37 % variability observed in the data [59]. In the case of protease production, the R2 value was 0.9944. The model can explain 99.44 % variability observed in the data. The lack of fit values were 3.17 and 0.9658 for CMCase and protease production, respectively, which were not significant. The signal-to-noise ratios of the models were 24.693 and 46.447, respectively, which indicated an adequate signal for both models. The data obtained from the models were fitted to the following second-order polynomial equation for both enzymes.
Table 3.
Central composite design of the medium component in coded units for CMCase and protease production
| Std | A:pH | B:Peptone | C:NaH2PO4 | Enzyme activity (/g) | |
|---|---|---|---|---|---|
| Cellulase | Protease | ||||
| 1 | 0 | 0 | 0 | 464 | 4040 |
| 2 | −1 | −1 | −1 | 270 | 2080 |
| 3 | 0 | 0 | 0 | 440 | 4163 |
| 4 | 0 | 0 | 1.682 | 348 | 3942 |
| 5 | 1.682 | 0 | 0 | 370 | 1798 |
| 6 | 1 | 1 | 1 | 418 | 4080 |
| 7 | 1 | −1 | −1 | 295 | 4019 |
| 8 | 0 | 0 | 0 | 442 | 4530 |
| 9 | −1 | −1 | 1 | 180 | 1728 |
| 10 | 0 | 0 | 0 | 462 | 4120 |
| 11 | −1 | 1 | 1 | 362 | 4686 |
| 12 | 1 | 1 | −1 | 298 | 3501 |
| 13 | 1 | −1 | 1 | 320 | 398 |
| 14 | 0 | 1.682 | 0 | 434 | 4000 |
| 15 | 0 | 0 | −1.682 | 152 | 4611 |
| 16 | 0 | 0 | 0 | 429 | 4200 |
| 17 | −1.682 | 0 | 0 | 127 | 1608 |
| 18 | −1 | −1 | −1 | 79 | 3263 |
| 19 | 0 | 0 | 0 | 462 | 4180 |
| 20 | 0 | −1.682 | 0 | 252 | 2109 |
Table 4.
ANOVA for the quadratic model for CMCase activity and protease activity of B. subtilis IND19
| Source | Sum of squares | df | Mean square | F value | p value | |
|---|---|---|---|---|---|---|
| CMCase activity of B. subtilis IND19 | ||||||
| Model | 2.75E+05 | 9 | 3.05E+04 | 67.14 | <0.0001 | Significant |
| A-pH | 5.27E+04 | 1 | 5.27E+04 | 116.04 | <0.0001 | |
| B-Peptone | 4.46E+04 | 1 | 4.46E+04 | 98.04 | <0.0001 | |
| C-NaH2PO4 | 3.26E+04 | 1 | 3.26E+04 | 71.81 | <0.0001 | |
| AB | 9.25E+03 | 1 | 9.25E+03 | 20.35 | 0.0011 | |
| AC | 2.88E+02 | 1 | 2.88E+02 | 0.63 | 0.4445 | |
| BC | 924 | 1 | 924 | 2.03 | 0.1843 | |
| A 2 | 69,800.17 | 1 | 69,800.17 | 153.57 | <0.0001 | |
| B 2 | 1.89E+04 | 1 | 1.89E+04 | 41.51 | <0.0001 | |
| C 2 | 6.87E+04 | 1 | 6.87E+04 | 151.24 | <0.0001 | |
| Residual | 4.55E+03 | 10 | 454.51 | |||
| Lack of fit | 3456.22 | 5 | 691.24 | 3.17 | Not significant | |
| Pure error | 1.09E+03 | 5 | 217.77 | |||
| Cor total | 2.79E+05 | 19 | ||||
| Protease activity of B. subtilis IND19 | ||||||
| Model | 2.95E+09 | 9 | 3.27E+06 | 197.54 | <0.0001 | Significant |
| A-pH | 2.30E+04 | 1 | 2.30E+04 | 1.39 | 0.266 | |
| B-Peptone | 4.82E+0.005 | 1 | 4.82E+0.005 | 291.22 | <0.0001 | |
| C-NaH2PO4 | 7.02E+05 | 1 | 7.02E+05 | 42.35 | <0.0001 | |
| AB | 2.412E+0.05 | 1 | 2.412E+0.05 | 14.55 | 0.0034 | |
| AC | 2.12E+06 | 1 | 2.12E+06 | 127.58 | <0.0001 | |
| BC | 8.70E+06 | 1 | 8.70E+06 | 524.67 | <0.0001 | |
| A 2 | 1.11E+07 | 1 | 1.11E+07 | 670.76 | <0.0001 | |
| B 2 | 2.31E+06 | 1 | 2.31E+06 | 139.47 | <0.0001 | |
| C 2 | 1.43E+04 | 1 | 1.43E+04 | 0.86 | 0.3744 | |
| Residual | 1.66E+05 | 10 | 16,575.16 | |||
| Lack of fit | 23,264.15 | 5 | 4652.83 | 0.16 | 0.9658 | Not significant |
| Pure error | 1.43E+05 | 5 | 28,497.5 | |||
| Cor total | 2.96E+07 | 19 | ||||
The final equations in terms of coded factors are as follows.
CMCase activity
Enzyme activity = +449.59 + 62.14A + 57.12B + 48.89C − 34AB − 6AC + 10.75BC − 69.59A2 − 36.18B2 − 69.06C2.
Protease activity
Enzyme activity = + 4204.52 + 41.04A + 594.52B − 226.71C + 173.63AB − 514.12AC + 1042.63BC − 878.34A2 − 400.51B2 + 31.53C2.
The 3D response surface curves in Fig. 1a–c show the interactions among pH, peptone, and NaH2PO4. These 3D graphs are helpful to identify the interaction between the variables and their levels. The increase in CMCase production was observed in peptone and pH (Fig. 1a), NaH2PO4 and pH (Fig. 1b), and NaH2PO4 and peptone (Fig. 1c). However, further increase in all of these three variables beyond the optimized level decreased the production of enzymes. Similarly, the protease production was increased by increasing the concentrations of peptone and NaH2PO4 (Fig. 2a–c) and was decreased after optimum concentrations of these factors. This was consistent with the fact that CMCase and protease were generally induced in the presence of carbon, nitrogen, minerals, and alteration of pH [18, 23, 36].
Fig. 1.
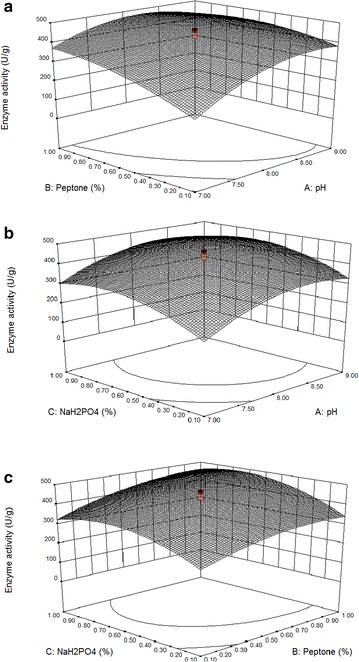
a Response surface curve showing the effects of pH and peptone on the CMCase activity of B. subtilis IND19 in SSF using cow dung substrate. b Response surface curve showing the effects of pH and NaH2PO4 on the CMCase activity of B. subtilis IND19 in SSF using cow dung substrate. c Response surface curve showing the effects of peptone and NaH2PO4 on the CMCase activity of B. subtilis IND19 in SSF using cow dung substrate
Fig. 2.
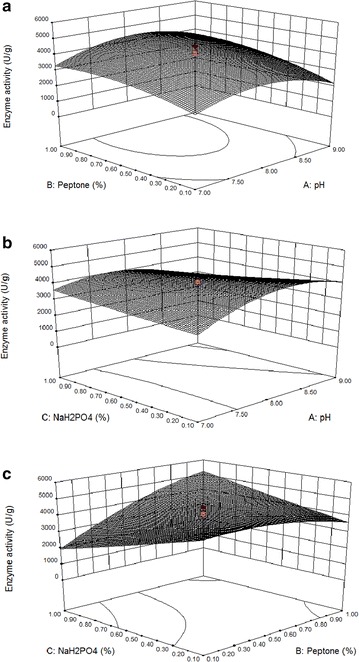
a Response surface curve showing the effects of pH and peptone on the protease activity of B. subtilis IND19 in SSF using cow dung substrate. b Response surface curve showing the effects of pH and NaH2PO4 on the protease activity of B. subtilis IND19 in SSF using cow dung substrate. c Response surface curve showing the effects of peptone and NaH2PO4 on the protease activity of B. subtilis IND19 in SSF using cow dung substrate
RSM has been widely used for the production of enzymes in SSF by various studies [60–63]. It helps to identify the interactive effects of selected parameters and requires the minimum number of experimental runs [64]. In this paper, the maximum CMCase and protease production were observed at 0.9 % peptone, 0.78 % NaH2PO4, and a substrate pH 8.41, and 1 % peptone, 0.72 % NaH2PO4, and a substrate pH of 8.11, respectively. Under the optimized conditions, the experimental yield of CMCase and protease reached 473.01 and 4643 U/g, which corresponded to the increase of 2.1-fold and 2.5-fold in CMCase and protease production. This finding could be observed because cow dung is a complex biomass already containing essential nutrients for the growth of microbes [24]. Hence, the addition of nutrient sources merely increased approximately twofold on CMCase and protease production.
Validation of the experimental model
The response surface model was validated with triplicate experiments under the predicted experimental conditions. The predicted response for CMCase production was 485.05 U/g, which was very close to the experimental value (473.01 U/g), thereby validating this model. The predicted response of the model for the production of protease was 4710 U/g, and the experimental value was 4643 U/g, which validated the model.
SDS-PAGE analysis of the extracellular protein from B. subtilis IND19
SDS-PAGE analysis revealed the protein pattern from the crude extract of B. subtilis IND19 (Fig. 3a). Zymogram analysis of the crude CMCase exhibited a band which corresponds to 44 kDa (Fig. 3b). This result was in accordance with the observations made with other Bacillus sp. [65]. The molecular weight of the protease was calculated and was found to be approximately 36.12 kDa (Fig. 3c). The molecular weight of protease was similar to that of the previous reports. Generally, the molecular masses of proteases from various Bacillus species range between 17 and 44 kDa [66, 67].
Fig. 3.
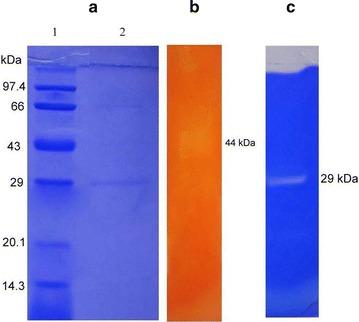
Electrophoresis analysis of the crude enzyme from B. subtilis IND19 (Lane 1: Protein marker, Lane 2: crude protein lysate) (a); Zymogram analysis of the crude enzyme for CMCase activity (b) and Zymogram analysis of the crude enzyme for protease activity (c)
Conclusions
This study aimed to optimize the simultaneous production of CMCase and protease by Bacillus subtilis IND19 with RSM. This report describes the first time that cow dung was applied as the substrate for the simultaneous production of these two enzymes in a single fermentation system. This cheap substrate could be useful for the production of CMCase and protease at industrial scale. RSM-mediated experimental design exhibited an increase of 2.1- and 2.5-fold, respectively, for CMCase and protease compared to non-optimized medium. This paper revealed that RSM is a suitable statistical tool in optimizing enzyme production with minimum experimental runs.
Methods
Microorganism
The CMCase- and protease-producing B. subtilis IND19 was isolated from the soil sample. The isolated B. subtilis IND19 was maintained on nutrient agar slants (in g/l) (peptic digest of animal tissue, 5.0; beef extract, 1.5; yeast extract, 1.5; sodium chloride, 5.0; and agar, 15) and stored at 4 °C for further experiments. This organism was subcultured every 30 days.
Screening of B. subtilis IND19 for cellulolytic and proteolytic enzyme
CMCase screening was carried out using carboxy methyl cellulose (CMC) agar medium (in g/l) (beef extract, 5.0; peptic digest of animal tissue, 5.0; yeast extract, 1.5; sodium chloride, 5.0; agar, 15; and CMC, 10). The bacterial growth was visible on these plates after 48 h incubation at 37 °C. To visualize the hydrolysis of CMC agar medium, the plate was stained with Gram’s iodine solution. This formed a bluish black complex with CMC and gave a distinct zone after 5 min [68]. The cellulolytic bacterial isolate, B. subtilis IND19 was further grown on skimmed milk agar medium (in g/l) (agar, 15; yeast extract, 5; peptone, 5; KH2PO4, 1.0; MgSO4, 0.2; NaCl, 10; skimmed milk, 10, and pH 10.0). The maximum enzyme-producing bacterial isolate was selected for further studies.
Molecular identification of the strain
The bacterial isolate was cultured for 18 h in the medium which contained (in g /l): (1) beef extract, 1.5; (2) peptic digest of animal tissue, 5; (3) yeast extract, 1.5; and (4) sodium chloride, 5 (pH 7.0). The genomic DNA of the selected bacterial isolate was purified using a QIAGEN DNA purification kit (Germany) according to the manufacturer’s instructions. The 16S rRNA gene of B. subtilis IND19 was amplified using the upstream primer (P1: 5′-AGAGTTTGATCMTGGCTAG-3′) and the downstream primer (P2: 5′-ACGGGCGG TGTGTRC-3′) (Sigma-Aldrich) [69]. The research gradient Peltier Thermal cycler machine PTC-225 and DNA polymerase (Sigma-Aldrich) were used to amplify the DNA. The following conditions were employed while amplifying DNA: denaturation at 95 °C for 3 min followed by 30 cycles at 95 °C for 1 min, 55 °C for 30 s, and 72 °C for 1 min and 50 s. The amplified 16S rDNA PCR product was sequenced. Further, the identity of the sequences was checked by BLAST through the NCBI server. The 831 bp 16S rDNA sequences of the bacterial isolate were submitted to GenBank and the accession number was assigned (KF688989).
Inoculum
B. subtilis IND19 was grown in the medium which contained (in g/l): (1) beef extract, 5; (2) peptic digest of animal tissue, 5; (3) yeast extract, 1.5; and (4) sodium chloride, 5). The medium was sterilized at 15 lbs for 30 min and cooled. Next, a loopful culture of B. subtilis IND19 was inoculated into the 100-ml Erlenmeyer flask. This was incubated on a rotary shaker (175 rpm) at 37 °C for 18 h. This culture was stored at 2–8 °C and was used as the inoculum.
Substrate
Cow dung was collected from a farm house (Nagercoil, Kanyakumari, Tamil Nadu, India). It was dried for 10 days and powdered. It was stored in an air tight container before further use.
Production of CMCase and protease under SSF
Cow dung substrate (5 g) was weighed in Erlenmeyer flask (250 ml) and a buffer solution (pH of 8.0, Tris–HCl buffer, 0.1 M) was added to maintain moisture content of the substrate and initial medium pH. Initial moisture content of the medium was maintained as 90 % (v/w). The solid substrate was mixed carefully with buffer and autoclaved at 15 lbs for 30 min and cooled. Then, 10 % inoculum (0.653 OD at 600 nm) was added to the culture medium. The contents were further mixed and incubated for 72 h under 37 °C.
Enzyme extraction
The fermented medium was stirred with double distilled water (1:10 ratio) and shaken at 175 rpm for 30 min in a rotary shaker. The mixed slurry was then completely filtered using cotton, followed by centrifugation at 10,000 rpm at 4 °C for 10 min. The cell free extract was used as the crude enzyme [49].
CMCase assay
CMCase activity was assayed using CMC as the substrate. Hundred microliter of crude enzyme was mixed with 100 μL of 1 % (w/v) CMC (pH 7.5) and incubated at 37 °C for 30 min. Next, 1.5 ml of dinitrosalicylate reagent was added, and the mixture was incubated at 100 °C for 10 min. The mixture was cooled, and the absorbance was measured against the reagent blank at 540 nm. One unit of CMCase activity was defined as the amount of enzyme that liberated 1 μmol of reducing sugars per minute under the above conditions [70].
Protease assay
Casein was used as the substrate for the determination of protease activity. The reaction mixture contained 1.0 ml casein which was prepared in Tris–HCl buffer (0.05 M, pH 8.0) and 0.1 ml of enzyme solution [71]. This mixture was incubated for 30 min at 37 °C and 2.5 ml trichloroacetic acid (0.11 M) was added to terminate the enzyme reaction. It was centrifuged at 10,000 g for 10 min, and the absorbance of the sample was read against sample blank at 280 nm. One unit of the protease activity was defined as 1 μg of tyrosine liberated min−1 under standard assay conditions.
Screening the optimal carbon, nitrogen, and mineral sources
The effect of carbon sources (1 %, w/w; sucrose, maltose, fructose, xylose, and glucose), nitrogen sources (1 %, w/w; peptone, yeast extract, oat meal, beef extract, and ammonium sulphate), and ionic sources (ferrous sulphate, di-sodium hydrogen phosphate, ammonium chloride, sodium nitrate, calcium chloride, and sodium dihydrogen phosphate) were screened for optimal production of CMCase and protease.
Elucidation of significant factors affecting CMCase and protease production by statistical approach
Two-level full factorial design (25) was used to identify the significant factors relative to CMCase and protease yield. In this paper, two important physical factors and three nutritional factors were selected. These variables and the selected ranges were based on the results obtained from one-variable-at-a-time approach. The factors selected were sucrose (carbon source), peptone (nitrogen source), NaH2PO4 (mineral), pH, and moisture (physical factors). Each variable was tested at two levels [high (+) and low (−1)]. In two-level full factorial design (25), a total of 32 experimental runs were generated and the enzyme activities (CMCase and protease) were determined from the crude sample. The variables and their levels are shown in Table 5. The other factors, namely, inoculum size and fermentation period, were kept at optimum level. Two-level full factorial design was based on the χ.
Table 5.
Variables and their levels for CMCase and protease production using 25 full factorial design
| Symbol | Variables | Units | Coded levels | |
|---|---|---|---|---|
| −1 | 1 | |||
| A | Sucrose | % | 0.1 | 1 |
| B | Peptone | % | 0.1 | 1 |
| C | NaH2PO4 | % | 0.01 | 0.1 |
| D | pH | % | 6 | 8 |
| E | Moisture | % | 90 | 110 |
where αij, αijk, αijkl, and αijklm are the ijth, ijkth, ijklth, and ijklmth interaction coefficients, respectively, αi is the ith linear coefficient, and α0 is an intercept.
Assays of CMCase and protease were carried out in triplicates, and the mean value was taken as response (Y) (Table 1). ANOVA was used to evaluate the significance of these models, and the p value <0.05 indicated that the model terms were significant. Statistical software Design-Expert 9.0.6.2 was used to design the experiments and analyse the results.
Central composite design and response surface methodology
The CCD was used to identify the optimum concentrations of the factors in order to obtain the maximum CMCase and protease production. The variables selected were analysed at five levels (−α, −1, 0, +1, +α) (Table 6). According to the Design-Expert 9.0.6.2, for these variables CCD consists of 20 experimental runs including, eight factorial, six axial, and six centre points. Five gram of substrate was taken in 250-ml Erlenmeyer flask, and the required quantities of peptone and NaH2PO4 were added according to the model. The pH of the medium was maintained according to the model design. The substrate and the supplemented nutrients were mixed carefully, sterilized (121 ± 1 °C for 20 min), and cooled. The Erlenmeyer flasks were inoculated with a 0.5-ml of inoculum (10 %, v/w) and incubated at 37 °C for 72 h. The enzyme was extracted as described previously in the materials and methods. After which, CMCase and protease assays were carried out individually in triplicate. The mean value of the experimental results was considered as response Y (Table 3). The fact that values of Prob(>F) are smaller than 0.05 would signify that the model terms were significant (Table 4). The experimental results of the CCD were fitted with a following second-order polynomial equation.
Table 6.
Experimental variables used for optimization of CMCase and protease production in B. subtilis IND19
| Variables | Symbol | Coded values | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | +α | ||
| pH | A | 6.32 | 7 | 8 | 9 | 9.68 |
| Peptone | B | −0.21 | 0.1 | 0.55 | 1 | 1.31 |
| NaH2PO4 | C | −0.21 | 0.1 | 0.55 | 1 | 1.31 |
where Y is the enzyme activity (U/g); A is the coded value of pH; B is the coded value of the peptone; C is the coded value of NaH2PO4; α1, α2, and α3 are the linear coefficients; α1α2, α1α3, and α2α3 are the interactive coefficients; and α1α1, α2α2, and α3α3 are the quadratic coefficients.
Response surface graphs were plotted to determine the optimum concentration of factors for the production of CMCase and protease. The fitted polynomial equation was expressed as 3D surface plots to visualize the relation between responses and the experimental levels of each factor used in the design. Validation of the model was performed under the conditions predicted by the model. The predicted response of the model was validated experimentally. Experiments were carried out in triplicates and validated.
SDS-PAGE and zymogram analysis for CMCase and protease activity
SDS-PAGE was performed using polyacrylamide gel (12 %) [72]. 25 µg crude protein sample was loaded on SDS-PAGE to determine the molecular weight of extracellular protein from B. subtilis IND19. A protein marker (97.4–14.3 kDa) was used to determine the molecular weight of proteins. CMCelluose (0.1 %) was co-polymerized to determine CMCase activity and casein (0.1 %) was co-polymerized with SDS-PAGE to determine the protease activity. The sample was not heated before electrophoresis. Zymography analysis was carried out as described previously [73, 74].
Authors’ contributions
PV and AA conducted the experiments. SGPV and KCC participated in the design of the study. NAA and MVV analysed the statistical data. All authors read and approved the final manuscript.
Acknowledgements
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this Prolific Research Group (PRG-1437-28).
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- CMCase
carboxy methyl cellulase
- RSM
response surface methodology
- SSF
solid state fermentation
- NaH2PO4
sodium dihydrogen phosphate
- Na2HPO4
di-sodium hydrogen phosphate
- 3D
three dimensional
- ANOVA
analysis of variance
- CMC
carboxy methyl cellulose
- PCR
polymerase chain reaction
- OD at 600 nm
optical density at 600 nm
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
Contributor Information
Ponnuswamy Vijayaraghavan, Email: venzymes@gmail.com.
Arumugaperumal Arun, Email: biotechstudent@gmail.com.
Naif Abdullah Al-Dhabi, Email: naldhabi@ksu.edu.sa.
Samuel Gnana Prakash Vincent, Email: enzyme.nanobiotechnology@gmail.com.
Mariadhas Valan Arasu, Email: mvalanarasu@gmail.com.
Ki Choon Choi, Email: choiwh@korea.kr.
References
- 1.Adeleke EO, Omafuvbe BO, Adewale IO, Bakare MK. Purification and characterisation of a cellulase obtained from cocoa (Theobroma cacao) pod-degrading Bacillus coagulans Co4. Turk J Biochem. 2012;37:222–230. doi: 10.5505/tjb.2012.47955. [DOI] [Google Scholar]
- 2.Ilmen M, den Haan R, Brevnova E, McBride J, Wiswall E, Froehlich A, et al. High level secretion of cellobiohydrolases by Saccharomyces cerevisiae. Biotechnol Biofuel. 2011;4:30. doi: 10.1186/1754-6834-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue H, Decker SR, TaylorII LE, Yano S, Sawayama S. Identification and characterization of core cellulolytic enzymes from Talaromyces cellulolyticus (formerly Acremonium cellulolyticus) critical for hydrolysis of lignocellulosic biomass. Biotechnol Biofuel. 2014;7:151. doi: 10.1186/s13068-014-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasetyo J, Naruse K, Kato T, Boonchird C, Harashima S, Park EY. Bioconversion of paper sludge to biofuel by simultaneous saccharification and fermentation using a cellulase of paper sludge origin and thermotolerant Saccharomyces cerevisiae TJ14. Biotechnol Biofuel. 2011;4:35. doi: 10.1186/1754-6834-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dienes D, Egyházi A, Réczey K. Treatment of recycled fiber with Trichoderma cellulases. Ind Crop Prod. 2004;20:11–21. doi: 10.1016/j.indcrop.2003.12.009. [DOI] [Google Scholar]
- 6.Duan XY, Liu SY, Zhang WC. Volumetric productivity improvement for endoglucanase of Trichoderma pseudokoingii S-38. J Appl Microbiol. 2004;96:772–776. doi: 10.1111/j.1365-2672.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Fatah OM, Hassan MM, Elshafei AM, Haroun BM, Atta HM, Othman AM. Physiological studies on carboxymethyl cellulase formation by Aspergillus terreus DSM 826. Braz J Microbiol. 2012;43:1–11. doi: 10.1590/S1517-83822012000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs MF. Expression of the subtilisin Carlsberg-encoding gene in Bacillus licheniformis and Bacillus subtilis. Gene. 1995;152:67–74. doi: 10.1016/0378-1119(94)00655-C. [DOI] [PubMed] [Google Scholar]
- 9.Binod P, Palkhiwala P, Gaikaiwari R, Nampoothiri K, Duggal A, Dey K, Pandey A. Industrial enzymes: present status and future perspectives for India: Present scenario and perspectives. J Sci Ind Res. 2013;72:271–286. [Google Scholar]
- 10.Joo HS, Kumar CG, Park GC, Paik SR, Chang CS. Oxidant and SDS-stable alkaline protease from Bacillus Clausii I-52: production and some properties. J Appl Microbiol. 2003;95:267–272. doi: 10.1046/j.1365-2672.2003.01982.x. [DOI] [PubMed] [Google Scholar]
- 11.Cen PL, Xia LM. Production of cellulase by solid-state fermentation. Adv Biochem Engin Biotechnol. 1999;65:68–92. [Google Scholar]
- 12.Vijayaraghavan P, Vijayan A, Arun A, Jenisha J, Vincent SGP. Cow dung: a potential biomass substrate for the production of detergent-stable dehairing protease by alkaliphilic Bacillus subtilis strain VV. SpringerPlus. 2012;2012(1):6. doi: 10.1186/2193-1801-1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrios-Gonzalez J. Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochem. 2012;47:175–185. doi: 10.1016/j.procbio.2011.11.016. [DOI] [Google Scholar]
- 14.Rajoka MI, Malik KA. Cellulase production by Cellulomonas biazotea cultured in media containing different cellulosic substrates. Bioresour Technol. 1997;59:21–27. doi: 10.1016/S0960-8524(96)00136-8. [DOI] [Google Scholar]
- 15.Kansoh AL, Essam SA, Zeinat AN. Biodegradation and utilization of bagasse with Trichoderma reesei. Polym Degrad Stab. 1999;62:273–278. doi: 10.1016/S0141-3910(98)00105-0. [DOI] [Google Scholar]
- 16.Johnvesly B, Manjunath BR, Naik GR. Pigeon pea waste as a novel, inexpensive, substrate for production of a thermostable alkaline protease from thermoalkalophilic Bacillus sp. JB-99. Bioresour Technol. 2002;82:61–64. doi: 10.1016/S0960-8524(01)00147-X. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen H, Olsson L. Production of cellulase by Penicillium brasilianum IBT 20888—effect of substrate on hydrolytic performance. Enzyme Microb Technol. 2006;38:381–390. doi: 10.1016/j.enzmictec.2005.06.018. [DOI] [Google Scholar]
- 18.Prakasham RS. Subba Rao C, Sarma PN: Green gram husk: an inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresour Technol. 2006;97:1449–1454. doi: 10.1016/j.biortech.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee AK, Adhikari H, Rai SK. Production of alkaline protease by a thermophilic Bacillus subtilis under solid-state fermentation (SSF) condition using Imperata cylindrical grass and potato peel as low cost medium: characterization and application of enzyme in detergent formulation. Biochem Eng J. 2008;39:353–361. doi: 10.1016/j.bej.2007.09.017. [DOI] [Google Scholar]
- 20.Oberoi HS, Chavan Y, Bansal S, Dhillon GS. Production of cellulases through solid state fermentation using kinnow pulp as a major substrate. Food Bioprocess Technol. 2010;3:528–536. doi: 10.1007/s11947-008-0092-8. [DOI] [Google Scholar]
- 21.Dhillon GS, Oberoi HS, Kaur S, Bansal S, Brar SK. Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid state tray fermentation employing mixed-culture of fungi. Ind Crops Prod. 2011;34:1160–1167. doi: 10.1016/j.indcrop.2011.04.001. [DOI] [Google Scholar]
- 22.Dhillon GS, Brar SK, Kaur S, Sabrine M, M’hamdi N. Lactoserum as a moistening medium and crude inducer for fungal cellulases and hemicellulase induction through solid-state fermentation of apple pomace. Biomass Bioen. 2012;41:165–174. doi: 10.1016/j.biombioe.2012.02.021. [DOI] [Google Scholar]
- 23.Dhillon GS, Brar SK, Kaur S, Valero JR. Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through SSF. Ind Crops Prod. 2012;8:6–13. doi: 10.1016/j.indcrop.2011.12.036. [DOI] [Google Scholar]
- 24.Misra RV, Roy RN, Hiraoka H. Farm Composting Method. Rome: FAO; 2003. [Google Scholar]
- 25.Huang XP. Purification and characterization of a cellulase (CMCase) from a newly isolated thermophilic aerobic bacterium Caldibacillus cellulovorans gen. nov. sp. World J Microbiol Biotechnol. 2004;20:85–92. doi: 10.1023/B:WIBI.0000013316.12730.e7. [DOI] [Google Scholar]
- 26.Macedo JMB, Gottschalk LMF, Bon EPS. Lignin peroxidase and protease production by Streptomyces viridosporus T7A in the presence of calcium carbonate, nutritional and regulatory carbon sources. Appl Biochem Biotechnol. 1999;77–79:735–744. doi: 10.1385/ABAB:79:1-3:735. [DOI] [PubMed] [Google Scholar]
- 27.Suto M, Tomita F. Induction and catabolite repression mechanisms of cellulase in fungi. J Biosci Bioeng. 2001;92(4):305–311. doi: 10.1016/S1389-1723(01)80231-0. [DOI] [PubMed] [Google Scholar]
- 28.Yang JK, Shih IL, Tzeng YM, Wang SL. Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microb Technol. 2000;26:406–413. doi: 10.1016/S0141-0229(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Li J, Meng D, Lu J, Gu G, Mao Z. Effect of pH, cultivation time and substrate concentration on the endoxylanase production by Aspergillus awamori ZH-26 under submerged fermentation using central composite rotary design. Food Technol Biotechnol. 2006;44:473–477. [Google Scholar]
- 30.Jeya M, Nguyen NPT, Moon HJ, Kim SM, Lee JK. Conversion of woody biomass into fermentable sugars by cellulase from Agaricus arvensis. Bioresour Technol. 2010;101:8742–8749. doi: 10.1016/j.biortech.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 31.Chen F, Cai T, Zhao G, Liao X, Guo L, Hu X. Optimizing conditions for the purification of crude octacosanol extract from rice bran wax by molecular distillation analyzed using response surface methodology. J Food Eng. 2005;70:47–53. doi: 10.1016/j.jfoodeng.2004.09.011. [DOI] [Google Scholar]
- 32.Zambare V. Optimization of amylase production from Bacillus sp. using statistics based experimental design. Emir J Food Agric. 2011;23:37–47. doi: 10.9755/ejfa.v23i1.5311. [DOI] [Google Scholar]
- 33.Liu BL, Tzeng YM. Optimization of growth medium for production of spores from Bacillus thuringiensis using response surface methodology. Bioprocess Eng. 1998;18:413–418. [Google Scholar]
- 34.Elibol M. Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3 (2) with response surface methodology. Process Biochem. 2003;39:1057–1062. doi: 10.1016/S0032-9592(03)00232-2. [DOI] [Google Scholar]
- 35.Billard H, Faraj A, Ferreira NL, Menir S, Heiss-Blanquet S. Optimization of a synthetic mixture composed of major Trichoderma reesei enzymes for the hydrolysis of steam-exploded wheat straw. Biotechnol Biofuel. 2012;5:9. doi: 10.1186/1754-6834-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijayaraghavan P, Vincent SGP, Dhillon GS. Solid-substrate bioprocessing of cow dung for the production of carboxymethyl cellulase by Bacillus halodurans IND18. Waste Manage. 2016;48:513–520. doi: 10.1016/j.wasman.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Zaghloul TI, Wahab AEA, Mostafa MH. Enhanced alkaline protease production in addition to α-amylase via constructing a Bacillus subtilis strain. Appl Biochem Biotechnol. 2000;84:319–327. doi: 10.1385/ABAB:84-86:1-9:319. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Xing XH, Liu MS. Production of multienzymes consisting of alkaline amylase and cellulase by mixed alkalophilic culture and their potential use in the saccharification of sweet potato. Biochem Eng J. 2004;19(2):181–187. doi: 10.1016/j.bej.2004.01.001. [DOI] [Google Scholar]
- 39.Negi S, Banerjee R. Optimization of culture parameters to enhance production of amylase and protease from Aspergillus awamori in a single fermentation. Afr J Biochem Res. 2010;4(3):73–80. [Google Scholar]
- 40.Abidi F, Limam F, Nejib MM. Production of alkaline proteases by Botrytis cinerea using economic raw materials: assay as biodetergent. Process Biochem. 2008;43(11):1202–1208. doi: 10.1016/j.procbio.2008.06.018. [DOI] [Google Scholar]
- 41.Sangeetha R, Geetha A, Arulpandi I. Concomitant production of protease and lipase by Bacillus licheniformsis VSG1: production, purification and characterization. Braz J Microbiol. 2010;41:179–185. doi: 10.1590/S1517-83822010000100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukhtar H, Haq I. Concomitant production of two proteases and alpha-amylase by a novel strain of Bacillus subtilis in a microprocessor controlled bioreactor. Braz J Microbiol. 2012;43(3):1072–1079. doi: 10.1590/S1517-83822012000300033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxena R, Singh R. Contemporaneous Production of Amylase and Protease through CCD Response Surface Methodology by Newly Isolated Bacillus megaterium Strain B69. Enzyme Res. 2014;2014:601046. doi: 10.1155/2014/601046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aboul-Enein A, Abou elalla F, Serour E, Hussien T. Purification and characterization of a novel thermoactive cellulase from thermophilic Actinomycetes isolated from soil sample of Egypt. Int J Acad Res. 2010;2:81–86. [Google Scholar]
- 45.Das A, Bhattacharya S, Murali L. Production of cellulase from Thermophilic Bacillus sp. isolated from cow dung. AM Eurasian J Agric Environ Sci. 2010;8:685–691. [Google Scholar]
- 46.Verma V, Verma A, Kushwaha A. Isolation and production of cellulase enzyme from bacteria isolated from agricultural fields in district Hardoi, Uttar Pradesh, India. Adv Appl Sci Res. 2012;3:171–174. [Google Scholar]
- 47.Sharma R, Chisti Y, Banerjee UC. Production, purification, characterization, and applications of lipases. Biotechnol Adv. 2001;19:627–662. doi: 10.1016/S0734-9750(01)00086-6. [DOI] [PubMed] [Google Scholar]
- 48.Pandey A, Soccol CR, Nigam P, Brand D, Mohan R, Roussos S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem Eng J. 2000;6:153–162. doi: 10.1016/S1369-703X(00)00084-X. [DOI] [PubMed] [Google Scholar]
- 49.Vijayaraghavan P, Vincent SGP. Cow dung as a novel, inexpensive substrate for the production of a halo-tolerant alkaline protease by Halomonas sp. PV1 for eco-friendly applications. Biochem Eng J. 2012;69:57–60. doi: 10.1016/j.bej.2012.08.014. [DOI] [Google Scholar]
- 50.Kapoor N, Tyagi M, Kumar H, Arya A, Siddiqui MA, Amir A, Malik AS. Production of cellulase enzyme by Chaetomium sp. using wheat straw in solid state fermentation. Res J Microbiol. 2010;5:1199–1206. doi: 10.3923/jm.2010.1199.1206. [DOI] [Google Scholar]
- 51.Wei ZJ, Zhou LC, Chen H, Chen GH. Optimization of the fermentation conditions for 1-deoxynojirimycin production by Streptomyces lavendulae applying the response surface methodology. Int J Food Eng. 2011;7:1–10. doi: 10.2202/1556-3758.2354. [DOI] [Google Scholar]
- 52.Umikalsom MS, Ariff AB, Shamsuddin ZH, Tong CC, Hassan MA, Karim MIA. Production of cellulase by a wild strain of Chaetomium globosum using delignified oil empty-fruit-bunch fibre as substrate. Appl Microbiol Biotechnol. 1997;47:590–595. doi: 10.1007/s002530050978. [DOI] [Google Scholar]
- 53.Shanmughapriya S, Kiran GS, Selvin J, Thomas TA, Rani C. Optimization, purification, nd characterization of extracellular mesophilic alkaline cellulase from sponge-associated Marinobacter sp. MSI032. Appl Biochem Biotechnol. 2010;162:625–640. doi: 10.1007/s12010-009-8747-0. [DOI] [PubMed] [Google Scholar]
- 54.Mazdak C, Treton B, Blanchin-Roland S. Strong hybrid promoters and integrative expression/secretion vectors for quasiconstitutive expression of heterologous proteins in the yeast Yarrowia lipolytica. J Mol Microbiol Biotechnol. 2000;2:207–216. [PubMed] [Google Scholar]
- 55.Puri S, Beg QK, Gupta R. Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Curr Microbiol. 2002;44:286–290. doi: 10.1007/s00284-001-0006-8. [DOI] [PubMed] [Google Scholar]
- 56.Chellappan S, Jasmin C, Basheer SM, Elyas KK, Bhat SG, Chandrasekaran M. Production, purification and partial characterization of a novel protease from marine Engyodontium album BTMFS10 under solid state fermentation. Process Biochem. 2006;41:956–961. doi: 10.1016/j.procbio.2005.10.017. [DOI] [Google Scholar]
- 57.Mrudula S, Murugammal R. Production of cellulose by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz J Microbiol. 2011;42:1119–1127. doi: 10.1590/S1517-83822011000300033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phadatare SU, Despande MV, Srinivasan MC. High activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20): enzyme production and compatibility with commercial detergents. Enzyme Microb Technol. 1993;15:72–76. doi: 10.1016/0141-0229(93)90119-M. [DOI] [Google Scholar]
- 59.Shabbiri K, Adnan A. Bio-statically optimized production of lipases by Brevibacterium linens DSM 20158. World Appl Sci J. 2011;13:1059–1066. [Google Scholar]
- 60.Levin L, Herrmann C, Papinutti VL. Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochem Eng J. 2008;39:207–214. doi: 10.1016/j.bej.2007.09.004. [DOI] [Google Scholar]
- 61.Liu CQ, Chen QH, Tang B, Ruan H, He GQ. Response surface methodology for optimizing the fermentation medium of alpha-galactosidase in solid-state fermentation. Lett Appl Microbiol. 2007;45:206–212. doi: 10.1111/j.1472-765X.2007.02173.x. [DOI] [PubMed] [Google Scholar]
- 62.Contesini FJ, da Silva VCF, Maciel RF, de Lima RJ, Barros FFC, de Oliveira Carvalho P. Response surface analysis for the production of an enantioselective lipase from Aspergillus niger by solid-state fermentation. J Microbiol. 2009;47:563–571. doi: 10.1007/s12275-008-0279-8. [DOI] [PubMed] [Google Scholar]
- 63.Xiao A, Huang Y, Ni H, Cai H, Yang Q. Statistical optimization for tannase production by Aspergillus tubingensis in solid-state fermentation using tea stalks. Electron J Biotechnol. 2015;18:143–147. doi: 10.1016/j.ejbt.2015.02.001. [DOI] [Google Scholar]
- 64.Mohana S, Shrivastava S, Divecha J, Madamwar D. Optimization of medium for decolorization of textile dye direct black 22 by a novel bacterial consortium. Bioresour Technol. 2008;99(3):562–569. doi: 10.1016/j.biortech.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 65.Aruwajoye GS, Ehigie LO, Agboola FK. Characterization of a cellulolytic enzyme from wood degrading bacteria. Bacillus circulans. Biokemistri. 2014;26(2):43–49. [Google Scholar]
- 66.Kim WJ, Kim SM. Purification and characterization of Bacillus subtilis JM-3 protease from anchovy sauce. J Food Biochem. 2005;29(5):591–610. doi: 10.1111/j.1745-4514.2005.00041.x. [DOI] [Google Scholar]
- 67.Ko JH, Yan JP, Zhu L, Qi YP. Identification of two novel fibrinolytic enzymes from Bacillus subtilis QK02. Comp Biochem Physiol C: Toxicol Pharmacol. 2004;137:65–74. doi: 10.1016/j.cca.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr Microbiol. 2008;57:503–507. doi: 10.1007/s00284-008-9276-8. [DOI] [PubMed] [Google Scholar]
- 69.Rejiniemon TS, Hussain RR, Rajamani B. In-vitro functional properties of Lactobacillus plantarum isolated from fermented ragi malt. South Ind J Biol Sci. 2015;1(1):15–23. [Google Scholar]
- 70.Wood TM, Bhat KM. Methods of measuring cellulase activities. Methods Ezymol. 1988;160:87–117. doi: 10.1016/0076-6879(88)60109-1. [DOI] [Google Scholar]
- 71.Kim DC, Oh NS, In MJ. Effect of carbon and nitrogen sources on cell growth and halotolerant alkaline protease production in Halomonas marisflava isolated from salt-fermented food. Food Sci Biotechnol. 2004;13:837–840. [Google Scholar]
- 72.Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 73.Jayashree S, Lalitha R, Vadivukkarasi P, Kato Y, Seshadri S. Cellulase production by pink pigmented facultative methylotrophic strains (PPFMs) Appl Biochem Biotechnol. 2011;164:666–680. doi: 10.1007/s12010-011-9166-6. [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Carreno FL, Dimes LE, Haard NF. Substrate gel electrophoresis for composition and molecular weight of proteinases and proteinaceous proteinase inhibitors. Anal Biochem. 1993;214:65–69. doi: 10.1006/abio.1993.1457. [DOI] [PubMed] [Google Scholar]


